Abstract
DNAzymes have enjoyed success as metal ion sensors outside cells. Their susceptibility to metal-dependent cleavage during delivery into cells has limited their intracellular applications. To overcome this limitation, we herein report a near-infrared (NIR) photothermal activation method for controlling DNAzyme activity in living cells. The system consists of a three-stranded DNAzyme precursor (TSDP) whose hybridization prevents the DNAzyme from being active. After conjugating the TSDP onto gold nanoshells and upon NIR illumination, the increased temperature dehybridizes the TSDP to release the active DNAzyme, which then carries out metal ion-dependent cleavage, resulting in releasing the cleaved product containing a fluorophore. Using this construct, detecting Zn2+ in living HeLa cells is demonstrated. This method has expanded the DNAzyme versatility for detecting metal ions in biological systems under NIR light that exhibits lower phototoxicity and higher tissue penetration ability.
Keywords: DNAzymes, Metal ions, Gold nanoshells, Photothermal activation, HeLa cells
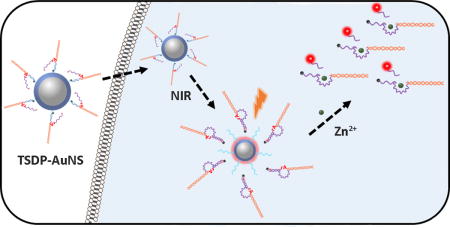
TOC image
Near-IR activated DNAzyme sensors: A near-IR light activated DNAzymes-AuNS system has been developed and applied for imaging metal ions in living cells.
Metal ions are vital components in biological systems, where they play important roles.[1] The disruptions in metal homeostasis are implicated in many severe diseases.[2] In order to understand the metal homeostasis in human health and disease, probes that can identify specific metal ion species and their subcellular locations are of significant interests.[3] Toward this goal, many fluorescent probes based on either small organic molecules or fluorescent proteins have been developed.[4] Despite numerous groups’ efforts for many years, there are only a limited number of metal ion sensors that were effective in sensing metal ions in cells, even though the large number of metal ions in the biological system demands a more generalizable approach to develop sensors for as many metal ions as possible. To meet such a demand, we and others have taken advantage of DNAzymes or deoxyribozymes, which are DNA molecules capable of performing enzymatic reactions in presence of metal cofactors.[5] In this complementary approach to rational design of fluorescent sensors, metal-specific DNAzymes can be obtained by a combinatorial process called in vitro selection starting from a large DNA library of up to 1015 combinations.[6] In this way, it is not necessary to have advanced knowledge to construct metal-binding site. The selection process allows the binding affinity and selectivity of the DNAzymes to be fine-tuned by introducing different levels of stringency. As a result, many DNAzymes with high selectivity have been obtained[5b,7] and then converted into metal sensors.[8] These sensors are playing key roles in environmental monitoring, including monitoring Pb2+ in municipal water system in public schools in the US.[9]
In contrast to much progress made in using DNAzymes to monitor metal ions in the environment, applications of DNAzyme in imaging of metal ions in living cells have begun to appear only recently.[10] A major barrier to the progress is that the DNAzymes can react with extracellular metal ions and cleave its substrate during the delivery process, before they reach the desired locations in cells. To overcome this barrier, we have developed a photochemical caging strategy to render the DNAzymes inactive during the delivery progress, and then activate the DNAzyme by UV light once the DNAzymes are inside the cells.[11] While this strategy has been demonstrated to work in living cells, it requires high-energy UV irradiation that can harm the cells. Therefore, it is highly desirable to develop methods to control DNAzymes activity without damaging the cells. We herein report a novel method of near-infrared (NIR) photothermal activated DNAzyme conjugated to gold nanoshells (AuNS) for detection of metal ions in living cells.
