Abstract
BACKGROUND
Measurement of arterial stiffness should be more available. Our aim was to show that aortic pulse wave velocity can be reliably measured with a bathroom scale combining the principles of ballistocardiography (BCG) and impedance plethysmography on a single foot.
METHOD
The calibration of the bathroom scale was conducted on a group of 106 individuals. The aortic pulse wave velocity was measured with the SphygmoCor in the supine position. Three consecutive measurements were then performed on the Withings scale in the standing position. This aorta-leg pulse transit time (alPTT) was then converted into a velocity with the additional input of the height of the person. Agreement between the SphygmoCor and the bathroom scale so calibrated is assessed on a separate group of 86 individuals, following the same protocol.
RESULTS
The bias is 0.25 m·s−1 and the SE 1.39 m·s−1. This agreement with Sphygmocor is “acceptable” according to the ARTERY classification. The alPTT correlated well with cfPTT with (Spearman) R = 0.73 in pooled population (cal 0.79, val 0.66). The aorta-leg pulse wave velocity correlated with carotid-femoral pulse wave velocity with R = 0.76 (cal 0.80, val 0.70).
CONCLUSION
Estimation of the aortic pulse wave velocity is feasible with a bathroom scale. Further investigations are needed to improve the repeatability of measurements and to test their accuracy in different populations and conditions.
Keywords: arterial stiffness, blood pressure, carotid-femoral pulse wave velocity, connected health, home monitoring, hypertension
There is a large body of evidence that central arteries stiffness is a marker of CV risk.1,2 Its predictive value of CV events and coronary heart events above and beyond traditional risk factors was demonstrated on specific groups3–6 and the general population.7–9 Accordingly, the monitoring of arterial stiffness has been included in the 2007 and 2013 ESH/ESC guidelines for the management of hypertension10 and the position paper from the ESC.11
Carotid-femoral pulse wave velocity (cfPWV) by applanation tonometry is considered as the reference noninvasive technique to measure central, mainly aortic, stiffness.1 It is simple and reliable enough to have been included in routine clinical examination, but it suffers from a number of limitations that prevent its widespread use. First, it must be handled by a trained physician. Indeed, 2 waveforms are recorded at the carotid and femoral arteries, simultaneously or gated by an ECG. The captation of the pulse waves, especially at the femoral site, may be delicate, in particular in patients with diabetes or obesity or in certain cultures. In addition, the measurement is also likely to be biased by a “white coat effect” and a added stress caused by the palpation of the femoral pulse. The recent years have therefore witnessed the emergence of a number of increasingly operator-independent apparatus to measure cfPWV more easily,12–18 but they are all aimed at a clinical use and some of them are disputable in term of validity. At the present stage, no device is adapted for nonmedical, self measurement of arterial stiffness.
We developed a novel device, a bathroom scale performing a direct measurement of aortic pulse wave velocity. In the following, we present the principles of the technique, the results of a validation study against the SphygmoCor, and describe the association of the pulse transit time with physiological variables known to be associated.
METHODS
Principle of the measurement on Withings scale
The bathroom scale uses the principle of impedance plethysmography (IPG) in a single foot combined with ballistocardiography (BCG). The ballistocardiogram corresponds to the recording of the small variations of body weight caused by the pulsatile displacement of blood generated by the left ventricle, see the study by Pinheiro et al.19 for a comprehensive review. The principle of BCG is known since 1937 and was primarily developed for monitoring LV contraction. The typical tracing of BCG comprises 2 extremal peaks H and I which correspond to the opening of the aortic valve, thus the ejection phase of systole. With simultaneous recordings of a ballistocardiogram and a phonocardiogram, the BCG has been shown20 to be a reliable marker of the opening of the aortic valve and consecutive ejection.
Local impedance-plethysmogram (IPG) detects the variations of the volume of blood at the measurement site. Tissues form a conducting electrolyte whose conductivity is roughly constant. Since conductivity of blood is higher than solid tissues, the flow wave induces changes in impedance proportional to blood flow. Flow is induced by instantaneous gradients in pressure, and 2 waves are perfectly in phase at least during the early phases of systole when the reservoir effect and wave reflections are not occurring. Practically, a very low intensity, high-voltage current of known amplitude is applied in between the toes and the heel through thin electrodes on the surface of the glass plate of the scale, and the corresponding voltage is measured. The impedance is calculated from the the amplitude of voltage though Ohm’s law. On time series, the small pulsatile component corresponds to the impedance-plethysmogram caused by blood flow changes, see Figure 1.
