Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency Medicine 2018. Other selected articles can be found online at https://www.biomedcentral.com/collections/annualupdate2018. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Background
Mechanical ventilation is needed to support respiratory function in different clinical conditions, from healthy to diseased lungs. However, in recent years, research has shown that mechanical ventilation may promote acute and chronic damage to pulmonary structures, the so-called ventilator-induced lung injury (VILI), especially in patients with acute respiratory distress syndrome (ARDS) [1]. ARDS is characterized by a loss of aerated lung tissue as a result of edema and atelectasis, which reduces respiratory system compliance and impairs gas exchange. Several mechanisms have been identified that may underlie VILI. Those considered most important are alveolar overdistension and the continuous opening and closing of atelectatic lung units during breath cycles [2]. As a consequence, clinical use of lower tidal volume (VT) to achieve reduced inspiratory stress and strain (‘gentle’ ventilation of the aerated lung), combined with higher levels of positive end-expiratory pressure (PEEP) to avoid repetitive collapse and reopening of alveolar units, have been suggested as a protective ventilatory strategy [3–5]. Furthermore, use of inspiratory pressures to open up atelectatic lung regions (so-called recruitment maneuvers) before setting PEEP has been proposed [6].
From a pathophysiological perspective, the “open lung approach” as originally recommended by Lachmann [7], now combined with protective VT, is considered the optimal ventilation strategy to minimize VILI in moderate to severe ARDS. However, clinical data regarding application of the open lung approach in patients with ARDS are conflicting. One meta-analysis showed that application of higher PEEP levels, as compared to low PEEP, was not associated with improved outcome (mortality rate before hospital discharge), did not affect barotrauma and only improved oxygenation within the first week of treatment in ARDS patients [8]. On the other hand, a more recent meta-analysis [9] showed that the open-lung strategy might reduce hospital mortality, 28-day mortality, and intensive care unit (ICU) mortality among patients with ARDS. However, the control group included studies with a combination of high VT and low PEEP, hindering any direct comparison of the potential beneficial effects of low VT and/or higher PEEP.
In the present chapter, we will discuss and address: 1) the ARDS lung and its characteristics; 2) controversial issues related to the open lung approach; and 3) the alternative concept of ‘permissive atelectasis’.
The ARDS lung
The ARDS lung is characterized by different degrees of damage to the alveolar-capillary membrane, depending on the pathogenetic pathway, which leads to increased lung edema [10]. The model that most comprehensively explains the pathophysiologic consequences of ARDS on the lungs is the so-called “sponge model”. The amount of edema depends on the severity of the injury, i.e., the worse the injury, the greater the alveolar edema, and vice versa [11]. Interstitial and alveolar edema promote collapse of the most dependent alveoli, as a result of increased superimposed pressure according to the density of fluids (plasma and blood) and the height of the lung. In general, the ARDS lung may be described as a ‘sponge’ filled with water, with best aeration of the non-dependent lung regions (the so-called “baby lung”), the dependent lung regions characterized by atelectatic areas, and consolidated lung regions widely distributed along the pulmonary structure or mainly localized in dependent areas [12]. Interestingly, even the aerated, non-dependent lung regions exhibit increased density, which suggests generalized, equally distributed injury of the alveolar-capillary barrier [13]. Additionally, small airway closure occurs mainly in the middle lung regions [14]. The reduction in aeration yields decreased lung compliance, while the presence of atelectatic and consolidated alveoli as well as peripheral airway closure might promote more or less severe alterations in gas exchange. These alterations are the result of a lower ventilation–perfusion ratio and the presence of true pulmonary shunt, according to the redistribution of regional perfusion. In fact, data suggest that regional perfusion is altered in ARDS as a result of intravascular clotting, collapse of capillaries and peripheral vessels, as well as edema of the endothelium and extracellular matrix [15].
Hubmayr [16] proposed an alternative mechanism for reduction in lung volume in ARDS, namely the presence of liquid and foam in conducting airways. In this model, increased edema occurs in the alveoli because of alterations in the alveolar-capillary barrier, rather than a predominance of alveolar collapse. This model completely changes and challenges the hypothesis that higher pressures in the lungs may be able to effectively re-open collapsed alveoli. In fact, if the model holds true, higher pressures may promote overstretching of partially aerated pulmonary zones.