We choose to use NIR light to control DNAzymes activity in living cells, because it exhibits lower photo-toxicity while providing higher tissue penetration ability than UV light.[12] AuNS are known to absorb NIR light and efficiently convert it into heat due to the large absorption cross-sectional inability to emit light.[13] This photothermal property of AuNS has been used in targeted drug delivery and cancer therapy.[14] One particular interesting feature of AuNS is that the NIR irradiation can release double stranded DNA (dsDNA) from AuNS by increasing the local temperature above the melting temperature of the dsDNA.[15] Taking advantage of these properties, we designed a three-stranded DNAzyme precursor (TSDP) consisting of an enzyme strand with a black hole quencher (BHQ2) at the 3′-end that can hybridize to a substrate strand with Cy5 at the 5′-end on the right arm (in orange, Scheme 1a), and to a Linker DNA strand with –SH group at the 5′-end on the left arm (in purple). The TSDP can be conjugated onto AuNS through thiol-gold chemistry. At 37 °C where the cells are cultured, the hybridization of the Linker DNA strand (in blue) with the left arm of the enzyme strand, which is designed to display a calculated melting temperature around 50 °C, prevents the enzyme strand from hybridizing to the left arm of the substrate strand and thus the DNAzyme from being active.
Scheme 1.
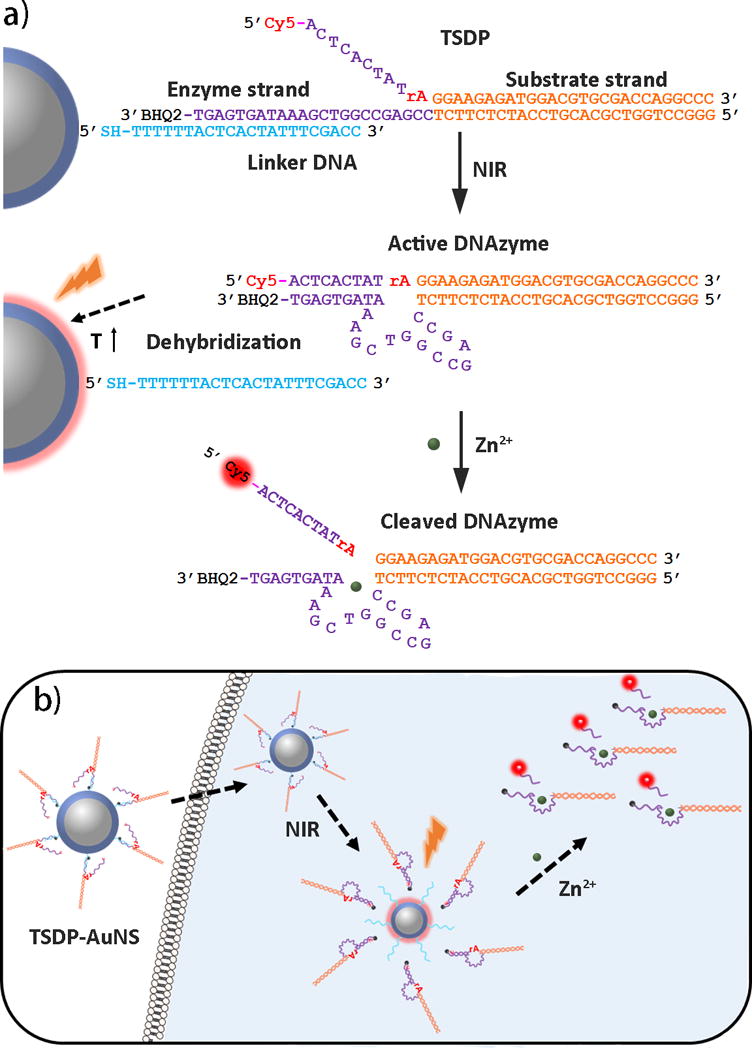
Design of NIR activated TSDP-AuNS for intracellular metal ion sensing.
Since Cy5 is close to AuNS, the Cy5 fluorescence is thereby quenched. Upon NIR irradiation, the local temperature around AuNS increased to above the melting temperature between the Linker strand and the enzyme strand, which causes a release the enzyme and substrate strands from AuNS. Once free of the Linker strand, the left arm of the enzyme strand can hybridize with the substrate strand to form an active DNAzyme. In this configuration, since Cy5 is next to BHQ2, the Cy5 fluorescence is quenched by BHQ2. In the presence of Zn2+, the substrate strand will be cleaved. Because the calculated melting temperature between the left arm of the DNAzyme and the cleaved product is around 20 °C, the cleaved product containing Cy5 will be released at 37 °C from the enzyme strand and away from BHQ2, resulting in an increased fluorescent signal.
