Abstract
BACKGROUND
Cancer survivors may experience long-term and late effects from treatment that adversely affect health and limit functioning. Few studies examine lost productivity and disease burden in cancer survivors compared with individuals who have other chronic conditions or by cancer type.
METHODS
We identified 4960 cancer survivors and 64,431 other individuals from the 2008–2010 Medical Expenditure Panel Survey and compared multiple measures of disease burden, including health status and lost productivity, between conditions and by cancer site for cancer survivors. All analyses controlled for the effects of age, sex, race/ethnicity, and number of comorbid conditions.
RESULTS
Overall, in adjusted analyses in multiple models, cancer survivors with another chronic disease (heart disease or diabetes) experienced higher levels of burden compared with individuals with a history of cancer only, chronic disease only, and neither cancer, heart disease, nor diabetes across multiple measures (P <.05). Among cancer survivors, individuals with short survival cancers and multiple cancers consistently had the highest levels of burden across multiple measures (P <.0001).
CONCLUSIONS
Cancer survivors who have another chronic disease experience more limitations and higher levels of burden across multiple measures. Limitations are particularly severe in cancer survivors with short survival cancer and multiple cancers.
Keywords: MEPS, quality of life, cancer survivors, chronic conditions, comorbidities
INTRODUCTION
As of 2009, there were nearly 13 million cancer survivors in the United States,1 and this number is projected to increase.2 Cancer survivors may experience long-term and late effects from their treatment that adversely affect their health and limit functioning and productivity, even many years after treatment.3,4 However, many studies of lost productivity and disease burden in cancer survivors have limited follow-up, have small sample sizes, or have been limited to a single cancer type.5,6 Although several older studies have used large national, population-based data to overcome these limitations,7,8 more recent national estimates are needed to understand the current impact on cancer survivors’ health and functioning. Treatment advances have improved cancer survival, but the prevalence of late effects have likely increased as well.9 Furthermore, available studies do not compare cancer survivors with individuals who have other medical conditions, making it challenging to understand how the burden from cancer compares to the impact of other chronic diseases in the United States adult population.5
A recent Institute of Medicine report highlights the need for research to assess health and functional status for individuals living with chronic illness.3 Furthermore, understanding the burden of cancer by specific cancer type is needed, because different treatments may lead to variations in outcomes.10,11 However, prior work in cancer survivors and comorbidities has been conducted primarily in the elderly population,11,12 which limits the ability to assess burden of illness from cancer on employment and other productivity measures. To address these research gaps, we 1) used national data to estimate disease burden by examining health, functional status, and lost productivity in cancer survivors and individuals without a history of cancer to understand how these burden and limitations estimates compare with the burden caused by other major chronic diseases in the United States and 2) compared burden of illness by cancer site to provide estimates for subgroups of the United States cancer survivor population.
MATERIALS AND METHODS
Data Source
The study sample was selected from the 2008–2010 Medical Expenditure Panel Survey (MEPS) Household Component. The MEPS is an annual, nationally representative survey conducted in the civilian noninstitutionalized population in the United States. The MEPS Household Component collects demographic, health status, employment, and health care use and medical expenditure data. It also collects information about medical conditions that have been specified as priority conditions due to prevalence, expense, or policy relevance. In-person interviews are conducted with an individual who responds for all household members. The average annual response rate was approximately 57%. More detailed information on the MEPS design and content has been provided elsewhere.13
Analytic Sample
We identified 69,391 adults aged ≥18 years. To make comparisons between cancer survivors and individuals with other chronic conditions, we categorized individuals into the following groups: having a personal history of cancer, having a personal history of heart disease, and having a personal history of diabetes. These groups were not mutually exclusive. These diseases were selected due to the prevalence and expense of these conditions in the United States population.14 Conditions were identified by questions asking whether a doctor or other health professional had ever told the person they had any of the MEPS priority conditions (arthritis, asthma, angina, cancer, coronary heart disease, diabetes, emphysema, heart attack, high blood pressure, high cholesterol, and stroke). For the history of cancer category, we excluded individuals diagnosed with only nonmelanoma skin cancer, as has been done elsewhere.8,15 We classified individuals with a history of coronary heart disease, angina, heart attack, stroke, or “other heart disease” as having a history of heart disease.16 The history of diabetes category included individuals who indicated they had a history of this condition. We also categorized cancer survivors by cancer site (breast, prostate, colorectal, short survival cancers, multiple cancers, and other single cancers). Short survival cancers were defined as those with a 5-year survival rate of <25% and included liver, lung, pancreas, and stomach cancers.