The open lung approach: Controversial issues
The putative beneficial effects of higher PEEP and the open lung approach in ARDS are the following: 1) maintenance of full opening of the lungs; 2) reduction of interfaces between open and closed lung regions; 3) minimization of injurious effects caused by continuous opening and closing of collapsed alveolar units during tidal breath, which promotes lung injury and inflammation leading to systemic release of inflammatory biomarkers, distal organ failure and death.
Lung edema: Controversial effects of PEEP
Most experimental studies have shown an increase in lung edema after application of increasing levels of PEEP [17, 18]. However, other studies reported possible protective effects of PEEP against edema formation, which seem to be mediated by decreased cardiac output and/or reduced pulmonary blood volume, and not by an action of PEEP per se [19, 20].
Reduced injury in Atelectatic-collapsed areas
In contrast to widespread belief, several experimental studies have shown less lung injury in atelectatic areas. Tsuchida et al. [21] investigated histological damage in lavaged rats subjected to non-injurious (with VT 8 ml/kg and PEEP 14 cmH2O) and injurious (VT 25 ml/kg and PEEP 4–7 cmH2O) mechanical ventilation. During injurious mechanical ventilation, lung injury was higher in non-dependent than in dependent lung regions. This finding was explained by the fact that injurious ventilation, and more particularly higher VT, was distributed mainly in non-dependent lung regions with potential risk of overdistension, while dependent lung regions were relatively protected because of possible intra-alveolar edema. Interestingly, the greatest damage was observed in peripheral airways, which were likely subjected to higher stress during injurious mechanical ventilation. In line with these findings, Wakabayashi et al. [22] investigated three different settings of mechanical ventilation in isolated, perfused lungs: 1) low VT (7 ml/kg) with PEEP (5 cmH2O) and regular sustained inflation; 2) high-stretch strategy consisting of high VT (30–32 ml/kg) with PEEP (3 cmH2O) and sustained inflation; and 3) atelectasis strategy with low tidal volume but no PEEP or sustained inflation. They found that the high-stretch strategy, but not atelectrauma (atelectasis), activated monocytes within the pulmonary vasculature, leading to cytokine release into the systemic circulation. Finally, Chu et al. [23] ventilated ex vivo rat lungs with an opening and closing strategy without PEEP (VT 7 ml/kg), opening and closing strategy with PEEP (VT 7 ml/kg and PEEP 5 cmH2O), and resting atelectasis. While the inflammatory process was more pronounced in animals ventilated with no PEEP, no differences were found between the PEEP-treated vs. resting atelectasis groups. Furthermore, the authors found that, at high volumes, cyclic stretch increased inflammatory mediators in the lungs compared to continuous stretch at a pressure equivalent to the mean airway pressure, but had no additional effect compared with continuous stretch at a pressure equal to the peak inspiratory pressure. These experimental data indicate that the degree of alveolar overdistension is a more important contributor to the release of pro-inflammatory cytokines than the cyclic nature of the ventilatory pattern. The authors suggested that “closing the lungs and keeping them closed” might be protective against VILI. These results differed from previous studies that observed increases in chemokines in the presence of overstretch of the lung, most likely because they used two-hit models, in which the lungs were injured before overstretch [24, 25].
In conclusion, experimental evidence suggests that atelectatic-collapsed lung regions, when not subjected to repetitive opening and closing, are not characterized by more tissue inflammation.
Reduced inflammation in Atelectatic-collapsed areas
A recent study investigated the severity of inflammation in atelectatic and non-atelectatic lung regions by using the 18F–fluorodesoxyglucose (FDG) positron emission tomography (PET) technique [26]. FDG-PET has been proposed as a means of evaluating the activation of lung neutrophils, which are a key feature of pathophysiologic mechanisms and inflammatory cell activity in ARDS [27]. In fact, pulmonary FDG kinetics are altered during both experimental and clinical ARDS. Therefore, FDG-PET has been proposed as a potential non-invasive method to provide comprehensive understanding of the mechanisms of ARDS, and help for early diagnosis and for evaluation of different therapeutic interventions [28]. Other techniques used PET with a sialic acid-binding immunoglobulin-like lectin 9 (siglec-9)-based imaging agent targeting vascular adhesion protein-1 (VAP-1) for quantification of regional pulmonary inflammation [29]. In anesthetized sheep receiving intravenous endotoxin, FDG-PET was performed during protective ventilation with low VT (8 ml/kg) and PEEP 17 cmH2O and compared with injurious ventilation with high VT (18 ml/kg) and no PEEP. During protective ventilation, there was a reduction in the amount and heterogeneity of pulmonary-cell metabolic activity, namely, inflammation formed mainly in the dependent lung regions [30]. In ARDS patients, independent of the type of mechanical ventilation, Bellani et al. [31] reported that lung metabolic activity was not directly related to the amount of atelectasis, being higher in the boundary areas between aerated and non-aerated lung regions (perhaps those with presence of airway closure) in most patients.
