Abstract
Subcortical white matter injury is often accompanied by orofacial motor dysfunction, but little is known about the structural substrates accounting for these common neurological deficits. We studied the trajectory of the corticobulbar projection from the orofacial region of the primary (M1), ventrolateral (LPMCv), supplementary (M2), rostral cingulate (M3) and caudal cingulate (M4) motor regions through the corona radiata (CR), internal capsule (IC) and crus cerebri of the cerebral peduncle (ccCP). In the CR each pathway was segregated. Medial motor area fibers (M2/M3/M4) arched over the caudate and lateral motor area fibers (M1/LPMCv) curved over the putamen. At superior IC levels, the pathways were widespread, involving the anterior limb, genu and posterior limb with the M3 projection located anteriorly, followed posteriorly by projections from M2, LPMCv, M4 and M1, respectively. Inferiorly, all pathways maintained this orientation but shifted posteriorly, with adjacent fiber bundles overlapping minimally. In the ccCP, M3 fibers were located medially and M1 fibers centromedially, with M2, LPMCv, and M4 pathways overlapping in between. Finally, at inferior ccCP levels, all pathways overlapped. Following CR and superior IC lesions, the dispersed pathway distribution may correlate with acute orofacial dysfunction with spared pathways contributing to orofacial motor recovery. In contrast, the gradually commixed nature of pathway representation inferiorly may enhance fiber vulnerability and correlate with severe, prolonged deficits following lower subcortical and midbrain injury. Additionally, in humans these findings may assist in interpreting orofacial movements evoked during deep brain stimulation, and neuroimaging tractography efforts to localize descending orofacial motor pathways.
Keywords: cerebral cortex, cingulate cortex, corticobulbar, dysarthria, dysphagia, frontal lobe, limbic system, RRID_004314, RRID: SCR_001775, stroke, tractography
1 | INTRODUCTION
Subcortical white matter damage produced by ischemic stroke or related cerebrovascular accidents often give rise to deficits associated with cranial nerve motor function. Common neurological impairments include facial paresis as well as tongue, mandibular, pharyngeal, and laryngeal dysfunction. In isolation, or in various combinations these symptoms can lead to dysphagia, dysarthria, malnutrition, dehydration, airway obstruction, and death (Bahia, Mourão, & Chun, 2016; Daniels, Brailey, Priestly, Weisberg, & Foundas, 1998; Daniels & Foundas, 1997; Galovic et al., 2013; Kumral, Celebisoy, Celebisoy, Canbaz, & Calli, 2007; Leder & Espinosa, 2002; Miller, 1999, 2008; Nunes et al., 2012; Smithard et al., 1996; Urban, Hopf, Fleischer, Zorowka, & Müller-Forell, 1997; Urban, Wicht, Hopf, Fleischer, & Nickel, 1999). Underlying these severe and debilitating clinical consequences is the presumed disruption of descending axons destined for the brainstem reticular formation, and corticobulbar fibers directly innervating cranial nerve motor nuclei. The latter is of particular importance because direct cortical input to brainstem motoneurons is a unique higher-order primate trait of motor control, and a critical substrate for the production of precise and highly coordinated orofacial movements (Kuypers, 1956, 1958a, 1958b, 1958c, 1981). However, little is known about the subcortical anatomical trajectory of the corticobulbar projection in the primate brain, particularly at the level of the corona radiata and internal capsule which are both common targets of subcortical white matter injury. Indeed, our understanding of somatotopic organization of axon fiber representation in the internal capsule (IC) remains limited, continuing to emphasize the traditional view that orofacial representation resides in the genu of the internal capsule (e.g., Adams, Victor, & Ropper, 1997; Brazis, Masdeu, & Biller, 2011; Campbell, 2013; Standring, 2016). Supporting this classical conception are clinical observations reporting that damage to the genu of the internal capsule results in facial muscle and tongue dysfunction and the allied emergence of dysarthria and dysphasia (e.g., Bogousslavsky & Regli, 1990; Chung et al., 2000; Rousseaux, Lesoin, & Quint, 1987; Tatemichi, Desmond, Prohovnik, Cross, Gropen, Mohr, & Stern, 1992; Tredici, Pizzini, Bogliun, & Tagliabue, 1982; Urban, Hopf, Connemann, Hundemer, & Koehler, 1996; Urban et al., 1997). Yet orofacial deficits also accompany internal capsule injury involving the anterior limb (Adams, Damasio, Putman, & Damasio, 1983; Caplan et al., 1990; Ichikawa & Kageyama, 1991; Kumral et al., 2007; Maestroni et al., 2006; Ozaki, Baba, Narita, Matsunaga, & Takebe, 1986) and posterior limb (Ghika, Bogousslavsky, & Regli, 1989; Gillingham, 1962; Helgason, Caplan, Goodwin, & Hedges, 1986; Northam et al., 2012; Titelbaum, Sodha, & Moonis, 2010; Tredici et al., 1982; Urban et al., 2001; Yim et al., 2013), indicating transiting pathway trajectories, and possibly, more widespread orofacial fiber representation than currently recognized.
Some support for this notion stems from a non-human primate study utilizing radiolabeled amino acids to trace descending projections to the pons (Schmahmann, Rosene, & Pandya, 2004). It was reported that fibers from the face representation of the primary motor cortex (M1) and face representation of the supplementary motor cortex (M2) occupy different regions of the internal capsule. The projection from M2 was described as passing through the anterior limb of the internal capsule, then genu, whereas the orofacial projection from M1 was reported to pass through the posterior limb of the internal capsule. Within the crus cerebri of the midbrain cerebral peduncle (ccCP), M2 face fibers were found to be more medial than fibers from the M1 face region. What remains unknown is the course of the descending corticobulbar projection from the orofacial regions of the ventrolateral premotor cortex (LPMCv), rostral cingulate motor cortex (M3), and caudal cingulate motor cortex (M4). Underscoring the importance of localizing all of these descending pathways is evidence from multiple non-human primate studies demonstrating that the orofacial region of all five cortical motor areas gives rise to terminal projections ending in multiple cranial nerve motor nuclei including the facial nucleus (Gong, DeCuypere, Zhao, & LeDoux, 2005; Jenny & Saper, 1987; Kuypers, 1958a; Morecraft, Louie, Herrick, & Morecraft-Stilwell, 2001; Morecraft, Schroeder, & Keifer, 1996; Simonyan & Jürgens, 2003), hypoglossal nucleus (Kuypers, 1958a; Morecraft, Stilwell-Morecraft, Solon-Cline, Ge, & Darling, 2014), and trigeminal motor nucleus (Kuypers, 1958a; Morecraft et al., in preparation). Furthermore, the spatial relationships among the pathways emanating from all five orofacial cortical representations, and the degree of potential subcortical pathway overlap remains unknown, as is the location of the corticobulbar projection from M3, M4, and LPMCv through the midbrain ccCP.
The implications of these questions relate not only to the fundamental problem of orofacial fiber localization, but also to recent experimental evidence on upper extremity pathway organization. Our previous non-human primate study has shown that fiber pathways emerging from the arm representation of the same five motor regions (M1, LPMCd, M2, M3, and M4) are located in widespread regions of the CR (Morecraft et al., 2002). Progressing into the territory of the IC, these fibers systems remain relatively dispersed occupying the anterior limb, genu, and posterior limb (Fries, Danek, Scheidtmann, & Hamburger, 1993; Morecraft et al., 2002). However, at lower IC levels and within the ccCP, these pathways gradually shift in location and the extent of overlap between neighboring fiber tracts increase (Morecraft et al., 2002; Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007). Thus, somatotopic organization of descending motor pathways may be more complex than currently interpreted and orofacial and arm pathways from different motor areas may overlap. Examining the spatial relationships between orofacial and arm representation in the IC with multiple tract tracers in the same animal experiment may shed light on this issue. Clinically, this information could be useful for interpreting motor response patterns accompanying deep brain stimulation (DBS) in the IC region, including overlapping somatotopic representation, co-activation of tongue, face and arm responses, and motor-related side effects (Beric et al., 2001; Bertrand, Blundell, & Musella, 1965; Costa, Valls-Solé, Valldeoriola, Rumià, & Tolosa, 2007; Duerden, Finnis, Peters, & Sadikot, 2011; Hanaway et al., 1977; Hardy, Bertrand, & Thompson, 1979; Wichmann & DeLong, 2006; Xu et al., 2011). Such information may also be beneficial for interpreting diffusion tractography findings in the human focusing on corticobulbar pathway organization (e.g., Holodny et al., 2005a,b; Pan, Peck, Young, & Holodny, 2012; Yim et al., 2013).
Therefore, the purpose of the current study was to investigate the course of descending axonal projections from the frontal and cingulate orofacial regions through the coronal radiata, internal capsule, and midbrain crus cerebri. We also compared the descending trajectory of orofacial representation to the descending trajectory of arm representation in M1, M2, and M3 using multiple tract tracing approaches in the same experimental brain. Furthermore, we examined the relationship of descending projections originating from the dorsal part of the M1 orofacial representation (primarily face region) and ventral part of the M1 orofacial representation (primarily tongue region) in the same manner. High-resolution anterograde dextran tract tracers were used in various combinations in the rhesus monkey brain to accomplish these goals.
2 | MATERIALS AND METHODS
The trajectory of the descending projection from the orofacial region of M1, LPMCv, M2, M3, and M4 was studied in the corona radiata, internal capsule, and midbrain crus cerebri using 17 cerebral cortical injection sites in the brain of 11 rhesus monkeys (Macaca mulatta) (Table 1). Localization of descending projections from the arm region of M1, M2, and M3 were also studied using four additional injection sites for comparisons to orofacial pathway representation (Table 1). The trajectories were examined from experimental material prepared in both horizontal and coronal planes. All applied experimental and surgical procedures followed United States Department of Agriculture, National Institutes of Health, and Society for Neuroscience guidelines for the ethical treatment of animals and were approved by the Institutional Animal Care and Use Committee at The University of South Dakota. A brief description of the experimental methods used to accomplish the goals of this study are provided below.
TABLE 1.
Description of the experimental and injection site parameters involved in each monkey case
| Case number (gender) | Area injected | Tracer/injections | Total volume (μL) | Survival (days) | Plane of section |
|---|---|---|---|---|---|
| SDM14 (F) | M1 face | PHAL/4 | 0.8 | 30 | Horizontal |
| LPMCv(ventral) | FD/4 | 0.8 | |||
| M3 face | BDA/2 | 0.6 | |||
| M4 face | FR/1 | 0.4 | |||
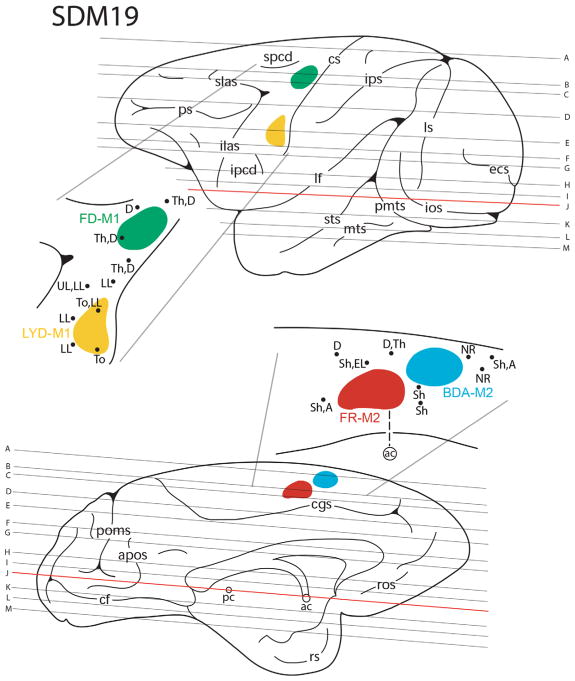
| SDM19 (M) | M1 face | LYD/4 | 1.2 | 26 | Horizontal |
| M1 arm | FD/4 | 0.9 | |||
| M2 face/arm | BDA/2 | 0.6 | |||
| M2 arm | FR/2 | 0.6 | |||
| SDM22 (M) | LPMCv(ventral) | BDA/4 | 1.2 | 29 | Coronal |
| M4 face | LYD/1 | 0.4 | |||
| SDM32 (M) | M1 face(dorsal) | BDA/1 | 0.4 | 30 | Coronal |
| M1 face(ventral) | FD/1 | 0.4 | |||
| SDM35 (F) | M2 face/preSMA | BDA/1 | 0.3 | 29 | Coronal |
| M2 arm/face | FD/2 | 0.6 | |||
| SDM36 (M) | M3 face | FD/2 | 0.6 | 35 | Coronal |
| M3 arm/face | BDA/2 | 0.6 | |||
| SDM39 (F) | M4 face | FD/3 | 1.2 | 32 | Coronal |
| SDM43 (F) | M3 face | FD/3 | 1.2 | 34 | Coronal |
| SDM57 (F) | LPMCv(dorsal) | BDA/3 | 1.2 | 32 | Coronal |
| SDM61 (F) | LPMCv(dorsal) | BDA/3 | 1.2 | 33 | Coronal |
| SDM66 (F) | M1 face(ventral) | LYD/3 | 1.2 | 33 | Coronal |
2.1 | Neurosurgical exposure and cortical tract tracer injection
All neurosurgical procedures were performed under aseptic conditions. Preoperatively, each monkey was immobilized with atropine (0.5 mg/kg) then ketamine hydrochloride (10 mg/kg). Each subject was intubated, placed on a mechanical ventilator and anesthetized with either pentobarbital (early cases), or a mixture of 1.0–1.5% isoflurane and surgical grade air/oxygen. Mannitol was administered intravenously (1.0–1.5 g/kg) to reduce overall cortical volume and enhance surgical accessibility of the cortex. The method of neurosurgical exposure of the medial and lateral surfaces of the cerebral cortex and subsequent wound closure have been reported (Morecraft et al., 2013, 2014, 2015a). Electrophysiological microstimulation was used in combination with ketamine (10 mg/kg) and diazepam (1.0 mg/kg) to map M1, LPMC, and M2 as described in previous reports (McNeal et al., 2010; Morecraft et al., 2013, 2014). Anatomical landmarks were used to localize the rostral (M3) and caudal (M4) cingulate motor regions in the lower bank of the cingulate sulcus (Morecraft et al., 1996, 2001, 2002).
In all experiments, multiple tract tracer injections were made 3–4 mm below the cortical surface using a surgical microscope and Hamilton microsyringe secured to a Kopf micromanipulator (Morecraft et al., 2013). The injected anterograde tracers included 10% biotinylated dextran amine (BDA) in 0.9% sterile saline, 10% lucifer yellow dextran (LYD) in 0.9% sterile saline, 10% fluorescein dextran (FD) in sterile distilled water, and 10% fluoro ruby (FR) in sterile distilled water (Invitrogen/Molecular Probes Inc, Eugene, OR) (Table 1). The FD solution was composed of an equal mixture of 3,000 and 10,000 MW volumes, as was the FR solution. In all experimental cases, total injection volumes ranged from 0.4 to 1.2 μL (Table 1).
2.2 | Tissue processing
Following a survival period of 26–35 days after tract tracer injection (Table 1), each monkey was deeply anesthetized with an IP overdose of pentobarbital (50 mg/kg or more) and perfused transcardially with 0.9% saline. For tissue fixation 2 L of 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 (PB) was infused, then 1 L each of 10 and 30% sucrose in 0.1 M PB for tissue cryoprotection. The CNS was removed, placed in 30% sucrose in 0.1 M PB and stored for 2–5 days at 4°C. In all cases the cerebral cortex was frozen sectioned in either the horizontal plane or coronal plane on a sliding microtome at a thickness of 50 μm in cycles of 10. Cases cut in the horizontal plane were sliced in parallel to the anterior-posterior commissure line (ac-pc line). This experimental design was implemented so that findings from our study could potentially be applied, or compared to tractography observations in the corona radiata, capsular region, and ventral midbrain obtained in the standard human MR axial plane (Kim, Ahn, Chung, & Kim, 2009; Talairach & Tournoux, 1988; Weiss et al., 2003). Each brainstem was blocked from the CNS, frozen with dry ice and cut horizontally on the microtome at a thickness of 50 μm in cycles of 5, 7, or 10. For both the cortex and brainstem, one series of tissue sections was mounted on subbed slides, dried and subsequently stained with thionin for Nissl substance.