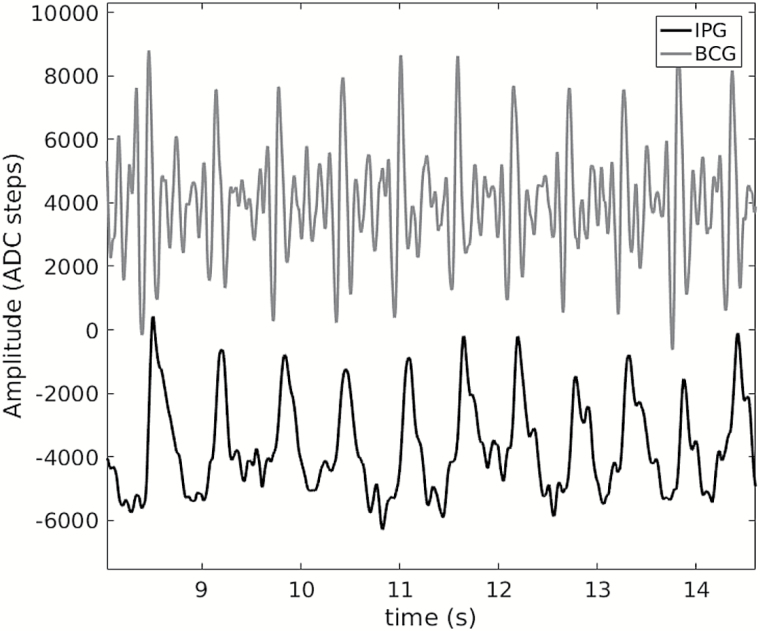
Figure 1.
Example of a BCG (top) and a IPG (bottom) acquired synchronously on a bathroom scale after smoothing. ADC steps correspond to arbitrary voltage units of the analogue to digital converter (ADC). Abbreviations: BCG, ballistocardiography; IPG, impedance plethysmography.
In brief, the BCG provides a hallmark of the onset of systole, while the foot of the rising slope of the IPG is a hallmark of the arrival time of the pulse in the foot. Hence, with the bathroom scale, we are able to measure the time interval aorta-leg pulse transit time (alPTT) between the ejection phase of systole from BCG and flow arrival in the foot.
Study protocol
Blood pressure was measured with an Omron 705C oscillometric device, in supine position for cfPWV measurement, then in orthostatic position. The cfPWV was measured by a physician trained with the SphygmoCor, (HK). Participants rested in supine position for 5 minutes before the measurement. Following,1 the travel distance was measured with a nonstretchable ruler between the measurement sites at the carotid and femoral arteries, and corrected by the multiplicative factor 0.8. Measurements were performed on the right side. The average of 2 consecutive measurements was taken. When these measurements differed by more than 0.5 m·s−1, a third measurement was made and cfPWV was taken equal to the central value.
Then, participants were asked to stand up. They remained in upright position for 5 minutes before measurement of the PWV on the bathroom scale. To ensure they reached a stable hemodynamic state within this lapse of time, they were allowed to move slightly while their upright brachial blood pressure was measured. Finally, 3 consecutive measurements of PWV on the bathroom scale were performed, each lasting 20 seconds. They were proposed to lay their hands on the wall facing them to ensure a better balance. The participants stepped down from the scale for a few seconds between each acquisition to avoid becoming fatigued.
Analysis of BCG and IPG signals
An example of BCG and IPG signals is shown on Figure 1.
The analysis of IPG signals was similar to the analysis of tonometric signals by the SphygmoCor. In particular, the foot of the rising slope of the IPG is calculated by the method of intersecting tangents.21 The BCG presents several characteristic points (known as F, G, etc. see e.g., study by Scarborough Talbot22). The apexes H, I, and J are stable markers of the onset of systole. For each of the 20-second long measurement, the time difference between a BCG marker and the foot of the IPG is calculated at each heart beat. An aorta-leg Pulse Transit Time alPTT is then defined from a combination of the various calculated time intervals. For each person, the result of 3 measurements is then averaged. A machine-learning algorithm (random forest) was developed to process automatically the signals. It takes as input the raw signals of IPG and BCG and outputs the labels at each beat successfully identified (F, G, etc. on the BCG, foot, and max of the IPG).
Population
Volunteers were recruited either through a specialized company (Stephenson études, http://stephenson-etudes.fr/), or through the outpatient clinic of HEGP for whom arterial measurements were done as routine test, including weighting with the Withings scale. Inclusion criteria were to volunteer for the study and having signed the informed consent. The procedure (weighting, blood pressure, and cfPWV) responding to standard care or entering the definition of minimal constrains and risk procedures, the Ethic Committee was consulted but did not have to give a formal answer. Subjects could be either normotensive or treated hypertensives of both sexes. Subjects were excluded if they present patent cardiovascular diseases, any chronic illness including psychiatric disorders. Patients were also excluded if pregnant or having morbid obesity (BMI > 35kg · m−2).
Definition of the Data Sets.
Three independent sets were used in this study.
The training data set was used to train the machine-learning algorithm (see section Analysis of BCG and IPG signals). The signals of the training set were labeled manually after preprocessing (mostly filtering) on a computer. The training set was composed of 191 recordings obtained in a home setting on 90 different beta-testers from the Withings company (mean age 30 years, with 50% women and no case of hypertension).