In conclusion, there is compelling experimental evidence suggesting that:
excessive inspiratory pressure and volume translate into overdistension and lung damage, stimulating inflammatory and fibrogenic responses;
studies in favor of a protective ventilatory strategy including low VT and higher PEEP, thus minimizing lung collapse and repetitive closing and opening of alveolar units with less inflammatory response, were always compared with strategies consisting of high or extremely high VT and no or low levels of PEEP. Thus, the possible beneficial effects of lower VT could not be separated from those of higher PEEP (or both). In other words, our hypothesis is that the beneficial effects of the so-called “open lung” strategy in different experimental models were mainly due to a reduction in VT and not to PEEP;
most of the studies were performed in models with apparently high recruitability. However, even in this case, conflicting results were observed regarding the beneficial effects for minimizing VILI;
hemodynamic impairment and its possible role in reducing lung injury was not considered in the majority of these experimental studies.
Recent experimental evidence of ‘permissive atelectasis’ to minimize VILI
Low PEEP combined with low VT, plateau pressure and Transpulmonary pressure minimizes lung injury
Recently, experimental studies challenged the common belief that atelectasis might be detrimental to protective ventilation, provided lungs are kept at rest. This protective strategy includes the following elements: 1) a minimal PEEP level to assure adequate gas exchange (a certain level of ‘permissive hypoxemia’ to allow for an oxygen saturation not below 88%) associated with low VT or a VT able to ventilate only the aerated lungs, while minimizing any detrimental effect on the collapsed alveoli and peripheral airways; and 2) the respiratory rate should be set to keep pHa within physiologic ranges, or even to allow a certain degree of permissive hypercapnia. This strategy of so-called “permissive atelectasis” should combine protective effects on the lungs as well as mitigate possible hemodynamic impairment. In endotoxin-induced lung ARDS, Samary et al. [32] investigated the impact of different mechanical ventilation strategies combining different VT and PEEP, aiming to reach different driving transpulmonary pressures (∆P): 1) low ∆P (VT = 6 ml/kg, PEEP = 3 cmH2O); high ∆P (VT = 22 ml/kg, PEEP = 3 cmH2O) and mean ∆P with a VT to reach a mean between low and high ∆P (VT = 13 ml/kg, PEEP = 3 cmH2O). Other groups, with low VT and moderate (9.5 cmH2O) and high (11 cmH2O) PEEP, were also investigated. In these experimental settings, PEEP was adjusted to obtain an inspiratory plateau pressure of the respiratory system similar to that achieved with mean and high ∆P while using high VT. This was the first experimental study to evaluate the individual effects of VT, PEEP, plateau pressure (Pplat) and ∆P on lung inflammation, fibrogenic response, endothelial and epithelial cell injury, and activation of cell stress. Ventilation with low VT and low PEEP was associated with greater atelectasis, while increased VT and low PEEP reduced the amount of atelectasis and low VT and higher PEEP promoted a progressive increase in hyperinflation, to similar degrees as with high VT with low PEEP.