Measures
Sample characteristics included age, sex, educational attainment, and race/ethnicity. Comorbid conditions were defined as known MEPS priority conditions (0, 1, 2, 3+) other than the primary conditions of the individual (ie, cancer, heart disease, or diabetes).
Health and functional limitation measures consisted of health status (excellent/very good, good, fair/poor); any limitations in physical functioning; and limitations in physical functioning that lasted for more than 3 months.
Lost productivity measures included whether the individual was employed within the past 12 months for individuals <65 years of age. We also measured any limitations among all individuals’ ability to work, do housework, or go to school because of a health problem; inability to do activities; and experience of cognitive limitations. Other measures included whether individuals accomplished less due to their physical health in the last 4 weeks or were limited in the kind of work or activities they engaged in due to their physical health in the last 4 weeks. Each health and lost productivity construct was obtained with a single item.
Data Analyses
Descriptive statistics were stratified by history of cancer and were compared using chi-square statistics. To compare health and functional status, employment, and lost productivity by chronic disease type, we used multivariate logistic regression in separate samples defined by history of cancer and 1) heart disease and 2) diabetes and controlled for the effects of age, race/ethnicity, sex, and comorbidities defined as number of other known MEPS priority conditions. We focused our discussion of results on consistent patterns of findings across multiple measures, rather than adjusting statistical significance thresholds for multiple comparisons. Results of the regression analyses are presented as predicted margins, which directly standardize the outcome of each group to the covariate distribution of the population.17 Standardized results from logit models can be compared like percentages and allow for ease of interpretation. All analyses used SUDAAN18 to account for the MEPS survey weights and complex design. Wald statistics were used to test the statistical significance of differences between groups, and all tests of statistical significance were 2-sided. We calculated 95% confidence intervals for the predicted margins using a logit transformation.17
RESULTS
The characteristics of the study subjects are presented in Table 1. Individuals with a history of cancer were more likely to be older, female, and non-Hispanic white and were more likely to report having 3 or more other MEPS priority conditions compared with individuals without cancer (P <.001).
TABLE 1.
Characteristics of Individuals With and Without a History of Cancer
