Abstract
Opioid use disorders (OUDs) are receiving significant attention as a public health crisis. Access to treatment for OUDs is essential and was expected to improve following implementation of the federal parity law and the Affordable Care Act. This study examines changes in coverage and management of treatments for OUDs (opioid treatment programs (OTPs) as a covered service benefit, buprenorphine as a pharmacy benefit) before, during and after parity and ACA implementation. Data are from three rounds of a nationally representative survey conducted with commercial health plans regarding behavioral health services in benefit years 2003, 2010, and 2014. Data were weighted to be representative of health plans’ commercial products in the continental United States (2003 weighted N= 7,469, 83% response rate; 2010 N=8,431, 89% response rate; and 2014 N=6,974, 80% response rate). Results showed treatment for OUDs was covered by nearly all health plan products in each year of the survey, but the types and patterns varied by year. Prior authorization requirements for OTPs have decreased over time. Despite the promise of expanded access to OUD treatment suggested by parity and the ACA, improved health plan coverage for treatment of OUDs, while essential, is not sufficient to address the opioid crisis.
Keywords: Addiction, treatment, insurance, ACA, opioid
INTRODUCTION
Opioid use disorders (OUDs) have an unprecedented focus as a public health crisis (U.S. DHHS 2016; U.S. Surgeon General 2016). In 2015, 2 million people in the U.S. ages 12 or older were estimated to have a substance use disorder (SUD) due to prescription opioid misuse and 0.6 million were estimated to have an SUD due to heroin; even higher numbers of people had misused opioids or used heroin in the past year (SAMHSA 2016). Most recently, fentanyl is a rapidly emerging threat and is contributing to the increasing overdose rate (Tamburro, Al-Hadidi & Dragovic 2016). OUDs are the cause of many overdoses and deaths (CDC 2016; Kanouse & Compton 2015; Rudd et al. 2016; SAMHSA 2013) and have many other adverse consequences, including criminal justice involvement, unemployment, and negative effects on relationships and families. Access to treatment for OUDs is essential, and it was anticipated that broad health care reforms, including the federal parity law and the Affordable Care Act (ACA) would lead to improved access for all who have SUDs by covering treatment and medications as a benefit.
Given the high rate of relapse for people with OUDs, medications such as methadone, buprenorphine, and extended-release naltrexone are considered front-line treatment and have been shown to be both effective and cost-effective (American Society of Addiction Medicine 2015; Fullerton et al. 2014; Gastfriend 2011; Mattick et al. 2014; Reif et al. 2016; Schackman et al. 2012; Thomas et al. 2014). Methadone has been available for decades, yet for treatment of OUDs is only available in “opioid treatment programs” (OTPs) subject to specific federal regulations regarding the dispensing of opioid agonist medications. For some people with OUDs the need to go to special programs daily, which also have stigma as identifying someone as a drug user, has been a barrier to accessing treatment. Buprenorphine is a prescription medication that may be prescribed in office settings (e.g., primary care), sometimes known as “office-based opioid treatment,” enabling treatment for OUDs in a less obtrusive environment and by obtaining medication at a pharmacy rather than a specialty addiction treatment program. Prescription of buprenorphine requires physicians to become trained and obtain a DEA waiver, and the number of patients they may treat at any time is capped. Buprenorphine may also be offered in OTPs and, if a waivered physician is available to prescribe it, in other specialty SUD treatment settings. In 2014, 40% of OTPs only offered methadone, 57% offered methadone and buprenorphine, and 3% did not offer methadone (SAMHSA 2015). Counseling is usually expected to accompany the use of medications to treat OUDs.
Despite the existence of effective treatment options, many individuals with OUDs go untreated (Oliva et al. 2011; Volkow et al. 2014). In general, a high proportion of people with SUDs do not want or are ambivalent about treatment (SAMHSA 2016). Others seek treatment but do not obtain it due to cost, lack of insurance, stigma or other issues (SAMHSA 2016). Further, for treatment of OUDs, structural barriers to access exist, such as proximity to an OTP or availability of waivered buprenorphine prescribers who are accepting new patients (Kasarabada et al. 2002; Oliva et al. 2011). Payment and financing have been identified as important barriers to accessing treatment for OUDs (Oliva et al. 2011).