The calibration data set was used to calibrate the bathroom scale, i.e., to adjust a model of aorta-leg pulse wave velocity (alPWV) as a function alPTT and distance estimation from height. A total of 106 participants were recruited for calibration, including 51 women (48%). It has an equal number of participants between 3 age groups ≤30, 31–60, and ≥61. Thirty-five participants had essential hypertension, including 20 with arterial hypertension of grade I, 13 of grade II, and 2 of grade III. Three of them had no treatment. The population also included 12 participants with high cholesterol and 28 smokers.
The validation data set was used for the agreement study with the SphygmoCor. The validation set comprises 99 patients, including 50 women. Forty-five participants had essential hypertension. The age distribution differs from that of the calibration sample: 13% are ≤30, 49% are between 31 and 60, and 38% are ≥61 years old.
The signals of the calibration and validation sets were labeled automatically by the random forest algorithm. The calibration and validation groups differ significantly by the proportion of hypertensive (resp. 31% and 45%), age (P = 0.001), heart rate in supine position (P = 0.006), and diastolic pressure in standing position (P = 0.03). The characteristics of the pooled calibration and validation sets are given in Table 1.
Table 1.
Descriptive statistics of the sample used for calibration and validation (n = 205)
| Variable | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 48.7 | 16.9 | 18–84 |
| Height (cm) | 171 | 9.4 | 152–196 |
| CF distance (cm) | 60.9 | 4.5 | 51–75 |
| Weight (kg) | 73.5 | 14.2 | 46–118 |
| BMI (kg m−2) | 25.1 | 3.6 | 18.4–34.8 |
| Supine SBP (mm Hg) | 125.8 | 18.6 | 88–189 |
| Supine DBP (mm Hg) | 75.3 | 10.2 | 55–104 |
| Supine heart rate (bpm) | 70.6 | 12.3 | 42–116 |
| Standing SBP (mm Hg) | 123.6 | 19.9 | 86–225 |
| Standing DBP (mm Hg) | 83.1 | 12.0 | 60–127 |
| Standing heart rate (bpm) | 78.5 | 12.0 | 48–110 |
| cfPWV (m·s−1) | 7.8 | 2.1 | 4.7–19.2 |
| cfPTT (ms) | 65.5 | 14.6 | 28.3–100.8 |
Abbreviations: BMI, body mass index; cfPWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Statistical analysis
The statistical analysis was conducted with the open source software Octave. Throughout, type-I error rate of hypothesis tests is fixed at 0.05. Normality was assessed with the Shapiro–Wilk test, normal probability plots, and tests on third and fourth sample moments.23 Homoscedasticity was checked by inspection of the scatter plot of the residuals. We used a t-test assuming equal variance to test equality of mean values, F-test for the equality of variances. Reliability of the recordings was assessed with the SD of the alPTT and cfPTT calculated at each beat.
Repeatability of the measurements was assessed with the difference between the largest and smallest values of alPTT and cfPTT between the 2 and 3 consecutive measurements.
The agreement with the SphygmoCor as measured by a Bland–Altman analysis was performed on the separate validation set.
Robust techniques were prefered for the analysis of the association of alPTT with other variables. Bivariate correlation was expressed with the Spearman rank-order correlation coefficients, and robust confidence intervals were calculated with Fisher’s z transform using conservative Fieller’s et al. estimate of the SE.24 Robust regressions using Andrew’s sine were made to analyze the relation between the pulse transit times alPTT of the bathroom scale and the other variables.
RESULTS
Data quality
Rate of failed measurements.
Signals from the calibration and validation sets are labeled automatically by the machine-learning algorithm. It outputs a value of alPTT only if there are at least 24 beats over 60 seconds of recordings. All the 318 (=3 × 106) acquisitions could be processes, but the algorithm discarded 13 out of the 99 participants in the validation set.
Reliability of the Measurements.
SphygmoCor built in quality control requires a SD of cfPTT <10% of the mean value cfPTT.
Calibration set.
This occurred in 6.7% of the 224 measurements with the SphygmoCor, all but 4 corresponding to cfPWV larger than 10 m·s−1. The average value of cfPTTSD was 6.3% of the mean value, or 4 ms. By comparison, the variability of the corresponding transit time in the scale alPTT was larger. The average value of alPTTSD was 23% of the mean value, or 15 ms, ranging from 5 to 31 ms over the calibration data set.
Validation set:
Results on the validation data set are similar. On the Sphygmocor, cfPTTSD exceeded 10% in 2.2% of the 182 recordings, all but one corresponding to velocities larger than 10 m·s−1. The average value of cfPTTSD was 5.5% of the mean value, or 3.5 ms. The average value of alPTTSD was 23% of the mean value, or 15 ms, ranging from 5 to 30 ms.
Repeatability of the method.
To assess the repeatability of the measurements, we calculated for each participant the difference between the largest and smallest values of the consecutive alPTT. We have a mean difference of 6 ms over the calibration set, and 8 ms over the validation set. For comparison on the SphygmoCor, the mean difference was about 3 ms on both sets.
Agreement with the SphygmoCor
Pulse wave velocity.
