Abstract
Background
This study aimed to investigate the added value of NeuroGam software analysis in the localization diagnosis of epileptogenic zone during interictal phase of seizures.
Material/Methods
The clinical data of 67 patients, clinically diagnosed as epilepsy, were analyzed retrospectively. Visual analysis and NeuroGam software analysis were used for independent analysis. The 2 methods were used to compare the efficacy indicator of the diagnosis of epileptogenic zone, and the receiver operating characteristic (ROC) curve evaluated the diagnostic efficacy.
Results
Through the final clinical diagnostic comprehensive localization, among 67 epilepsy patients, the epileptogenic zone in 51 cases could be located distinctly, and those in 16 cases could not be located. Compared to the visual analysis, the NeuroGam software analysis was more sensitive in the location of epileptogenic zone (χ2=4.876, P=0.027). The area under the ROC curve (AUC) and 95% confidence interval (CI) of the NeuroGam software and visual analyses was 0.760 and 0.689, (0.613, 0.908) and (0.547, 0.832), respectively. However, the consistency of the 2 methods was poor (Kappa=0.367, P=0.001). Compared to visual analysis, the NeuroGam software analysis exerted more advantages in the localization diagnosis of the epileptogenic zone (P<0.001).
Conclusions
In the location diagnosis of brain perfusion, single photon emission computed tomography (SPECT) epileptogenic zone was used in interictal phase of seizures, and NeuroGam software analysis exerted a distinct added value for visual analysis.
MeSH Keywords: Brain; Epilepsy; Tomography, Emission-Computed, Single-Photon
Background
Epilepsy is a group of syndromes characterized by the dysfunction of central nervous system caused by excessive abnormal brain neurons or synchronous discharge [1]. As some patients are not sensitive to antiepileptic drugs and develop drug resistant epilepsy, surgical treatments, such as epileptogenic zone resection, are critical for effectively controlling epilepsy; the key to the success of operation is the diagnostic localization of the epileptogenic zone [2]. Although invasive intracranial electroencephalogram recording is the gold standard for epilepsy localization [3], the method is invasive and has surgical risks.
In recent years, single photon emission computed tomography (SPECT) and positron emission computed tomography (PET) have shown unique and crucial roles in the evaluation and diagnosis of epilepsy [4]. The application of localization diagnosis utilizes radionuclide imaging, which is suitable for negative or indefinite MRI (magnetic resonance imaging) or EEG (electroencephalogram) results that are ambiguous or inconsistent with the structural image [5].
PET might have an excellent prospective and applicative value in the study of epilepsy; however, there are high requirements for the preparation of positron radiopharmaceuticals, and the examination is cost-ineffective, which limits its routine clinical application [6,7].
The localization value of SPECT on epileptogenic zone has been widely recognized. By comparing the examination results during the ictal phase and interictal phase of seizures, the accuracy of localization diagnosis of epileptogenic zone was found to be increased distinctly [8]. However, owing to the rapid spread in the epileptic seizure, it is challenging to capture the epileptogenic zone, which requires precise timing of the injection of the imaging agent. Therefore, the routine SPECT examination in the ictal phase of seizures is greatly limited [9]. Nevertheless, the usage of drug induction might exhibit an adequate application prospect [10]; the use of this technique is still limited, and the diagnostic utility remains to be demonstrated.
The sensitivity and specificity of SPECT in locating the epileptogenic zone during interictal phase are lower than during the ictal phase. The brain function analysis software can restore the deficiency and analyze the brain SPECT data based on the voxel level. This approach is advantageous with respect to objectivity and repeatability [11]. The present study used NeuroGam brain function analysis software and performed localization analysis of the epileptogenic zone during the interictal phase of seizures based on the SPECT cerebral blood flow perfusion image. In addition, the method investigated added value of the localization ability of the conventional visual analysis.