For tract tracer visualization under brightfield illumination, one series of tissue sections through the cortex and brainstem was immunohistochemically processed for BDA alone, then several additional series of tissue sections were processed for double labeling of tract tracer (e.g., BDA +LYD; BDA +FD) as described in our previous studies (Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007; Morecraft et al. 2013, 2014). In double labeled preparations one tracer was stained brown, and the other was stained either blue or black (Figures 1 and 2). Once processed, the tissue sections were mounted on subbed slides, dried overnight, and coverslipped using Permount.
FIGURE 1.
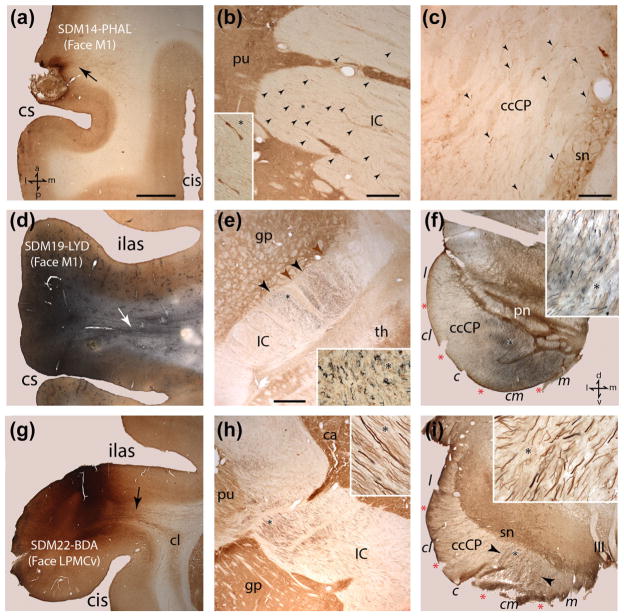
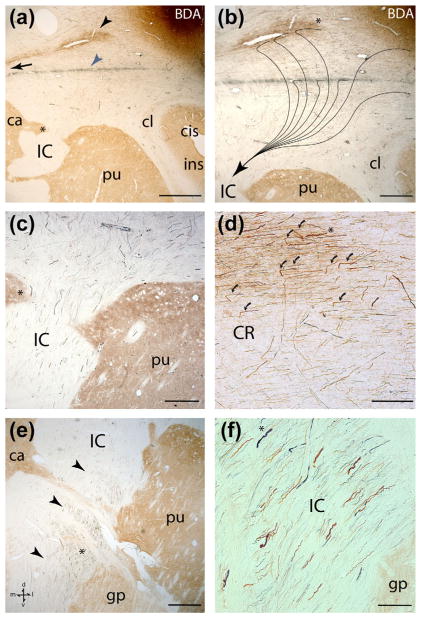
Photomicrographic montage of representative examples of lateral motor cortex injection sites and labeled fibers captured under brightfield illumination from immunohistochemically developed tissue sections. (a) Horizontal section showing the PHAL injection site in the M1 orofacial representation in case SDM14. The black arrow identifies a coalesced fiber bundle emerging from the injection site that is coursing medially. (b) Horizontal section through mid-levels of the internal capsule (IC) in case SDM14 showing PHAL labeled fibers (black arrowheads) in the lateral half of the IC. The inset is a higher magnification of labeled fibers from the IC location marked by the asterisk. (c) Horizontal section in SDM14 though superior levels of the midbrain showing labeled PHAL fibers (black arrowheads) in the crus cerebri of the cerebral peduncle (ccCP). (d) Horizontal section of the LYD injection site in the orofacial region of M1 in case SDM19. The white arrow identifies a band of labeled fibers emerging from the injection site coursing medially. (e) Horizontal section through inferior levels of the internal capsule in case SDM19 showing LYD labeled fibers (blue) from the M1 orofacial injection site and BDA labeled fibers (brown) from the orofacial/arm injection site in M2. The IC region occupied by the main bundle of M1 (blue) labeled fibers is denoted by the black arrowheads, and the M2 (brown) labeled fibers by the brown arrowheads. Note the zone of overlap between the two labeled fascicles. The inset is a higher magnification of labeled fibers from the IC location marked by the asterisk. (f) Horizontal section though the lower midbrain in case SDM19 showing labeled LYD and BDA fibers in the ccCP. Note the extensive overlap of LYD and BDA labeled fibers throughout the centromedial (cm) and medial (m) sectors of the ccCP, with some commixed fiber labeling occupying the posterior region of the central (c) sector. Pontine nuclei (pn) are visible demarcating the juncture between the midbrain and pons. The inset is a higher magnification of labeled fibers marked by the asterisk. (g) Coronal section showing the BDA injection site in LPMCv in case SDM22. Note that the injection site was largely confined to the cortical surface and did not spread to involve area 44 in the depths of the arcuate sulcus which has been shown by comparative cytoarchitectonic analysis (Petrides & Pandya, 2002) and by functional microstimulation (Petrides, Cadoret, & Mackey, 2005) to be homologous to Broca’s area. The black arrow identifies a distinct band of labeled BDA fibers emerging from the injection site that is coursing medially and over the claustrum (cl) to eventually enter the corona radiata. (h) Coronal section from case SDM22 showing descending BDA labeled fibers originating from the LPMCv orofacial region passing through mid-levels of the internal capsule. Note the primarily lateral disposition of the main bundle lying adjacent to the putamen and globus pallidus. The inset is a higher magnification of labeled fibers from the IC location marked by the asterisk. (i) Transverse section showing descending BDA labeled fibers passing through mid-levels of the midbrain in case SDM22. Note the primary concentration of labeled fibers is located within the centromedial sector of the ccCP (external limits marked by the black arrowheads). The inset is a higher magnification of labeled fibers marked by the asterisk. The anatomical orientation shown in bottom left of panel a applies to panels a–e and the anatomical orientation shown in bottom right of panel f applies to panels f–i. Scale bar =2 mm in a (applies to d, g); 500 μm in b; 200 μm in c; 1 mm in e (applies to f, h, i). c =central sector of the ccCP; ccCP =crus cerebri of the cerebral peduncle; cis =circular sulcus; cl =claustrum; cm =centromedial sector of the ccCP; cs =central sulcus; gp =globus pallidus; l =lateral sector of the ccCP; IC =internal capsule; III =occulomotor nerve; ilas =inferior limb of the arcuate sulcus; m =medial sector of the ccCP; pn =pontine nuclei; pu =putamen; sn =substantia nigra; th =thalamus.
FIGURE 2.
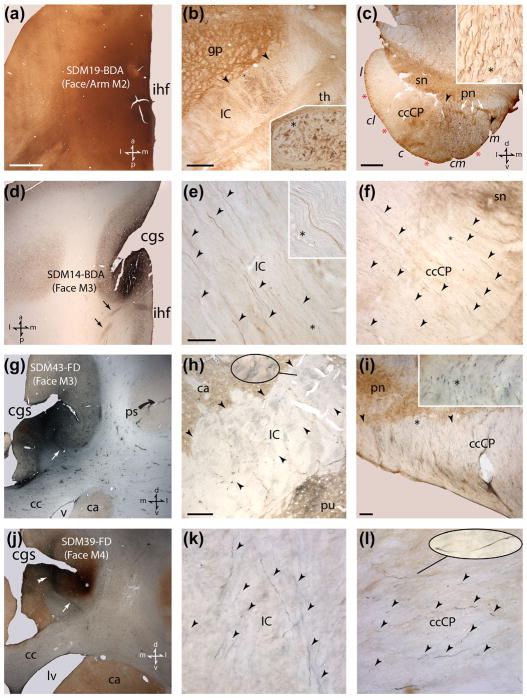
Photomicrographic montage of representative examples of medial motor area injection sites and labeled fibers taken from immunohistochemically developed tissue sections under brightfield illumination. (a) Horizontal section through the M2 BDA injection site in case SDM19. The anatomical orientation shown in the bottom right applies to panels a and b. (b) Horizontal section through inferior levels of the internal capsule in case SDM19 showing BDA labeled fibers (brown) from the orofacial/arm injection site in M2. The IC region occupied the main BDA labeled fascicle is demarcated by the black arrowheads. The inset is a higher magnification of labeled fibers from the IC location marked by the asterisk. (c) Transverse section though inferior levels of the midbrain showing labeled BDA fibers in the cerebral peduncle occupying both centromedial (cm) and medial (m) sectors of the ccCP. Note the emergence of pontine nuclei (pn) marking the presence of pontine structures. The inset is a higher magnification of BDA labeled fibers from the ccCP location marked by the asterisk. The anatomical orientation depicted in the bottom right applies to panels c and f. (d) Low power image from case SDM14 of a representative horizontal section through the BDA injection site in the orofacial region of M3. The black arrows identify coalesced groups of labeled fibers emerging from the injection site coursing posteriorly in the corona radiata. The anatomical orientation shown in the bottom left applies to panels d and e. (e) Photomicrograph of a representative horizontal section through mid-levels of the internal capsule showing BDA labeled fibers (black arrowheads) in case SDM14 in the medial half of the IC. The inset is a higher magnification of BDA labeled fibers from the IC location marked by the asterisk. (f) Transverse section though superior levels of the midbrain in case SDM14 showing labeled BDA fibers (black arrowheads) in the medial sector of the ccCP. (g) Coronal section showing the FD injection site in the orofacial region of M3 in case SDM43. The white arrow identifies a distinct band of FD labeled fibers arching over the cingulum bundle which coursed posteriorly to pass over the caudate and into the anterior limb of the IC. The anatomical orientation shown in the bottom right applies to panels g–i. (h) Coronal section through mid-levels of the internal capsule in case SDM43 showing FD labeled fibers in the medial half (see black arrowheads) of the IC. The oblong pullout is a higher magnification of labeled FD fibers from the location marked by the extended line. Note the distinct patch of corticostriate FD labeling in part of the putamen bordering the lateral region of the IC (directly above “pu”). (i) Transverse section showing FD labeled fibers passing through the medial sector of the ccCP at inferior levels of the midbrain in case SDM43. Note the pontine nuclei, demarcating the midbrain-pontine transition region. The overall extent of the ccCP region occupied by FD labeled fibers is demarcated by the two black arrowheads. The inset is a higher magnification of labeled fibers from the IC location marked by the asterisk. (j) Coronal section showing the blue labeled FD injection site (double arrowhead) in the orofacial region of M4 in case SDM39. The white arrow identifies a distinct band of labeled FD fibers arching over the cingulum bundle. This labeled fascicle coursed posteriorly to eventually arch over the caudate nucleus and pass into the anterior quarter of the ICp. The anatomical orientation shown in the bottom right applies to panels j–l. The white asterisk is over the BDA injection site which extends anterior to this coronal level (i.e., into the M3 arm area). (k) Coronal section through inferior-levels of the internal capsule in case SDM39 showing FD labeled fibers (arrowheads) in the medial half of the IC. (l) Transverse section showing FD labeled fibers (arrowheads) passing through the centromedial sector of the ccCP at inferior levels of the midbrain in case SDM39. The oblong pullout is a higher magnification of labeled FD fibers from the location marked by the extended line. Scale bar =2 mm in a (applies to a, d, g, j); 1 mm in b; 1 mm in c; 50 μm in e (applies to e, f, k, l); 500 μm in h; 100 μm in i. cc =corpus callosum; cgs =cingulate sulcus; ihf =interhemispheric fissure; lv =lateral ventricle; ps =principal sulcus; v =ventricle. For other abbreviations see Figure 1.
In cases where brain tissue was processed for fluorescent visualization (Figure 3), one additional series of tissue sections was mounted on subbed slides and dried overnight at 4°C. For the present report, these cases included SDM14 (for visualizing and plotting the FD and FR labeled fibers) (Figure 3a–d) and case SDM19 (for visualizing and plotting the FD and FR labeled fibers) (Figure 3e–h). The following day the slides were removed from refrigeration, allowed to reach room temperature, then coverslipped with D.P.X. mounting medium (Aldrich Chemical Company, Milwaukee, WI) and stored at 4°C (Morecraft, Geula, & Mesulam, 1992; Morecraft et al., 2012; Morecraft, Stilwell-Morecraft, Ge, Cipolloni, & Pandya, 2015b).
FIGURE 3.
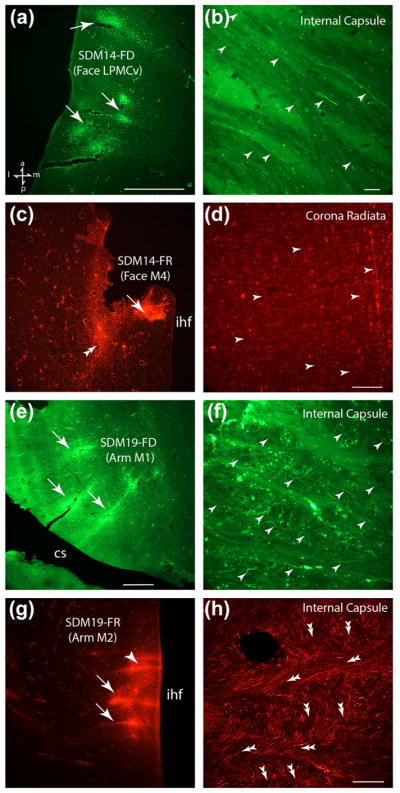
Photographic montage of representative examples of injection sites and labeled fibers from lateral and medial motor cortex captured under fluorescent illumination. All sections are in the horizontal plane and the anatomical orientation shown in the bottom left of panel a applies to all panels. (a) Low power image in case SDM14 depicting the FD injection site in the orofacial region of LPMCv. The white arrows show the core areas of the injection site. (b) FD labeled axons (white arrowheads) in case SDM14 passing over the putamen and into the superior region of the internal capsule (see horizontal level f in Figure 5). (c) Low power image in case SDM14 showing the FR injection in the orofacial region of M4. The double white arrow head denotes a coalesced fiber bundle emerging from the injection site and curving posteriorly to enter the coronal radiata. The white arrow shows the core area of the FD injection site. (d) Example of elongated FR labeled fibers (white arrowheads) in case SDM14 coursing posteriorly in the corona radiata toward the internal capsule (see horizontal level b in Figure 5). (e) Low power image in case SDM19 showing the FD injection in the arm region of M1 in the anterior bank of the central sulcus (cs). The white arrows show the core areas of the injection site. (f) FD labeled axons (white arrowheads) in case SDM19 within the superior region of the internal capsule just after passing over the putamen (see horizontal level f in Figure 7). (g) Low power image in case SDM19 showing the FR injection in the arm region of M2 on the medial surface of the superior frontal lobule. The white arrows show the core areas of the injection site and the arrowhead some microstimulation tracks. (h) Tissue section from case SDM19 showing the extensive matrix of interlaced FR labeled axon bundles in the superior region of the internal capsule just after passing around the caudate nucleus to gain access into the IC (see horizontal level f in Figure 7). The double headed arrows indicate the general trajectory of the FR labeled fascicles with those coursing horizontally pointing to the left and those fascicles coursing inferiorly pointing downward and slightly to the right. Scale bar =1 mm in a (also applies to c); 50 μm in b; 100 μm in d (also applies to f); 1 mm in e (also applies to g); 200 μm in h. cs =central sulcus; ihf =interhemispheric fissure.
2.3 | Data analysis
Localization of the cortical injection site and descending labeled fibers was accomplished using brightfield or fluorescent illumination on a BX-60 or BX-51 Olympus microscope (Leeds Precision Instruments, Minneapolis, MN). Attached to the microscope was a high resolution MAC 5000 motorized stage (Ludl Electronic Products, Hawthorne, NY) which was interfaced with Neurolucida (Neurolicida, RRID: SCR_001775) neuroanatomical data collection software system (MBF Bioscience, Williston, VT, RRID: SCR_004314). For a tract tracer experiment to be included in the analysis of the current study, at least every other tissue section (spaced 1 mm apart) through the corona radiata and internal capsule was required. Using the software system, 4×–40× microscope objectives, and the coverslipped tissue sections, we recorded the external contour of the brain, the boundary between cortical layer VI and subcortical white matter, the location of each injection site, all major anatomical landmarks, and the locations of labeled fibers within the CR, IC, and ccCP. Matching Nissl stained sections were consulted to confirm the cytoarchitectonic affiliation of each cortical injection site and the major anatomical regions and boundaries in the plotted tissue sections.