| Individuals With History of Cancer | Individuals Without History of Cancer | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristics | n | Wtd % | n | Wtd % | Pa |
| Age, y | |||||
| 18–39 | 462 | 7.4 | 28,082 | 41.9 | <.0001 |
| 40–44 | 215 | 4.2 | 6265 | 9.4 | |
| 45–49 | 296 | 5.4 | 6391 | 9.6 | |
| 50–54 | 405 | 7.8 | 6104 | 9.9 | |
| 55–59 | 507 | 10.0 | 5136 | 8.2 | |
| 60–64 | 606 | 12.8 | 3994 | 6.8 | |
| 65–69 | 598 | 12.2 | 2861 | 4.6 | |
| 70–74 | 533 | 11.5 | 1968 | 3.2 | |
| 75–79 | 524 | 10.5 | 1524 | 2.6 | |
| ≥80 | 814 | 18.1 | 2106 | 3.7 | |
| Sex | |||||
| Men | 1941 | 41.3 | 30,088 | 49.1 | <.0001 |
| Women | 3019 | 58.7 | 34,343 | 50.9 | |
| Education when first entered MEPSb | |||||
| Less than high school graduate | 1062 | 15.9 | 15,825 | 17.4 | .0350 |
| High school graduate | 1578 | 32.4 | 19,736 | 29.9 | |
| Some college or more | 2303 | 51.4 | 28,550 | 52.3 | |
| Race/ethnicity | |||||
| Non-Hispanic white | 3523 | 84.8 | 28,835 | 66.5 | <.0001 |
| Non-Hispanic black | 704 | 7.1 | 12,399 | 11.9 | |
| Hispanic | 502 | 5.1 | 16,968 | 14.7 | |
| Non-Hispanic other/multiple | 231 | 3.1 | 6229 | 7.0 | |
| Number of other known MEPS priority conditions, excluding cancer | |||||
| 0 | 781 | 15.7 | 31,114 | 46.6 | <.0001 |
| 1 | 925 | 19.1 | 14,144 | 22.7 | |
| 2 | 1090 | 21.6 | 8579 | 14.1 | |
| 3+ | 2164 | 43.6 | 10,594 | 16.6 | |
| Conditions | |||||
| Heart disease | 1793 | 36.8 | 8502 | 14.2 | <.0001 |
| Diabetes | 940 | 17.4 | 5946 | 8.3 | <.0001 |
Chi-square test.
Missing not reported. Abbreviations: Wtd, Weighted.
GENERAL HEALTH, FUNCTIONAL STATUS AND LOST PRODUCTIVITY BY CHRONIC DISEASE HISTORY
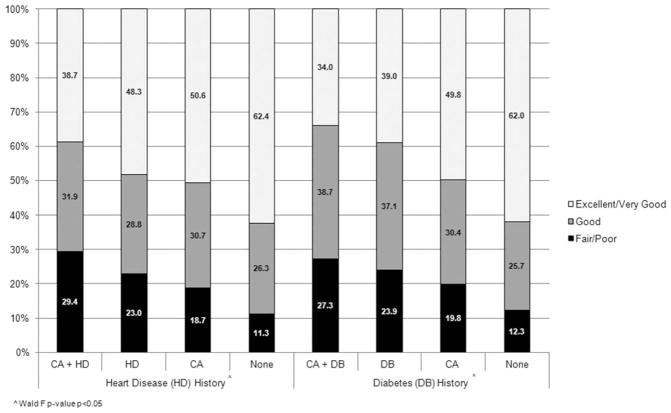
Figure 1 displays health status in individuals with and without a history of cancer, heart disease, or diabetes. Overall, individuals who had a history of cancer plus history of heart disease or diabetes were more likely to have fair/poor health compared with individuals who had a history of cancer, history of heart disease or diabetes, or none of these conditions in separate multivariate analyses (P <.05).
Figure 1.
General health in individuals with and without a history of cancer, heart disease, and diabetes. Predicted margins were determined using logistic regression models with age, comorbidities, race/ethnicity, and sex as covariates (P <.05 [Wald test]). Abbreviations: CA, cancer; DB, diabetes; HD, heart disease.
Table 2 presents functional status and lost productivity by chronic disease history. Individuals with a history of cancer plus another chronic disease had poorer functional status than individuals with a history of cancer or a history of another chronic disease, or without cancer or the other chronic conditions, across multiple measures.
TABLE 2.