Material and Methods
Patients
A retrospective analysis of patients diagnosed with epilepsy by neurological physicians was conducted at the Third Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China, from April 2006 to 2016. The diagnosis of epilepsy was carried out according to the standards of International League Against Epilepsy [12]. All patients were examined by SPECT before admission and the administration of antiepileptic drugs was ceased 24 h before admission. The inclusion criteria of the patients for the study were as follows: 1) the quality of brain SPECT image was good; 2) no epileptic seizure occurred within 24 h before the SPECT examination; 3) the patient was followed up for >1 year after discharge. The exclusion criteria were as follows: occurrence of epilepsy during brain SPECT examination, and epilepsy combined with brain diseases including cerebrovascular diseases, trauma, tumor, infection, or inflammation. Finally, 67 cases (36 males and 31 females, aged 23–61 years, mean 42.2±10.7 years) were enrolled. This study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University. All human studies were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Informed consent was waived by the committee because of the retrospective nature of the study.
Examination method
99Tcm-ethyl cysteinate dimer (ECD) cerebral blood perfusion imaging was performed. The imaging instrument was a GE Infinia Vc Hawkeye SPECT instrument with low energy and high-resolution parallel hole collimator. The imaging agent was 99Tcm-ECD with >90%, radiochemical purity; 99TcmO4− was provided by Beijing Atom-Hitech Co., Ltd., whereas ECD was provided by the Beijing Normal University Shihong Pharmaceutical Co., Ltd. Imaging methods had the patients at quiet rest, avoiding light stimulation; the patients received an intravenous injection of 99Tcm-ECD 740~925MBq 1 h after oral administration of 400 mg potassium perchlorate. Then, the patients were placed in a supine position and cerebral perfusion tomography was performed after 30 min. Acquisition conditions: 128×128 matrix, magnification 1.5, 6º/frame, rotating 360º, the acquisition was performed in 30 s/frame, and a total of 60 frames were collected. The energy peak was 140 keV, and the window width was 20%. The original data were processed by computer; wave filtering was performed using the Butterworth method. The ramp filter back projection was utilized to reconstruct the image. The attenuation correction was performed by the Chang method. The image reconstruction was performed on the transverse, coronal, and sagittal sections in parallel with the orbitomeatal (OM) line, respectively. The thickness of the section was 2.95 mm.
SPECT visual analysis
The films were visually inspected and interpreted by 2 experienced nuclear medicine physicians. Based on the transverse section, and referring to the coronal and sagittal sections, the visual local sparse or defective radioactive distribution appeared in >2 sections, and ≥2 layers in the same section were confirmed as positive lesions [13]. The localization of epileptogenic zone was divided into 3 grades: clear, uncertain, and unable to locate. The clear location for the focal lesions was visually recognizable. The uncertain location was scattered, or uneven cerebral perfusion changes were observed. The unable to locate grade indicated clinically diagnosed epileptic patients with negative SPECT results in visual analysis.
NeuroGam software analysis method
NeuroGam software (Segami Co., USA) was used for the data analysis of image reconstruction [14]. By affine registration of the data blocks, the raw data of 99Tcm-ECD cerebral blood flow perfusion imaging was mapped to Talairach standard space. In the vertical section, the horizontal deviation of the data was corrected using interhemispheric fissure as the baseline; the horizontal sectional image was automatically generated with the occipital lower pole and inferior margin of the frontal lobe as baselines; the anterior and posterior commissure lines were defined as the medium-level position between the anterior temporal lobe and the pons; the brain volume range for the analysis was defined with the front, back, upper, lower, and both sides of the brain. After normalization of the image, standardization was performed with the cerebellum as a reference area in qualitative analysis. The local cerebral blood flow perfusion was represented by the continuous tone from orange to blue. In the semi-quantitative analysis, the normal database on the identical age and gender provided by the software was used as control and analyzed independently; the computer program calculated the Z values of each voxel using the following formula: Z value=(average value of the control group in the database–individual value)/standard deviation of the control group in the database. The Z value in different regions was represented as χ̄±s. The difference of more than 2-fold of the standard deviation was considered as the abnormal value, and the Z value was represented using special color gradation. In order to accurately locate the abnormal perfusion area, the functional areas of the cerebral cortex were analyzed using the Brodmann area. The blood perfusion in both lobes of the left and right cerebral hemisphere and special Brodmann area was assessed. The localizations of epileptogenic zone were divided into 3 grades: clear, uncertain, and unable to locate. The clear location comprised of the hypoperfusion focal lesions with Z-value ≥2-fold of the standard deviation; the uncertain location represented the multiple scattered or uneven cerebral perfusion changes with the Z-value <1–2-fold of the standard deviation, and the unable to locate was comprised of clinically diagnosed epileptic patients with negative results in the analysis by NeuroGam software.