The TSDP was prepared by heating and annealing the -SH modified Linker DNA (Lane 1, Figure 1) with the enzyme strand (Lane 2), resulting in a band (Lane 4) with higher molecular weight (MW) consistent with hybridization between the two strands. The thiolated DNA contains a poly T spacer to avoid the steric interference of the AuNS to DNA hybridization. The construct was then annealed with the substrate strand (Lane 3), resulting in a strong top band (Lane 6) with MW similar to that of the TSDP and a minor lower band consistent with hybridization of enzyme and substrate strand, like in Lane 5. In the presence of Zn2+, the enzyme/substrate strand in Lane 5 displayed a lower MW band (Lane 7), suggesting that the substrate band was cleaved. In contrast, the top band, assigned to TSDP in Lane 6 remained the same in the presence of Zn2+ (Lane 8), indicating that, even in the presence of Zn2+, the DNAzyme is blocked from being active. To test the long-term stability of the TSDP, the construct was incubated for 24 h in the presence of Zn2+. The TSDP remained the same with little degradation (Figure S1), suggesting that this TSDP is “caged” without being cleaved.
Figure 1.
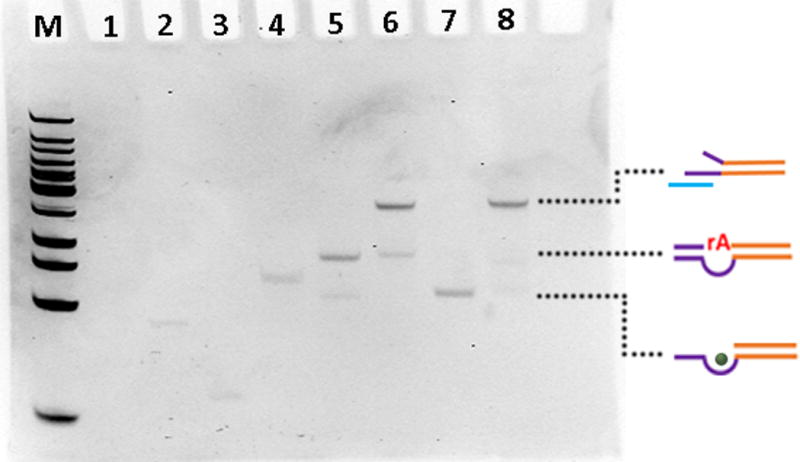
Native PAGE of TSDP formation and stability in the presence of metal ion. The gel was stained with ethidium bromide for visualization. M: DNA ladder; Lane 1: Linker DNA (the ethidium bromide does not stain short single stranded Linker DNA); Lane 2: Enzyme strand; Lane 3: Substrate strand; Lane 4: Linker DNA+ Enzyme strand; Lane 5: Enzyme strand+ Substrate strand; Lane 6: Linker DNA+ Enzyme strand+ Substrate strand; Lane 7: lane 5+ Zn2+; Lane 8: lane 6+ Zn2+. The dotted lines (from top to bottom) indicate TSDP, DNAzymes and cleaved DNAzymes.
Having demonstrated successful preparation of the TSDP, we synthesized the AuNS according to the reported protocol.13 As the transmission electron microscopy (TEM, Figure 2a) and scanning electron microscopy (SEM) (Figure S2) images shown, the size of AuNS is ~150 nm. Illuminating the AuNS with 808 nm laser (1W/cm2) for 10 min caused an increase in temperature that depended on the particle concentration (Figure 2b). The TSDP was conjugated onto AuNS using the modified salt-aging method.[16] The TSDP-functionalized AuNS (called TSDP-AuNS hereafter) are similar in the shape and dispersity to those of bare AuNS (Figure 2c). While suspending bare AuNS in the buffer containing 100 mM NaCl caused them to aggregate in several minutes, as indicated by the broad optical extinction spectrum (Figure 2d, blue line), redispersion of TSDP-AuNS in buffer caused minimal change to the UV spectrum, suggesting that the stability of the AuNS was significantly enhanced after the TSDP functionalization. The number of TSDP on each AuNS was calculated to be 3635±534 using fluorescence method (Figure S3).[13,15] In addition, the Cy5 fluorescence of TSDP-AuNS was completely quenched (Figure S4).
Figure 2.