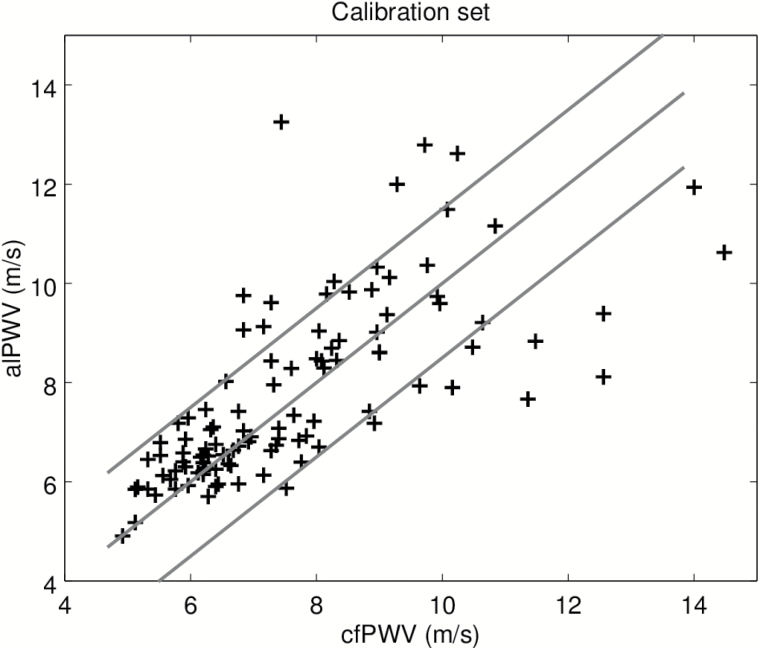
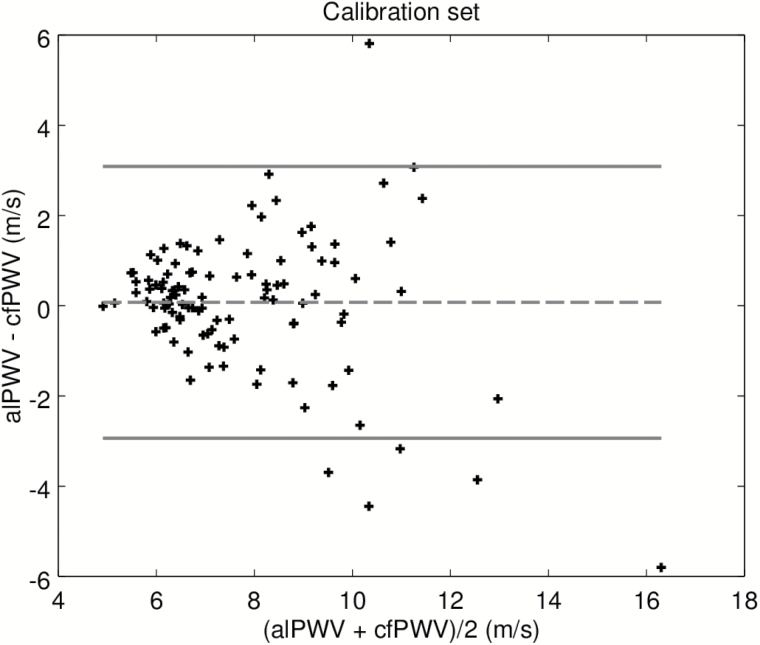
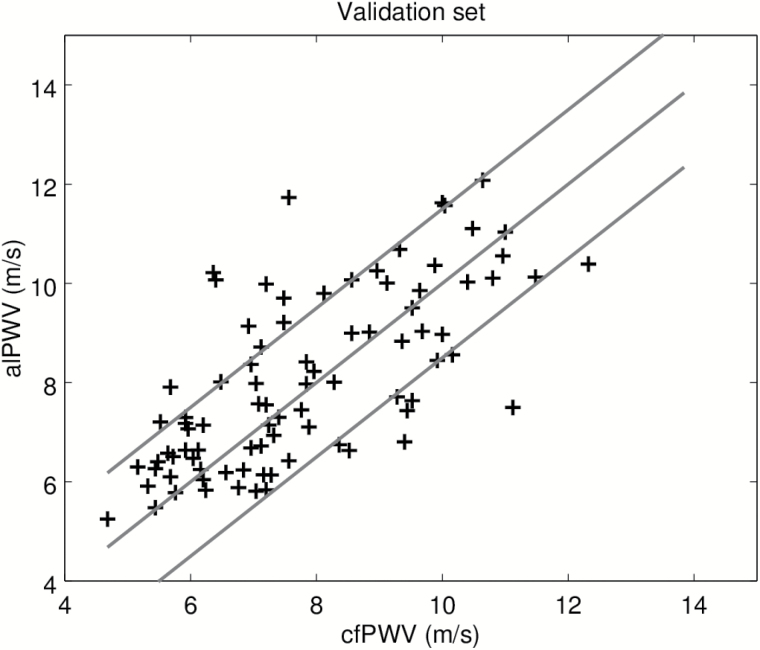
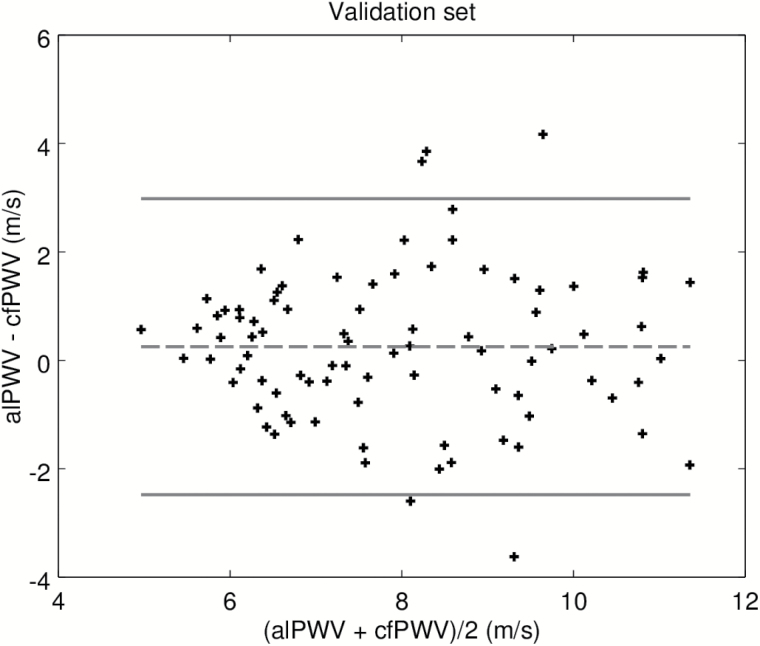
In order to test separately, the machine-learning algorithm used to label the BCG and IPG signals, we first present results on the calibration set. Recall that this data set was used to adjust the model converting alPTT into alPWV, where alPTT is the output of the machine-learning algorithm. Figure 2 is the scatter plot of the predicted alPWV of the 106 participants against the corresponding cfPWV measured with the SphygmoCor. The Spearman correlation coefficient is 0.80. Figure 3 shows the agreement between alPWV and cfPWV measured with the SphygmoCor. The bias is 0.07 m·s−1 ([−0.22, 0.39]) and 95% the limits of agreement are ([−2.93, 3.09]). The SD of the error is 1.54 m·s−1. Data from the validation set were processed by the final algorithm combining the signal processing from the machine-learning algorithm and the model calibrated on the calibration set. Figure 4 is the scatter plot of the 86 predicted alPWV against the corresponding cfPWV. The Spearman correlation coefficient is 0.70. Figure 5 shows the agreement between alPWV and cfPWV measured with the Sphygmocor. The bias is 0.25 m·s−1 ([−0.04, 0.55]) and 95% the limits of agreement are ([−2.48, 2.98]). The SD of the error is 1.39 m·s−1.
Figure 2.