Final clinical localization results
The diagnosis and analysis of the final location of the epileptogenic zone were conducted by 2 experienced neurological physicians according to the clinical manifestations, EEG, imaging data, and follow-up results of the patients [15].
Statistical method
The positive predictive rate (PPV), negative predictive rate (NPV), accuracy (Ac), sensitivity (Se), specificity (Sp), and uncertainty rate were calculated by visual analysis and NeuroGam software analysis methods.
The SPSS17.0 software was used for data analysis. The rate of the 2 methods used in this study was calculated using the 4-fold table method, and the consistency of the 2 methods was assessed by the Kappa test. In addition, the diagnostic level of the 2 methods was evaluated using Fisher’s exact probability method. The receiver operating characteristic curve (ROC) was plotted, and the area under curve (AUC) and 95% confidence intervals (CI) were computed for the evaluation of diagnostic efficiency. P<0.05 was considered as statistically significant.
Results
The baseline characteristics of all the patients are listed in Table 1. Based on the diagnostic analysis of the final clinical localization, 51 cases showed a clear localization of the epileptogenic zone among 67 patients with epilepsy with a total of 68 epileptic foci (including 7 patients with at least 2 epileptogenic zone). The location of the epileptogenic zone in the other 16 cases could not be diagnosed. NeuroGam analysis showed 47 cases with clear location, of which, 12 were graded as unable to locate and 8 cases were uncertain. SPECT visual analysis method showed 32 cases with clear location, 19 cases unable to locate, and 16 were uncertain (Table 2).
Table 1.
Baseline data of all subjects.
| Epileptic patients | |
|---|---|
| Number of cases | 67 |
| Age (y) | 42.2±10.7 (23–61) |
| Gender (M/F) | 36/31 |
| Handedness (L/R) | 11/56 |
| Course of disease (y) | 9.4±4.8 |
| Seizure frequency (n/y) | 8.3±2.5 |
| Medication (y) | 5.2±3.7 |
Table 2.
Comparison of the localization results of the epileptogenic foci by SPECT visual analysis and NeuroGam software analysis, and the final clinical localization results of epileptogenic zone in 67 cases of epilepsy patients.
| Final result | Visual analysis | NeuroGam software analysis | ||||
|---|---|---|---|---|---|---|
| Clearly localized | Could not be located | Uncertain | Clearly localized | Could not be located | Uncertain | |
| Clearly localized | 29 | 12 | 10 | 42 | 5 | 4 |
| Could not be located | 3 | 7 | 6 | 5 | 7 | 4 |
| Total | 32 | 19 | 16 | 47 | 12 | 8 |
PPV, NPV, Ac, Se, Sp, and the uncertain proportion of visual analysis method for the localization diagnosis of epileptogenic zone in patients with interictal phase of seizures were: 90.6% (29/32), 36.8% (7/19), 70.6% (36/51), 70.7% (29/41), 70% (7/10), and 23.9% (16/67), respectively. The AUC and 95% CI were 0.689 and 0.547–0.832, respectively (Figure 1).
Figure 1.
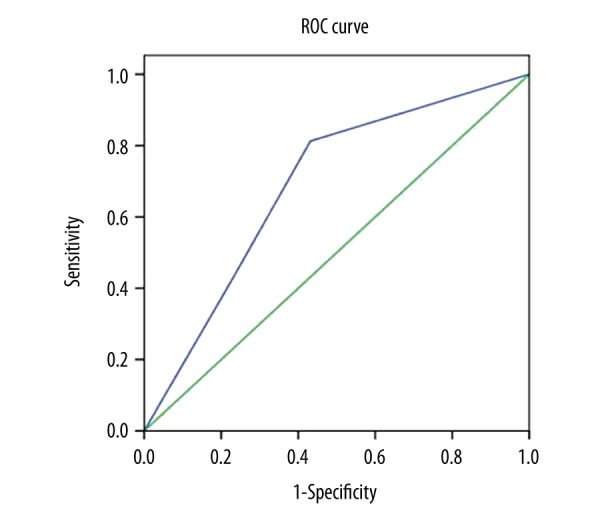
ROC curve of SPECT visual analysis in 67 cases of epileptic patients. (AUC: 0.689; P=0.023; 95% CI: 0.547–0.832). ROC – receiver operating characteristic; AUC – area under ROC curve; SPECT – single photon emission computed tomography.