The first question is, therefore, whether it is more injurious to the lungs to adopt a ventilation strategy with more atelectasis but lower inspiratory pressures with low VT, designed to ventilate only the aerated lungs without promoting excessive opening and closing of alveolar units or stressing the peripheral airways? In agreement with our primary hypothesis, ventilation with low VT, low PEEP and low ∆P resulted in reduced expression of interleukin (IL)-6, receptor for advanced glycation end products (RAGE), and amphiregulin. Interestingly, mechanical ventilation with low VT and higher PEEP combined with higher ∆P and plateau pressure, a situation in which lungs were fully open, resulted in reduced expressions of IL-6 and RAGE, but was associated with increased amphiregulin expression and lung hyperinflation. Therefore, our data suggest that a ventilation strategy aimed to keep the lung fully open and then gently ventilated with low VT might effectively reduce lung inflammation, However, mechanical ventilation with low VT but a PEEP level not high enough to keep the lung fully open induced alveolar instability, thus resulting in increased expression of IL-6, RAGE and amphiregulin. Overall, IL-6 and amphiregulin expressions correlated better with plateau pressure and ∆P, highlighting the major influence of inspiratory stress as compared to other pressures in determining VILI. In a secondary analysis of these data, we investigated the impact of energy and power on VILI. IL-6 and amphiregulin expressions correlated better with power compared to energy of mechanical ventilation [33]. In conclusion, in experimental pulmonary ARDS, both mechanical ventilation strategies – 1) low VT and PEEP, yielding low transpulmonary ∆P, plateau pressure, energy, and power, and 2) low VT combined with a PEEP level sufficient to keep the lungs fully open – mitigated VILI. It is noteworthy that non-optimal PEEP might have negative effects on lung injury (Fig. 1).
Fig. 1.

Lung aeration at expiration (left) and inspiration (right) using different ventilation strategies. Very light blue: normally aerated regions; light blue: poorly aerated regions; middle blue: collapsed regions; dark blue: hyper-aerated regions. VT: tidal volume; PEEP: positive end-expiratory pressure; Pplat: plateau pressure; ∆P: driving transpulmonary pressure
Low static strain is less injurious
Another important and underevaluated effect of PEEP is its possible injurious effects related to excessive static strain. In fact, as discussed above, only dynamic strain (such as ∆P) has been considered as a potential factor determining lung injury. In a study by Güldner et al. [34], pigs that had undergone saline lung lavage were separately ventilated with a double-lumen tube: the left lung with a very low VT (3 ml/kg predicted body weight [PBW]) according to an atelectrauma or volutrauma strategy, while the right lung was ventilated with a continuous positive airway pressure (CPAP) of 20 cmH2O. The volutrauma strategy included high PEEP set above the level where dynamic compliance increased more than 5% during a PEEP trial, and the atelectrauma strategy included low PEEP to achieve driving pressures comparable with those of volutrauma. The potential increase in CO2 and decrease in pHa due to the extremely low VT was controlled by extracorporeal removal. This experiment separated the potential beneficial or detrimental effects of higher or lower static strain on VILI, i.e., higher or lower PEEP. In both conditions, atelectrauma and volutrauma, the tidal breath was extremely low. Regional lung aeration was assessed by computed tomography (CT), and inflammation by FDG-PET. Contrary to general belief regarding ultraprotective ventilation, volutrauma (i.e., higher static strain) yielded higher inflammation as compared to atelectrauma (i.e., lower static strain). Volutrauma decreased the blood fraction at similar perfusion and increased normally and hyperaerated lung compartments and tidal hyperaeration. Atelectrauma yielded more poorly and non-aerated lung compartments, and tidal recruitment, as well as increased ∆P. These data suggested that volutrauma and static strain may promote even greater lung inflammation than atelectrauma at comparable low VT values and lower driving pressures, suggesting again that static stress and strain are major determinants of VILI. Mechanical power was higher in volutrauma compared to atelectrauma groups. However, the intensity, i.e., mechanical power normalized to lung tissue, was comparable between volutrauma and atelectrauma, with negligible differences. Thus, we exclude any influence of differences in intensity to explain our results regarding the potential injurious effects of excessive static strain. In conclusion, higher PEEP increases static strain, thus promoting lung inflammation.