Functional Status and Lost Productivity in Individuals with and without a History of Cancer, Heart Disease, or Diabetes
| History of Heart Disease | History of Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Yes | No | Yes | No | |||||
|
|
|
|
|
|||||
| CA + HD (n = 1787) | HD (n = 8484) | CA (n = 3160) | None (n = 55,903) | CA + DB (n = 936) | DB (n = 5936) | CA (n = 4011) | None (n = 58,451) | |
| Functional status | ||||||||
| Limitation in physical functioning | 20.4 (18.6–22.2) | 17.4 (16.3–18.4) | 13.3 (12.0–14.7) | 10.5 (9.9–11.1) | 19.7 (17.4–22.2) | 16.1 (15.0–17.2) | 14.2 (13.0–15.5) | 11.6 (11.0–12.3) |
| Limitation in physical functioning >3 mo | 18.2 (16.6–19.9) | 16.0 (15.1–17.0) | 12.0 (10.8–13.3) | 9.2 (8.6–9.8) | 17.6 (15.5–20.0) | 14.7 (13.7–15.8) | 12.7 (11.7–13.9) | 10.4 (9.8–11.0) |
| Lost productivity | ||||||||
| Employed in past 12 mo (age <65 y) | 65.5 (59.5–70.9) | 74.1 (72.1–76.0) | 77.4 (74.6–80.0) | 82.3 (81.6–82.9) | 70.2 (63.6–76.1) | 76.3 (74.0–78.4) | 76.1 (73.4–78.6) | 81.8 (81.1–82.4) |
| Any limitation in work, housework, or school | 17.1 (15.2–19.3) | 14.0 (12.9–15.1) | 10.1 (8.9–11.4) | 6.5 (6.1–7.0) | 15.7 (13.5–18.2) | 12.4 (11.4–13.4) | 10.9 (9.8–12.1) | 7.7 (7.2–8.3) |
| Completely unable to do activities | 10.5 (9.1–12.0) | 8.4 (7.6–9.2) | 6.0 (5.1–7.0) | 3.9 (3.6–4.2) | 9.7 (8.1–11.6) | 7.7 (7.0–8.5) | 6.6 (5.7–7.5) | 4.6 (4.3–5.0) |
| Cognitive limitation | 8.4 (7.0–10.0) | 7.7 (7.0–8.6) | 4.8 (4.0–5.8) | 3.2 (3.0–3.5) | 7.5 (6.0–9.3) | 5.6 (4.9–6.4) | 5.1 (4.3–6.0) | 4.2 (3.9–4.5) |
Data are presented as % (95% confidence interval). Predicted margins were determined using logistic regression models with age, comorbidities, race/ethnicity, and sex as covariates. Some samples were smaller based on missing data on specific outcomes. Heart disease and diabetes comparisons between chronic disease categories were statistically significant (P<.05).
Abbreviations: CA, cancer; DB, diabetes; HD, heart disease.
With respect to employment status, adults <65 years of age with a history of cancer plus heart disease were less likely to be employed in the past 12 months compared with individuals who had a history of cancer or heart disease or individuals without a history of cancer or heart disease in adjusted models (65.5%, 77.4%, 74.1, and 82.3%, respectively; P <.0001). Results were similar for individuals with a history of diabetes. Other measures of lost productivity also followed a similar pattern across multiple measures for the majority of the heart disease and diabetes comparisons (P <.001).
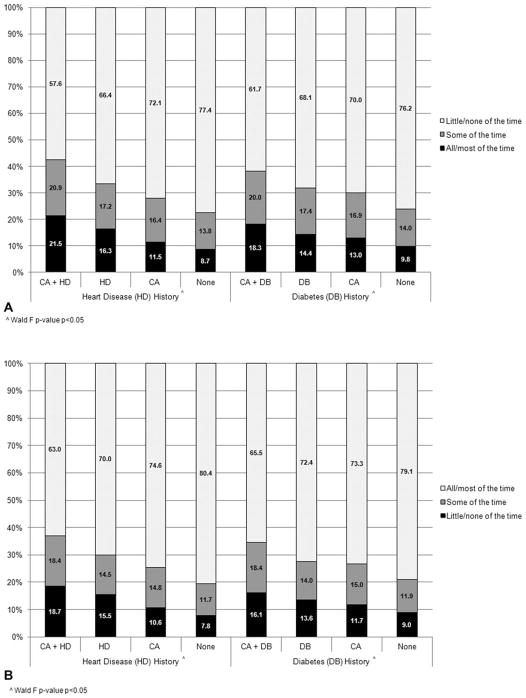
Within the heart disease and diabetes comparisons, individuals with a history of cancer plus another chronic disease were the most likely to accomplish less because of their physical health (Fig. 2A) and be more limited in the kind of work or activities they engaged in because of physical health in the past 4 weeks (Fig. 2B) compared with individuals who had specific chronic disease, cancer, or neither cancer nor other chronic disease in adjusted analyses (P <.05).