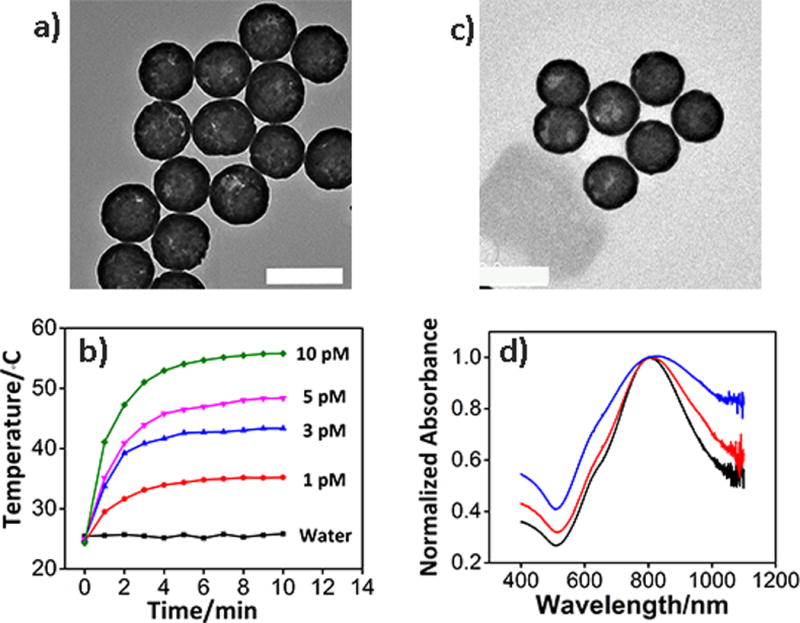
Characterization of AuNS and TSDP-AuNS. a) TEM image of bare AuNS; b). Photothermal response of bare AuNS at different concentrations.; c) TEM image of TSDP-AuNS; d). Absorption spectra of bare AuNS in water (black line), bare AuNS in buffer (blue line) and TSDP-AuNS in buffer (red line). Scale bar: 200 nm.
After conjugating the TSDP onto AuNS, we evaluated the feasibility of TSDP-AuNS as a biosensor in a buffer (50 mM Tris, 100 mM NaCl, pH 7.4). As shown in Figure 3a, the TSDP-AuNS showed minimum fluorescence, indicating Cy5 has been nearly completely quenched by AuNS. The construct was irradiated for 15 min. using a laser with a power density of 1 W/cm2, which is commonly used for cellular study.[15b] After NIR irradiation, in the absence of Zn2+, the construct displayed an enhanced fluorescence signal around 660 nm, consistent with the DNAzyme being released from AuNS, as shown in Scheme 1a. Since BHQ2 is a poorer quencher compared with AuNS, the fluorescence of Cy5 was recovered. Upon addition of Zn2+ into the supernatant, the fluorescence was enhanced further by nearly 4 folds, demonstrating that cleaved product containing Cy5 has been released from the enzyme strand containing BHQ2. To support the above findings, we further used polyacrylamide gel electrophoresis (PAGE). As shown in Figure 3b, without NIR irradiation or addition of Zn2+, no DNA band was observed on the supernatant (Lane 1 and 4), suggesting that the DNAzymes stayed on the AuNS. NIR irradiation resulted in observation of a DNA band with MW consistent with that of the active DNAzymes (Lane 5). A band with a similar MW was observed when the same system is treated with a hot water bath (Lane 6), suggesting that the NIR photothermal decaging has the same effect as thermal decaging in releasing the active DNAzymes. Upon addition of Zn2+, the above NIR irradiated or thermally decaged constructs showed an even lower band (see Lane 2 and 3, respectively) whose MW is similar to the cleaved products. Together, these results indicate successful controlled NIR photothermal caging and decaging of the TSDP-AuNS. The response of released DNAzymes to different concentrations of Zn2+ was tested (Figure S5), which showed similar kinetics to free DNAzymes.
Figure 3.
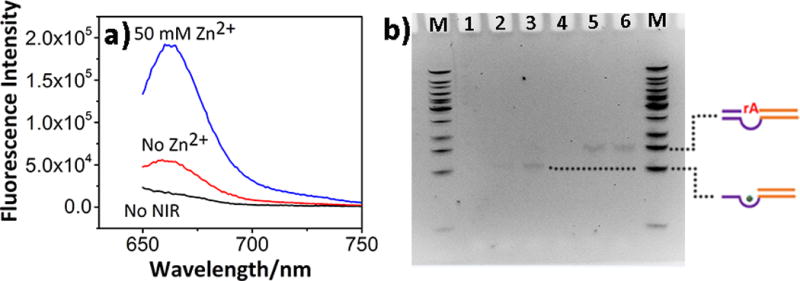
DNAzyme release study from TSDP-AuNS under NIR irradiation. a). Fluorescence spectra of TSDP-AuNS before NIR irradiation and supernatant of TSDP-AuNS after NIR irradiation under different conditions; b). 12% PAGE. M: DNA ladder; Lane 1 and 4: TSDP-AuNS without NIR irradiation or addition of Zn2+; Lane 2: NIR irradiation for 15 min, with Zn2+; Lane 3: 70 °C water bath for 15 min, with Zn2+; Lane 5: NIR irradiation for 15 min, without Zn2+; Lane 6: 70 °C water bath for 15 min, without Zn2+. The dotted lines (from top to bottom) indicate released active DNAzymes and cleaved DNAzymes.