With financing of treatment for OUDs as a key issue, healthcare reforms that address this may be an important lever for increasing access and treatment, and ultimately improving outcomes for OUDs. Payers of treatment for OUDs include shifting combinations of public funds including the federal block grant, public and private health insurance, and out-of-pocket spending by individuals or their families (Mark et al. 2016). Compared to mental health, SUD treatment has been less likely to be paid for by health insurance and more likely to receive funding directly from public agencies. In 2014, only 18% of substance use treatment expenditures were paid for by private insurance (Mark et al. 2016). Nonetheless, private insurance remains an important funding source, and with recent reforms such as the 2008 Mental Health Parity and Addiction Equity Act (MHPAEA) and the 2010 Affordable Care Act (ACA), it has been anticipated that private insurance coverage would expand (Volkow et al. 2014) for people with SUDs. In addition, the ACA specified SUD treatment as an essential health benefit that must be covered by public and private insurance sources that were expanded under the ACA. The combined effect of MHPAEA and the ACA is that many more Americans now have access to a broader and more affordable range of treatment options for OUDs by virtue of improved access to and benefits under insurance plans and reduced financial burden (Beronio, Glied & Frank 2014; Ettner et al. 2016; Thalmayer et al. 2016). The federal and state governments continue to target funding for prevention and treatment services to address the opioid crisis (e.g., the 21st Century Cures Act, passed in December 2016). However, it should be noted that these expansions do not necessarily translate to increased numbers of people who seek or receive SUD treatment. (Ali, Teich & Mutter 2015; Busch et al. 2014; Creedon & Cook 2016; Saloner et al. 2016; Thalmayer et al. 2016). Other barriers such as stigma, variation in treatment availability, and the unique degree to which SUD treatment is paid for by sources other than private health insurance were not immediately addressed by MHPAEA and the ACA (Ali, Teich & Mutter 2015; Mark et al. 2016).
The ACA should reduce the number of people who fully pay out of pocket for treatment of SUDs, removing that barrier. Yet, even if insured, managed care policies often require copayments or other cost-sharing, which in the past have been higher for SUDs than for general medical care (Horgan et al. 2016; Oliva et al. 2011). Public and private insurers also commonly use managed care techniques, such as prior authorization or treatment limits, which may affect access to treatment services or medications. MHPAEA was designed to eliminate disparate cost-sharing and utilization management techniques, compared to general medical care, thus improving access to SUD treatment over time (Horgan et al. 2016).
In addition to the changing insurance environment over time, the treatment landscape for OUDs has been changing. Historically, methadone maintenance therapy was the primary treatment for OUDs and was covered by insurance only under the treatment service benefit. Since then, however, buprenorphine has grown to overtake methadone as the most commonly prescribed treatment for OUDs. More recently, extended-release naltrexone was approved for OUDs in 2010. Treatment for OUDs are now covered by health plans through the treatment service benefit (e.g., OTPs), the pharmacy benefit (e.g., buprenorphine prescription), or the medical benefit (e.g., injectable naltrexone). Some have implied that methadone is no longer necessary, but it is clearly still the best treatment for some, especially those with more severe OUDs (American Society of Addiction Medicine 2015; Knopf 2016; Mattick et al. 2014). Pharmacological treatment for OUDs should be accompanied by counseling (and other services) in most cases (SAMHSA 2015). Counseling, regardless of the type of SUD, is covered as a treatment service benefit.
In this study, we examined how commercial health plans managed coverage for treatment of OUDs including OTPs covered as a treatment service benefit and buprenorphine covered as a pharmacy benefit, before, during and after MHPAEA and ACA implementation. Drawing on national survey data of commercial health plans from 2003, 2010, and 2014, we investigated the following research questions: (1) How has private insurance coverage of opioid treatment programs and buprenorphine pharmacy changed over time, in the midst of MHPAEA and ACA insurance reforms? (2) How have private health plans approached managed care factors beyond coverage, such as prior authorization and continuing review, over this period? And, (3) what health plan characteristics are associated with coverage and utilization management policies for treatment of OUDs? The findings from this study will indicate whether trends in availability and management of OUD treatment over time reflect improved coverage for those who are privately insured.
METHODS
Data source and population
Data were collected through three rounds of a nationally representative survey of commercial health plans regarding alcohol, drug and mental health services, fielded to cover benefit years 2003, 2010, and 2014 [cites blinded]. The 2010 and 2014 surveys were designed to capture changes during and following implementation of MHPAEA (implemented in 2010, with interim and final regulations available in 2011 and 2014) and the ACA (implemented in phases between 2010 and 2014). The telephone survey was administered to senior health plan executives. Typically, one respondent answered administrative questions and referred interviewers to the medical director or behavioral health medical director for clinical questions and, rarely, to the pharmacy director for pharmacy questions (asked within the clinical module). Plans occasionally referred interviewers to their managed behavioral health organization (MBHO) contractor for additional information.
For some national or regional plans, respondents at corporate headquarters responded for multiple sites. Health plans typically offer multiple products such as a health maintenance organization (HMO) or a preferred provider organization (PPO). Items were asked at the product level within each market-area-specific plan. Each plan was asked about its top three commercial products.
This study employed a panel survey design with replacement and has been described previously [cites blinded]. The primary sampling units were the 60 market areas identified in the Community Tracking Study (Kemper et al. 1996) to be nationally representative. The second stage sampled plans within market areas. Plans serving multiple market areas were defined separately and data were collected with respect to a specific market area.
For each round of the survey a sample frame of commercial health plans was built up by identifying all health plans operating in the study sites, adding newly operating plans and removing plans that closed or merged. Eligibility screening verified health plan operation in the market area and coverage of behavioral health services for a commercial population with more than 300 subscribers or 600 covered lives. In 2003, this approach identified 441 plans of which 368 responded (83%) reporting on 812 products. For the clinical portion of the survey 347 plans (79%) responded, reporting on 771 products. In 2010, 438 eligible plans were identified, of which 389 responded (89%) and reported on 939 insurance products for the administrative module, while 385 plans (88%) responded to the clinical module, reporting on 925 products. In 2014, 344 eligible plans were identified, of which 274 plans responded (80%) and reported on 705 products. Our Institutional Review Board approved this study.