Low PEEP minimally impairs lymphatic drainage
Higher PEEP may also have negative effects on fluid drainage from pulmonary structures. The dynamic of fluids in the pulmonary interstitium is carefully regulated by the pressures inside and outside the capillaries, the extracellular matrix, and pulmonary lymphatics, and differs between spontaneous breathing and mechanical ventilation. The lymphatics collect fluids through three routes: hilar, transpleural, and transabdominal [35]. In normal conditions, a continuous leak of fluids occurs from the capillaries to the interstitium, because of the overall balance between hydrostatic and oncotic pressures in the capillaries and interstitium. The lymphatics maintain a negative pressure in the interstitium, which is important to prevent changes in the mechanical and functional properties of the respiratory system. Furthermore, fluids are also drained from the pleural space to the interstitium in the parietal side through specific foramina or, again, a combination of hydrostatic and oncotic pressures. Finally, drainage occurs through lymphatics positioned in the diaphragm, which play a relevant role during spontaneous breathing and mechanical ventilation.
Unlike the situation for more central diaphragmatic lymphatic vessels, optimization of lymphatic drainage through the diaphragm depends on anatomical location and functional physiological properties. In fact, central diaphragmatic lymphatic vessels are passively activated by muscular contraction, and thus become partially ineffective during controlled mechanical ventilation. On the other hand, lymphatic loops located at the extreme diaphragmatic periphery additionally require an intrinsic pumping mechanism to propel lymph centripetally [36]. Such active lymph propulsion is attained by means of a complex interplay among sites and is able to organize lymph flow in an ordered way. More recently, it has been shown that spontaneous contraction of lymphatics located in the extreme diaphragmatic periphery might involve hyperpolarization-activated cyclic nucleotide-gated channels in lymphatics equipped with muscle cells [37]. Hence, the three-dimensional arrangement of the diaphragmatic lymphatic network seems to be finalized to efficiently exploit the stresses exerted by muscle fibers during the contracting inspiratory phase to promote lymph formation in superficial submesothelial lymphatics and its further propulsion in deeper intramuscular vessels [38]. In the presence of diffuse damage of the alveolar capillary membrane, the role of lymphatics is even more important, to avoid progression and provide at least partial cleaning of lung edema. The increase in pressure in the alveoli, with increased inspiratory, mean or end-expiratory pressure, may markedly impair the function of lymphatics, determining a reduction in fluid drainage capability. During spontaneous breathing, the pressure in the interstitium is higher than the pressure in the lymphatics, resulting in a negative gradient (around 3–4 mmHg) which facilitates continuous drainage of fluids [39]. By contrast, during positive pressure, an increase in interstitial and lymphatic pressures occurs, to a similar degree (10 mmHg). In this case, the gradient between the interstitium and lymphatics becomes around zero or even positive, impairing possible fluid drainage. In addition, the increase in pressure in the respiratory system increases pressures in the pulmonary vessels and, as a consequence, on the venous side. This increase in hydrostatic pressures promotes fluid leak from capillaries on the abdominal and diaphragmatic side, thus potentially increasing pressure in the abdomen and further worsening respiratory and circulatory function as well as lymphatic drainage from the lungs (Fig. 2). In conclusion, mechanical ventilation with higher PEEP negatively affects lymphatic drainage from the lung, possibly impairing fluid exchange from the interstitial lung tissue.
Fig. 2.
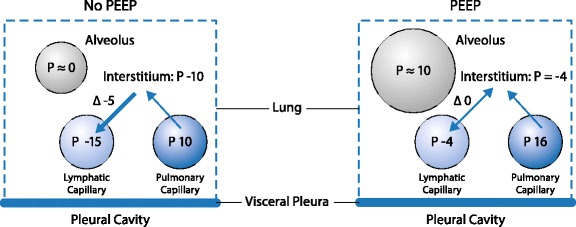
Relation between alveolar, capillary, lymphatic and interstitial pressures in a lung ventilated without (left) or with (right) positive end-expiratory pressure (PEEP)
Low PEEP improves right ventricular function
Patients with ARDS are characterized by a moderate-to-severe impairment of right ventricular (RV) function, which impacts on systemic hemodynamics [40]. Several devices and modalities, such as echocardiography, are now available to monitor respiratory settings according to RV tolerance. Acute cor pulmonale is defined as a persistent increase in pulmonary vascular resistance and, from an echocardiographic point of view, is characterized by paradoxical septal motion [40]. In patients with ARDS, the severity of the pulmonary disease involving the microvasculature influences development of acute cor pulmonale, which may also be caused or exacerbated by an aggressive ventilatory strategy. In fact, even minor overload in pulmonary vascular resistance may impair RV function. In this context, the use of lower VT has been associated with a decreased rate of RV impairment and acute cor pulmonale, with possible beneficial effects on outcome [41]. However, PEEP may negatively affect RV function [42]. In fact, the decrease in cardiac output is more often associated with a preload decrease and no change in RV contractility, whereas the increased RV volumes with PEEP may be associated with a reduction in RV myocardial performance. Acidosis and hypercapnia induced by VT reduction and increase in PEEP with constant plateau pressure have been found to be associated with impaired RV function despite positive effects on oxygenation and alveolar recruitment [43]. It has been suggested that respiratory system ∆P ≥ 18 cmH2O, PaCO2 ≥ 48 mmHg, and PaO2/FiO2 < 150 mmHg are three factors independently associated with acute cor pulmonale. Thus, extended sessions of prone positioning instead of increasing PEEP have been proposed in patients with moderate to severe ARDS [44]. In conclusion: 1) increased RV afterload during ARDS may induce acute cor pulmonale; 2) higher PEEP and plateau pressure, as well as increased ∆P, worsens RV function and systemic hemodynamics.