PPV, NPV, Ac, Se, Sp, and uncertain proportion of the NeuroGam software analysis method for the localization diagnosis of epileptogenic zone in patients with interictal phase of seizures were: 89.4% (42/47), 58.3% (7/12), 83.1% (49/59), 89.4% (42/47), 58.3% (7/12), and 11.9% (8/67), respectively. The AUC and 95% CI were 0.760 and 0.613–0.908, respectively (Figure 2).
Figure 2.
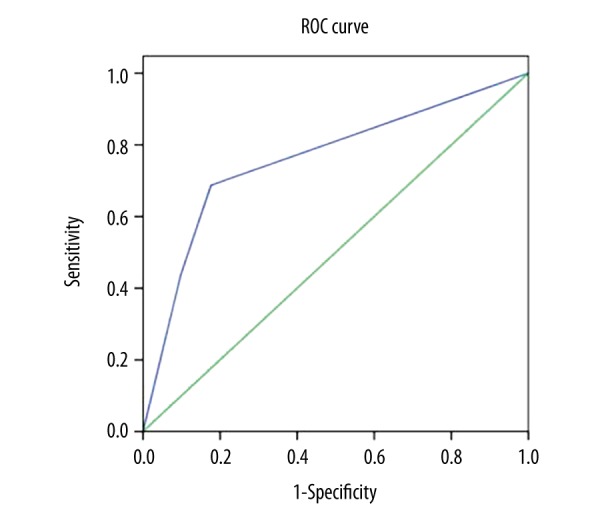
ROC curve of NeuroGam software analysis in 67 cases of epileptic patients (AUC: 0.760; P=0.002; 95% CI: 0.613–0.908). ROC – receiver operating characteristic; AUC – area under ROC curve.
Compared to the visual analysis method, NeuroGam software analysis method exhibited greater sensitivity in localizing the epileptogenic zone (χ2=4.876, P=0.027), and the difference in PPV, NPV, Ac, Se, Sp, and uncertain proportion did not show any statistical significance between the 2 methods (Table 3).
Table 3.
Comparison of each diagnostic efficacy parameter by SPECT visual analysis and NeuroGam software analysis in 67 cases of epilepsy patients.
| PPV | NPV | Ac | Se* | Sp | Uncertainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | + | − | + | − | |
| Visual analysis | 29 | 3 | 7 | 12 | 36 | 15 | 29 | 12 | 7 | 3 | 16 | 51 |
| NeuroGam software analysis | 42 | 5 | 7 | 5 | 49 | 10 | 42 | 5 | 7 | 5 | 8 | 59 |
Se represented statistical significance, χ2=4.876, P=0.027.
All the 32 cases of epilepsy with clear location of the epileptogenic zone, as assessed by the visual analysis method, could be clearly localized using the NeuroGam software analysis method. Among the 19 epilepsy patients in whom the location of the epileptogenic zone could not be diagnosed by the visual analysis method, the NeuroGam software analysis method detected the localization, while in the remaining 6 cases it could not be localized. The consistency of the 2 methods was poor (Kappa=0.367, P=0.001). The positive rate of NeuroGam software analysis method was 88.2% (45/51), and that of the SPECT visual analysis method was 62.7% (32/51). The former was higher than that of the latter with a statistical significant difference (P=0.000). Compared to the visual analysis method, NeuroGam software analysis method presented several advantages in the localization diagnosis of epileptogenic zone (Table 4).
Table 4.
Consistency test of localization results of epileptogenic zone by SPECT visual analysis and NeuroGam software analysis in 67 cases of epilepsy patients.
| SPECT visual analysis | Total | |||
|---|---|---|---|---|
| Clearly localized | Could not be located | |||
| NeuroGam software analysis | Clearly localized | 32 | 13 | 45 |
| Could not be located | 0 | 6 | 6 | |
| Total | 32 | 19 | ||
The consistency of the localization diagnosis results of epileptogenic zone by SPECT visual analysis and NeuroGam software analysis is poor (Kappa=0.367, P=0.001). The positive rates of NeuroGam software analysis and visual analysis were 88.2% (45/51) and 62.7% (32/51), respectively with a statistically significant difference (P<0.001).