Variables
Treatment for Opioid Use Disorders
Methadone for the treatment of opioid use disorders is offered only in regulated OTPs and as such is considered by health plans to be a treatment service (not a medication). OTPs may also dispense buprenorphine in the same regulated environment as methadone. The survey thus asked about coverage of OTPs as a treatment service benefit along with other specialty addiction treatment services such as residential and outpatient care. In 2003, the survey specifically asked only about outpatient methadone maintenance programs; in 2010, it asked about methadone and “other opioid replacement” programs; in 2014, it asked about “opioid treatment programs.” For the purposes of this paper, each is considered to be an OTP. In 2010, a question was asked about the length of treatment covered, only for methadone maintenance.
Buprenorphine is treated as any other medication when prescribed by waivered physicians in office-based opioid treatment, thus was asked about in the pharmacy section of the 2003 and 2010 surveys. The 2014 survey was abbreviated to focus on changes due to MHPAEA and ACA, and did not include the pharmacy section. Extended-release naltrexone was not approved for OUDs at the time of the 2010 survey, and as for buprenorphine, was not asked in the 2014 survey; it is thus omitted from this analysis.
To better understand trends over time, as buprenorphine became more commonly used, we created a variable to indicate if a health plan product covered only OTPs, only buprenorphine pharmacy, both or neither, for 2003 and 2010 data.
Treatment Management
Health plans can use a range of techniques to manage access to treatment services. A common managed care technique is prior authorization, in which the provider or enrollee needs to obtain approval for treatment prior to admission in order for the health plan to pay for care. Authorization is often accompanied by a specific number of services (e.g., days or length of time) after which continuing review is required by the health plan for additional services to be covered.
Health Plan Characteristics
Health plan products were characterized by product type: health maintenance organization (HMO), preferred provider organization (PPO), and point of service (POS) products. In 2010, consumer-directed products (CDP), which are typically products with high deductibles and health savings accounts, were first represented in the most common product types of some health plans, and their prevalence continued to increase in 2014. Products were also characterized by the contracting arrangements for behavioral health services. External contracting reflects those products that contracted with a specialty managed behavioral health organization. In internal arrangements, the health plan manages both medical and behavioral health benefits, and services are provided by plan employees or through a network of providers directly administered by the plan. Comprehensive arrangements involve a health plan contracting with a single vendor for both general medical and behavioral health provider networks, but are extremely rare; therefore, they are not reported separately.
Statistical Analysis
Findings reported are national estimates. The data are weighted to be representative of health plans’ private managed care products in the continental U.S., for each survey year. Statistical analyses used SUDAAN software for accurate estimation of the sampling variance given the complex sampling design. Significant differences are based on pairwise t-tests with a .05 significance level, adjusted for multiple tests (three pairwise tests for each variable by year and product type (2003) and six pairwise tests for product type (2010 (2014)) using the Bonferroni correction.
RESULTS
Overview of Treatment for Opioid Use Disorders
Treatment for OUDs was covered by nearly all health plan products in each year of the survey, but the types and patterns of treatment varied over time (Figures 1 and 2). OTPs were a covered service for 64.5% of health plan products in 2003, 69.0% in 2010 and, in a marked increase (p<.05), 97.0% in 2014 (Figure 1). Buprenorphine was covered under the pharmacy benefit by 70.0% of products in 2003, soon after approval for treatment of OUDs. In 2010, all health plan products covered buprenorphine under the pharmacy benefit, a significant increase from 2003 (p<.05). This was not asked in 2014, although it was presumed to remain universally covered.
Figure 1.
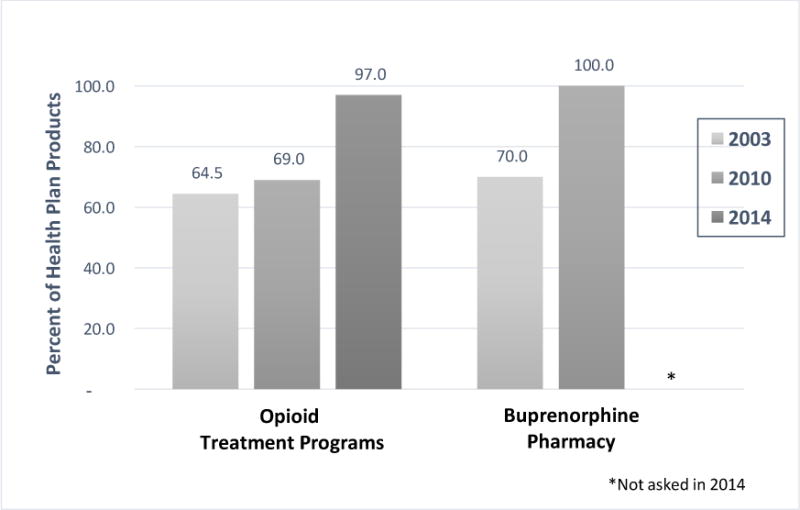
Health Plan Coverage of Treatment for OUDs, by year
Figure 2.