2.4 | Data reconstruction and presentation
Using the coverslipped tissue sections, publication quality images of injection sites and labeled fibers were captured under appropriate lighting conditions (brightfield or fluorescent) using a Spotflex 64 Mp shifting pixel camera, (Diagnostic Instruments Inc., Sterling Heights, MI, version 4.6), mounted on a Olympus BX51 microscope (Figures 1–3). Photographic montages of the injection sites and labeled fibers were made with this material using Adobe PhotoShop 7.0 (Adobe Systems Inc., San Jose, CA). Only brightness and contrast were adjusted in the original images. To illustrate the locations of the injection sites, cortical reconstructions were developed as previously described using metrically calibrated photographic images of the cortical surface (Morecraft & Van Hoesen, 1992, 1993).
3 | RESULTS
The majority of injection sites used in the present study were located in the orofacial region of the frontal or cingulate motor cortex (Table 1). This somatotopic affiliation was determined by anatomical or physiological criteria (Morecraft & Van Hoesen, 1992; Morecraft, Louie, Herrick, & Morecraft-Stilwell, 2001; Morecraft et al., 2014) and verified in all cases by the presence of terminal axon labeling in the facial nucleus (Morecraft et al., 2001), hypoglossal nucleus (Morecraft et al., 2014), and trigeminal motor nucleus (Morecraft et al., in preparation). Detailed descriptions of each injection site have been published (i.e., for cases SDM14, SDM19, and SDM22 see Morecraft et al., 2001; for cases SDM32, SDM35, SDM36, SDM43, and SDM66 see Morecraft et al., 2014; for cases SDM57 and SDM61 see Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007). Below we describe the descending orofacial pathways originating from the lateral motor areas followed by the medial motor areas. Finally, we describe the outcome of experiments designed to compare the locations of orofacial pathways to arm pathways.
3.1 | Trajectory of M1 orofacial fibers
3.1.1 | Corona radiata and internal capsule
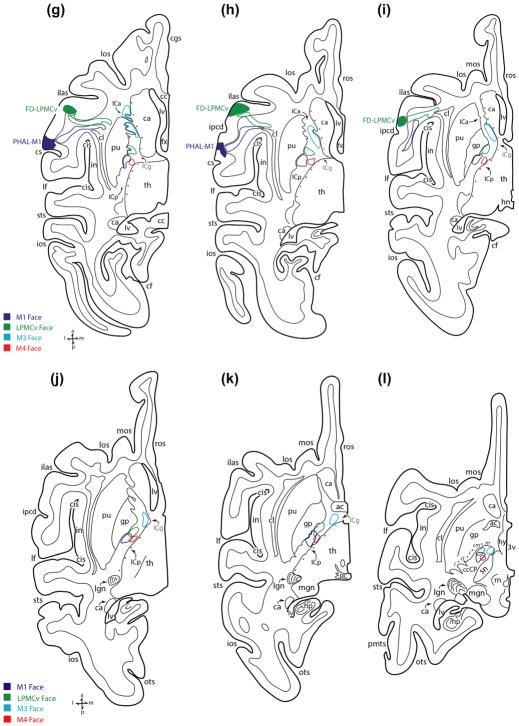
The findings from injection case SDM14-PHAL, which was processed in the horizontal plane, were largely representative of the other M1 orofacial cases and will be presented first (Figures 4 and 5). A dense bundle of labeled fibers emerged directly below the M1 orofacial region in the ventral precentral gyrus (Figure 1a) and turned medially to assume a course directed toward the midline (Figure 5e,f). Fibers emerging from lower levels of the injection site, near the opercular region, also coursed medially while ascending and arching directly over the claustrum/anterior insular/circular sulcus region (Figure 5g–i). While continuing its medial/midline migration, the main trajectory of the bundle gradually coursed superiorly (Figure 5d) then curved over the dorsal surface of the putamen to enter the internal capsule (IC) (Figure 5e,f). At this location the M1 fibers abruptly curved posteriorly, to occupy the anterior-most part of the posterior limb (Figure 5e). Superiorly, and from a medio-lateral perspective, the main bundle of descending fibers (excluding fibers leaving the main bundle to enter the thalamus) occupied the lateral half of the ICp (Figures 1b and 5f,g). Progressing inferiorly, the bundle of fibers gradually shifted posteriorly to completely occupy the anterior quarter of the posterior limb of the internal capsule (ICp) (Figure 5g). The descending M1 fibers continued to migrate posteriorly to occupy the second and third quarter of the ICp (Figure 5h–j). At the level of the anterior commissure-posterior commissure (ac-pc) line the fibers occupied the second quarter of the ICp (Figure 5k). As the labeled fascicle advanced to the level of the subthalamic nucleus/superior midbrain, it shifted slightly anteriorly to accommodate the anatomical transition into the midbrain ccCP region (Figure 5l).
FIGURE 4.
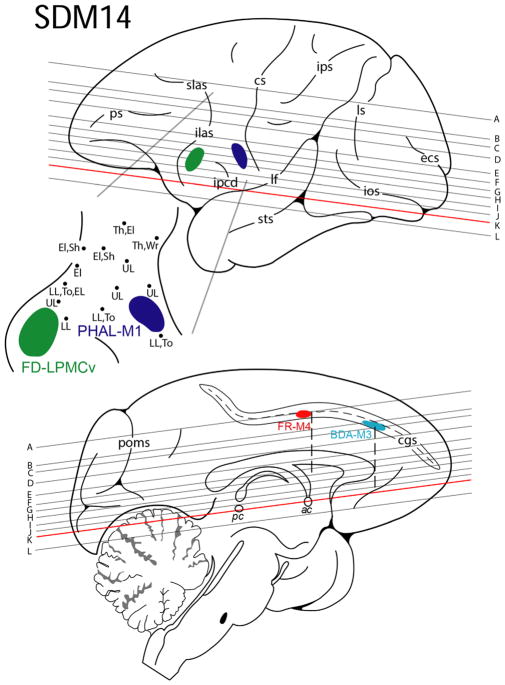
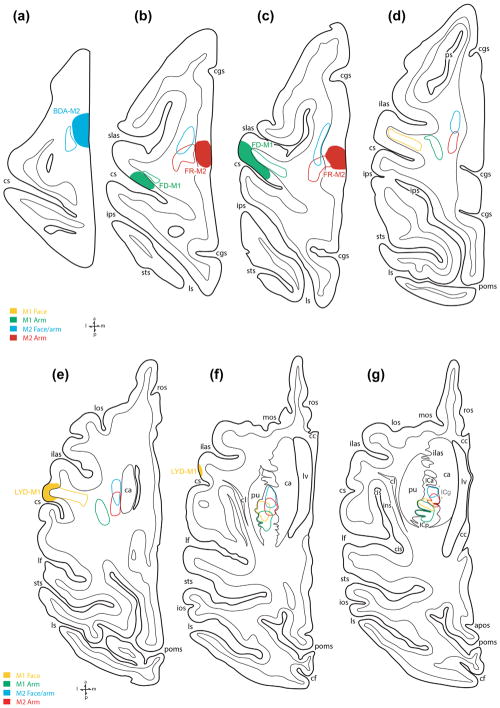
Line drawing of the lateral (top) and medial (bottom) surfaces of the cerebral cortex illustrating the experimental design in case SDM14. Cortical injections (colored irregular spheres) were made into the orofacial representation of four different motor areas in the left hemisphere. The course of the corticobulbar pathway was mapped in the corona radiata, internal capsule and cerebral peduncle using immunohistochemical and fluorescent processed tissue sections. The top pullout depicts the location of the phaseolus vulgaris leucoagglutinin (PHAL) injection site in M1 (dark blue) and the fluorescein dextran (FD) injection site in LPMCv (green) in relation to physiological mapping on the lateral cortical surface. Each black dot represents a stimulation point labeled with the corresponding body part where the evoked movement was observed. Shown on the medial surface is the fluoro ruby (FR) injection site in M4 (red) and biotinylated dextran amine (BDA) injection site in M3 (light blue) in the opened cingulate sulcus (cgs). The horizontal lines indicate the level of each representative tissue section shown graphically in Figure 5 and are in reference to the anterior (ac) and posterior (pc) commissural plane (see horizontal section k). For abbreviations: see list
FIGURE 5.
Line drawings of representative horizontal sections depicting the cortical injection sites and descending trajectories of orofacial pathways emanating from M1, LPMCv, M3, and M4 in case SDM14 studied through the corona radiata and internal capsule upper-most level of the midbrain. Sections are shown from superior (a) to inferior (l) and the location of each level with respect to the intact cerebral cortex is depicted in Figure 4. Each injection site is color coded and specifically identified by tract tracer and motor area (e.g., dark blue, PHAL-M1). Each respective descending fiber bundle is identified by a corresponding colored outline that matches the appropriate color-coded motor area injection site. Note the vacated space in the IC between the M3 and LPMCv fiber pathway (horizontal levels g–k), which based upon the results from case SDM19 (Figure 7) would likely have accommodated the M2 orofacial tract. For abbreviations: see list
In general, the M1 orofacial pathway in case SDM19-LYD (Figures 1d, 6, and 7) which was also prepared in the horizontal plane, followed a similar path in the corona radiata as reported above for case SDM14-PHAL in that it coursed medially and arched superiorly over the anterior region of the putamen to enter the IC (Figure 7d–f). Like case SDM14-PHAL, at the most superior level of the IC (Figure 7f) the projection occupied the anterior quarter of the ICp (Figure 7f). In the IC there were some minor variances between the cases in that the M1 orofacial projection in SDM19-LYD spread medially at middle as well as inferior levels of the IC (i.e., to occupy part of the IC adjacent to the lateral border of the thalamus) (Figure 7i–k). At middle and inferior ICp levels the M1 pathway in SDM19-LYD primarily occupied the second quarter of the ICp (Figure 7i–k) as in case SDM14-PHAL (Figure 5i–k).
FIGURE 6.
Line drawing of the lateral (top) and medial (bottom) surfaces of the cerebral cortex illustrating the experimental design in case SDM19. Cortical injections (colored irregular spheres) were made into two different somatotopic representations (orofacial and arm) in M1 and M2 in the left hemisphere. The course of each corresponding corticobulbar and corticospinal projection was mapped in the corona radiata, internal capsule, and cerebral peduncle using immunohistochemical and fluorescent processed tissue sections. The top pullout depicts the location of the lucifer yellow dextran (LYD) injection site in orofacial representation of M1 (yellow) and the fluorescein dextran (FD) injection site in arm representation of M1 (green) in relation to physiological mapping on the lateral cortical surface. Each black dot represents a stimulation point labeled with the corresponding body part where the evoked movement was observed. The bottom pullout demonstrates the location of the fluoro ruby (FR) injection site in the arm representation of M2 (red) and the biotinylated dextran amine (BDA) injection in the M2 face/arm representation (blue) in relation to physiological mapping. The horizontal lines indicate the level of each representative tissue section shown graphically in Figure 7 (see horizontal plates a–m) and are in reference to the anterior (ac) and posterior (pc) commissural plane (see horizontal section j). For abbreviations: see list
FIGURE 7.
Line drawings of representative horizontal sections depicting the cortical injection sites and descending trajectories of the M1 and M2 orofacial and arm pathways in the corona radiata, internal capsule, and upper levels of the cerebral peduncle in case SDM19. Sections are shown from superior (a) to inferior (m) and the location of each horizontal level with respect to the intact cerebral cortex is shown in Figure 6. Each injection site is color coded and specifically identified by tract tracer and motor area (e.g., yellow, LYD-M1). Each respective descending fiber bundle is identified by a corresponding colored outline that matches the appropriate color-coded motor area injection site. For abbreviations: see list
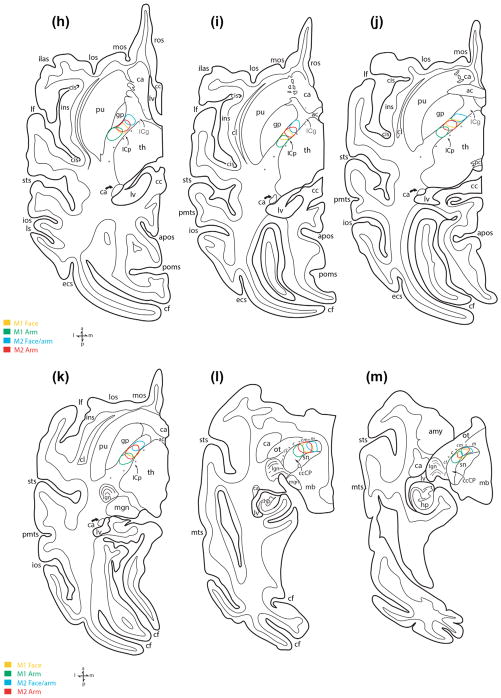
The M1 orofacial pathway was also studied in three additional monkey cases (SDM22, SDM32, and SDM66; Figure 8 (top); Table 1) histologically prepared in the coronal plane and corroborated the major features found in cases SDM14-PHAL and SDM19-LYD described above. What was particularly prominent in the coronal dimension was the anatomical organization of fibers coursing medially in the corona radiata and over the putamen to enter the IC. Notably, in case SDM32, 2 different high-resolution tracers were placed in the orofacial region of M1. One tracer was placed dorsally that involved primarily the M1 face region (SDM32-BDA), and the second tracer (SDM32-FD) was injected ventrally, that involved primarily the M1 tongue region (Figure 8; Table 1; see also Morecraft et al., 2014, Figure 4a). In the CR, BDA labeled fibers emerging from the more dorsally located injection slightly descended to take a medial course toward the corpus callosum (Figure 9a). Fibers from the ventrally located FD injection site arched directly over the insula/circular sulcus region and claustrum with BDA labeled fibers from the dorsal injection site positioned more dorsal in the CR (Figure 9a, large arrowheads: see also Morecraft et al., 2014, Figure 4a). What was clearly evident from this plane of section was the curvilinear trajectory assumed by fibers leaving the ventral pathway in close proximity to the injection site (Figure 9b). As both fiber bundles passed through the territory of the superior longitudinal fasciculus I and II (of Petrides & Pandya, 1984; see also Schmahmann & Pandya, 2006), fibers systematically branched from the main bundle of labeled axons (Figure 9a,b). Specifically, directly over the IC region numerous labeled fibers were found leaving the main bundle, bending sharply at 60–120° angles to descend into the IC (Figure 9b,d—see curved arrows in panel d). As the labeled fibers from both injection sites entered the IC, there was an immediate intermingling of FD/blue and BDA/brown labeled fibers which continued throughout the descending course of this merged pathway (Figure 9c,e,f). At superior and mid-levels of the IC, the main group of labeled fibers were concentrated laterally against the lentiform nucleus (Figure 9c,e), but at inferior levels of the IC the bundle widened to also occupy the medial region of the IC bordering the thalamus, as found at inferior IC levels in experimental cases SDM14-PHAL (Figure 5k) and SDM19-LYD (Figure 7i,j).
FIGURE 8.
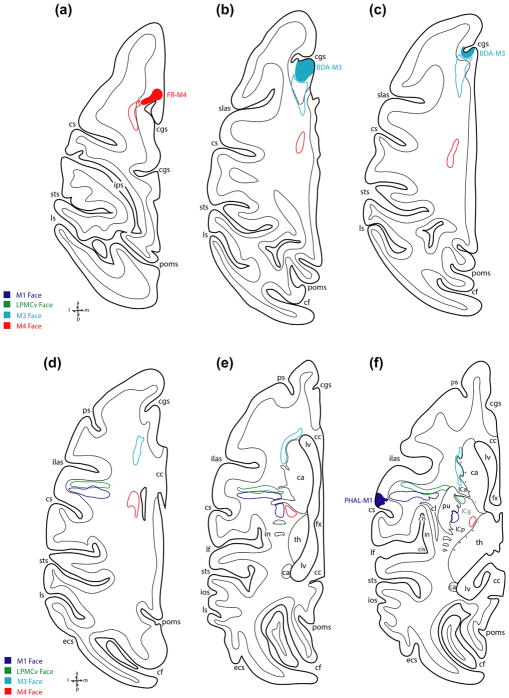
Line drawings of lateral (top) and medial (bottom) surfaces of monkey cases with tract tracer injection sites that were used to evaluate subcortical orofacial pathway organization. All cases shown were processed in the coronal plane. For abbreviations: see list
FIGURE 9.