To achieve the best performance of the biosensor, we optimized the length of the Linker DNA (Figure S6). While the release efficiencies were very similar, the SH-16 sequence consistently shows higher release efficiency and thus chosen for further studies. Before we explored TSDP-AuNS for cellular studies, we tested its stability and feasibility in cell lysate (Figure S7). After a 7 h incubation, there was no fluorescence signal increase, indicating that the construct is stable in cell lysate. After NIR irradiation, adding Zn2+ enhanced the fluorescence by 2.4 folds, which is similar to the result observed in the buffer, suggesting TSDP-AuNS can be used as a biosensor in cells.
Finally, we tested the performance of TSDP-AuNS for sensing Zn2+ in HeLa cells. The probe was incubated with the cells for 6 h to allow sufficient uptake. Upon NIR irradiation, followed by the incubation of Zn2+, confocal microscopy was used to compared samples with and without NIR irradiation and with and without Zn2+ incubation. As shown in Figure 4, without NIR irradiation and even with addition of Zn2+, there was minimal fluorescent signal in the Cy5 channel, indicating successful photothermal caging of the DNAzymes. After NIR irradiation, but without addition of Zn2+, some fluorescence of Cy5 was recovered, and this recovery may come from the released DNAzymes that reacted with endogenous mobile Zn2+. To further corroborate this finding, we added 10 μM Zn2+ into the cells after NIR irradiation, and observed dramatic increase of the fluorescent intensity. A co-localization study (Figure S8) indicated that the fluorescence comes mainly from cytoplasm, suggesting that the AuNS can escape from lysosomes into cytoplasm (for discussion of mechanisms, please see legend of S8), making this biosensor system an excellent choice for imaging metal ions in different compartments of the cell.
Figure 4.
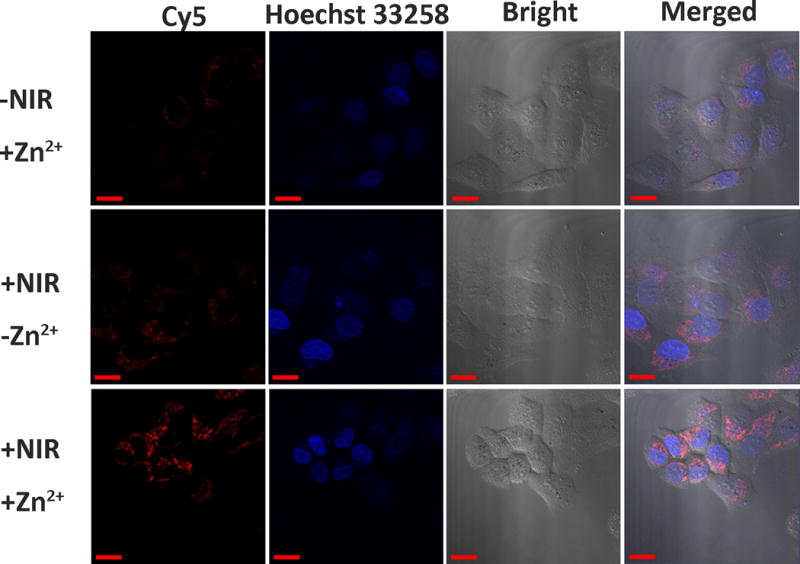
Confocal microscopy images of HeLa cells. Scale bar: 20 μm.