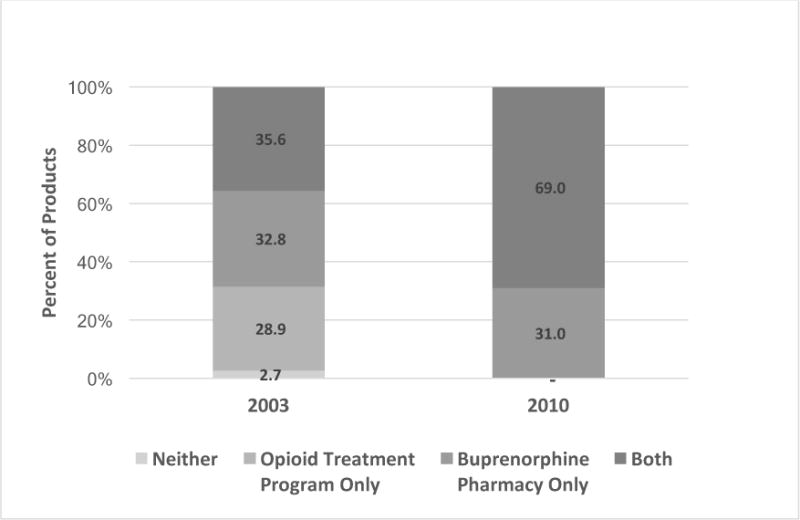
Change in Coverage Patterns for Treatment of OUDs, 2003 to 2010
Figure 1 might lead to the conclusion that treatment for OUDs hovered around 70% in 2003. However, when the pattern of coverage of treatment for OUDs was examined by determining if products covered OTP only, buprenorphine only, both or neither, only 2.7% of health plan products in 2003 did not cover treatment for OUDs (Figure 2). In 2010, all products covered OUD treatment. Yet, in 2003, 28.9% of products only covered OTPs, not buprenorphine pharmacy; conversely, 32.8% of products only covered buprenorphine pharmacy and not OTPs. Both types of treatment for OUDs were covered by just over a third (35.6%) of products in 2003; by 2010 this doubled to 69.0%. The remaining 31% of products in 2010 only covered buprenorphine under the pharmacy benefit, very similar to 2003.
Opioid Treatment Programs
Coverage for treatment for OUDs varied by product type and contracting arrangement (Table 2). In 2003 OTPs were slightly less often covered by PPO products (57.9%) than by POS (68.3%) products. In 2010, HMO products more often covered OTPs (80.2%) than PPO (70.7%) or POS (67.4%) products. Almost no CDP products covered OTPs in 2010. By 2014, OTPs were covered nearly universally, including by CDP products. In terms of behavioral health contracting arrangements, in 2003, products with external behavioral health contracts were more likely to cover OTPs (67.7%) than those with internal arrangements (35.9%). In 2010, the pattern reversed for OTPs; products with external contracting were less likely to cover these programs (34.9%) than those with internal behavioral health arrangements (74.6%). Near universal coverage in 2014 meant that no variation was found by contracting arrangement.
Table 2.
Treatment for Opioid Use Disorders – Coverage, Prior Authorization, and Continuing Review, by product type, contracting and year
| Product Type | Contracting Arrangement* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| HMO N |
PPO N |
POS N |
CDP** N |
External N |
Internal N |
||||||||
| Number of Products | |||||||||||||
| 2003 | 2,209 | 2,619 | 2,702 | – | 5,471 | 1,147 | |||||||
| 2010 | 2,420 | 3,004 | 2,613 | 390 | 1,219 | 7,177 | |||||||
| 2014 | 2,355 | 2,478 | 900 | 1,241 | 701 | 6,273 | |||||||
|
| |||||||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | ||
|
| |||||||||||||
| Opioid Treatment Programs | |||||||||||||
| Coverage | |||||||||||||
| 2003 | 66.6 | 3.9 | 57.9a | 3.5 | 68.3a | 2.4 | – | – | 67.7f | 2.6 | 35.9f | 7.6 | |
| 2010 | 80.2a | 2.8 | 70.7a | 2.3 | 67.4a | 2.0 | 0.4a | 0.3 | 34.9f | 6.7 | 74.6f | 1.2 | |
| 2014 | 96.4 | 1.2 | 97.9 | 0.6 | 96.4 | 2.2 | 96.7 | 2.4 | 98.0 | 0.9 | 96.8 | 1.3 | |
| Prior Authorization | |||||||||||||
| 2003 | 93.5a | 3.5 | 42.0a,b | 8.8 | 94.3b | 3.5 | – | – | 94.0 | 1.6 | 57.3 | 18.7 | |
| 2010 | 72.2a | 2.0 | 69.7bc | 2.3 | 77.1bd | 1.7 | 99.6acd | 0.3 | 93.2f | 2.4 | 71.2f | 1.7 | |
| 2014 | 35.9ab | 2.7 | 36.4cd | 2.4 | 15.2ace | 3.8 | 53.1bde | 3.2 | 78.7f | 8.4 | 31.8f | 2.1 | |
| Continuing Review | |||||||||||||
| 2003 | 91.5ab | 2.2 | 54.3bc | 5.6 | 98.9ac | 0.4 | – | – | 95.0 | 1.4 | 82.2 | 7.4 | |
| 2010 | 70.2ab | 2.6 | 70.2cd | 1.9 | 73.9ace | 1.6 | 100.0bde | – | 100.0f | – | 68.7f | 1.4 | |
| 2014 | 44.9a | 1.4 | 45.9b | 2.4 | 23.4abc | 5.0 | 50.8c | 3.2 | 96.4f | 1.4 | 38.2f | 1.9 | |
|
| |||||||||||||
| Buprenorphine Pharmacy*** | |||||||||||||
| Coverage | |||||||||||||
| 2003 | 63.0a,b | 2.5 | 94.3b,c | 1.6 | 55.2a,c | 3.0 | – | – | 64.3f | 1.9 | 84.5f | 5.2 | |