Photomicrographic plate showing immunohistochemically labeled fibers from case SDM32, which had two injection sites in the M1 orofacial representation (see Figure 8, top right, for lateral cortical surface view of the injection sites). All sections are in the coronal plane and the anatomical orientation shown in the bottom left of panel e applies to all panels. The brown colored fibers are from the more dorsally located BDA injection site and the blue fibers are from the more ventrally located FD injection site. (a) Low power coronal section depicting the main BDA labeled fiber bundle (black arrowhead) and FD labeled fiber bundle (blue arrowhead) traveling in the corona radiata and arching over the putamen (pu). Note the dorsal to ventral location of the respective labeled fiber bundles and distinct spatial separation between the main fascicles. The black arrow identifies the path direction toward the corpus callosum where both fiber bundles eventually cross the midline. The asterisk is for anatomical orientation of panel c, which shows greater detail of the descending labeled fibers in this location but at a higher magnification. (b) Higher magnification of panel a showing the complex, and multifaceted nature of fibers leaving the main BDA labeled fiber bundle and FD labeled fiber bundle to enter the IC. Although the fibers emerge from each main bundle from many micro orientations, the superimposed trajectories (fine black lines leading to the arrowhead) illustrate some curvilinear and angulated trajectories taken by fibers destine to reach the IC. The asterisk adjacent to the main BDA (brown) labeled fiber pathway is for anatomical orientation of panel d, which shows greater detail of the descending labeled fibers in this location but at a higher magnification. (c) Photomicrograph showing FD labeled (blue) and BDA labeled (brown) fibers at superior levels of the IC. The anatomical location of this image is demarcated in panel a (see asterisk). (d) Higher power photomicrograph of BDA labeled fibers leaving the main fiber bundle in the corona radiata (CR). The anatomical location of this image is demarcated in panel b (see asterisk). Note the sharp angles taken by the fibers (curved arrows) as they abruptly changed their trajectory downward en route to the IC. This general pattern of fiber trajectory was also characteristic of fibers traveling from the LPMCv injection sites as they coursed in the CR to enter the IC. (e) Photomicrograph showing the descending fibers from the M1 orofacial injection sites at mid-levels of the IC as they descend near the medial border of the putamen and globus pallidus. Note how the main bundle of fibers occupy the lateral half of the IC (arrow heads). The asterisk is for anatomical orientation of panel f, which shows higher magnification of the labeled fibers in this location. (f) High power photomicrograph of fibers in the IC (anatomical location shown in panel e) illustrating the commixed nature of blue and brown labeled fibers from tracer injections in the ventral and dorsal regions of the M1 orofacial representation. Scale bar =2 mm in a; 1 mm in b; 500 μm in c; 250 μm in d; 1 mm in e; 100 μm in f. BDA =biotinylated dextran amine; ca =caudate nucleus; cis =circular sulcus; cl =claustrum; CR =corona radiata; gp =globus pallidus; IC =internal capsule; ins =insula; pu =putamen
3.1.2 | Midbrain cerebral peduncle
Entering the midbrain, and within the crus cerebri of the cerebral peduncle (ccCP), M1 orofacial fibers occupied the medial part of the central sector and adjacent centromedial sector (Figures 1c, 5l, 7l, and 10a). Coursing further inferiorly the fibers continued to occupy primarily the centromedial sector (Figures 7m and 10b,c). Inferiorly in the midbrain, M1 orofacial fibers spread medially to involve both centromedial and medial sectors as well as posterior part of the central sector, particularly at the level of the midbrain-pontine isthmus (Figures 1f and 10d). In the double labeling experiment (case SDM32), there was extensive intermingling of FD and BDA labeled fibers from the orofacial region of M1 that followed the same general course described above.
FIGURE 10.
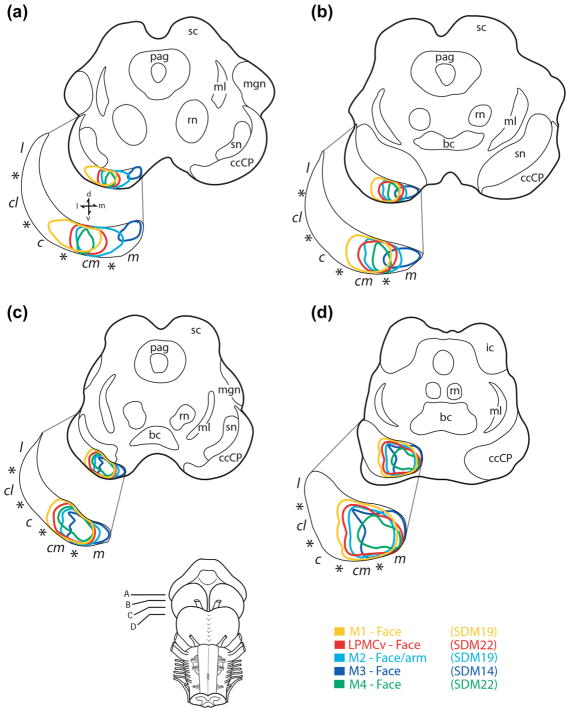
Line drawings of horizontal sections through the midbrain showing the location of descending orofacial pathways from M1 (yellow), LPMCv (red), M2 (light blue), M3 (dark blue), and M4 (green). To obtain each pathway location, individually labeled fibers in the ccCP were plotted using immunohistochemically processed tissue preparations in three animal cases (SDM14, SDM19, and SDM22) and the data was superimposed at equivalent horizontal midbrain levels (panel a, superior- panel d, inferior). Note that at superior midbrain levels (a) the M1 orofacial pathway was relatively lateral, and M3 pathway medial, with significant overlap of all other pathways in between. Progressing inferiorly (b, c) pathway overlap gradually increased. At inferior midbrain levels (d) complete overlap occurred and the space occupied by each pathway expanded as a result of the significant commixed nature of fiber organization. Anatomical subsectors of the ccCP are shown separated by each asterisk (c, central; cl, central lateral; cm, centromedial; l, lateral; m, medial). For other abbreviations see list
3.2 | Trajectory of LPMCv orofacial fibers
3.2.1 | Corona radiata and internal capsule
Four experimental cases were used to evaluate the descending projection from LPMCv, one histologically prepared in the horizontal plane (injection SDM14-FD) (Figures 3a and 4), and three prepared in the coronal plane (injection SDM22-BDA, SDM57-BDA, and SDM61-BDA) (Figures 1g and 8). First, the results of injections placed into the ventral (orofacial) region of LPMCv will be described (cases SDM14-BDA, case SDM22-BDA). Then we will describe the findings from the injections placed in the dorsal (orofacial/arm) region of LPMCv (cases SDM57-BDA and SDM61-BDA).
A large and dense bundle of labeled fibers emerged below the ventral LPMCv orofacial area and turned medially to arch over the claustrum/anterior insula/circular sulcus region (Figures 1g and 5g–i). From a horizontal view, the position was directly anterior to the M1 orofacial pathway with little to no overlap as determined in monkey SDM14 (Figure 5g–i). While continuing its medial/midline migration, the main trajectory of the LPMCv axon bundle gradually coursed superiorly (Figure 5d), then curved over the dorsal surface of the putamen (Figure 5e) to enter the internal capsule (IC) (Figures 3b and 5f). In the coronal plane (case SDM22-BDA), and much like what was found in the M1 orofacial experiments, fibers leaving the main fascicle, as it arched directly over the IC, abruptly redirected their trajectory by bending at sharp angles (60–120° angles), to descend into the IC. From the horizontal perspective, once in the IC, the LPMCv fibers curved posteriorly (Figure 5f), to occupy the genu of the internal capsule (ICg), positioned anterior to the M1 orofacial pathway (Figure 5g). From a medio-lateral perspective (i.e., width of the IC), at superior and mid-levels of the IC, the main bundle of descending fibers occupied the lateral half of the internal capsule posterior limb (ICp) (Figures 1h and 5f–h). Progressing inferiorly, the dense bundle of fibers gradually shifted posteriorly to occupy the first (anterior) quarter of the ICp (Figure 5i), then first and second quarter of the ICp region including the level of the anterior commissure-posterior commissure (ac-pc) line (Figure 5j,k). As the main fiber bundle advanced to the horizontal level marked by the presence of the superior midbrain/subthalamic nucleus, the M1 fibers entered the ccCP of the midbrain (Figure 5l).
We then compared the pathway trajectory of cases processed in the coronal dimension in which the injection was placed in the ventral region of LPMCv (Figure 8-SDM22-BDA), to cases with the injection site placed in the dorsal part of LPMCv (Figure 8-cases SDM57-BDA and SDM61-BDA). This vantage point demonstrated slightly different planes of trajectory consistent with the general anatomical location of each injection site. That is, the fiber path in the CR from the dorsal LPMCv injection site coursed dorsal to the ventral LPMCv pathway (Figure 1g). Although we did not have a double labeling experiment to precisely determine the extent of CR overlap, it appeared likely that some interdigitating among these two pathways would occur directly over the putamen, with extensive overlap potential within the IC. It is again noteworthy to mention that as the fiber bundle from the dorsal LPMCv region arched over the IC region labeled fibers abruptly redirected their trajectory by bending at sharp angles (60–120°), en route to the IC. Within the IC the dorsal LPMCv fibers appeared to be located in the anterior part of the posterior limb but the lack of a horizontal plane perspective proved difficult to determine exactly how much posterior shift occurred. From a medio-lateral perspective, dorsal LPMCv fibers were largely positioned in the lateral half at superior and mid-levels of the IC, but at inferior IC levels occupied both lateral and medial regions as noted for the ventral LPMCv pathway.
3.2.2 | Midbrain cerebral peduncle
In the midbrain, ventral LPMCv fibers occupied the centromedial sector of the ccCP at superior levels (Figures 5l and 10a). Progressing inferiorly the fibers shifted medially to occupy the medial part of the centromedial sector and lateral part of the adjacent medial sector (Figures 1i and 10b,c). At inferior levels of the ccCP, the territory occupied by the fiber bundle slightly enlarged to occupy extensive part of both centromedial and medial subsectors of the ccCP (Figure 10d). Fibers from the dorsal region of LPMCv took a similar course through the ccCP as the ventral LPMCv pathway with modest differences including some presence of labeled fibers in the medial part of the central sector at superior ccCP levels as previously reported (Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007), with substantial labeling in the centromedial sector as found for the ventral LPMCv pathway (Figure 10a,b). At middle and inferior levels of the ccCP the dorsal LPMCv fiber path migrated medially (as described for the ventral LPMCv path) to involve the medial and centromedial sectors as previously reported (Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007).
3.3 | Trajectory of M2 orofacial fibers
3.3.1 | Corona radiata and internal capsule
Two cases were used to evaluate the descending projection from the orofacial region of M2, one prepared in the horizontal plane (injection SDM19-BDA) (Figures 2a and 6) and one prepared in the coronal plane (injection SDM35-BDA) (Figure 8). Although each injection site has been described in detail in previous reports (i.e., SDM19, see Morecraft et al., 2001; SDM35, see Morecraft et al., 2014) we would like to point out that in addition to the M2 orofacial representation involvement, the BDA injection site in case SDM19 spread caudally to involve the rostral part of the M2 shoulder/arm region, whereas the BDA injection in case SDM35 spread minimally in the rostral direction to involve a small portion of the adjacent pre-SMA region. Although both injection sites extended outside the intended M2 orofacial area, they were generally representative of the rostral (orofacial) region of M2 because caudal to each injection site another tract tracer was injected into the major portion of the M2 arm representation (Figures 6 and 8). The spatial relationship between the descending pathways arising from these paired injection sites (e.g., the M2 arm area trajectory versus M2 arm/orofacial area trajectory in Case SDM19) will be reported later in the “Results” section.
As noted in the horizontal dimension, the major fiber bundle arising from the M2 orofacial injection site curved caudally in the CR (Figure 7a,b). Continuing on this course, the pathway gradually descended, occupying a position immediately deep to the gray matter lining the medial wall of the hemisphere (Figure 7c,d). Approaching the region of the caudate nucleus, the fiber path curved slightly laterally, bending around and over the caudate head to assume a position on its lateral surface (Figure 7e) to enter the posterior region of the anterior limb of the internal capsule (ICa) and genu of the internal capsule (ICg) (Figure 7f,g). From this location the fiber bundle migrated inferiorly to occupy the IC genu, with the bulk of labeled fibers favoring the medial half of the IC adjacent to the caudate (Figure 7g). Passing through middle to inferior levels of the IC, the M2 orofacial pathway shifted from the IC genu to occupy the anterior quarter of the posterior limb where it remained for most of its descending course (Figure 7h–j). Inferiorly and in the medial-lateral dimension, fibers spread laterally to occupy both medial and lateral IC regions (Figures 2b and 7h–j). At the most inferior level of the IC, just below the ac-pc line, the pathway reached it maximal posterior extent, where it occupied in part, both the first and second quadrant of the ICp (Figure 7k) before advancing into the crus cerebri of the midbrain (Figure 7l).
3.3.2 | Midbrain cerebral peduncle
At superior midbrain levels, orofacial M2 fibers occupied the centromedial sector of the ccCP and lateral half of the medial sector (Figures 7l and 10a). As the labeled fascicle passed downward, the pathway remained in this position (Figures 7m and 10b,c) until it approached the midbrain-pontine isthmus where it spread, or expanded in size, to occupy a significant portion of both centromedial and medial sectors of the ccCP, with minimal involvement of the medial edge of the central sector (Figures 2c and 10d).
3.4 | Trajectory of M3 orofacial fibers
3.4.1 | Corona radiata and internal capsule
The descending projection from the M3 orofacial representation was studied in three cases, one prepared in the horizontal plane (injection SDM14-BDA) (Figures 2d and 4), and two additional cases prepared in the coronal plane (injection cases SDM36-FD and SDM43-FD) (Figures 2g and 8). In all cases, the M3 injection site was placed in the lower bank of the cingulate sulcus (Figures 2d,g and 8) and originated from most rostral position of the cortex involved in this study, corresponding to coronal levels including the genu of the corpus callosum (Figures 4 and 8). Labeled axons emerged from the M3 injection site in a tightly coalesced bundle (Figure 2d—see black arrows, g—see white arrow). Arching over the cingulum bundle, and with an immediate downward trajectory, the fibers coursed posteriorly in the CR (Figure 5b–d). At a similar stage of subcortical passage, the M3 labeled fibers appeared much lower in the CR when compared to the path taken by orofacial fibers from M2 (Figure 7c,d). As the labeled bundle approached the rostral pole, or head of the caudate nucleus, the M3 fibers arched over the caudate (as verified in cases prepared in the coronal dimension—see Figure 11), then curved slightly laterally, bending around the gray matter mass (Figure 5e) to enter the ICa (Figures 2e and 5f). At superior IC levels, the M3 pathway occupied the anterior half of the anterior limb (Figure 5f,g), spanning across medial and lateral regions of the ICa (Figure 5g). Although the medial occupation of this fiber bundle was anticipated, the emergence of numerous corticostriate projection fibers appeared to correlate with the lateral expansion on this fiber system, based upon relatively prominent terminal corticostriate projection patches noted in the head of the caudate. From this point, and quite distinguishable in the horizontal view, the bundle began descending at a very gradual angle of trajectory. In the horizontal plane, this correlated with the bundle appearing distinctly elongated, as it passed through upper levels of the anterior limb (Figure 5f,g). At IC mid-levels, the labeled fascicle continued on a very gradual, descending course through the posterior half of the anterior limb, assuming a position favoring the medial side of the IC bordering the caudate nucleus (Figures 2h and 5h). Passing inferiorly, the fibers remained in the caudal part of the ICa, but also entered the genu (Figure 5i). At lower horizontal levels, including the ac-pc line, the fibers occupied the ICg and a small portion of the first quadrant of the ICp (Figure 5j,k).