PEEP and CT scan
As discussed above, it is likely that it is necessary to keep the lungs fully open to achieve the potential positive effects of the ‘open-lung’ strategy. CT scan studies in ARDS patients have shown that the potential of recruitment in this population varies widely [45]. Moreover, the level of PEEP required to keep the lungs fully open is extremely high, especially in moderate to severe ARDS [46]. Additionally, even 15 cmH2O PEEP has been shown not to be enough to keep the lung open [47] and to be associated with overdistension [48]. In conclusion, therefore, CT scan studies have shown that high PEEP levels (> 15 cmH2O) are needed to keep the lungs fully open and are always associated with increased overdistension and hemodynamic impairment.
Conclusion
Several studies have reported that the open lung approach, as achieved with high PEEP and recruitment maneuvers, is important to reduce VILI and improve outcomes. However, experimental and clinical evidence does not fully support the hypothesis that PEEP per se might reduce lung injury. Indeed, PEEP may have negative effects resulting in overdistension, edema formation, poor lymphatic drainage and impairment of RV function, as well as an impact on systemic hemodynamics. Furthermore, atelectatic lung regions exhibit reduced lung inflammation if kept at rest. Most studies have compared ventilatory strategies with high PEEP combined with low VT to high VT and no PEEP. Thus, it was impossible to separate the possible protective effects of low VT from those of PEEP or the combination thereof. Moreover, moderate PEEP levels might induce even greater injury as compared to high PEEP. Thus, we propose the following ventilation strategies:
keep the lungs partially collapsed;
avoid opening and closing of collapsed alveoli;
mitigate injury in the peripheral airways, thus resulting in gentle ventilation of the aerated lungs while keeping collapsed and consolidated lung tissues at rest.
We believe that permissive atelectasis might be at least as effective as a strategy aimed at opening the lungs and keeping them open, but with the advantage of minimizing overstretch of the lung parenchyma and mitigating hemodynamic consequences. In short, we believe that, in order to minimize VILI, we should consider moving away from the classical concept of ‘open up the lungs and keep them open’ towards ‘close down the lungs and keep them closed’. A ventilatory strategy that leaves as much lung derecruited as possible, so that gas exchange is still adequate and opening and closing of collapsed alveoli is avoided could potentially minimize lung injury.
Acknowledgements
We aknowledge Dr. Lorenzo Ball for his support in the preparation of figures and support in the preparation of the paper.
Funding
Publication was funded by the Department of Surgical Sciences and Integrated Diagnostics, University of Genoa.
Availability of data and materials
Not applicable.