To make sure that the fluorescent signal indeed comes from the catalytic activity of the DNAzyme, instead of intracellular DNA or RNA nucleases-mediated substrate cleavage, we employed either a non-cleavable substrate or an inactive mutant DNAzyme as negative controls (Figure S9). Under the same condition, neither of two negative controls samples gave visible fluorescent signals, while the active DNAzyme with cleavable substrate showed enhance fluorescent signal, demonstrating that the fluorescent signal came from metal-ion specific catalytic activity of the DNAzyme. To confirm that the observations from the above confocal images apply to the whole cell population, flow cytometry was employed (Figure S10). HeLa cells treated with both NIR irradiation and Zn2+ had a higher level of fluorescence intensity than those with either no NIR irradiation or no Zn2+ addition. Together, these results indicate that TSDP-AuNS can be employed to image metal ions in living cells.
In conclusion, we have developed a NIR light activated DNAzymes-AuNS system, and demonstrated its application in detecting metal ions in living cells. During their delivery into cells, the activity of DNAzymes could be inhibited, protecting the substrate strand from being cleaved by extracellular metal ions. The AuNS allows NIR light-activated photothermal dehybridization, which activate the DNAzymes for metal-dependent cleavage and release of products with fluorophore attached. This NIR activated DNAzyme-based sensing system broadens the range of applications of DNAzymes in living cells, making it a powerful tool for cellular imaging applications.
Supplementary Material
Acknowledgments
This material is based on work supported by the US National Institutes of Health (NIH) under Award R21MH110975 (to Y.L.). J.-J.Z acknowledges support from National Natural Science Foundation (21335004). We wish to thank Drs. Jingjing Zhang, Kevin Hwang, Peiwen Wu for their insightful discussions, Bruno Giuliano Nicolau for the help with laser power density measurement, Ryan Lake for the help with cell culture, and Dr. Ryan Huschka from Prof. Naomi J. Halas’s lab for suggestions in AuNS synthesis and functionalization. W.W. and Z.W acknowledge financial support from Chinese Scholarship Council.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Outten CE, O’Halloran TV. Science. 2001;292:2488. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]; b) Andrews NC, Schmidt PJ. Annu Rev Physiol. 2007;69:69. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]; c) Clapham DE. Cell. 2007;131:1047. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]; d) Kim BE, Nevitt T, Thiele DJ. Nat Chem Biol. 2008;4:176. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]; e) Wegner SV, Sun F, Hernandez N, He C. Chem Commun. 2011;47:2571. doi: 10.1039/c0cc04292g. [DOI] [PubMed] [Google Scholar]; f) Wang J, Luo C, Shan C, You Q, Lu J, Elf S, Zhou Y, Wen Y, Vinkenborg JL, Fan J, Kang H, Lin R, Han D, Xie Y, Karpus J, Chen S, Ouyang S, Luan C, Zhang N, Ding H, Merkx M, Liu H, Chen J, Jiang H, He C. Nat Chem. 2015;7:968. doi: 10.1038/nchem.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Hainaut P, Milner J. Cancer Res. 1993;53:1739. [PubMed] [Google Scholar]; b) Barnham KJ, Bush AI. Curr Opin Chem Biol. 2008;12:222. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]; c) Grasso G, Salomone F, Tundo GR, Pappalardo G, Ciaccio C, Spoto G, Pietropaolo A, Coletta M, Rizzarelli EJ. Inorg Biochem. 2012;117:351. doi: 10.1016/j.jinorgbio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 3.a) Finney L, Mandava S, Ursos L, Zhang W, Rodi D, Vogt S, Legnini D, Maser J, Ikpatt F, Olopade OI, Glesne D. Proc Natl Acad Sci USA. 2007;104:2247. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Proc Natl Acad Sci USA. 2011;108:5980. doi: 10.1073/pnas.1009932108. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rodriguez-Haas B, Finney L, Vogt S, Gonzalez-Melendi P, Imperial J, Gonzalez-Guerrero M. Metallomics. 2013;5:1247. doi: 10.1039/c3mt00060e. [DOI] [PubMed] [Google Scholar]