FIGURE 11.
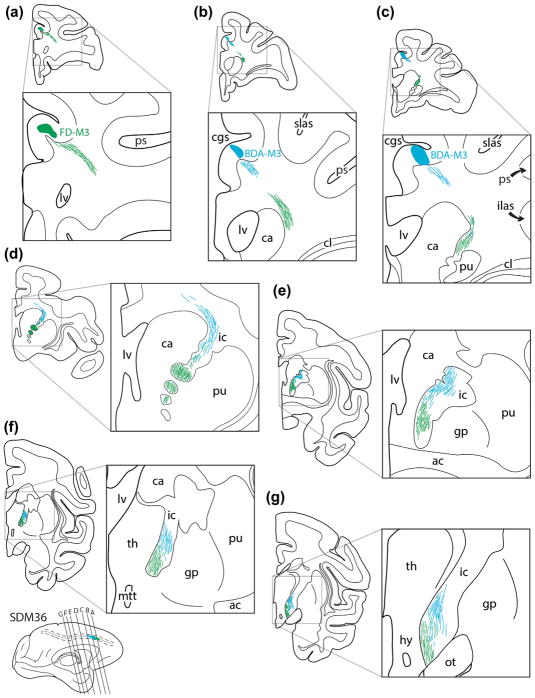
Line drawings of representative rostral (a) to caudal (g) coronal sections from case SDM36 depicting the descending course of labeled axon pathways in the CR and IC following injections of tract tracer into M3. Specifically, FD was injected into the central part of the orofacial region and BDA was injected into the caudal part of the orofacial area and rostral part of the M3 arm area (see Figure 8, bottom). The green fiber bundles represent axons originating from the FD injection site and the blue fiber bundles represent axons from the BDA injection site. Note how the respective fiber bundles arch around the head of the caudate (ca), enter the internal capsule (IC), and remain relatively spatially separate throughout the descending route, with a label sparse zone in between that contains a light mixture of FD and BDA labeled fibers. For other abbreviations see list
3.4.2 | Midbrain cerebral peduncle
Transitioning from the IC to the midbrain, the M3 orofacial fibers immediately coursed medially to occupy the medial sector of the ccCP where they remained at superior and mid-levels of the midbrain (Figures 2f,i, 5l, and 10a,b). At inferior levels of the ccCP the fiber bundle slightly expanded in the horizontal dimension, continuing to occupy the medial sector, but also part of the adjacent centromedial sector (Figure 10c,d).
3.5 | Trajectory of M4 orofacial fibers
3.5.1 | Corona radiata and internal capsule
Like all other pathways, the descending M4 orofacial pathway was studied in both horizontal (injection SDM14-FR) and coronal planes (SDM22-LYD) (Figures 4 and 8). All injection sites were located in the lower bank of the cingulate sulcus at coronal levels immediately posterior to the midline crossing of the anterior commissure (Figures 2j, 3c, 4, and 8). Like the other experimental medial wall cases (i.e., M2 and M3), the main fiber bundle emerged from the injection site and abruptly coursed posteriorly in the CR (Figures 3d and 5a–c). Then, the labeled M4 fibers arched over the caudate (Figure 5d,e), which was quite evident in the coronal sections of case SDM22-LYD, to briefly enter the superior-most part of the IC genu then ICp (Figure 5f). Traveling through superior and middle IC levels, the M4 bundle occupied the medial side of the first quarter of the ICp (Figure 5f–h). At inferior IC levels, the pathway maintained its medial orientation but moved posteriorly to occupy the second quarter of the ICp, in close proximity to the M1 orofacial pathway (Figure 5i–k).
3.5.2 | Midbrain cerebral peduncle
Once within the midbrain, the M4 orofacial fiber pathway occupied the centromedial sector of the ccCP (Figures 2l, 5l, and 10a). Progressing inferiorly, the fiber bundle gradually expanded medially to occupy the centromedial and medial sectors (Figure 10b,c). At the midbrain-pontine isthmus the M4 pathway primarily occupied the medial sector and to some extent the posterior region of the centromedial sector of the ccCP (Figure 10d).
3.6 | Comparison of orofacial fiber trajectory with arm fiber trajectory
Four experiments in three animals were designed to examine the spatial relationship of orofacial and arm representation from the same motor area through the CR, IC, and ccCP. These experimental cases included SDM-19 for M1 and M2 (Figures 3e–h and 6), SDM35 for M2, and SDM36 for M3 (Figure 8). The anatomical locations and somatotopic affiliation of the SDM19 and SDM35 injection sites have previously been summarized. With regard to the M3 experiment (SDM36) the two injection sites were located rostral and caudal to one another, with some overlap (Figure 8). The rostral M3 injection site was clearly in the orofacial region as verified by its anatomical position located above the genu of the corpus callosum, in addition to resultant terminal labeling found in the facial nucleus (Morecraft, McNeal, Stilwell-Morecraft, Gedney, et al., 2007—see Figure 9 bottom right), hypoglossal nucleus (Morecraft et al., 2014), and trigeminal motor nucleus (Morecraft et al., in preparation). The caudal injection site in case SDM36 involved the rostral part of the M3 arm area as supported by the labeling of corticospinal projection axons and terminals (Morecraft, McNeal, Stilwell-Morecraft, Gedney, et al., 2007—see Figure 9 bottom left), but also involved the caudal part of the M3 orofacial region as determined by terminal labeling in the facial nucleus (Morecraft, McNeal, Stilwell-Morecraft, Gedney, et al., 2007—see Figure 9 bottom right), hypoglossal nucleus (Morecraft et al., 2014), and trigeminal motor nucleus (Morecraft et al., in preparation).
3.6.1 | Primary motor cortex experiment
In case SDM19 which was prepared in the horizontal plane, the M1 orofacial and arm pathways maintained a parallel course in the CR, moving toward the middle third of the CR with arm fibers located dorsal to orofacial fibers (Figure 7b–e). Once within the superior region of the IC, M1 arm fibers were located posterior to M1 orofacial fibers with partial overlap (Figure 7f,g). Overlap of the M1 orofacial pathway was also noted with the M2 arm pathway (Figure 7f,g). At IC mid-levels to inferior levels, both M1 pathways continued to move in parallel, shifting to more a posterior region of the ICp, maintaining the same spatial relationship (Figure 7h–k). In the midbrain ccCP M1 arm fibers were lateral to M1 orofacial fibers with some overlap (Figure 7l,m) but at inferior midbrain levels there was complete overlap.
3.6.2 | Supplementary motor cortex experiments
With regard to the M2 experiments (SDM19 and SDM36), the orofacial and arm pathways in the CR were located rostral and caudal, respectively, to one another with some overlap (Figure 7b–e). Within the superior region of the IC, M2 arm fibers were located posterior to M2 orofacial fibers with an intervening area of overlap which was quite evident in the horizontal plane (Figure 7f,g). In the coronal view (case SDM36), at superior IC levels the region of overlap between the two pathways was found to be minimal, containing few blue (FD - arm) and brown (BDA-orofacial) commixed fibers. At IC mid-levels to inferior levels, both pathways moved posteriorly in the ICp, but the same spatial relationship was maintained (Figure 7h–k). From the coronal view, and at inferior IC levels the zone of overlap contained considerable numbers of commixed blue and brown fibers. Once in the midbrain ccCP M2 arm fibers were lateral to M2 orofacial fibers with some overlap (Figure 7l,m) whereas at inferior midbrain levels there was considerable fiber system overlap.
3.6.3 | Rostral cingulate motor cortex experiment
In the M3 double labeling experiment (Figure 8, see SDM36) the brain was sectioned in the coronal plane, so the results will be described progressing from anterior to posterior levels of the brain (Figure 11). Fibers emerging from the more rostrally located FR injection site arched over the cingulum bundle and took a steep, inferiorly and laterally directed trajectory, passing over the head of the caudate and were first to enter the superior part of the ICa (Figure 11a–c). From a mediolateral perspective FR labeled fibers occupied both regions (Figure 11c,d). Progressing caudally in the brain, the FD fibers began to descend in the ICa when the posteriorly located BDA injection site appeared in cortex forming the lower bank of the cingulate sulcus (Figure 11b,c). BDA fibers emerged from the site and coursed posteriorly, then inferiorly to also arch over the caudate nucleus to enter the ICa (Figure 11d). When both fiber bundles were within the IC, the FD (orofacial) fibers were clearly ventral (inferior) to the more dorsally (superior) located bundle of BDA (orofacial and arm) fibers demonstrating a more advanced existence of the FD labeled fiber system (Figure 11d,e). Notably, there was a label-sparse gap between dorsal edge of the main FD labeled fascicle, and the ventral edge of the main BDA labeled fascicle. Within the label-sparse zone, separating the two densely labeled fascicles, there were a few intermingled FD and BDA labeled fibers (Figure 11d,e). Moving posteriorly through the coronal sections, both fiber systems continued their descent in the IC, but also sustained their spatial separation (Figure 11e). However the number of labeled fibers found between the main fascicles of labeled fibers (i.e., in the intervening “label-sparse” zone) gradually increased in number, characterized by a significant commixture of both FD and BDA labeled axons (Figure 11f). As the fiber bundles passed from the IC and into the midbrain, the pathways assumed a lateral (arm/orofacial fibers) to medial (orofacial fibers) orientation with some overlap (Figure 11g). At inferior ccCP levels both pathways completely overlapped.
3.7 | Summary of orofacial pathway results
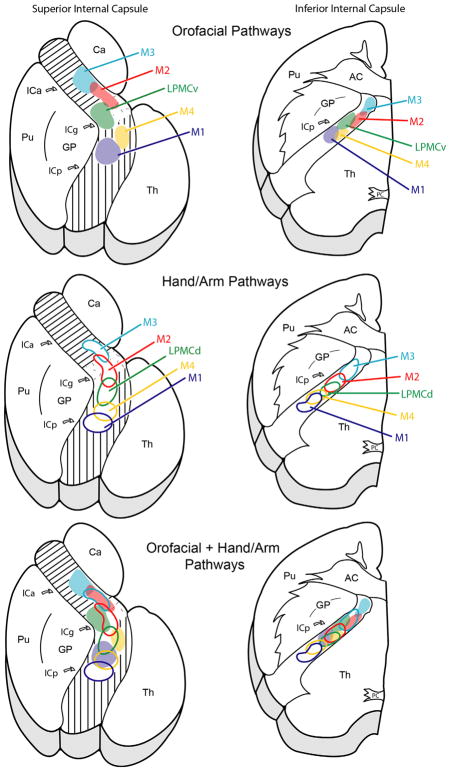
In the CR, each pathway was segregated as medial motor area fibers arched over the caudate nucleus and lateral motor area fibers arched over the putamen nucleus to enter the IC. Within the IC, the individual corticobulbar pathways were found to be widespread and topographically organized (Figure 12, top). At superior IC levels, the corticobulbar projection from M3 coursed through the ICa and the projection from M2 passed through the ICa then ICg. The projection from LPMCv initially occupied the ICg then entered the anterior portion of the ICp. The projection from M4 and M1 was posterior to the other fiber systems as they passed into the anterior region of the ICp. At middle to inferior IC levels, all descending tracts shifted posteriorly, maintaining their relative anterior to posterior orientation (e.g., M3, M2, LPMCv, M4, M1) with adjacent fiber bundles overlapping to some degree. Upon entering the midbrain cerebral peduncle, M3 fibers were located medially, M1 fibers primarily in the centromedial sector with M2/M4/LPMCv fibers pathways located in between and overlapping extensively (Figure 10). At the midbrain-pontine isthmus, all fiber pathways overlapped medially.
FIGURE 12.
Top: simplified summary diagram showing general locations of orofacial (corticobulbar) representation at superior (left) to inferior (right) levels of the internal capsule in the horizontal plane. Middle: simplified summary diagram showing general locations of arm (corticospinal) representation at superior (left) to inferior (right) levels of the internal capsule (modified from Morecraft et al., 2002). Bottom: composite diagram showing representative locations of orofacial and arm representation at superior (left) to inferior (right) levels of the internal capsule. The primary intent of this template is to emphasize the widespread distribution of orofacial and arm representation in the IC and potential for overlapping somatotopy. It is important to stress that inter-subject variations occur in the locations of these pathways as minor positional shifts along the main pathway trajectory (see Results and Figures 5 and 7 for details). For abbreviations: see list
4 | DISCUSSION
Understanding motor pathway organization in the corona radiata, internal capsule, and midbrain cerebral peduncle is critical for interpreting motor deficits following subcortical white matter injury, predicting subsequent motor recovery outcomes and designing therapeutic intervention strategies to improve recovery. This information is also indispensable for planning subcortical neurosurgical interventions and interpreting undesirable side-effects of deep brain stimulation and stereotaxic lesions. Prior to our investigation, little information was available in the non-human primate model on the descending projection in the CR, IC, and ccCP from cortical orofacial representations, and reported observations were limited to parts of the descending projection from M1 and M2. Our findings confirm some previously reported information on the descending projection from orofacial region of M1 and M2 in the IC and ccCP and extend this knowledge by accurately documenting the location and migration of these pathways throughout the CR, IC, and ccCP. Furthermore, we present new information on the spatial relationships between the descending M1 and M2 orofacial and arm pathways. Finally, experiments conducted in the present study provide novel information on the subcortical organization of orofacial pathways arising from LPMCv, M3, and M4. Although it is not currently known if non-human primate orofacial pathway organization is similar to human brain organization, some diffusion tractography observations, as well as clinical reports from ischemic lesion and DBS studies indicate some structural parallels may occur. Additionally, the fact that some patients show rapid recovery from acute and subacute orofacial deficits following localized supratentorial injury imply that widespread orofacial pathway representation, as found in our study, may exist in the human brain. In the following discussion, we will compare our findings to what is known from non-human primate studies, and when appropriate, we would like to draw attention to some of the tractography and clinical observations suggesting fundamental similarities may exist between the non-human primate and human brain.
4.1 | Localization of orofacial representation in the CR, IC, and CP
4.1.1 | Lateral cortical pathways (M1 and LPMCv)
Primary motor cortex
Our scientific observations show that the M1 orofacial pathway in the CR courses medially toward the midline, occupying the mid-coronal brain region (Figure 4c–f). This appears to be similar to the proposed location in the human brain based upon clinicopathological observations correlating lesion location with cranial nerve motor dysfunction. In particular, CR lesions in patients located near, or just caudal to the midway point between the rostral and caudal poles of the lateral ventricle (i.e., central region of the middle third of the CR), result in bulbar/facial paresis or dysarthria (Bradley, Hannon, Lebus, O’Brien, & Khadjooi, 2014; Kim & Pope 2005; Kwon et al., 2016; Tohgi, Takahashi, Takahashi, Tamura, & Yonezawa, 1996; Urban et al., 1999, 2001; Song, 2007). Not surprisingly, in the CR we found fibers from the dorsal part of the M1 orofacial representation course dorsal to fibers emerging from the ventral part of the orofacial region (Figure 9a,b). Diffusion imaging observations have similarly shown segregated dorsal and ventral pathways emerging from the M1 orofacial region in the human brain (Jenabi, Peck, Young, Brennan, & Holodny, 2015; Liégeois, Tournier, Pigdon, Connelly, & Morgan, 2013). A number of relevant investigations indicate these pathways may mediate similar, but somewhat distinguishable functional properties. Physiologically, stimulation of the dorsal M1 orofacial region in monkey primarily evokes movements in the face whereas the ventral region evokes tongue movements more so than facial movements (Cure & Rasmussen, 1954; Hatanaka, Tokuno, Nambu, Inoue, & Takada, 2005; Huang, Hiraba, Murray, Sessle, 1989; Huang, Hiraba, & Sessle, 1989; Huang, Sirisko, Hiraba, Murray, & Sessle, 1988; McGuinness, Sivertsen, & Allman, 1980; Murray & Sessle, 1992; Woolsey et al., 1952). Related to these observations, we have shown that axons forming the ventral orofacial pathway give rise to more axon terminals in the hypoglossal nucleus than the dorsal pathway (Morecraft et al., 2014, see cases SDM32 and SDM66). Although we found that the dorsal and ventral tracts are spatially segregated in the CR (Figure 9a,b), once within the superior region of the ICp these bundles overlapped extensively (Figure 9c). This identical fiber pattern of M1 orofacial dorsal and ventral tract segregation then IC pathway integration, as determined by MRI tractography, has been observed in the human subcortical white matter (Jenabi et al., 2015; Liégeois et al., 2013). Possibly related to the finding of merged orofacial pathways are DBS observations obtained from neurosurgical exploration of the IC at the AC-PC level demonstrating that co-activation of face and tongue muscle contractions occur extensively (59–62%) (Duerden et al., 2011).