| 2010 | 100.0 | – | 100.0 | – | 100.0 | – | 100.0 | – | 100.0 | – | 100.0 | – | |
| Prior Authorization | |||||||||||||
| 2003 | 8.3 | 2.4 | 9.8 | 1.7 | 4.7 | 1.9 | – | – | 7.3 | 1.7 | 12.5 | 8.5 | |
| 2010 | 31.3abc | 2.1 | 36.6ad | 3.0 | 39.6bd | 2.4 | 99.6cd | 0.3 | 63.0f | 5.4 | 34.9f | 2.7 | |
Excludes “comprehensive” products
CDP was not asked in 2003
Buprenorphine pharmacy was not asked in 2014
Significance testing compared categories within a year (row). Pairs with the same superscript within a row and section indicate significant differences at p<.05.
Almost no health plan products (7%) placed a time limit, when asked specifically about methadone maintenance therapy in 2010. For those that did so, the typical time limits were 3 months (1%), 6 months (1%), “standard medical criteria” (4%) and other (1%) (data not shown).
Prior authorization requirements for OTPs, if covered, have decreased over time, from 79.1% in 2003 to 74.3% in 2010, and even more rapidly to 36.5% in 2014 (Table 1). Variations are again seen by product type and behavioral health contracting arrangements (Table 2). In 2003, nearly all HMO (93.5%) and POS (94.3%) products required prior authorization for OTPs in 2003, compared to just 42.0% of PPO products. By 2010, the differences evened out, with fewer HMO (72.2%) and POS (77.1%) products requiring prior authorization than in 2003, and more PPO (69.7%) products doing so. If OTPs were covered by CDP products in 2010, all required prior authorization. By 2014, just over one third of HMO (35.9%) and PPO (36.4%) products had prior authorization requirements, and only 15.2% of POS products did so. Just over half (53.1%) of CDP products in 2014 had prior authorization requirements for OTPs. Products with external contracting arrangements were more likely to require prior authorization for OTPs in each year.
Table 1.
Treatment for Opioid Use Disorders – Coverage, Prior Authorization, and Continuing Review, by year
| All Products | ||||
|---|---|---|---|---|
|
| ||||
| N | ||||
| Number of Products | ||||
| 2003 | 7530 | |||
| 2010 | 8427 | |||
| 2014 | 6974 | |||
|
| ||||
| N | % | SE | ||
|
| ||||
| Opioid Treatment Programs | ||||
| Coverage | ||||
| 2003 | 6650 | 64.5 | 2.4 | |
| 2010 | 8301 | 69.0a | 2.0 | |
| 2014 | 5440 | 97.0a | 1.2 | |
| Prior Authorization | ||||
| 2003 | 3990 | 79.1 | 4.6 | |
| 2010 | 7555 | 74.3a | 1.6 | |
| 2014 | 6813 | 36.5a | 2.3 | |
| Continuing Review | ||||
| 2003 | 4056 | 83.7a | 2.9 | |
| 2010 | 6179 | 73.2a,b | 1.6 | |
| 2014 | 6632 | 43.5b | 1.8 | |
|
| ||||
| Buprenorphine Pharmacy* | ||||
| Coverage | ||||
| 2003 | 6965 | 70.0a | 2.4 | |
| 2010 | 8422 | 100.0a | – | |
| Prior Authorization | ||||
| 2003 | 4797 | 7.9a | 1.5 | |
| 2010 | 8402 | 38.9a | 2.6 | |
Significance testing compared 2003 to 2010 and 2010 to 2014. Pairs with the same letter, within a variable, were significantly different at p<.05.
Buprenorphine pharmacy was not asked in 2014
Continuing review was fairly common for OTPs, if covered, across time periods, but decreased over time (Table 1). In 2003, 83.7% of products had continuing review requirements for OTPs. In 2010, continuing review was required for 73.2% of products, and by 2014, it dropped to 43.6% of products for OTPs. Continuing review requirements had similar patterns by product type as for prior authorization requirements (Table 2). Products with external behavioral health contracting arrangements were less likely to have continuing review requirements than those with internal arrangements in 2010 and 2014.