Authors’ contributions
PP, PRMR, MGDA equally contributed in the preparation and writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Disclaimer
Reference 8: Cruz SR, Rojas JI, Nervi R, Heredia R, Ciapponi A (2013) High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev CD009098, should also include the volume ID: (Jun 6;(6)).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Rocco PRM, Dos Santos C, Pelosi P. Pathophysiology of ventilator-associated lung injury. Curr Opin Anaesthesiol. 2012;25:123–130. doi: 10.1097/ACO.0b013e32834f8c7f. [DOI] [PubMed] [Google Scholar]
- 3.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–230. doi: 10.1164/rccm.201212-2169OC. [DOI] [PubMed] [Google Scholar]
- 6.Constantin JM, Godet T, Jabaudon M, Bazin JE, Futier E. Recruitment maneuvers in acute respiratory distress syndrome. Ann Transl Med. 2017;5:290. doi: 10.21037/atm.2017.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18:319–321. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 8.Cruz SR, Rojas JI, Nervi R, Heredia R, Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013:CD009098. [DOI] [PMC free article] [PubMed]
- 9.Lu J, Wang X, Chen M, et al. An open lung strategy in the management of acute respiratory distress syndrome: a systematic review and meta-analysis. Shock. 2017;48:43–53. doi: 10.1097/SHK.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 10.Rezoagli E, Fumagalli R, Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med. 2017;5:282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L. The “baby lung” became an adult. Intensive Care Med. 2016;42:663–673. doi: 10.1007/s00134-015-4200-8. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Pesenti A, Carlesso E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med. 2013;39:1909–1915. doi: 10.1007/s00134-013-3066-x. [DOI] [PubMed] [Google Scholar]
- 13.Pelosi P, D’Andrea L, Vitale G, Pesenti A, Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- 14.Pelosi P, Rocco PRM. Airway closure: the silent killer of peripheral airways. Crit Care. 2007;11:114. doi: 10.1186/cc5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesenti A, Musch G, Lichtenstein D, et al. Imaging in acute respiratory distress syndrome. Intensive Care Med. 2016;42:686–698. doi: 10.1007/s00134-016-4328-1. [DOI] [PubMed] [Google Scholar]
- 16.Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165:1647–1653. doi: 10.1164/rccm.2001080-01CP. [DOI] [PubMed] [Google Scholar]
- 17.Caldini P, Leith JD, Brennan MJ. Effect of continuous postive-pressure ventilation (CPPV) on edema formation in dog lung. J Appl Physiol. 1975;39:672–679. doi: 10.1152/jappl.1975.39.4.672. [DOI] [PubMed] [Google Scholar]
- 18.Demling RH, Staub NC, Edmunds LH. Effect of end-expiratory airway pressure on accumulation of extravascular lung water. J Appl Physiol. 1975;38:907–912. doi: 10.1152/jappl.1975.38.5.907. [DOI] [PubMed] [Google Scholar]
- 19.Colmenero-Ruiz M, Fernández-Mondéjar E, Fernández-Sacristán MA, Rivera-Fernández R, Vazquez-Mata G. PEEP and low tidal volume ventilation reduce lung water in porcine pulmonary edema. Am J Respir Crit Care Med. 1997;155:964–970. doi: 10.1164/ajrccm.155.3.9117033. [DOI] [PubMed] [Google Scholar]
- 20.Russell JA, Hoeffel J, Murray JF. Effect of different levels of positive end-expiratory pressure on lung water content. J Appl Physiol. 1982;53:9–15. doi: 10.1152/jappl.1982.53.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchida S, Engelberts D, Peltekova V, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174:279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 22.Wakabayashi K, Wilson MR, Tatham KC, O’Dea KP, Takata M. Volutrauma, but not atelectrauma, induces systemic cytokine production by lung-marginated monocytes. Crit Care Med. 2014;42:e49–e57. doi: 10.1097/CCM.0b013e31829a822a. [DOI] [PubMed] [Google Scholar]
- 23.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004;32:168–174. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- 24.Narimanbekov IO, Rozycki HJ. Effect of IL-1 blockade on inflammatory manifestations of acute ventilator-induced lung injury in a rabbit model. Exp Lung Res. 1995;21:239–254. doi: 10.3109/01902149509068830. [DOI] [PubMed] [Google Scholar]
- 25.Takata M, Abe J, Tanaka H, et al. Intraalveolar expression of tumor necrosis factor-alpha gene during conventional and high-frequency ventilation. Am J Respir Crit Care Med. 1997;156:272–279. doi: 10.1164/ajrccm.156.1.9607072. [DOI] [PubMed] [Google Scholar]