Figure 2.
(A) Percent of individuals with and without a history of cancer, heart disease, and diabetes who accomplished less because of physical health in last 4 weeks. (B) Percent of individuals with and without a history of cancer, heart disease, and diabetes who had limited ability to work in last 4 weeks. Predicted margins were determined using logistic regression models with age, comorbidities, race/ethnicity, and sex as covariates (P <.05 [Wald test]). Abbreviations: CA, cancer; DB, diabetes; HD, heart disease.
GENERAL HEALTH, FUNCTIONAL STATUS AND LOST PRODUCTIVITY BY CANCER SITE
In adjusted analyses, individuals with a history of cancer, particularly those with a history of short survival cancers or multiple cancers, had poorer health and greater productivity loss across multiple measures compared with individuals without cancer (Table 3; P <.05). Generally, adults with a history of breast cancer were least likely to have limitations and were more similar to individuals without cancer across these measures.
TABLE 3.
General Health, Functional Status, and Lost Productivity in Individuals With and Without a History of Cancer
| Cancer | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Breast (n = 825) | Prostate (n = 598) | Colorectal (n = 293) | Short Survival Cancersa (n = 193) | Multiple Cancers (n = 339) | Other Single Cancers (n = 2699) | No Cancer (n = 64,387) | Pb | |
| General health status | ||||||||
| Excellent/Very good | 51.2 (46.8–55.5) | 49.7 (44.6–54.9) | 49.8 (42.9–56.6) | 38.7 (30.4–47.8) | 38.6 (31.3–46.5) | 49.6 (47.3–51.9) | 60.0 (59.2–60.8) | <.0001 |
| Good | 31.4 (27.9–35.0) | 31.8 (27.1–37.0) | 28.7 (22.5–35.8) | 26.0 (19.0–34.4) | 26.9 (21.2–33.4) | 31.3 (29.1–33.6) | 26.2 (25.7–26.8) | |
| Fair/Poor | 17.5 (14.8–20.5) | 18.4 (15.6–21.6) | 21.5 (16.4–27.8) | 35.3 (27.0–44.5) | 34.5 (28.3–41.3) | 19.1 (17.4–20.9) | 13.7 (13.2–14.2) | |
| Functional status | ||||||||
| Limitation in physical functioning | 12.4 (10.3–14.8) | 11.9 (9.4–14.9) | 13.0 (9.8–17.0) | 20.5 (15.8–26.0) | 20.5 (16.2–25.5) | 16.3 (14.8–17.8) | 12.4 (11.8–13.0) | <.0001 |
| Limitation in physical functioning >3 mo | 10.6 (8.8–12.8) | 10.5 (8.1–13.5) | 12.4 (9.3–16.2) | 17.7 (13.7–22.6) | 18.9 (14.7–23.9) | 14.6 (13.3–16.0) | 11.1 (10.6–11.7) | <.0001 |
| Lost productivity | ||||||||
| Employed in past 12 months (age <65 y) | 81.6 (77.0–85.4) | 73.6 (62.8–82.2) | 79.2 (67.0–87.8) | 66.8 (52.0–78.9) | 57.1 (45.2–68.2) | 75.2 (72.3–77.9) | 81.4 (80.7–82.0) | <.0001 |
| Any limitation in work, housework, or school | 8.8 (7.1–11.0) | 9.4 (6.8–12.9) | 10.4 (7.6–14.1) | 17.5 (12.8–23.4) | 15.1 (11.5–19.7) | 12.5 (11.2–14.0) | 8.5 (8.0–9.0) | <.0001 |
| Completely unable to do activities | 4.7 (3.5–6.2) | 6.3 (4.4–9.1) | 7.4 (5.2–10.6) | 11.7 (8.3–16.4) | 10.3 (7.7–13.8) | 7.4 (6.3–8.6) | 5.2 (4.8–5.6) | <.0001 |
| Cognitive limitation | 3.4 (2.4–4.8) | 4.9 (3.5–6.9) | 4.6 (2.9–7.4) | 6.8 (4.5–10.1) | 8.5 (6.1–11.6) | 6.1 (5.1–7.2) | 4.4 (4.1–4.7) | <.0001 |
Data are presented as % (95% confidence interval). Predicted margins were determined using logistic regression models with age, comorbidities, race/ethnicity, and sex as covariates. Some sample sizes were smaller based on missing data on specific outcomes.