Buprenorphine
Buprenorphine had significant increase in coverage from 70.0% of health plan products in 2003 to 100% in 2010 (Table 1). In 2003, buprenorphine as a pharmacy benefit was nearly always covered by PPO products and less often covered by HMO and POS products (Table 2). Buprenorphine as a pharmacy benefit had little variation in 2010, since coverage was universal. In 2003, products with external behavioral health contracts were less likely to cover buprenorphine as a pharmacy benefit. As noted, coverage for buprenorphine in 2010 and OTPs in 2014 were near universal, thus no variation was found by contracting arrangement.
Buprenorphine pharmacy increased prior authorization requirements from 7.9% in 2003 to 38.9% in 2010, if covered (Table 1). Prior authorization for buprenorphine pharmacy was similar for PPO (4.7%),HMO (8.3%) and POS (9.8%) products in 2003 (Table 2). In 2010, the pattern shifted, with prior authorization least likely for HMO (31.3%) compared to PPO (36.6%),POS (39.6%) and CDP (99.6%) products. In 2010, prior authorization was more likely to be required by products with external contracting arrangements than by those without.
DISCUSSION
This study reports on trends in commercial insurance coverage for treatment for opioid use disorders during a time of great change in health care overall, as evidenced by MHPAEA and ACA, as well as enhanced focus on the opioid crisis. It is encouraging to see that treatment options for OUDs were covered by nearly all private health plan products at each of our time periods (2003, 2010, and 2014), although the type of treatment that was covered varied. It seems likely that the decrease in methadone-specific coverage from 2003 to 2010 was directly related to the increase in buprenorphine and broader OTP coverage. These findings are likely to reflect the increasing comfort level with buprenorphine as a medication to treat opioid use disorders and the increasing number of prescribers from 2003 to 2010. Also during this time, OTPs were allowed to start incorporating buprenorphine into what were previously solely methadone programs. Counseling services for OUDs were not addressed in these analyses, but results from this same survey indicate that outpatient therapy was covered by 100% of health plan products [cite blinded].
Coverage for both OUD treatment services and medications is necessary to begin to address the opioid crisis. However, it is important to note that coverage through insurance is not sufficient. For example, if a person lives in a geographic area without an OTP easily accessible on a daily basis, coverage is unlikely to ensure access. Similarly, if a person lives in an area without enough (or any) trained and waivered physicians who can and will prescribe buprenorphine, and are accepting new patients, coverage is also unlikely to ensure access (Dick et al. 2015; Jones et al. 2015; Knudsen 2015; Stein et al. 2015).
Parity seems to have played a role in improving treatment management approaches. The 2011 interim regulations of MHPAEA highlighted the need to ensure that treatment management approaches are equivalent, not just the overall benefit. A focus on “non-quantitative treatment limits,” such as prior authorization and continuing review, was expected to result in a lowering of such limits for most addiction treatment services. This study demonstrated that effect for OTPs, an encouraging finding. Although this trend seemed to already be occurring from 2003 to 2010, a more rapid decrease of treatment management requirements happened from 2010 to 2014. Such loosening of requirements is also likely to reflect the heightened focus on the opioid crisis, where calls for access to treatment have been loud and sustained (ONDCP, 2016; U.S. Surgeon General 2016; Volkow et al. 2014), with an awareness that barriers such as authorization requirements may make the difference in whether someone accesses care.
Health plan characteristics also mattered for the treatments for OUDs that were not yet universally available in each year. The pattern of coverage varied by whether the product was an HMO, PPO or POS; it is not clear why these would vary, or why the product type patterns would change over time. CDPs, an increasingly common product design with an emphasis on reduced costs, did not offer coverage for OTPs until 2014, and then universally did so; they did universally cover buprenorphine pharmacy in 2010. Prior authorization and continuing authorization also varied by product type, perhaps in the ways expected by the inherent characteristics of the products, but not always. For instance, POS products are often considered the least restrictive, and were least likely to have authorization requirements for methadone/opioid treatment programs in 2014, but not earlier. One possibility for patterns by product type that were not as expected is that there was great flux in the industry response to the opioid epidemic and to MHPAEA and ACA in general during this time. It is possible that these findings reflect the different pace at which health plans were implementing new responses (or removing restrictions) within their products.
The use of external behavioral health contractors, or the traditional MBHO carveout model, did not seem to improve coverage, except that health plan products with external contracting were more likely to cover buprenorphine in 2003. The products with external contracting were also more likely to use prior authorization and continuing authorization for OTPs, in particular in 2014. This was also true for buprenorphine pharmacy, but only in 2010.
The ACA is widely considered to be a way in which to increase the numbers of people who have access to treatment by increasing access to health insurance. These results indicate that if you have insurance through private health plans, your coverage for opioid use disorder treatment has been consistent since 2003, but over time the options have increased.
The reduction in authorization requirements for OTPs over time could be a reflection of MHPAEA or could be a reflection of the opioid crisis. Since these occurred simultaneously, it is near impossible with these data to speak to the specific drivers of change. It is worth noting, however, that prior authorization requirements for treatment of OUDs are still fairly common in 2014, and much more so than is seen in these same data for non-methadone outpatient treatment [cites blinded]. It would be interesting to evaluate how private health plans consider methadone for the purposes of ensuring compliance with MHPAEA.