- 4.a) Taki M, Wolford JL, O’Halloran TV. J Am Chem Soc. 2004;126:712. doi: 10.1021/ja039073j. [DOI] [PubMed] [Google Scholar]; b) Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. J Am Chem Soc. 2006;128:10. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Domaille DW, Que EL, Chang CJ. Nat Chem Biol. 2008;4:168. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]; d) Cheng T, Xu Y, Zhang S, Zhu W, Qian X, Duan L. J Am Chem Soc. 2008;130:16160. doi: 10.1021/ja806928n. [DOI] [PubMed] [Google Scholar]; e) Marbella L, Serli-Mitasev B, Basu P. Angew Chem Int Ed. 2009;48:3996. doi: 10.1002/anie.200806297. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Qian F, Zhang C, Zhang Y, He W, Gao X, Hu P, Guo Z. J Am Chem Soc. 2009;131:1460. doi: 10.1021/ja806489y. [DOI] [PubMed] [Google Scholar]; g) Wegner SV, Arslan H, Sunbul M, Yin J, He C. J Am Chem Soc. 2010;132:2567. doi: 10.1021/ja9097324. [DOI] [PubMed] [Google Scholar]; h) Au-Yeung HY, New EJ, Chang CJ. Chem Commun. 2012;48:5268. doi: 10.1039/c2cc31681a. [DOI] [PubMed] [Google Scholar]; i) Deibler K, Basu P. Eur J Inorg Chem. 2013;2013:1086. doi: 10.1002/ejic.201200997. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Liu J, Karpus J, Wegner SV, Chen PR, He C. J Am Chem Soc. 2013;135:3144. doi: 10.1021/ja3106779. [DOI] [PubMed] [Google Scholar]; k) Guo Z, Kim GH, Yoon J, Shin I. Nat Protoc. 2014;9:1245. doi: 10.1038/nprot.2014.086. [DOI] [PubMed] [Google Scholar]
- 5.a) Li Y, Breaker RR. Curr Opin Struct Biol. 1999;9:315. doi: 10.1016/S0959-440X(99)80042-6. [DOI] [PubMed] [Google Scholar]; b) Feldman AR, Sen D. J Mol Biol. 2001;313:283. doi: 10.1006/jmbi.2001.5058. [DOI] [PubMed] [Google Scholar]; c) Lu Y. Chem Euro J. 2002;8:4588. doi: 10.1002/1521-3765(20021018)8:20<4588::AID-CHEM4588>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]; d) Lu Y, Liu J, Li J, Bruesehoff PJ, Pavot CMB, Brown AK. Biosensors & Bioeletronics. 2003;18:529. doi: 10.1016/s0956-5663(03)00013-7. [DOI] [PubMed] [Google Scholar]; e) Feldman AR, Leung EKY, Bennet AJ, Sen D. ChemBioChem. 2006;7:98. doi: 10.1002/cbic.200500264. [DOI] [PubMed] [Google Scholar]; f) Lu Y. Inorg Chem. 2006;45:9930. doi: 10.1021/ic052007t. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Lau PS, Li Y. Curr Org Chem. 2011;15:557. [Google Scholar]; h) Hwang K, Hosseinzadeh P, Lu Y. Inorg Chim Acta. 2016;452:12–24. doi: 10.1016/j.ica.2016.04.017. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Breaker RR, Joyce GF. Chem Biol. 1994;1:223. doi: 10.1016/1074-5521(94)90014-0. [DOI] [PubMed] [Google Scholar]; b) Li J, Zheng W, Kwon AH, Lu Y. Nucleic Acids Res. 2000;28:481. doi: 10.1093/nar/28.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Joyce GF. Annu Rev Biochem. 2004;73:791. doi: 10.1146/annurev.biochem.73.011303.073717. [DOI] [PubMed] [Google Scholar]; d) Nelson KE, Bruesehoff PJ, Lu Y. J Mol Evol. 2005;61:216. doi: 10.1007/s00239-004-0374-3. [DOI] [PubMed] [Google Scholar]; e) Hollenstein M, Hipolito C, Lam C, Dietrich D, Perrin DM. Angew Chem Int Ed. 2008;47:4346. doi: 10.1002/anie.200800960. [DOI] [PubMed] [Google Scholar]