Cancers with <25% 5-year survival rate (liver, lung, pancreas, and stomach).
Wald test.
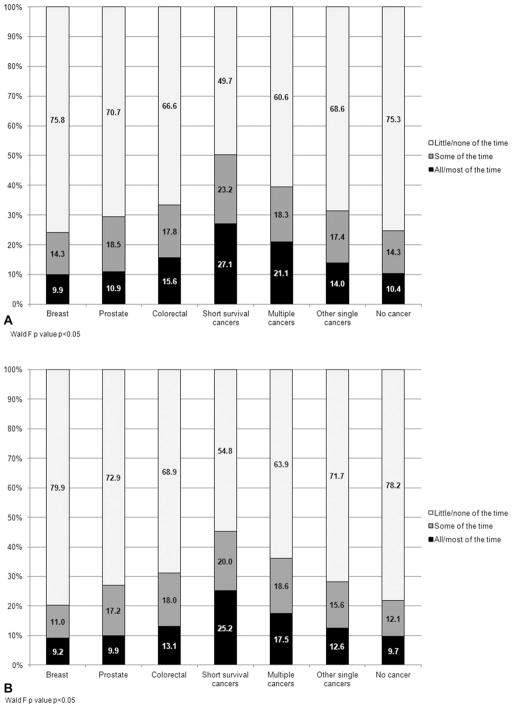
Compared with individuals who had a history of other single cancers or had no history of cancer, individuals with a history of short survival cancers or multiple cancers were most likely to accomplish less (Fig. 3A; P <.0001) and be limited in the kind of work or activities they engaged in due to their physical health (Fig. 3B; P <.0001) in the past 4 weeks.
Figure 3.
(A) Percent of individuals with and without a history of cancer who accomplished less because of physical health in last 4 weeks by cancer type. (B) Percent of individuals with and without a history of cancer who had limited ability to work in last 4 weeks by cancer type. Predicted margins were determined using logistic regression models with age, comorbidities, race/ethnicity, and sex as covariates (P <.05 [Wald test]).
DISCUSSION
In this study, we used a nationally representative sample to assess health status, employment, and lost productivity in cancer survivors compared with adults with and without other chronic diseases. The goals of our study were to provide current population-based estimates of health status and lost productivity for cancer survivors compared with individuals without cancer to 1) understand how these estimates compare with the burden caused by other major chronic diseases in the United States, 2) understand any additional impact of specific comorbidity for cancer survivors, and 3) examine these measures by specific cancer sites. This study was conducted using the most recently available data in a sample of adults ≥18 years of age, complementing previous studies of comorbidities in cancer survivors that were primarily conducted in the elderly population.19
As expected, we found that cancer survivors continue to experience significant levels of burden, and that having an additional history of heart disease or diabetes is associated with even greater burden. While other studies have documented poorer outcomes, diminished physical capacity and productivity of cancer survivors,5,7 few have provided estimates of these outcomes in cancer survivors with and without other types of chronic disease.