Despite health plan coverage of treatment for OUDs, it is also clear from the literature that treatment of SUDs overall remains very low in 2014 – under 10% of people deemed to need it – and this has remained unchanged for many years (SAMHSA 2016). For coverage to matter, people need to be willing to use treatment. Many of those who do not access treatment cite cost and access as factors (SAMHSA 2016); prior authorization requirements could play a role here. But many other barriers are also relevant, in particular geographic accessibility and availability of waivered buprenorphine prescribers.
As a survey of health plans, our data does have limitations. It reports only what health plans said they were doing, but cannot offer information about the numbers of people who accessed or tried to access these services. Due to the changing addiction treatment environment and limitations in the length of the survey, the treatment options differed in each year, making the trends more difficult to understand. In particular, this study could not evaluate buprenorphine prior authorization in 2014 or continuing review at any year, nor extended-release naltrexone in any detail. Further, in considering buprenorphine through the pharmacy benefit, this study did not specifically examine office visits for medication management or counseling as it relates to buprenorphine beyond those that might occur in OTP settings. Other analyses from this study indicate universal coverage of outpatient counseling [cites blinded] thus it is unlikely to be a specific barrier for individuals who are prescribed buprenorphine.
Because of our focus on commercial health plans, this study could not lend guidance to our understanding of Medicaid in areas where it expanded under the ACA or to marketplace plans that were developed under the ACA. It is encouraging that coverage for treatment of OUDs is universal in commercial health plans by 2014, and perhaps that would be reflected in other types of public and private insurance.
CONCLUSION
This study offers a snapshot of treatment for opioid use disorders by commercial health plans over a decade that has seen much churn in the health care industry, regulations of insurers and treatment options, growth in the availability of treatments, changes in views about addiction, and treatment and increased access to health insurance. MHPAEA and the ACA have leveled the playing field as well, reducing disparities in coverage by depending on what health plans individuals are enrolled in and increasing the numbers of people insured overall. Despite the promise of expanded access to OUD treatment suggested by these reforms and the findings reported here, the continued increase in people with OUDs during our nation’s opioid crisis suggest that improved health plan coverage for treatment of OUDs, while essential, is not sufficient.
Acknowledgments
The authors wish to acknowledge the contributions of Pat Nemeth, Frank Potter and staff at Mathematica Policy Research, Inc. (survey design, statistical consultation and data collection), Grant Ritter (statistical consultation), Galina Zolutusky (statistical programming), and Ann-Marie Matteucci (research support) at Brandeis University. This work was funded by the National Institute on Alcohol Abuse and Alcoholism (R01AA010869) and the National Institute on Drug Abuse (R01DA029316). The funding agencies did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Preliminary findings were presented at the American Association for the Treatment of Opioid Disorders, March 2015.
Contributor Information
Sharon Reif, Senior Scientist, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University.
Timothy B. Creedon, Associate Research Scientist, Cambridge Health Alliance; Research Associate, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University (when studied was conducted)
Constance M. Horgan, Professor and Director, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University
Maureen T. Stewart, Scientist, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University
Deborah W. Garnick, Professor, Institute for Behavioral Health, Heller School for Social Policy and Management, Brandeis University
References
- ONDCP. The Epidemic of Prescription Drug and Heroin Abuse in the United States. Committee on Oversight and Government Reform United States House of Representatives. 2016 https://oversight.house.gov/wp-content/uploads/2016/03/Botticelli-ONDCP-Statement-3-22-Heroin-Opioid-Abuse.pdf.
- Ali MM, Teich JL, Mutter R. The role of perceived need and health insurance in substance use treatment: implications for the Affordable Care Act. J Subst Abuse Treat. 2015;54:14–20. doi: 10.1016/j.jsat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- American Society of Addiction Medicine. National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use 2015 [Google Scholar]
- Beronio K, Glied S, Frank R. How the affordable care act and mental health parity and addiction equity act greatly expand coverage of behavioral health care. J Behav Health Serv Res. 2014;41(4):410–28. doi: 10.1007/s11414-014-9412-0. [DOI] [PubMed] [Google Scholar]
- Busch SH, Epstein AJ, Harhay MO, Fiellin DA, Un H, Leader D, Jr, Barry CL. The effects of federal parity on substance use disorder treatment. Am J Manag Care. 2014;20(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- CDC. Injury Prevention & Control: Opioid Overdose 2016 [Google Scholar]