Rhesus monkey experiments indicate that the M1 orofacial projection descends in the posterior limb of the internal capsule (Schmahmann et al., 2004) which corresponds to our findings. However, at superior levels of the IC, we found the M1 orofacial pathway courses in the anterior-most region (first quarter) of the ICp. Progressing inferiorly, our experiments further show that the projection moves from the first quarter to the second quarter of the ICp (Figures 5g–k and 7f–i). Collectively, these observations do not lend support the classical anatomical description of M1 orofacial fibers passing through the genu of the IC (e.g., Adams et al., 1997; Brazis et al., 2011; Campbell, 2013; Standring, 2016). This would be in accord with classical DBS observations who found corticobulbar pathways were located posterior to the genu (Bertrand et al., 1965; Guiot, Rougerie, Sachs, & Hertzog, 1958). On a more contemporary note, similar conclusions have been reached in a clinicopathological/tractography study suggesting that the human M1 corticobulbar pathway travels through the ICp but not within the ICg (Yim et al., 2013). Further conclusions derived from the classical stimulation studies mentioned above, and those of Yim and colleagues suggest the corticobulbar pathway resides near the ICp midpoint, and/ or slightly posterior to that position. Our non-human primate results show the M1 orofacial pathway resides immediately anterior to the ICp midpoint (Figures 5i–k and 7h–k). In humans, the posterior shift of the M1 corticobulbar pathway could be a manifestation of an enhanced contribution of prefrontal fibers in the IC compared to the macaque (Ramnani et al., 2006). In the midbrain, we found the M1 orofacial projection resides primarily in the centromedial sector of the ccCP in accord with previous evidence (Barnard & Woolsey, 1956; Schmahmann et al., 2004) (Figures 7l–m and 10a,b). Additionally, our findings show the M1 fiber bundle slightly expands to occupy the medial sector as well as the centromedial sector of the ccCP at the midbrain-pontine isthmus (Figure 10d).
Lateral premotor cortex
Over the years, there have been a considerable number of studies reporting the afferent and efferent connections of the ventral region of LPMCv. However, we are unaware of experimental work characterizing the anatomical course of the descending projection from this brain region. Our findings in the CR reveal that the ventral LPMCv projection is spatially separate from the M1 orofacial projection, residing directly anterior to the M1 projection (Figure 5d–f). This relative relationship continues throughout the ICp (Figure 5g–k). Additionally, we also found the ventral LPMCv projection in the ccCP resides medial to the M1 projection with overlap that increases as these pathways approach the midbrain-pontine junction, where there is extensive overlap (Figure 10).
We are aware of one report that has characterized the descending projection from the dorsal part of LPMCv through the IC (Fries et al., 1993) and it appears as if our findings are quite similar. Specifically, Fries et al. reported the dorsal LPMCv projection occupied “the anterior most part of the posterior limb of the internal capsule close to its knee.” Comparing this with our ventral LPMCv data, it would appear that the ventral LPMCv path (Figure 5i–k) may be slightly rostral to the dorsal path with significant overlap potential. However, a double labeling study in the same animal experiment processed in the horizontal plane would best resolve the overlap issue.
4.1.2 | Medial cortical pathways (M2, M3, and M4)
Supplementary motor cortex
Only one other study has provided information on the descending course of the orofacial projection from M2. It was mentioned that the M2 orofacial projection coursed through the anterior limb of the IC then genu to directly enter the ccCP (Schmahmann et al., 2004). Our findings support this when considering the tract position at upper IC levels, but not at middle and inferior IC levels. For example, we found the M2 orofacial projection passes over the caudate then through the ICa then ICg at superior levels (Figure 7f,g). However, the main bundle then enters the anterior quarter of the ICp where it resides for most of its descending course (Figure 7h–j). This subcortical route appears to be similar to the human pathway as determined using diffusion weighted imaging and probabilistic tractography methods in control subjects. For example, Newton and colleagues describe the supplementary motor cortex tract as passing close to the head of the caudate nucleus, entering the anterior limb then genu at superior IC levels, then shifting to occupy the ICp inferiorly (Newton et al., 2006). Within the midbrain ccCP we found the M2 orofacial projection to be positioned medial to the M1 orofacial projection (Figures 7l,m and 10a). A similar conclusion was reached by Schmahmann et al. (2004). Our studies further demonstrate that overlap between these tracts gradually increase inferiorly in the ccCP (10b,c), attaining complete overlap at the midbrain-pontine isthmus (Figure 10d).
Cingulate motor cortex
This report is the first to document the anatomical location of the descending projection from the orofacial region of the rostral and caudal cingulate motor areas. Overall, the M3 projection maintained the most rostral position in the CR and IC of all motor pathways studied, whereas the M4 projection traveled posteriorly, in close proximity to the M1 orofacial projection. Specifically, the M3 orofacial projection coursed within the ICa (Figure 5f–i) until approaching the AC-PC level where it moved to primarily occupy the ICg (Figure 5j,k). It has been shown in the monkey that both M3 and M4 orofacial regions have direct projections to the facial nucleus (Morecraft et al., 2001) and hypoglossal nucleus (Morecraft et al., 2014). If the rostral cingulate motor system (M3) is present in the human brain as suggested by functional imaging and stimulation studies (Chassagnon, Minotti, Kremer, Hoffmann, & Kahane, 2008; Hanakawa, Dimyan, & Hallett, 2008; Paus, Petrides, Evans, & Meyer, 1993; Picard & Strick, 1996; Sohn et al., 2004; Talairach et al., 1973), this may account for orofacial movements elicited during DBS of the ICa for the treatment of neuropsychiatric disorders (Okun et al., 2004; Springer, Bowers, Goodman, Shapira, Foote, & Okun, 2006) and orofacial motor deficits reported following localized ICa injury (Adams et al., 1983; Caplan et al., 1990; Ichikawa & Kageyama, 1991; Kumral et al., 2007; Maestroni et al., 2006; Ozaki et al., 1986). Additionally, since we found the M2 orofacial projection to pass through the ICa, this may also factor into these clinically relevant movement-related observations. Further consideration the musculotopic-related organization of the corticofacial projection from M2 and M3 (Morecraft et al., 2001) suggests that some movements elicited from the ICa, and related motor deficits following ICa injury, may present in the upper facial musculature. Within the crus cerebri, the M3 projection assumed the most medial position of all fiber systems studied, corresponding to the same location harboring descending fibers from the prefrontal cortex (Beck, 1950; Brodal, 1981; Bucy, Ladpli, & Ehrlich, 1966) (Figure 10). By contrast, the M4 projection overlapped considerably with the M1 projection in the centromedial region at upper and middle ccCP levels (Figure 10). Thus, cingulate motor cortex orofacial projections occupy extensive parts of the midbrain ccCP.
4.2 | Subcortical localization of orofacial and arm representation
We examined the spatial relationship between the orofacial and arm pathways in M1 and M2 due to their robust corticobulbar and cortico-spinal projections (Dum & Strick, 1996; Maier et al., 2002; McNeal et al., 2010; Morecraft et al., 2001, 2013, 2014), and potential importance of their spared descending axon pathways in recovery of motor function following unilateral capsular ischemia and superficial middle cerebral artery infarction (McNeal et al., 2010; Morecraft et al., 2015a, 2016). We found the M1 arm pathway entered the ICp at superior levels to occupy a location between the second and third quarter of the ICp throughout its descent (Figure 7f–k). This evidence brings further confirmation of the macaque M1 arm tract location as previously reported (Fries et al., 1993; Morecraft et al., 2002). In contrast, we found the M2 arm pathway to briefly pass through the ICg region superiorly, then reside between the first and second quarter of the ICp (Figure 7h–j) as previously described (Morecraft et al., 2002). The only minor discrepancy was with case SDM19, where the M2 arm tract shifted slightly posteriorly to occupy the second quarter of the ICp just below the AC-PC line (Figure 7k).
Oriented along the long axis of the ICp, we found the corresponding orofacial pathway from both motor areas (M1 and M2) residing anterior to their respective arm pathways (Figure 7f–k). This supporting early non-human primate observations on the general capsular arrangement of M1 orofacial and arm pathway trajectories gathered using the degeneration tract tracing method (Mellus, 1899). This spatial orientation parallels human tractography studies showing the M1 orofacial pathway resides anterior to the M1 arm pathway (Liégeois et al., 2013; Pan et al., 2012; Park et al., 2008). Importantly, a novel finding in case SDM19 was the significant overlap between the M1 orofacial pathway and the M2 arm pathway throughout the IC (Figure 7h–k). If this fiber tract arrangement occurs in the human brain, this spatial relationship may contribute to observations of overlapping face, tongue, and arm movements reported following DBS of the IC (Bertrand et al., 1965; Duerden et al., 2011; Hanaway & Young, 1977; Hardy et al., 1979). Indeed in the monkey, the M1 orofacial projection to the facial nucleus and hypoglossal nucleus is robust (Jenny & Saper, 1987; Kuypers, 1958a; Morecraft et al., 2001, 2014). Although not as dense as the M1 arm corticospinal projection (Boudrias et al., 2006; Boudrias, Lee, Svojanovsky, & Cheney, 2010; Dum & Strick, 1996; Lemon, Maier, Armand, Kirkwood, & Yang, 2002; Maier et al., 2002; Morecraft et al., 2014), the M2 arm corticospinal projection is characterized by heavily distributed terminal boutons in the spinal intermediate zone and moderate numbers of terminal boutons in motoneuron laminae IX of the anterior horn (Dum & Strick, 1996; Lemon et al., 2002; Maier et al., 2002; McNeal et al., 2010; Rouiller, Moret, Tanne, & Boussaoud, 1996). While we did not conduct additional double labeling experiments addressing potential pathway overlap among other motor area orofacial and arm representations (e.g., LPMCv vs. M2; M2 vs. M3), similar overlapping relationships would likely occur (Figure 12, bottom) and may contribute to DBS induced muscular co-activations and fractured somatotopy found in the region of the ICa, ICg, and anterior part of the ICp.
4.3 | Additional clinical considerations
Clinocopathological studies have advanced the suggestion that upper extremity motor recovery potential is likely to decrease as localized injury involves progressively lower white matter locations along the CR/IC axis (Chamorro et al., 1991; Shelton & Reding, 2001). In our previous report on subcortical white matter organization in monkey, we presented strong support for this proposal due to the widespread distribution of arm pathway organization found in the CR and at upper IC levels (Morecraft et al., 2002). Hypothetically, this dispersed pathway organization, if present in the human brain, would increase the probability of spared descending arm pathways, and correlate with acute upper extremity dysfunction and favorable levels of motor recovery. In further agreement with the clinicopathological interpretations, our data suggested that motor deficit severity would increase, and motor recovery potential would concurrently decrease following isolated injury seated at lower IC levels. This premise is based upon the progressively compact and commixed relationship of arm fiber pathway organization at lower IC locations (Morecraft et al., 2002). The present findings of dispersed orofacial pathway distribution in the CR followed by the gradual convergence of these fiber systems at middle and lower IC levels suggest the same predictions can be advanced for recovery potential of cranial motor function. That is, localized injury to the CR and upper IC may correlate with acute motor deficits and significant recovery potential of craniofacial movements, while injury of similar magnitude but sustained at lower IC levels may correlate with more severe and prolonged orofacial deficits, as well as poorer recovery prognosis due to the potential destruction of a large number of orofacial motor fibers located in a confined subcortical space.
We advanced an additional clinocoanatomical concept related to the anterior-posterior organization of arm representation within the IC, suggesting that motor deficits would gradually increase with lesions located progressively more posteriorly in the IC, and be maximal at the location where the M1 arm area tract resides (Morecraft et al., 2002). Factored into this prediction were the relative number of corticospinal soma present within the various arm-related motor areas of interest (Dum & Strick, 1991; Galea & Darian Smith, 1994; He, Dum, & Strick, 1993; He, Dum, & Strick, 1995; Murray & Coulter, 1981; Nudo & Masterton, 1990), and corresponding terminal density of each respective corticospinal projection (Dum & Strick, 1996; Maier et al., 2002; McNeal et al., 2010; Morecraft, Louie, Schroeder, & Avramov, 1997; Morecraft et al. 2013). This supposition has since received significant clinical support from several groups applying diverse experimental approaches in patients presenting with upper extremity dysfunction and localized IC damage (e.g., Fridman et al., 2004; Schaechter, Perdue, & Wang, 2008; Schiemanck, Kwakkel, Post, Kappelle, & Prevo, 2008; Wenzelburger et al., 2005). Based upon our new orofacial pathway findings, and the relative strength of each motor areas terminal projection to the facial and hypoglossal nuclei (Morecraft et al., 2001, 2014), we can advance a similar clinicoanatomical prediction. Namely, more anteriorly seated capsular lesions that include the ICa, ICg, and anterior-most region of the ICp, may result in moderate to mild orofacial deficits accompanied by promising recovery potential. As mentioned, because we found M2 and M3 orofacial tracts to be located in this capsular region, upper facial muscles may be affected to a greater degree compared to lower facial muscles. On the contrary, posterior ICp lesions that involve the M1 orofacial pathway may give rise to more severe orofacial deficits (specifically in the lower facial muscles) and correlate with relatively prolonged and less complete levels of recovery. Finally, it is important to note that the orofacial tract from LPMCv, which primarily innervates lower facial muscles as does M1 (Morecraft et al., 2001; Morecraft, Stilwell-Morecraft, & Rossing, 2004), was also found to pass through the IC genu (Figure 5f–h). Thus, injury affecting superior ICg levels may compromise lower facial movements by disruption of LPMCv corticobulbar axons. This may underlie the traditional teaching of IC genu orofacial representation and possibly correspond to the clinical observations reporting orofacial deficits following ICg injury (Bogousslavsky & Regli, 1990; Chung et al., 2000; Rousseaux et al., 1987; Tatemichi et al., 1992; Tredici et al., 1982; Urban et al., 1996, 1997).
In terms of midbrain fiber passage, we discovered orofacial pathways collectively occupy the medial 1/3 of peduncle at upper and middle midbrain levels, with the M1 tract positioned most lateral (in the centromedial sector) and M3 tract most medial (in the medial sector) (Figure 10a–c). These findings may have relevance to neurological symptomology accompanying posterior circulation ischemia, and specifically isolated midbrain injury. Motor symptoms following midbrain injury typically accompany anterior medial and anterior lateral vascular lesions involving the cerebral peduncle and its descending motor tracts, whereas sensory disturbance is associated with lateral vascular midbrain lesions disrupting the ascending sensory pathways (Alvarez-Sabin, Montalbán, Tintoré, & Codina, 1991; Bogousslavsky, Maeder, Regli, & Meuli, 1994; Caplan, 1980; Hommel & Besson, 2001; Kim & Kim, 2005; Tuttle & Reinmuth, 1984). Thus, damage to orofacial fiber tracts located medially in the ccCP (e.g., M2 and M3) may contribute to the marked presence of dysarthria following anterior medial vascular occlusions (Kim & Kim, 2005), whereas dysarthria and facial paresis that accompany anterior lateral ischemia (Kim & Kim, 2005) may be a manifestation of compromised M1 orofacial tract fibers. Indeed, the M1 orofacial pathway location as identified in the present report, would theoretically be positioned in the medial most part of the anterior lateral vascular territory. Finally, we noted a particularly vulnerable region of fiber passage at the level of the midbrain-pontine isthmus where all five orofacial pathways overlapped medially (Figure 10d). This is the identical anatomical location and overlap pattern that we previously found for all five descending cortical motor arm tracts (Morecraft, McNeal, Stilwell-Morecraft, Dvanajscak, et al., 2007). Taken together, these observations suggest that bilateral destruction of this critical mesencephalic region could result in comprehensive loss of all major corticobulbar and corticospinal projection systems, and account for the absence of orofacial and upper extremity movement that in part, characterize midbrain “locked in syndrome” (Bauer, Gerstenbrand, & Rumpl, 1979; Carrai et al., 2009; Chia, 1991; Dehaene & Dom, 1982; Fujimoto, Ohnishi, Wakayama, & Yoshimine, 2011; Meienberg, Mumenthaler, & Karbowski, 1979; Park, Sohn, & Kim, 1997; Zakaria & Flaherty, 2006).