Scatter plot of alPWV with cfPWV in the calibration set (n = 106). The blue line is the identity. The red lines are 1.5 m·s−1 away from the identity. Abbreviations: cfPWV, carotid-femoral pulse wave velocity.
Figure 3.
Bland–Altman plot of alPWV and cfPWV on the calibration set. Abbreviations: cfPWV, carotid-femoral pulse wave velocity.
Figure 4.
Scatter plot of alPWV with cfPWV in the validation set (n = 86). The blue line is the identity. The red lines are 1.5 m·s−1 away from the identity. Abbreviations: cfPWV, carotid-femoral pulse wave velocity.
Figure 5.
Bland–Altman plot of alPWV and cfPWV on the validation set. Abbreviations: cfPWV, carotid-femoral pulse wave velocity.
In summary, these results show the good performance of the machine-learning algorithm and an agreement between the estimates alPWV and cfPWV of the aortic PWV which is acceptable according to ARTERY guidelines.25
Association between alPTT and cfPTT.
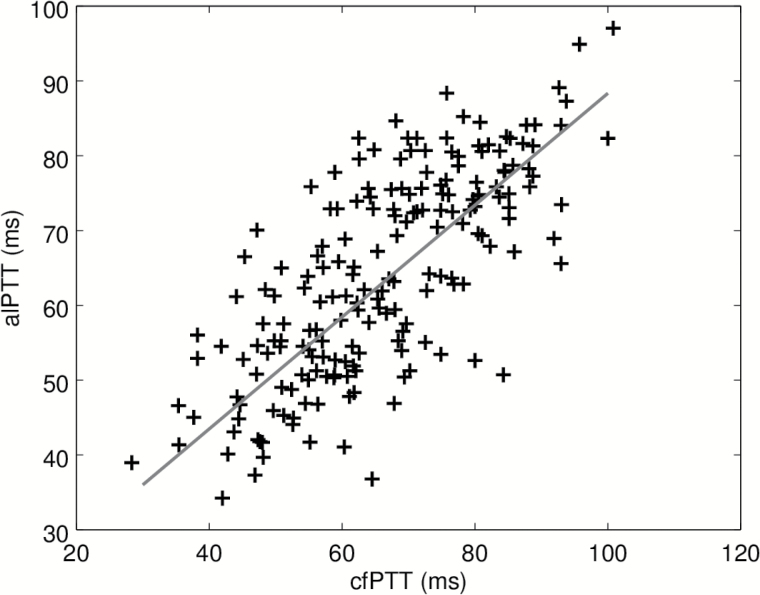
Calibration and validation data sets are pooled for this statistical analyses. Spearman correlation coefficient (95% confidence interval) is 0.72 ([0.64, 0.79]). In univariate robust regression, cfPTT explained 52% of the variance of alPTT. The scatter plot and regression line are shown on Figure 6. The value of the slope (95% confidence interval) is 0.75 ([0.65, 0.85]). The SD of the error on the estimated pulse transit time is 11 ms.