These findings help contextualize the estimates for cancer survivors more broadly to the burden caused by other chronic diseases in the United States. Notably, the increased burden and limitations for cancer survivors appear similar for survivors who additionally have either heart disease or diabetes, possibly suggesting a generalized disease effect of multiple comorbidities. These findings underscore the value of working across chronic diseases in clinical care and preventive services, especially due to the growing population of cancer survivors who are older adults and at risk for multiple comorbidities.3,20 There is ongoing debate regarding the best models of care to meet cancer survivors’ needs.21 This study highlights the importance of assessing and managing other comorbidities as part of survivorship care, especially our findings that suggest 37% of cancer survivors also have a history of heart disease and 17% have a history of diabetes. Given the observed functional limitations for cancer survivors and individuals with a history of other chronic diseases, applying evidence-based interventions or models of care from other disease models (eg, cardiac care’s rehabilitation approach)20 may improve cancer care delivery and quality of life in cancer survivors.
Consistent with other studies,7,8 we observed that employment, lost productivity, and burden of illness vary substantially across subgroups of cancer survivors. However, our findings provide current estimates of burden and productivity losses by cancer type for cancer survivors. As expected, survivors with cancers associated with short survival or who had multiple cancers consistently had the highest levels of burden compared with other single site cancers. Approximately 320,120 people in the United States are diagnosed with liver, lung, pancreas, or stomach cancers,1 and at least 750,000 people are diagnosed with multiple cancers, accounting for almost 8% of the overall cancer survivorship population.22 While our results were consistent with the few population-based studies on individuals with multiple cancer diagnoses,15,23 more research is needed to measure and understand the burden of illness in individuals with multiple cancer diagnoses, particularly as they become more prevalent with improved survival following diagnosis,24 increased risk of second cancers associated with treatment,9 and the aging population.22
Our results also indicate that breast cancer survivors had the lowest levels of burden reported among cancer survivors and are similar on many measures to adults without cancer. In our sample, breast cancer survivors were generally younger than other cancer survivors, and a substantial proportion were long-term survivors (ie, greater than 10 years since diagnosis). These variations in burden of illness and limitations in cancer survivors across cancer site highlight the importance of detailed examination by specific cancer sites, particularly as cancer treatment becomes more specified for individuals, which may result in differing outcomes.
Therefore, our findings suggest that significant levels of burden and limitations persist after treatment among cancer survivors that affect physical functioning and ability to work or perform usual activities. Notably, approximately 30% of cancer survivors accomplished less in the past 4 weeks or were limited in work or usual activities because of physical health, with about 12% being limited all the time. Several approaches have been developed to quantify the impact of activity limitations, including wage- and preference-based approaches to valuing time.25 Further development and application of approaches to quantifying limitations in productivity will improve our current understanding of disease burden in cancer survivors, including costs of care and financial issues. Helping inform future research, the MEPS Experiences with Cancer Survivorship Supplement will provide nationally representative data to address many of these issues.26
Despite the strengths of having a large population-based sample and the ability to assess multiple chronic diseases, there are several limitations to this study. We were unable to assess some commonly diagnosed cancer sites separately, such as lung cancer, due to shorter survival and therefore limited sample size. However, we were able to group several cancer types associated with short survival separately and evaluate burden for these individuals. Data on history of cancer and chronic disease were based on self-report or household proxy; however, validation studies have found strong agreement between household-reported and physician-reported conditions.27 Although false positive reports of cancer and other chronic diseases are low,28 some individuals with a history of cancer, heart disease or diabetes may have been classified in the other comparison group (ie, false negative reports), which may have underestimated the differences between these groups.29 Additionally, we could not assess the severity of the diseases reported, and cancer stage or treatment information was not available. Because of our interest in evaluating multiple measures of burden for individuals with and without heart disease or diabetes, we conducted many separate analyses. Finally, the cancer survivor population was disproportionately older and non-Hispanic white compared with the population of individuals without a history of cancer. Future studies should replicate these findings among more diverse populations of cancer survivors.