5 | CONCLUDING REMARKS
Understanding the complexity of subcortical white matter organization remains a priority in the clinical setting for neurosurgical planning and understanding stroke symptoms and recovery of function. Our results, derived in the non-human model, provide a theoretical template to assist in advancing our understanding of human subcortical orofacial representation for prospective diagnosis and therapeutic intervention following subcortical white matter injury. Potentially relevant are our findings suggesting that white matter lesions involving the superior region of the CR/IC/ccCP axis may lead to favorable prognosis of orofacial recovery due to the dispersed nature of pathway organization. In contrast, inferior lesions may be associated with greater motor deficits and poorer recovery due to the compact nature of orofacial pathway organization. Efforts mapping the anatomical trajectory of descending orofacial pathways using evolving tractography methodologies may also benefit from these observations. Last, our capability to determine axon pathway position and spatial inter-relationships of multiple descending orofacial representations in the non-human primate, particularly in the region of the internal capsule may aid in neurosurgical preparation, and interpreting movement responses elicited from deep brain stimulation during stereotaxic navigation for the treatment of intractable movement disorders and psychiatric illness.
Acknowledgments
Funding information
National Institutes of Health Grants, Grant/ Award Number: NS 097450, NS 046367, NS 33003; The Benign Essential Blepharospasm Research Foundation
Abbreviations
- III
oculomotor complex/nerve
- 3v
third ventricle
- a
anterior
- A
arm
- ac
anterior commissure
- amy
amygdala
- apos
anterior parieto-occipital sulcus
- as
aqueduct of Sylvius
- bc
brachium conjunctivum
- BDA
biotinylated dextran amine
- c
caudal
- c
central subsector of the crus cerebri
- ca
caudate nucleus
- cc
corpus callosum
- ccCP
crus cerebri of the cerebral peduncle
- cf
calcarine fissure
- cgs
cingulate sulcus
- cis
circular sulcus
- cl
claustrum
- cl
centrolateral subsector of the crus cerebri
- cm
centromedial subsector of the crus cerebri
- CP
cerebral peduncle
- CR
corona radiata
- cs
central sulcus
- d
dorsal
- D
digit
- DBS
deep brain stimulation
- ecs
ectocalcarine sulcus
- El
elbow
- FD
fluorescein dextran
- FR
fluoro-ruby
- fx
fornix
- gp
globus pallidus
- hf
hippocampal fissure
- hn
habenular nucleus
- hp
hippocampus
- hy
hypothalamus
- ic
inferior colliculus
- IC
internal capsule
- ICa
anterior limb of the internal capsule
- ICg
genu of the internal capsule
- ICp
posterior limb of the internal capsule
- icpd
inferior precentral dimple
- ihf
interhemispheric fissure
- ilas
inferior limb of the arcuate sulcus
- in
insula
- ios
inferior occipital sulcus
- ips
intraparietal sulcus
- l
lateral
- l
lateral subsector of the crus cerebri
- lf
lateral fissure
- lgn
lateral geniculate nucleus
- LL
lower lip
- los
lateral occipital sulcus
- LPMCd
dorsal lateral premotor cortex
- LPMCv
ventral lateral premotor cortex
- ls
lunate sulcus
- lv
lateral ventricle
- LYD
lucifer yellow dextran
- m
medial
- m
medial subsector of the crus cerebri
- M1
primary motor cortex
- M2
supplementary motor cortex
- M3
rostral cingulate motor cortex
- M4
caudal cingulate motor cortex
- mb
midbrain
- mgn
medial geniculate nucleus
- ml
medial lemniscus
- mos
medial occipital sulcus
- MRI
magnetic resonance imaging
- mtt
mammillothalamic tract
- NR
no response
- oc
optic chiasm
- ot
optic tract
- ots
occipital temporal sulcus
- p
posterior
- pag
periaqueductal gray
- pc
posterior commissure
- PHAL
phaseolus vulgaris leucoagglutinin
- pmts
posterior middle temporal sulcus
- pn
pontine nuclei
- poms
medial parieto-occipital sulcus
- ps
principal sulcus
- pu
putamen
- r
rostral
- rn
red nucleus
- ros
rostral sulcus
- rs
rhinal sulcus
- sc
superior colliculus
- Sh
shoulder
- slas
superior limb of the arcuate sulcus
- sn
substantia nigra
- stn
subthalamic nucleus
- sts
superior temporal sulcus
- th
thalamus
- Th
thumb
- To
tongue
- UL
upper lip
- v
ventral
- Wr
wrist
References
- Adams HP, Damasio HC, Putman SF, Damasio AR. Middle cerebral artery occlusion as a cause of isolated subcortical infarction. Stroke. 1983;14:948–952. doi: 10.1161/01.str.14.6.948. [DOI] [PubMed] [Google Scholar]
- Adams RD, Victor M, Ropper AH. Principles of neurology. 6. New York, NY: McGraw-Hill; 1997. [Google Scholar]
- Alvarez-Sabin J, Montalbán J, Tintoré M, Codina A. Pure sensory stroke due to midbrain haemorrhage. Journal of Neurology, Neurosurgery, and Psychiatry. 1991;54:843. doi: 10.1136/jnnp.54.9.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia MM, Mourão LF, Chun RY. Dysarthria as a predictor of dysphagia following stroke. NeuroRehabilitation. 2016;38:155–162. doi: 10.3233/NRE-161305. [DOI] [PubMed] [Google Scholar]
- Barnard JW, Woolsey CN. A study of localization in the corticospinal tracts of monkey and rat. Journal of Comparative Neurology. 1956;105:25–50. doi: 10.1002/cne.901050103. [DOI] [PubMed] [Google Scholar]
- Bauer G, Gerstenbrand F, Rumpl E. Varieties of the locked-in syndrome. Journal of Neurology. 1979;221:77–91. doi: 10.1007/BF00313105. [DOI] [PubMed] [Google Scholar]
- Beck E. The origin, course and termination of the prefronto-pontine tract in the human brain. Brain. 1950;73:368–391. doi: 10.1093/brain/73.3.368. [DOI] [PubMed] [Google Scholar]
- Beric A, Kelly PJ, Rezai A, Sterio D, Mogilner A, Zonenshayn M, Kopell B. Complications of deep brain stimulation surgery. Stereotactic and Functional Neurosurgery. 2001;77:73–78. doi: 10.1159/000064600. [DOI] [PubMed] [Google Scholar]
- Bertrand G, Blundell J, Musella R. Electrical exploration of the internal capsule and neighboring structures during stereotaxic procedures. Journal of Neurosurgery. 1965;22:333–343. doi: 10.3171/jns.1965.22.4.0333. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Maeder P, Regli F, Meuli R. Pure midbrain infarction: Clinical syndromes, MRI, and etiologic patterns. Neurology. 1994;44:2032–2040. doi: 10.1212/wnl.44.11.2032. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Regli F. Capsular genu syndrome. Neurology. 1990;40:1499–1502. doi: 10.1212/wnl.40.10.1499. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Belhaj-Saïf A, Park MC, Cheney PD. Contrasting properties of motor output from the supplementary motor area and primary motor cortex in rhesus macaques. Cerebral Cortex. 2006;16:632–638. doi: 10.1093/cercor/bhj009. [DOI] [PubMed] [Google Scholar]
- Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Fore-limb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cerebral Cortex. 2010;20:704–719. doi: 10.1093/cercor/bhp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley N, Hannon N, Lebus C, O’Brien E, Khadjooi K. Bilateral corona radiata infarcts: A new topographic location of Foix-Chavany-Marie syndrome. International Journal of Stroke. 2014;9:E39. doi: 10.1111/ijs.12387. [DOI] [PubMed] [Google Scholar]
- Brazis PW, Masdeu JC, Biller J. Localization in clinical neurology. 6. Philadelphia, IL: Lippincott, Williams and Wilkins; 2011. pp. 8–9. [Google Scholar]
- Brodal A. Neurological anatomy: In relation to clinical medicine. 3. New York, NY: Oxford University Press; 1981. [Google Scholar]
- Bucy PC, Ladpli R, Ehrlich A. Destruction of the pyramidal tract in the monkey. The effects of bilateral section of the cerebral peduncles. Journal of Neurosurgery. 1966;25:1–23. doi: 10.3171/jns.1966.25.1.0001. [DOI] [PubMed] [Google Scholar]
- Campbell WW. De Jong’s The Neurological Examination. 7. Philadelphia, PA: Lippincott, Williams and Wilkins; 2013. p. 59. [Google Scholar]
- Caplan LR. “Top of the basilar” syndrome. Neurology. 1980;30:72–79. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, … Hier DB. Caudate infarcts. Archives of Neurology. 1990;47:133–143. doi: 10.1001/archneur.1990.00530020029011. [DOI] [PubMed] [Google Scholar]
- Carrai R, Grippo A, Fossi S, Campolo MC, Lanzo G, Pinto F, … Amantini A. Transient post-traumatic locked-in syndrome: A case report and a literature review. Neurophysiologie Clinique. 2009;39:95–100. doi: 10.1016/j.neucli.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Sacco RL, Mohr JP, Foulkes MA, Kase CS, Tatemichi TK, … Hier DB. Clinical-computed tomographic correlations of lacunar infarction in the Stroke Data Bank. Stroke. 1991;22:175–181. doi: 10.1161/01.str.22.2.175. [DOI] [PubMed] [Google Scholar]
- Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P. Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. Journal of Neurosurgery. 2008;109:593–604. doi: 10.3171/JNS/2008/109/10/0593. [DOI] [PubMed] [Google Scholar]
- Chia LG. Locked-in syndrome with bilateral ventral midbrain infarcts. Neurology. 1991;41:445–446. doi: 10.1212/wnl.41.3.445. [DOI] [PubMed] [Google Scholar]
- Chung CS, Caplan LR, Yamamoto Y, Chang HM, Lee SJ, Song HJ, … Yoo KM. Striatocapsular haemorrhage. Brain. 2000;123:1850–1862. doi: 10.1093/brain/123.9.1850. [DOI] [PubMed] [Google Scholar]
- Costa J, Valls-Solé J, Valldeoriola F, Rumià J, Tolosa E. Motor responses of muscles supplied by cranial nerves to subthalamic nucleus deep brain stimuli. Brain. 2007;130:245–255. doi: 10.1093/brain/awl336. [DOI] [PubMed] [Google Scholar]
- Cure C, Rasmussen T. Effects of altering the parameters of electrical stimulating currents upon motor responses from the pre-central gyrus of Macaca mulatta. Brain. 1954;77:18–33. doi: 10.1093/brain/77.1.18. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Brailey K, Priestly DH, Weisberg LA, Foundas AL. Aspiration in patients with acute stroke. Archives of Physical Medicine and Rehabilitation. 1998;79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- Dehaene I, Dom R. A mesencephalic locked-in syndrome. Journal of Neurology. 1982;227:255–259. doi: 10.1007/BF00313393. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Finnis KW, Peters TM, Sadikot AF. Three-dimensional somatotopic organization and probabilistic mapping of motor responses from the human internal capsule. Journal of Neurosurgery. 2011;114:1706–1714. doi: 10.3171/2011.1.JNS10136. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. Journal of Neuroscience. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. Journal of Neuroscience. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain. 1993;116:369–382. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Ohnishi Y, Wakayama A, Yoshimine T. Transient total mesencephalic locked-in syndrome after bilateral ptosis due to basilar artery thrombosis. Journal of Stroke and Cerebrovascular Diseases. 2011;21:909, e7–8. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cerebral Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Galovic M, Leisi N, Müller M, Weber J, Abela E, Kägi G, Weder B. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;22:2760–2767. doi: 10.1161/STROKEAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Ghika J, Bogousslavsky J, Regli F. Infarcts in the territory of the deep perforators from the carotid system. Neurology. 1989;39:507–512. doi: 10.1212/wnl.39.4.507. [DOI] [PubMed] [Google Scholar]
- Gillingham FJ. Small localized surgical lesions of the internal capsule in the treatment of the dyskineasias. Confinia Neurologica. 1962;22:385–392. doi: 10.1159/000104390. [DOI] [PubMed] [Google Scholar]
- Gong S, DeCuypere M, Zhao Y, LeDoux MS. Cerebral cortical control of orbicularis oculi motoneurons. Brain Research. 2005;1047:177–193. doi: 10.1016/j.brainres.2005.04.045. [DOI] [PubMed] [Google Scholar]
- Guiot G, Rougerie J, Sachs M, Hertzog E. La stimulation capsularie chez I’homme. Son intérêt dans la stéréotaxie pallidale pour syndromes parkinsoniens. Revue Neurologique. 1958;98:222–224. [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. The representation of blinking movement in cingulate motor areas: A functional magnetic resonance imaging study. Cerebral Cortex. 2008;18:930–937. doi: 10.1093/cercor/bhm129. [DOI] [PubMed] [Google Scholar]
- Hanaway J, Young RR. Localization of the pyramidal tract in the internal capsule of man. Journal of the Neurological Sciences. 1977;34:63–70. doi: 10.1016/0022-510x(77)90092-2. [DOI] [PubMed] [Google Scholar]
- Hardy TL, Bertrand G, Thompson CJ. The position and organization of motor fibers in the internal capsule found during stereotactic surgery. Applied Neurophysiology. 1979;42:160–170. doi: 10.1159/000102360. [DOI] [PubMed] [Google Scholar]
- Hatanaka N, Tokuno H, Nambu A, Inoue T, Takada M. Input-output organization of jaw movement-related areas in monkey frontal cortex. Journal of Comparative Neurology. 2005;492:401–415. doi: 10.1002/cne.20730. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the lateral surface of the hemisphere. Journal of Neuroscience. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the medial surface of the hemisphere. Journal of Neuroscience. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason C, Caplan LR, Goodwin J, Hedges T. Anterior choroidal artery-territory infarction. Report of cases and review. Archives of Neurology. 1986;43:681–686. doi: 10.1001/archneur.1986.00520070039015. [DOI] [PubMed] [Google Scholar]
- Holodny AI, Watts R, Korneinko VN, Pronin IN, Zhukovskiy ME, Gor DM, Ulug A. Diffusion tensor tractography of the motor white matter tracts in man: Current controversies and future directions. Annals of the New York Academy of Sciences. 2005a;1064:88–97. doi: 10.1196/annals.1340.016. [DOI] [PubMed] [Google Scholar]
- Holodny AI, Gor DM, Watts R, Gutin PH, Ulug AM. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: Initial anatomic results in contradistinction to prior reports. Radiology. 2005b;234:649–653. doi: 10.1148/radiol.2343032087. [DOI] [PubMed] [Google Scholar]
- Hommel M, Besson G. Midbrain infarcts. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2. Cambridge: Cambridge University Press; 2001. pp. 512–519. [Google Scholar]
- Huang CS, Hiraba H, Murray GM, Sessle BJ. Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis) Journal of Neurophysiology. 1989;61:635–650. doi: 10.1152/jn.1989.61.3.635. [DOI] [PubMed] [Google Scholar]
- Huang CS, Hiraba H, Sessle BJ. Input-output relationships of the primary face motor cortex in the monkey (Macaca fascicularis) Journal of Neurophysiology. 1989;61:350–362. doi: 10.1152/jn.1989.61.2.350. [DOI] [PubMed] [Google Scholar]
- Huang CS, Sirisko MA, Hiraba H, Murray GM, Sessle BJ. Organization of the primate face motor cortex as revealed by intracortical microstimulation and electrophysiological identification of afferent inputs and corticobulbar projections. Journal of Neurophysiology. 1988;59:796–818. doi: 10.1152/jn.1988.59.3.796. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Kageyama Y. Clinical anatomic study of pure dysarthria. Stroke. 1991;22:809–812. doi: 10.1161/01.str.22.6.809. [DOI] [PubMed] [Google Scholar]
- Jenabi M, Peck KK, Young RJ, Brennan N, Holodny AI. Identification of the corticobulbar tracts of the tongue and face using deterministic and probabilistic DTI fiber tracking in patients with brain tumor. American Journal of Neuroradiology. 2015;36:2036–2041. doi: 10.3174/ajnr.A4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny AB, Saper CB. Organization of the facial nucleus and corticofacial projection in the monkey: A reconsideration of the upper motor neuron facial palsy. Neurology. 1987;37:930–939. doi: 10.1212/wnl.37.6.930. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim J. Pure midbrain infarction: Clinical, radiologic, and pathophysiologic findings. Neurology. 2005;64:1227–1232. doi: 10.1212/01.WNL.0000156520.46056.6B. [DOI] [PubMed] [Google Scholar]
- Kim JS, Pope A. Somatotopically located motor fibers in corona radiata: Evidence from subcortical small infarcts. Neurology. 2005;64:1438–1440. doi: 10.1212/01.WNL.0000158656.09335.E7. [DOI] [PubMed] [Google Scholar]