Case example
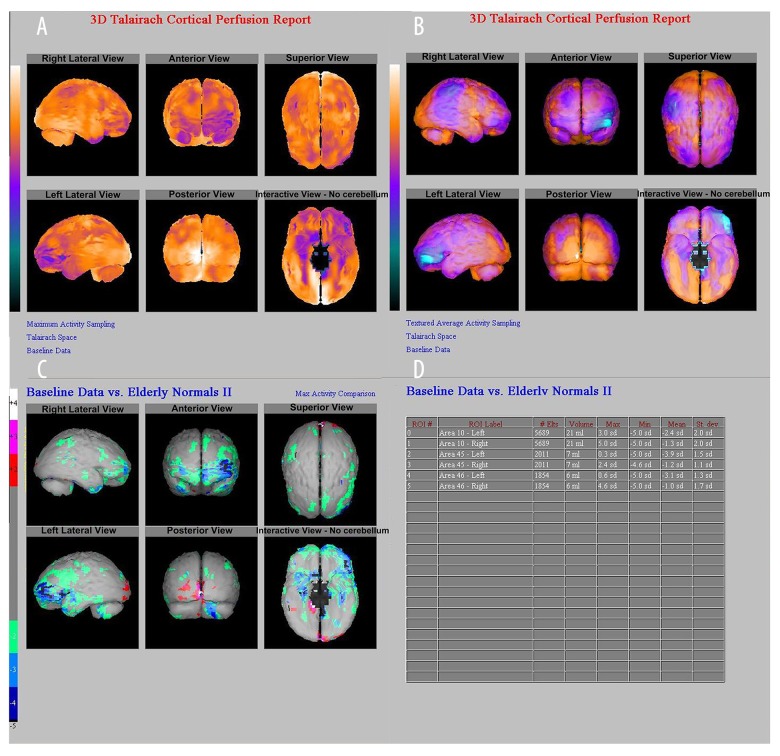
A male patient, aged 48 years, with paroxysmal unconsciousness accompanied by limb twitching for more than 4 years, up to 7 times per day did not undergo any anti-epileptic treatment before SPECT examination. No obvious abnormalities in EEG and MRI were observed. The SPECT cerebral perfusion imaging showed a hypoperfusion area in the left frontal cortex. NeuroGam analysis showed that the regional cerebral blood flow (rCBF) hypoperfusion area was located in the left frontal lobe (Brodmann 10, 45, and 46); the semi-quantitative analysis of the rCBF hypoperfusion area in the left frontal lobe (Brodmann 10, 45, and 46) showed that the difference of Z values was decreased by 2.4, 3.9, and 3.1 standard deviations (SD) respectively, when compared with the normal database (Figure 3).
Figure 3.
A 48-years-old male patient. (A, B). Z-value map of semi-quantitative analysis by NeuroGam software. (C) Comparison of rCBF hypoperfusion area in Brodmann 10, 45, 46 zones of the left frontal lobe and normal databases (represented by standard deviation). (D). The left frontal Brodmann 10, 45, and 46 zones were decreased by 2.4, 3.9, and 3.1 SD, respectively, and the right frontal Brodmann 10, 45, and 46 zones were decreased by 1.3, 1.2, and 1.0 SD, respectively, suggesting that the lesion was located in the left frontal lobe (Brodmann 10, 45, 46 zone). rCBF – regional cerebral blood flow.
Discussion
SPECT often appears as a lower local cerebral blood perfusion during the interictal phase of seizures. Although the pathological mechanism is not yet clear, it might be related to gliosis, cortical atrophy, neural loss, decreased synaptic density, and decreased activity caused by the relatively healthy neurons in the region that were otherwise absent in the epileptogenic zone [16,17]. Brain SPECT during the interictal phase is often used to assess the baseline levels of epileptic patients in order to aid in the localization of the epileptogenic zone by combining the brain SPECT findings during the ictal phase of seizures. A large number of studies have shown that the diagnostic efficacy of localizing the epileptogenic zone by interictal brain SPECT is low and that its sensitivity to temporal lobe epilepsy and extratemporal epilepsy was only 66% and 60% [18], respectively. In the present study, the sensitivity of SPECT in localizing the epileptogenic zone was 70.7% by visual analysis method, which was slightly higher than that reported in the literature, which could be attributed to the small sample size and patients who were clinically diagnosed as epileptic patients.