- Creedon TB, Cook BL. Access to mental health care increased but not for substance use, while disparities remain. Health Aff (Millwood) 2016;35(6):1017–21. doi: 10.1377/hlthaff.2016.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie D, Stein BD. Growth in buprenorphine waivers for physicians increased potential access to opioid agonist treatment, 2002-11. Health Aff (Millwood) 2015;34(6):1028–34. doi: 10.1377/hlthaff.2014.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettner SL, J MH, Thalmayer A, Ong MK, Xu H, Bresolin MJ, Wells KB, Tseng CH, Azocar F. The Mental Health Parity and Addiction Equity Act evaluation study: Impact on specialty behavioral health utilization and expenditures among “carve-out” enrollees. J Health Econ. 2016;50:131–143. doi: 10.1016/j.jhealeco.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton CA, Kim M, Thomas CP, Lyman DR, Montejano LB, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65(2):146–57. doi: 10.1176/appi.ps.201300235. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR. Intramuscular extended-release naltrexone: current evidence. Ann N Y Acad Sci. 2011;1216:144–66. doi: 10.1111/j.1749-6632.2010.05900.x. [DOI] [PubMed] [Google Scholar]
- Horgan CM, Hodgkin D, Stewart MT, Quinn A, Merrick EL, Reif S, Garnick DW, Creedon TB. Health Plans’ Early Response to Federal Parity Legislation for Mental Health and Addiction Services. Psychiatr Serv. 2016;67(2):162–8. doi: 10.1176/appi.ps.201400575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55–63. doi: 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanouse AB, Compton P. The epidemic of prescription opioid abuse, the subsequent rising prevalence of heroin use, and the federal response. J Pain Palliat Care Pharmacother. 2015;29(2):102–14. doi: 10.3109/15360288.2015.1037521. [DOI] [PubMed] [Google Scholar]
- Kasarabada ND, Hser YI, Boles SM, Huang YC. Do patients’ perceptions of their counselors influence outcomes of drug treatment? J Subst Abuse Treat. 2002;23(4):327–34. doi: 10.1016/s0740-5472(02)00276-3. [DOI] [PubMed] [Google Scholar]
- Kemper P, Blumenthal D, Corrigan JM, Cunningham PJ, Felt SM, Grossman JM, Kohn LT, Metcalf CE, St Peter RF, Strouse RC, Ginsburg PB. The design of the community tracking study: a longitudinal study of health system change and its effects on people. Inquiry. 1996;33(2):195–206. [PubMed] [Google Scholar]
- Knopf A. Methadone remains vital in buprenorphine era. Addiction Professional Magazine 2016 [Google Scholar]
- Knudsen HK. The supply of physicians waivered to prescribe buprenorphine for opioid use disorders in the united states: a state-level analysis. J Stud Alcohol Drugs. 2015;76(4):644–54. doi: 10.15288/jsad.2015.76.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Yee T, Levit KR, Camacho-Cook J, Cutler E, Carroll CD. Insurance Financing Increased For Mental Health Conditions But Not For Substance Use Disorders, 1986-2014. Health Aff (Millwood) 2016;35(6):958–65. doi: 10.1377/hlthaff.2016.0002. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep. 2011;13(5):374–81. doi: 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Current psychiatry reports. 2011;13(5):374–381. doi: 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Horgan CM, Hodgkin D, Matteucci AM, Creedon TB, Stewart MT. Access to Addiction Pharmacotherapy in Private Health Plans. J Subst Abuse Treat. 2016;66:23–9. doi: 10.1016/j.jsat.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R. Increases in drug and opioid overdose deaths—United States, 2000–2014. American Journal of Transplantation. 2016;16(4):1323–1327. [Google Scholar]
- Saloner B, Bandara SN, McGinty EE, Barry CL. Justice-involved adults with substance use disorders: coverage increased but rates of treatment did not in 2014. Health Aff (Millwood) 2016;35(6):1058–66. doi: 10.1377/hlthaff.2016.0005. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables 2016 [Google Scholar]
- SAMHSA. National Survey of Substance Abuse Treatment Services (N-SSATS) 2014: Data on Substance Abuse Treatment Facilities. Rockville, MD: SAMHSA; 2015. [Google Scholar]
- SAMHSA. Federal Guidelines for Opioid Treatment Programs. Rockville, MD: 2015. (HHS Publication No. (SMA) PEP15-FEDGUIDEOTP). [Google Scholar]
- SAMHSA. Drug Abuse Warning Network, 2011: National estimates of drug-related emergency department visits. 2013. (HHS publication no. (SMA) 13-4760, DAWN series D-39). [Google Scholar]
- Schackman BR, Leff JA, Polsky D, Moore BA, Fiellin DA. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med. 2012;27(6):669–76. doi: 10.1007/s11606-011-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Gordon AJ, Dick AW, Burns RM, Pacula RL, Farmer CM, Leslie DL, Sorbero M. Supply of buprenorphine waivered physicians: the influence of state policies. J Subst Abuse Treat. 2015;48(1):104–11. doi: 10.1016/j.jsat.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburro LP, Al-Hadidi JH, Dragovic LJ. Resurgence of fentanyl as a drug of abuse. J Forensic Sci Med. 2016;2:111–4. [Google Scholar]
- Thalmayer AG, Friedman SA, Azocar F, Harwood JM, Ettner SL. The Mental Health Parity and Addiction Equity Act (MHPAEA) Evaluation Study: Impact on Quantitative Treatment Limits. Psychiatr Serv. 2016 doi: 10.1176/appi.ps.201600110. appips201600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Fullerton CA, Kim M, Montejano L, Lyman DR, Dougherty RH, Daniels AS, Ghose SS, Delphin-Rittmon ME. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv. 2014;65(2):158–70. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- U.S. DHHS. The Opioid Epidemic: By the Numbers 2016 [Google Scholar]
- U.S. Surgeon General. Turn the Tide: The Surgeon General’s Call to End the Opioid Crisis 2016 [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]