- Kim YI, Ahn KJ, Chung YA, Kim BS. A new reference line for the brain CT: The tuberculum sellae-occipital protuberance line is parallel to the anterior/posterior commissure line. American Journal of Neuroradiology. 2009;30:1704–1708. doi: 10.3174/ajnr.A1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral E, Celebisoy M, Celebisoy N, Canbaz DH, Calli C. Dysarthria due to supratentorial and infratentorial ischemic stroke: A diffusion-weighted imaging study. Cerebrovascular Diseases. 2007;23:331–338. doi: 10.1159/000099131. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Cortical projections to the pons and medulla oblongata in the cat and man. Anatomical Record. 1956;124:322–323. [Google Scholar]
- Kuypers HGJM. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. Journal of Comparative Neurology. 1958a;110:221–251. doi: 10.1002/cne.901100205. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Corticobulbar connexions to the pons and lower brain-stem in man: An anatomical study. Brain. 1958b;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. An anatomical analysis of corticobulbar connexions to the pons and lower brain stem in cat. Journal of Anatomy. 1958c;92:198–218. [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM. Anatomy of the descending motor pathways. In: Brooks VB, editor. Handbook of physiology. Section I: The nervous system. Vol II: Motor control, Pt I. Bethesda, MD: American Physiological Society; 1981. pp. 567–666. [Google Scholar]
- Kwon HG, Lee J, Jang SH. Injury of the corticobulbar tract in patients with dysarthria following cerebral infarct: Diffusion tensor tractography study. International Journal of Neuroscience. 2016;126:361–365. doi: 10.3109/00207454.2015.1020536. [DOI] [PubMed] [Google Scholar]
- Leder SP, Espinosa JF. Aspiration risk after acute stroke: Comparison of clinical examination and fiberoptic endoscopic evaluation of swallowing. Dysphagia. 2002;17:214–218. doi: 10.1007/s00455-002-0054-7. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Maier MA, Armand J, Kirkwood PA, Yang HW. Functional differences in corticospinal projections from macaque primary motor cortex and supplementary motor area. Advances in Experimental Medicine and Biology. 2002;508:425–434. doi: 10.1007/978-1-4615-0713-0_48. [DOI] [PubMed] [Google Scholar]
- Liégeois F, Tournier JD, Pigdon L, Connelly A, Morgan AT. Corticobulbar tract changes as predictors of dysarthria in childhood brain injury. Neurology. 2013;80:926–932. doi: 10.1212/WNL.0b013e3182840c6d. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: An anatomical and electrophysiological study. Cerebral Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- Maestroni A, Mandelli C, Zecca B, Rossi P, Isalberti M, Manganaro D, … Torgano G. Minor stroke and major vascular occlusion. A case report. Neurological Sciences. 2006;27:183–186. doi: 10.1007/s10072-006-0666-z. [DOI] [PubMed] [Google Scholar]
- McGuinness E, Sivertsen D, Allman JM. Organization of the face representation in macaque motor cortex. Journal of Comparative Neurology. 1980;193:591–608. doi: 10.1002/cne.901930302. [DOI] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, … Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. Journal of Comparative Neurology. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienberg O, Mumenthaler M, Karbowski K. Quadriparesis and nuclear oculomotor palsy with total bilateral ptosis mimicking coma: A mesencephalic ’locked-in syndrome”? Archives of Neurology. 1979;36:708–710. doi: 10.1001/archneur.1979.00500470078016. [DOI] [PubMed] [Google Scholar]
- Mellus LM. Motor paths in the brain and cord of the monkey. Journal of Nervous and Mental Disease. 1899;26:197–209. [Google Scholar]
- Miller AJ. The neuroscientific principles of swallowing and dysphagia. San Diego, CA: Singular Publishing Group; 1999. [Google Scholar]
- Miller AJ. The neurobiology of swallowing. Developmental Disabilities Research Reviews. 2008;14:77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, Hynes SM, Pizzimenti MA, Rotella DL, Darling WG. Frontal and frontoparietal injury differentially affect the ipsilateral corticospinal projection from the non-lesioned hemisphere in monkey (Macaca mulatta) Journal of Comparative Neurology. 2016;524:380–407. doi: 10.1002/cne.23861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, … Darling WG. Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury in rhesus monkey. Journal of Comparative Neurology. 2015a;523:669–697. doi: 10.1002/cne.23703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Ge J, Cipolloni PB, Pandya DN. Cytoarchitecture and cortical connections of the anterior insula and adjacent frontal motor fields in the rhesus monkey. Brain Research Bulletin. 2015b;119:52–72. doi: 10.1016/j.brainresbull.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. Journal of Comparative Neurology. 2013;521:4205–4235. doi: 10.1002/cne.23410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick J, Stilwell-Morecraft KS, Louie JL, Ottenbacher J, Schroeder CM, Schoolfield M. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Herrick J, Morecraft-Stilwell KS. Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–203. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Louie JL, Schroeder CM, Avramov K. Segregated parallel inputs to the brachial spinal cord from the cingulate motor cortex in the monkey. Neuroreport. 1997;8:3933–3938. [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. Journal of Comparative Neurology. 2007;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal D, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. Journal of Comparative Neurology. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Schroeder CM, Keifer K. Organization of face representation in the cingulate cortex of the rhesus monkey. NeuroReport. 1996;7:1343–1348. doi: 10.1097/00001756-199605310-00002. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Research Bulletin. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: New insights from neuroscience. Neurologist. 2004;10:235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Solon-Cline KM, Ge J, Darling WG. Cortical innervation of the hypoglossal nucleus in the non-human primate (Macaca mulatta) Journal of Comparative Neurology. 2014;522:3456–3484. doi: 10.1002/cne.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to primary and supplementary motor cortices in the rhesus monkey: Evidence for somatotopy in areas 24c and 23c. Journal of Comparative Neurology. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (M1) motor cortices in the rhesus monkey. Journal of Comparative Neurology. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. Journal of Comparative Neurology. 1981;195:339–365. doi: 10.1002/cne.901950212. [DOI] [PubMed] [Google Scholar]
- Murray GM, Sessle BJ. Functional properties of single neurons in the face primary motor cortex of the primate. I. Input and output features of tongue motor cortex. Journal of Neurophysiology. 1992;67:747–758. doi: 10.1152/jn.1992.67.3.747. [DOI] [PubMed] [Google Scholar]
- Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, Frackowiak RS. Non-invasive mapping of corticofugal fibres from multiple motor areas–relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liégeois F, Chong WK, Baker K, Tournier JD, Wyatt JS, … Morgan A. Speech and oromotor outcome in adolescents born preterm: Relationship to motor tract integrity. Journal of Pediatrics. 2012;160:402–408. doi: 10.1016/j.jpeds.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: Sites of origin of the corticospinal tract. Journal of Comparative Neurology. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Jurkiewicz AL, Santos RS, Furkim AM, Massi G, Pinto GS, Lange MC. Correlation between brain injury and dysphagia in adult patients with stroke. International Archives of Otorhinolaryngology. 2012;16:313–321. doi: 10.7162/S1809-97772012000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR, … Goodman WK. What’s in a “smile?” Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki I, Baba M, Narita S, Matsunaga M, Takebe K. Pure dysarthria due to anterior internal capsule and/or corona radiata infarction: A report of five cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:1435–1437. doi: 10.1136/jnnp.49.12.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Peck KK, Young RJ, Holodny AI. Somatotopic organization of motor pathways in the internal capsule: A probabilistic diffusion tractography study. American Journal of Neuroradiology. 2012;33:1274–1280. doi: 10.3174/ajnr.A2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JK, Kim BS, Choi G, Kim SH, Choi JC, Khang H. Evaluation of the somatotopic organization of corticospinal tracts in the internal capsule and cerebral peduncle: Results of diffusion-tensor MR tractography. Korean Journal of Radiology. 2008;9:191–195. doi: 10.3348/kjr.2008.9.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SA, Sohn YH, Kim WC. Locked-in syndrome with bilateral peduncular infarct. Journal of Neuroimaging. 1997;7:126–128. doi: 10.1111/jon199772126. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. Journal of Neurophysiology. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, … Matthews PM. The evolution of pre-frontal inputs to the corticopontine system: Diffusion imaging evidence from Macaque monkeys and humans. Cerebral Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Moret V, Tanne J, Boussaoud D. Evidence for direct connections between the hand region of the supplementary motor area and cervical motoneurons in the macaque monkey. European Journal of Neuroscience. 1996;8:1055–1059. doi: 10.1111/j.1460-9568.1996.tb01592.x. [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Lesoin F, Quint S. Unilateral pseudobulbar syndrome with limited capsulothalamic infarction. European Neurology. 1987;27:227–230. doi: 10.1159/000116161. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39:1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemanck SK, Kwakkel G, Post MW, Kappelle LJ, Prevo AJ. Impact of internal capsule lesions on outcome of motor hand function at one year post-stroke. Journal of Rehabilitation Medicine. 2008;40:96–101. doi: 10.2340/16501977-0130. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Rosene DL, Pandya DN. Motor projections to the basis pontis in rhesus monkey. Journal of Comparative Neurology. 2004;478:248–268. doi: 10.1002/cne.20286. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U. Efferent subcortical projections of the laryngeal motor cortex in the rhesus monkey. Brain Research. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- Smithard DG, O’Neill PA, Park C, Morris J, Wyatt R, England R, Martin DF. Complications and outcome after stroke: Does dysphagia matter? Stroke. 1996;27:1200–1204. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Voller B, Dimyan M, St Clair Gibson A, Hanakawa T, Leon-Sarmiento FE, … Hallett M. Cortical control of voluntary blinking: A transcranial magnetic stimulation study. Clinical Neurophysiology. 2004;115:341–347. doi: 10.1016/j.clinph.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Song YM. Somatotopic organization of motor fibers in the corona radiata in monoparetic patients with small subcortical infarct. Stroke. 2007;38:2353–2355. doi: 10.1161/STROKEAHA.106.480632. [DOI] [PubMed] [Google Scholar]
- Springer US, Bowers D, Goodman WK, Shapira NA, Foote KD, Okun MS. Long-term habituation of the smile response with deep brain stimulation. Neurocase. 2006;12:191–196. doi: 10.1080/13554790600646995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring S. Gray’s Anatomy: The anatomical basis of clinical practice. 41. New York, NY: Elsevier; 2016. p. 395. [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas Ferrer M, Bonis A, Szikla G, Rusu M. Electroencephalography and clinical neurophysiology: The cingulate gyrus and human behaviour. Electroencephalography and Clinical Neurophysiology. 1973;34:45–52. doi: 10.1016/0013-4694(73)90149-1. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thiem Medical Publishers, Inc; 1988. [Google Scholar]
- Tatemichi TK, Desmond DW, Prohovnik I, Cross DT, Gropen TI, Mohr JP, Stern Y. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- Titelbaum DS, Sodha NB, Moonis M. Transient hemiglossal denervation during acute internal capsule infarct in the setting of dysarthria-clumsy hand syndrome. American Journal of Neuroradiology. 2010;31:1266–1267. doi: 10.3174/ajnr.A1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Takahashi H, Tamura K, Yonezawa H. The side and somatotopical location of single small infarcts in the corona radiata and pontine base in relation to contralateral limb paresis and dysarthria. European Neurology. 1996;36:338–342. doi: 10.1159/000117290. [DOI] [PubMed] [Google Scholar]
- Tredici G, Pizzini G, Bogliun G, Tagliabue M. The site of motor corticospinal fibers in the internal capsule of man. A computerised tomographic study of restricted lesions. Journal of Anatomy. 1982;134:199–208. [PMC free article] [PubMed] [Google Scholar]
- Tuttle PV, Reinmuth OM. Midbrain hemorrhage producing pure sensory stroke. Archives of Neurology. 1984;41:794–795. doi: 10.1001/archneur.1984.04050180120033. [DOI] [PubMed] [Google Scholar]
- Urban PP, Hopf HC, Connemann B, Hundemer HP, Koehler J. The course of corticohyopglossal projections in the human brainstem: Functional testing using transcranial magnetic stimulation. Brain. 1996;119:1031–1038. doi: 10.1093/brain/119.3.1031. [DOI] [PubMed] [Google Scholar]
- Urban PP, Hopf HC, Fleischer S, Zorowka PG, Müller-Forell W. Impaired corticobulbar tract function in dysarthria due to hemispheric stroke: Functional testing using transcranial magnetic stimulation. Brain. 1997;120:1077–1084. doi: 10.1093/brain/120.6.1077. [DOI] [PubMed] [Google Scholar]
- Urban PP, Wicht S, Hopf HC, Fleischer S, Nickel O. Isolated dysarthria due to extracerebellar lacunar stroke: A central monoparesis of the tongue. Journal of Neurology, Neurosurgery, and Psychiatry. 1999;66:495–501. doi: 10.1136/jnnp.66.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban PP, Wicht S, Vukurevic G, Fitzek C, Fitzek S, Stoeter P, … Hopf HC. Dysarthria in acute ischemic stroke. Lesion topography, clinicoradiologic correlation, and etiology. Neurology. 2001;56:1021–1027. doi: 10.1212/wnl.56.8.1021. [DOI] [PubMed] [Google Scholar]
- Weiss KL, Pan H, Storrs J, Strub W, Weiss JL, Jia L, Eldevik OP. Clinical brain MR imaging prescriptions in Talairach space: Tzechnologist- and computer-driven methods. American Journal of Neuroradiology. 2003;24:922–929. [PMC free article] [PubMed] [Google Scholar]
- Wenzelburger R, Kopper F, Frenzel A, Stolze H, Klebe S, Brossmann A, … Deuschl G. Hand coordination following capsular stroke. Brain. 2005;128:64–74. doi: 10.1093/brain/awh317. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Delong MR. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron. 2006;52:197–204. doi: 10.1016/j.neuron.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Research Publications - Association for Research in Nervous and Mental Disease. 1952;30:238–264. [PubMed] [Google Scholar]
- Xu W, Miocinovic S, Zhang J, Baker KB, McIntyre CC, Vitek JL. Dissociation of motor symptoms during deep brain stimulation of the subthalamic nucleus in the region of the internal capsule. Experimental Neurology. 2011;228:294–297. doi: 10.1016/j.expneurol.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim SH, Kim JH, Han ZA, Jeon S, Cho JH, Kim GS, … Lee JH. Distribution of the corticobulbar tract in the internal capsule. Journal of the Neurological Sciences. 2013;334:63–68. doi: 10.1016/j.jns.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Zakaria T, Flaherty ML. Locked-in syndrome resulting from bilateral cerebral peduncle infarctions. Neurology. 2006;67:1889. doi: 10.1212/01.wnl.0000229160.49552.b0. [DOI] [PubMed] [Google Scholar]