Figure 6.
Scatter plot of alPTT measured by Body Cardio scale vs cfPTT measured by the SphygmoCor. The red line is the robust univariate regression. Abbreviations: alPTT, aorta-leg pulse transit time; cfPTT, carotid-femoral pulse transit time.
Associations of PTT with age, height, heart rate, and blood pressure
The β-coefficients of the robust regressions are given in Table 2 for standardized variables so that they can be directly compared with one another.
Table 2.
Coefficients (95% CI) of the robust multivariate regressions
| Variable | Estimate, 95% CI | P | R 2 |
|---|---|---|---|
| Dependent var. alPTT—model 1 | |||
| Age | −0.58 [0.71, 0.0.46] | 10−16 | 0.58 |
| Height | 0.20 [0.009, 0.30] | 2·10−4 | |
| Dependent var. alPTT—model 1ʹ | |||
| Age | −0.43 [0.56, 0.31] | 2·10−10 | |
| cfPTT | 0.40 [0.26, 0.53] | 4·10−8 | 0.65 |
| Height | 0.10 [0.00, 0.20] | 0.046 | |
| Dependent var. cfPTT—model 2 | |||
| Age | −0.39 [0.52, 0.26] | 4·10−8 | |
| Height | 0.26 [0.15, 0.37] | 10−6 | 0.57 |
| Standing SBP | −0.33 [0.54, 0.11] | 4·10−3 | |
| Dependent var. cfPTT—model 2ʹ | |||
| alPTT | 0.41 [0.27, 0.55] | 7·10−8 | |
| Height | 0.18 [0.08, 0.28] | 5·10−4 | 0.63 |
| Standing SBP | −0.29 [0.09, 0.49] | 5·10−3 | |
| Age | −0.15 [0.01, 0.30] | 0.04 | |
The variables are standardized. Only statistically significant variables are given. Abbreviations: alPTT, aorta-leg pulse transit time; cfPTT, carotid-femoral pulse transit time; cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval; SBP, systolic blood pressure.
alPTT.
First, in a robust multivariate regression excluding cfPTT (model 1), only age was statistically significantly associated alPTT. This model explains 58% of the variance. The association with age alone is strong since it explains 52% of the variance (coefficient −0.73) in a univariate model.
Second, including cfPTT in the list of independent variables (model 1′), a robust multivariate regression selected age, cfPTT, marginally the height, thus explaining 65% of the variance
cfPTT.
A robust multivariate regression showed an association with age, SBP in standing position, and height, explaining 57% of the variance (model 2). Among all blood pressure components, the association was strongest with SBP in standing position. Adjusting also for alPTT (model 2′), the association was significant with alPTT, height, SBP in standing position, but only marginally with age.
This analysis shows 2 interesting results. First, after adjustment of the other variables, the association between alPTT and age is stronger than between cfPTT and age: the standardized β-coefficients in models 1′ and 2′ are −0.43 for alPTT and −0.15 for cfPTT. It is noteworthy that the 95% confidence intervals do not overlap. Second, the association with blood pressure, in particular SBP in standing position, is much stronger with cfPTT than alPTT (for which no statistically significant association was found) after adjustment of the other variables. Finally, we also note that the association of alPTT with height is slightly weaker than between cfPTT and height.
Associations of PWV with age, height, heart rate, and blood pressure
alPWV.
In a model excluding cfPWV (model 3), age was the only variable associated with alPWV in a robust multivariate regression (Table 3). This model explains 57% of the variance. Age alone explains 47% of the variance (β-coefficient 0.66) in a robust univariate regression. Including cfPWV (model 3ʹ), we have a significant association with cfPWV, age, and borderline for height (P = 0.07). This model explained 64% of the variance of alPWV.
Table 3.
Coefficients (95% CI) of the robust multivariate regressions
| Variable | estimate, 95% CI | P | R2 |
|---|---|---|---|
| Dependent var. alPWV—model 3 | |||
| Age | 0.55 [0.43, 0.68] | 3·10−16 | 0.57 |
| Dependent var. alPWV—model 3ʹ | |||
| cfPWV | 0.40 [0.27, 0.53] | 6·10−9 | 0.64 |
| Age | 0.38 [0.26, 0.50] | 4·10−9 | |
| Dependent var. cfPWV—model 4 | |||
| Age | 0.38 [0.26, 0.50] | 4·10−9 | 0.55 |
The variables are standardized. Only statistically significant variables are given. Abbreviations: cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval.
cfPWV.