The SPECT visual analysis method is limited by the intrinsic spatial resolution of the instrument, and poorly shows slight changes in the regional blood flow. The evaluation of these changes relies primarily on the experience of nuclear medicine physicians and is susceptible to the subjective factors of the observers, thereby greatly affecting the diagnostic efficacy [19]. The computer post-processing method statistically analyzed the voxel level of the images that could distinctly improve the diagnostic efficiency. Among them, the brain function analysis software, such as subtraction ictal SPECT co-registered to MRI (SISCOM) and the statistical parametric mapping (SPM), can directly and statistically analyze the original data on the voxel level and has been widely used [20,21]. With respect to the accuracy in the localization diagnosis of epilepsy, SPM is considered superior to SISCOM [11]. However, some studies have suggested that SPM required programming experience and complex operation, and exerted a specific impact on the efficiency and operation ability of the diagnosis, which rendered it unsuitable for individual diagnosis and evaluation in routine clinical practice [20,22].
In the current study, the application of NeuroGam software analysis could clearly localize not only 32 epilepsy patients diagnosed by visual analysis but also the 13 cases that could not be diagnosed similarly, indicating that NeuroGam software analysis method had superior localization diagnosis ability. The software could perform the specific image correction processing of the original data on the patients’ cerebral blood perfusion images and display the results in the Talairach standard atlas by 3D reconstruction. This normalized image processing method could effectively eliminate the difference executed by the observer with objectivity and reproducibility. The voxel-based analysis method was similar to the principle of SPM, and it could show the subtle abnormality in the blood flow distribution with statistical significance. Moreover, the difference that could not be distinguished by human eyes could be discriminated by different color gradations that aid the physicians in completing the qualitative and localization analysis. On the other hand, the conventional visual analysis failed to distinguish between different types of dementia. Conversely, the NeuroGam software analysis method could observe, compare, and analyze the characteristics of cerebral perfusion model of different types of dementia patients, which not only aided in the differential diagnosis of frontotemporal dementia and other types of dementia but also provided vital diagnostic information to recognize and distinguish the various subtypes of frontotemporal dementia [23,24].
In this study, the NeuroGam software analysis showed a unique advantage in the ability of localization diagnosis of epileptogenic zone, and the PPV, NPV, Ac, Se, and Sp of localization diagnosis of epileptogenic zone were higher than those of visual analysis, among which Se displayed a statistical significance. The operation of NeuroGam software is simple, and its health database could provide reference data to match the age and gender of patients for comparison (the data were represented as standard deviation), which rendered it optimal for the comparison between individuals and groups, thereby contributing to the semi-quantitative analysis of the different parts, regions, and Brodmann brain function areas of each leaf of the cerebral cortex. Therefore, this software had been widely used in Alzheimer disease (AD), Parkinson disease, and cerebrovascular disease as a major image analysis and research tool for nuclear medicine physicians [25–27]. Valotssiou et al. [26] speculated that the application of NeuroGam software analysis could objectively show the abnormal cerebral perfusion region in AD patients and could be used as an assistant tool in the evaluation of the early cerebral perfusion injury in AD patients in clinical practice. The combination of the aforementioned method with Brodmann brain functional area analysis could improve the understanding about the particular brain regions in AD patients and patients with frontotemporal dementia, which were markedly significant for an accurate differential diagnosis [28]. Therefore, the semi-quantitative analysis method of NeuroGam software could obviously improve its ability of localization diagnosis of epileptogenic zone.