- 26.Bellani G, Rouby JJ, Constantin JM, Pesenti A. Looking closer at acute respiratory distress syndrome: the role of advanced imaging techniques. Curr Opin Crit Care. 2017;23:30–37. doi: 10.1097/MCC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 27.Ball L, Vercesi V, Costantino F, Chandrapatham K, Pelosi P. Lung imaging: how to get better look inside the lung. Ann Transl Med. 2017;5:294. doi: 10.21037/atm.2017.07.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues RS, Bozza FA, Hanrahan CJ, et al. (18)F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury. Nucl Med Biol. 2017;48:52–62. doi: 10.1016/j.nucmedbio.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Retamal J, Sörensen J, Lubberink M, et al. Feasibility of (68)Ga-labeled Siglec-9 peptide for the imaging of acute lung inflammation: a pilot study in a porcine model of acute respiratory distress syndrome. Am J Nucl Med Mol Imaging. 2016;6:18–31. [PMC free article] [PubMed] [Google Scholar]
- 30.de Prost N, Costa EL, Wellman T, et al. Effects of ventilation strategy on distribution of lung inflammatory cell activity. Crit Care. 2013;17:R175. doi: 10.1186/cc12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellani G, Messa C, Guerra L, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. Crit Care Med. 2009;37:2216–2222. doi: 10.1097/CCM.0b013e3181aab31f. [DOI] [PubMed] [Google Scholar]
- 32.Samary CS, Santos RS, Santos CL, et al. Biological impact of transpulmonary driving pressure in experimental acute respiratory distress syndrome. Anesthesiology. 2015;123:423–433. doi: 10.1097/ALN.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 33.Samary CS, Silva PL, Gama de Abreu M, Pelosi P, Rocco PRM. Ventilator-induced lung injury: power to the mechanical power. Anesthesiology. 2016;125:1070–1071. doi: 10.1097/ALN.0000000000001297. [DOI] [PubMed] [Google Scholar]
- 34.Güldner A, Braune A, Ball L, et al. Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit Care Med. 2016;44:e854–e865. doi: 10.1097/CCM.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malbrain M, Pelosi P. Open up and keep the lymphatics open: they are the hydraulics of the body! Crit Care Med. 2006;34:2860–2862. doi: 10.1097/01.CCM.0000239420.64405.29. [DOI] [PubMed] [Google Scholar]
- 36.Moriondo A, Solari E, Marcozzi C, Negrini D. Spontaneous activity in peripheral diaphragmatic lymphatic loops. Am J Physiol Heart Circ Physiol. 2013;305:H987–H995. doi: 10.1152/ajpheart.00418.2013. [DOI] [PubMed] [Google Scholar]
- 37.Negrini D, Marcozzi C, Solari E, et al. Hyperpolarization-activated cyclic nucleotide-gated channels in peripheral diaphragmatic lymphatics. Am J Physiol Heart Circ Physiol. 2016;311:H892–H903. doi: 10.1152/ajpheart.00193.2016. [DOI] [PubMed] [Google Scholar]
- 38.Moriondo A, Solari E, Marcozzi C, Negrini D. Diaphragmatic lymphatic vessel behavior during local skeletal muscle contraction. Am J Physiol Heart Circ Physiol. 2015;308:H193–H205. doi: 10.1152/ajpheart.00701.2014. [DOI] [PubMed] [Google Scholar]
- 39.Moriondo A, Mukenge S, Negrini D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289:H263–H269. doi: 10.1152/ajpheart.00060.2005. [DOI] [PubMed] [Google Scholar]
- 40.Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147:259–265. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 41.Mekontso Dessap A, Boissier F, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 42.Dambrosio M, Fiore G, Brienza N, et al. Right ventricular myocardial function in ARF patients. PEEP as a challenge for the right heart. Intensive Care Med. 1996;22:772–780. doi: 10.1007/BF01709520. [DOI] [PubMed] [Google Scholar]
- 43.Mekontso Dessap A, Charron C, Devaquet J, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med. 2017;5:295. doi: 10.21037/atm.2017.06.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 46.Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1807–1814. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 47.Cressoni M, Chiumello D, Algieri I, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43:603–611. doi: 10.1007/s00134-017-4754-8. [DOI] [PubMed] [Google Scholar]
- 48.Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT scan ARDS study group. Intensive Care Med. 2000;26:857–869. doi: 10.1007/s001340051274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