Only age was statistically significantly associated with cfPWV in a robust multivariate regression (model 4). This model explained 55% of the variance, and a robust regression on age alone explained 47% of the variance (β-coefficient 0.55).
DISCUSSION
In the present study, the combined use of IPG in a single foot and BCG, with additional input of the height of the person, estimated the carotid-femoral PWV with acceptable accuracy and precision.
We stress the nontrivial character of this result. Indeed, there are several important differences between the standard technique of applanation tonometry and our setup on a bathroom scale. A first difference of importance is the position of the subject: supine for tonometry, upright on the bathroom scale.
A second major difference is the path of the pulse wave. With the applanation tonometry, the pulse wave follows a complex path where the main segments are the carotid (retrograde), the descending aorta and the iliac artery and femoral arteries (anterograde). With the bathroom scale, the path of the pulse wave is unidirectional, but includes distal arteries of the lower limbs. It starts at the heart and includes the ascending aorta, the aortic arch, the descending aorta, the iliac artery, and the arteries of the lower limbs down to the foot (femoral, tibial and peronal, and plantar arch). Despite that more than half of the path length comprises these distal arteries, the fact that they are stiffer than the aorta and exhibit a relatively slow aging26,27 is presumed to make them fairly neutral, but still has to be proven in different conditions. By contrast, the inclusion of the most proximal part of the aorta is an advantage of the bathroom scale compared to the reference technique.
Despite these differences, a good correlation was found between the pulse transit times measured by the two devices. The variability between measurements on the scale is twice as large as with the SphygmoCor, but limitation can be compensated by the fact that this device is destined to repeated measures in individuals. Indeed, given the ease and rapidity of the measurement on a bathroom scale, more than 3 measurements can be made and averaged to yield a meaningful result.
The estimation of the carotid-femoral distance from the height of the person is a limitation of both the study and the technique.28 Although the measurement of the carotid to femoral distance is also subject to many criticisms, an estimation of this distance from body height has been used in the past.29 One critic might be the validity of self-reported body height. Indeed, we noticed in the course of the validation protocol that people are often unaware of their exact height, sometimes by more than 10 cm. We should have included a measure of the height of each participant in our protocol. At least, this corresponds to the future usage by the public of the scale. Measuring height was associated with minimal changes in precision in the subgroup of patients where we actually measured height (data not shown). Some discrepancies between cfPWV and alPWV might correspond to true changes in PWV between supine and standing position. A few studies investigated the impact of standing on PWV and found a BP related increase. Here, this explanation is unlikely because the difference between cfPWV and alPWV was not related to BP changes between supine and standing (data not shown).
The technique is also potentially limited by the gait instability which affects more frail elderly and certain neurological diseases. Other diseases might affect the pertinence of the measurement, such as atrial fibrillation or premature heart beats, lower limb atherosclerosis, skin diseases, severe heart failure etc. One has to remind that this device is primarily made for self-assessment of risk in relatively healthy subjects, and not for clinical use. Visual inspection of Bland and Altman plots shows a high degree of heteroscedasticity. This can be considered as a measure of imprecision and is a clear limitation of the method.
We found a closer association of age on alPWV than on cfPWV, suggesting that cfPWV is more strongly influenced than alPWV by known variables other than age. This is to our knowledge the first time that an arterial parameter has stronger association with age than cfPWV, suggesting that alPWV contains information additional to cfPWV. This is an exciting prospect worthy of further investigations.
One important point is the communication made around this value of pulse wave velocity. In order to be interpreted, age has to be taken into account. For the moment, afPWV is returned to the user as the mean of 5 measurements, and in 3 categories (optimal, normal, and elevated) according to percentiles of the reference value population.30 The application is providing advices based on physical activity, diet, and stress control. Whether this translates into a reduction of risk factors and a better prevention of cardiovascular diseases remains to be studied.31
In conclusion, we showed that IPG in a single foot combined to BCG to measure the PWV on a bathroom scale is possible and may be promising for widespread use in the home setting. However, further investigations are needed to improve the repeatability of measurements, and it is necessary to test their accuracy in different populations and conditions.
DISCLOSURE
David Campo, Roger Yu, Nicolas Genain, Paul Edouard, and Nadine Buard are employees of Withings, which develops the bathroom scale. Hakim Khettab received payment for his work. Pierre Boutouyrie received honorarium for scientific counseling.