In conclusion, we observed that cancer survivors continue to experience significant burden of illness across multiple measures of productivity and functioning. Additionally, cancer survivors who have an additional chronic disease generally experience higher levels of burden compared with other individuals. We also identify cancer survivors who might be at higher risk for functional limitations and inform efforts to develop more efficacious and targeted interventions.8,10,11
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the positions of the National Cancer Institute, the Centers for Disease Control and Prevention, or the US Department of Health and Human Services.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2009 (vintage 2009 populations) National Cancer Institute; Bethesda, MD: [Accessed June 5, 2012]. Available at: http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Living well with chronic illness: a call for public health action. Washington, DC: Institute of Medicine; 2012. [Google Scholar]
- 4.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16:197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 6.Yabroff KR, McNeel TS, Waldron WR, et al. Health limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care. 2007;45:629–637. doi: 10.1097/MLR.0b013e318045576a. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 8.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine. From cancer patient to cancer survivor: lost in transition. Washington, DC: Institute of Medicine; 2005. [Google Scholar]
- 10.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113(12 suppl):3519–3529. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
- 11.Bellizzi KM, Mustian KM, Palesh OG, Diefenbach M. Cancer survivorship and aging: moving the science forward. Cancer. 2008;113(12 suppl):3530–3539. doi: 10.1002/cncr.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. [Accessed June 20, 2012];MEPS survey background. Available at: http://meps.ahrq.gov/mepsweb/about_meps/survey_back.jsp.
- 14.Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by source of payment: United States, 2010. Medical Expenditure Panel Survey Household Component Data. [Accessed March 13, 2013];Generated interactively. http://meps.ahrq.gov/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2010&Table=HCFY2010_CNDXP_D&_Debug=
- 15.Andrykowski MA. Physical and mental health status of survivors of multiple cancer diagnoses: findings from the national health interview survey. Cancer. 2012;118:3645–3653. doi: 10.1002/cncr.26678. [DOI] [PubMed] [Google Scholar]
- 16.Soni A. Aspirin use among the adult U.S. noninstitutionalized population, with and without indicators of heart disease, 2005. Statistical Brief #179. Available at: http://meps.ahrq.gov/mepsweb/data_files/publications/st179/stat179.shtml.
- 17.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 18.Research Triangle Institute. SUDAAN language manual: release 9.0. 2004. [Google Scholar]
- 19.Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53:2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Alfano CM, Ganz PA, Rowland JH, Hahn EE. Cancer survivorship and cancer rehabilitation: revitalizing the link. J Clin Oncol. 2012;30:904–906. doi: 10.1200/JCO.2011.37.1674. [DOI] [PubMed] [Google Scholar]
- 21.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 22.Mariotto AB, Rowland JH, Ries LA, Scoppa S, Feuer EJ. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 23.Burris JL, Andrykowski MA. Physical and mental health status and health behaviors of survivors of multiple cancers: a national, population-based study. Ann Behav Med. 2011;42:304–312. doi: 10.1007/s12160-011-9290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 25.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 26.Yabroff K, Dowling E, Rodriguez J, et al. The medical expenditure panel survey (MEPS) experiences with cancer survivorship supplement. J Cancer Surviv. 2012;6:407–419. doi: 10.1007/s11764-012-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss N, Kass B. Comparison of household and medical provider reports of medical conditions. Paper presented at: Joint Statistical Meetings; August 13–17, 2000; Indianapolis, IN. [Google Scholar]
- 28.Harlow SD, Linet MS. Agreement between questionnaire data and medical records. The evidence for accuracy of recall. Am J Epidemiol. 1989;129:233–248. doi: 10.1093/oxfordjournals.aje.a115129. [DOI] [PubMed] [Google Scholar]
- 29.Desai MM, Bruce ML, Desai RA, Druss BG. Validity of self-reported cancer history: a comparison of health interview data and cancer registry records. Am J Epidemiol. 2001;153:299–306. doi: 10.1093/aje/153.3.299. [DOI] [PubMed] [Google Scholar]