In the comparison between NeuroGam software analysis and visual analysis, the consistency of the localization ability of the epileptogenic zone was found to be poor. The diagnostic positive rate and AUC of the NeuroGam software analysis were both higher than those of visual analysis. Therefore, we speculated that NeuroGam software analysis was more advantageous for the localization diagnosis of epileptogenic zone as compared to the visual analysis. However, in this study, the small sample size might exert some bias, and hence, it is essential to expand the sample size in future studies. In addition, the limitations of this study also include the retrospective analysis of the patients and single-center nature. Since most patients did not undergo surgical treatment, the results of this study necessitate further confirmation by epilepsy surgery. In future, the subgroups of analysis in ictal SPECT and operated patients, which should be added in the in-depth study, will be of great significance.
Conclusions
In conclusion, in the localization diagnosis of epilepsy, NeuroGam software performs the analysis based on the voxel level. Compared to the conventional vision analysis method, the software is more objective with the characteristics of satisfactory reproducibility and high credibility. It can improve PPV and NPV and decrease the ratio of uncertainty diagnosis and false negative diagnosis; the overall diagnosis efficiency is better than that of the visual analysis. NeuroGam software analysis detects the epileptogenic zone that cannot be recognized by naked eyes in visual analysis; hence, it can be used for the semi-quantitative evaluation of the brain lobes and different brain functional areas, which has distinct added value for visual analysis. Moreover, the software can be used conveniently without technical difficulty. Thus, it can be utilized as a valuable clinical tool for the evaluation of epilepsy.
Footnotes
Conflict of interest
None.
Source of support: None
References
- 1.Fisher RS, Scharfman HE, deCurtis M. How can we identify ictal and interictal abnormal activity? Adv Exp Med Biol. 2014;813:3–23. doi: 10.1007/978-94-017-8914-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki M, Jin K, Nakasato N, Tominaga T. Non-invasive evaluation for epilepsy surgery. Neurol Med Chir (Tokyo) 2016;56:632–40. doi: 10.2176/nmc.ra.2016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducis K, Guan J, Karsy M, Bollo RJ. Preoperative evaluation and surgical decision-making in pediatric epilepsy surgery. Transl Pediatr. 2016;5:169–79. doi: 10.21037/tp.2016.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, Part 1: Sporadic temporal and extratemporal lobe epilepsy. J Nucl Med. 2013;54:1775–81. doi: 10.2967/jnumed.112.114397. [DOI] [PubMed] [Google Scholar]
- 5.Willmann O, Wennberg R, May T, et al. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure. 2007;16:509–20. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Hodolic M, Topakian R, Pichler R. (18)F-fluorodeoxyglucose and (18)F-flumazenil positron emission tomography in patients with refractory epilepsy. Radiol Oncol. 2016;50:247–53. doi: 10.1515/raon-2016-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XT, Li L, Yan B, et al. How to effectively constrain the cost of presurgical evaluation for resective surgery in low-income population: Clinically oriented opinions. Seizure. 2011;20:425–27. doi: 10.1016/j.seizure.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Theodore WH. Presurgical focus localization in epilepsy: PET and SPECT. Semin Nucl Med. 2017;47:44–53. doi: 10.1053/j.semnuclmed.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Holder DL, Laymon CM, et al. Clinical value of the first dedicated, commercially available automatic injector for ictal brain SPECT in presurgical evaluation of pediatric epilepsy: Comparison with manual injection. J Nucl Med. 2013;54:732–38. doi: 10.2967/jnumed.112.105189. [DOI] [PubMed] [Google Scholar]
- 10.Barba C, Di Giuda D, Policicchio D, et al. Correlation between provoked ictal SPECT and depth recordings in adult drug-resistant epilepsy patients. Epilepsia. 2007;48:278–85. doi: 10.1111/j.1528-1167.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 11.Sulc V, Stykel S, Hanson DP, et al. Statistical SPECT processing in MRI-negative epilepsy surgery. Neurology. 2014;82:932–39. doi: 10.1212/WNL.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr M, Linehan C, Thompson R, et al. A White Paper on the medical and social needs of people with epilepsy and intellectual disability: The Task Force on Intellectual Disabilities and Epilepsy of the International League Against Epilepsy. Epilepsia. 2014;55:1902–6. doi: 10.1111/epi.12848. [DOI] [PubMed] [Google Scholar]