REFERENCES
- 1. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos Ch, Wilkinson I, Struijker-Boudier H; on behalf of the European Network for Non-invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27: 25882605. [DOI] [PubMed] [Google Scholar]
- 2. Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:12361241. [DOI] [PubMed] [Google Scholar]
- 4. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39:10–15. [DOI] [PubMed] [Google Scholar]
- 5. Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003; 34:1203–1206. [DOI] [PubMed] [Google Scholar]
- 6. Meaune S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects > 70 years of age. Arterioscler Thromb Vasc Biol 2001; 21: 2046–2050. [DOI] [PubMed] [Google Scholar]
- 7. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005; 111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 8. Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial stiffness and risk of coronary heart disease and stroke the Rotterdam Study. Circulation 2006; 113:657–663. [DOI] [PubMed] [Google Scholar]
- 9. Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006; 113:664–670. [DOI] [PubMed] [Google Scholar]
- 10. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members The task force for the management of arterial hypertension of the European Society of H, The task force for the management of arterial hypertension of the European Society of C. 2013 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 54:21592219. [DOI] [PubMed] [Google Scholar]
- 11. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt-Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O’Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241:507–532. [DOI] [PubMed] [Google Scholar]
- 12. Horv′ath IG, N′emeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Czir′aki A. Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens 2010; 28:2068–2075. [DOI] [PubMed] [Google Scholar]
- 13. Baulmann J, Schillings U, Rickert S, Uen S, Düsing R, Illyes M, Cziraki A, Nickering G, Mengden T. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens 2008; 26:523–528. [DOI] [PubMed] [Google Scholar]
- 14. Hickson SS, Butlin M, Broad J, Avolio AP, Wilkinson IB, McEniery CM. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res 2009; 32:1079–1085. [DOI] [PubMed] [Google Scholar]
- 15. Salvi P, Lio G, Labat C, Ricci E, Pannier B, Benetos A. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens 2004; 22:2285–2293. [DOI] [PubMed] [Google Scholar]
- 16. Stea F, Bozec E, Millasseau S, Khettab H, Boutouyrie P, Laurent S. Comparison of the Complior Analyse device with Sphygmocor and Complior SP for pulse wave velocity and central pressure assessment. J Hypertens 2014; 32:873–880. [DOI] [PubMed] [Google Scholar]
- 17. Salvi P, Magnani E, Valbusa F, Agnoletti D, Alecu C, Joly L, Benetos A. Comparative study of methodologies for pulse wave velocity estimation. J Hum Hypertens 2008; 22:669–677. [DOI] [PubMed] [Google Scholar]
- 18. Alivon M, Vo-Duc Phuong T, Vignon V, Bozec E, Khettab H, Hanon O, Briet M, Halimi JM, Hallab M, Plichart M, Mohammedi K, Marre M, Boutouyrie P, Laurent S. A novel device for measuring arterial stiffness using finger-toe pulse wave velocity: Validation study of the pOpmètre®. Arch Cardiovasc Dis 2015; 108:227–234. [DOI] [PubMed] [Google Scholar]
- 19. Pinheiro E, Postolache O, Girão P. Theory and developments in an unobtrusive cardiovascular system representation: ballistocardiography. Open Biomed Eng J 2010; 4:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horwitz O, Mayock RL, Starr I. Direct experiments on the relation between the form of the ballistocardiogram and the shape of the systolic velocity curve in the aorta of man. Fed Proc 1948; 7:57. [PubMed] [Google Scholar]
- 21. Millasseau SC, Stewart AD, Patel SJ, Redwood SR, Chowienczyk PJ. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension 2005; 45:222–226. [DOI] [PubMed] [Google Scholar]
- 22. Scarborough WR, Talbot SA. Proposals for ballistocardiographic nomenclature and conventions: revised and extended report of Committee on Ballistocardiographic Terminology. Circulation 1956; 14:435–450. [DOI] [PubMed] [Google Scholar]
- 23. D’Agostino RB, Belanger A, D’Agostino RB., Jr A suggestion for using powerful and informative tests of normality. The American Statistician 1990; 44:316–321. [Google Scholar]
- 24. Fieller EC, Hartley HO, Pearson ES. Tests for rank correlation coefficients: I. Biometrika 1957; 44:470–481. [Google Scholar]
- 25. Wilkinson IB, McEniery CM, Schillaci G, Boutouyrie P, Segers P, Donald A, Chowienczyk PJ. ARTERY Society guidelines for validation of non-invasive haemodynamic measurement devices: Part 1, arterial pulse wave velocity. Artery Res 2010; 4:3440. [Google Scholar]
- 26. Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 1985; 71:202–210. [DOI] [PubMed] [Google Scholar]
- 27. Lemogoum D, Ngatchou W, Janssen C, Leeman M, Van Bortel L, Boutouyrie P, Degaute JP, Van de Borne P. Effects of hunter-gatherer subsistence mode on arterial distensibility in Cameroonian pygmies. Hypertension 2012; 60:123–128. [DOI] [PubMed] [Google Scholar]
- 28. Aasvee K, Rasmussen M, Kelly C, Kurvinen E, Giacchi MV, Ahluwalia N. Validity of self-reported height and weight for estimating prevalence of overweight among Estonian adolescents: the Health Behaviour in School-aged Children study. BMC Res Notes 2015; 34:1587–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 30. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts), European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Heart Journal 2012; 33:16351701. [DOI] [PubMed] [Google Scholar]