- 7.a) Navani NK, Li Y. Curr Opin Chem Biol. 2006;10:272. doi: 10.1016/j.cbpa.2006.04.003. [DOI] [PubMed] [Google Scholar]; b) Liu J, Cao Z, Lu Y. Chem Rev. 2009;109:1948. doi: 10.1021/cr030183i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Li J, Lu Y. J Am Chem Soc. 2000;122:10466. [Google Scholar]; b) Liu J, Lu Y. J Am Chem Soc. 2005;127:12677. doi: 10.1021/ja053567u. [DOI] [PubMed] [Google Scholar]; c) Xiao Y, Rowe AA, Plaxco KW. J Am Chem Soc. 2007;129:262. doi: 10.1021/ja067278x. [DOI] [PubMed] [Google Scholar]; d) Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y. Proc Natl Acad Sci USA. 2007;104:2056. doi: 10.1073/pnas.0607875104. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Liu J, Lu Y. J Am Chem Soc. 2007;129:9838. doi: 10.1021/ja0717358. [DOI] [PubMed] [Google Scholar]; f) Li T, Dong S, Wang E. Anal Chem. 2009;81:2144. doi: 10.1021/ac900188y. [DOI] [PubMed] [Google Scholar]; g) Yin BC, Ye BC, Tan W, Wang H, Xie CC. J Am Chem Soc. 2009;131:14624. doi: 10.1021/ja9062426. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Xiang Y, Lu Y. Nat Chem. 2011;3:697. doi: 10.1038/nchem.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Zhang XB, Kong RM, Lu Y. Annu Rev Anal Chem. 2011;4:105. doi: 10.1146/annurev.anchem.111808.073617. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Zhang J, Cheng F, Li J, Zhu JJ, Lu Y. Nano Today. 2016;11:309. doi: 10.1016/j.nantod.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Huang PJ, Liu MVJ. Biochemistry. 2016;55:2518. doi: 10.1021/acs.biochem.6b00132. [DOI] [PubMed] [Google Scholar]
- 9.Chaloux E, Altmann E. Lead in Water: St Paul Schools Delayed Fixes. 2016 http://www.kaaltv.com/article/stories/S4265242.shtml [Accessed 15 Jan 2017]
- 10.a) Wu P, Hwang K, Lan T, Lu Y. J Am Chem Soc. 2013;135:5254. doi: 10.1021/ja400150v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang L, Huang H, Xu N, Yin Q. J Mater Chem B. 2014;2:4935. doi: 10.1039/c4tb00680a. [DOI] [PubMed] [Google Scholar]; c) Torabi SF, Wu P, McGhee CE, Chen L, Hwang K, Zheng N, Cheng J, Lu Y. Proc Natl Acad Sci USA. 2015;112:5903. doi: 10.1073/pnas.1420361112. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Cui L, Peng R, Fu T, Zhang X, Wu C, Chen H, Liang H, Yang CJ, Tan W. Anal Chem. 2016;88:1850. doi: 10.1021/acs.analchem.5b04170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang K, Wu P, Kim T, Lei L, Tian S, Wang Y, Lu Y. Angew Chem Int Ed. 2014;53:13798. doi: 10.1002/anie.201408333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Weissleder R. Nat Biotech. 2001;19:316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]; b) Dvir T, Banghart MR, Timko BP, Langer R, Kohane DS. Nano Lett. 2010;10:250. doi: 10.1021/nl903411s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barhoumi A, Huschka R, Bardhan R, Knight MW, Halas NJ. Chem Phys Lett. 2009;482:171. [Google Scholar]
- 14.a) Poon L, Zandberg W, Hsiao D, Erno Z, Sen D, Gates BD, Branda NR. ACS Nano. 2010;4:6395. doi: 10.1021/nn1016346. [DOI] [PubMed] [Google Scholar]; b) Huang X, Pallaoro A, Braun GB, Morales DP, Ogunyankin MO, Zasadzinski J, Reich NO. Nano Lett. 2014;14:2046. doi: 10.1021/nl500214e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Barhoumi A, Wang W, Zurakowski D, Langer RS, Kohane DS. Nano Lett. 2014;14:3697. doi: 10.1021/nl403733z. [DOI] [PubMed] [Google Scholar]; d) Zhang K, Zhu X, Jia F, Auyeung E, Mirkin CA. J Am Chem Soc. 2013;135:14102. doi: 10.1021/ja408465t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Huschka R, Zuloaga J, Knight MW, Brown LV, Nordlander P, Halas NJ. J Am Chem Soc. 2011;133:12247. doi: 10.1021/ja204578e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Huschka R, Neumann O, Barhoumi A, Halas NJ. Nano Lett. 2010;10:4117. doi: 10.1021/nl102293b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Goodman AM, Hogan NJ, Gottheim S, Li C, Clare SE, Halas NJ. ACS Nano. 2016;11:171. doi: 10.1021/acsnano.6b06510. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Lu Y. Nat Protocols. 2006;1:246. doi: 10.1038/nprot.2006.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