- 13.Asl MT, Yousefi F, Nemati R, Assadi M. 99mTc-ECD brain perfusion SPECT imaging for the assessment of brain perfusion in cerebral palsy (CP) patients with evaluation of the effect of hyperbaric oxygen therapy. Int J Clin Exp Med. 2015;8:1101–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Paschali A, Messinis L, Lyros E, et al. Neuropsychological functions and rCBF SPECT in Parkinson’s disease patients considered candidates for deep brain stimulation. Eur J Nucl Med Mol Imaging. 2009;36:1851–58. doi: 10.1007/s00259-009-1168-z. [DOI] [PubMed] [Google Scholar]
- 15.von Ellenrieder N, Pellegrino G, Hedrich T, et al. Detection and magnetic source imaging of fast oscillations (40–160 Hz) recorded with magnetoencephalography in focal epilepsy patients. Brain Topogr. 2016;29:218–31. doi: 10.1007/s10548-016-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferys JG. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure. 2010;19:638–46. doi: 10.1016/j.seizure.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Lason W, Chlebicka M, Rejdak K. Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol Rep. 2013;65:787–801. doi: 10.1016/s1734-1140(13)71060-0. [DOI] [PubMed] [Google Scholar]
- 18.Desai A, Bekelis K, Thadani VM, et al. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54:341–50. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 19.Rocha ET, Buchpiguel CA, Nitrini R, et al. Diagnosis of regional cerebral blood flow abnormalities using spect: Agreement between individualized statistical parametric maps and visual inspection by nuclear medicine physicians with different levels of expertise in nuclear neurology. Clinics (Sao Paulo) 2009;64:1145–53. doi: 10.1590/S1807-59322009001200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Liu T, Zhao X, et al. Comparative study of voxel-based epileptic foci localization accuracy between statistical parametric mapping and three-dimensional stereotactic surface projection. Front Neurol. 2016;7:164. doi: 10.3389/fneur.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newey CR, Wong C, Wang ZI, et al. Optimizing SPECT SISCOM analysis to localize seizure-onset zone by using varying z scores. Epilepsia. 2013;54:793–800. doi: 10.1111/epi.12139. [DOI] [PubMed] [Google Scholar]
- 22.Schrouff J, Rosa MJ, Rondina JM, et al. PRoNTo: Pattern recognition for neuroimaging toolbox. Neuroinformatics. 2013;11:319–37. doi: 10.1007/s12021-013-9178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santra A, Sinha GK, Neogi R, Thukral RK. (99m)Tc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography in characterization of dementia: An initial experience in Indian clinical practice. World J Nucl Med. 2014;13:120–27. doi: 10.4103/1450-1147.139143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valotassiou V, Papatriantafyllou J, Sifakis N, et al. Brain perfusion SPECT with Brodmann areas analysis in differentiating frontotemporal dementia subtypes. Curr Alzheimer Res. 2014;11:941–54. doi: 10.2174/1567205011666141107125104. [DOI] [PubMed] [Google Scholar]
- 25.Han JH, Park YS, Lee WH, et al. Cerebral-perfusion-based single-photon emission computed tomography (SPECT) staging using NeuroGam(R) in patients with moyamoya disease. Childs Nerv Syst. 2016;32:1471–77. doi: 10.1007/s00381-015-2974-1. [DOI] [PubMed] [Google Scholar]
- 26.Valotassiou V, Papatriantafyllou J, Sifakis N, et al. Clinical evaluation of brain perfusion SPECT with brodmann areas mapping in early diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2015;47:773–85. doi: 10.3233/JAD-150068. [DOI] [PubMed] [Google Scholar]
- 27.Paschali A, Constantoyannis C, Angelatou F, Vassilakos P. Perfusion brain SPECT in assessing motor improvement after deep brain stimulation in Parkinson’s disease. Acta Neurochir (Wien) 2013;155:497–505. doi: 10.1007/s00701-012-1610-z. [DOI] [PubMed] [Google Scholar]
- 28.Valotassiou V, Papatriantafyllou J, Sifakis N, et al. Perfusion SPECT studies with mapping of Brodmann areas in differentiating Alzheimer’s disease from frontotemporal degeneration syndromes. Nucl Med Commun. 2012;33:1267–76. doi: 10.1097/MNM.0b013e3283599983. [DOI] [PubMed] [Google Scholar]