ABSTRACT
Influenza virus is a common pathogen implicated in respiratory tract infections, annually affecting up to 20% of the general population, and pneumonia is a leading cause of death after influenza infection. Post-influenza pneumonia is especially common in the elderly and chronically ill patients. The risk of post-influenza pneumonia is significantly increased according to the number of concurrent comorbidities. Vaccination is the primary measure used to abate influenza epidemics and associated complications. In meta-analyses, influenza vaccine significantly reduces pneumonia- and influenza-related hospitalizations, with a vaccine effectiveness of 25–53%. However, considering the poor effectiveness of conventional influenza vaccines in the elderly, several highly immunogenic influenza vaccines have been developed. Further evaluations of the comparative effectiveness of diverse vaccine formulations are warranted to assess their utility for preventing influenza infection, post-influenza pneumonia, and related hospitalization/mortality. Based on cost-effectiveness and budget impact analysis, influenza vaccination strategies should be tailored in the elderly.
KEYWORDS: Aged, Influenza, Immunization, Pneumonia, Vaccine effectiveness
Introduction
Influenza virus is a common pathogen implicated in respiratory tract infections, annually affecting up to 20% of the general population, and pneumonia is a leading cause of death after influenza infection.1,2 The incidence (0.1 – ≥10%) and case-fatality (<0.1 – 60%) of influenza pneumonia vary depending on antigenic variation and the virulence of circulating viruses.3 Seasonal influenza viruses cause 200,000–400,000 deaths per year during annual epidemics, while the 1918 H1N1 Spanish pandemic influenza virus was responsible for >40 million deaths.1,4,5
There are three types of influenza-related pneumonia, although clear distinctions may not be feasible: primary viral pneumonia, concomitant viral-bacterial pneumonia and secondary bacterial pneumonia (Fig. 1). Primary viral pneumonia is the least common type of influenza-related pneumonia, although it is more common in cases of avian influenza pneumonia (H5N1 and H7N9 viruses).6 Primary viral pneumonia can progress rapidly to severe pneumonia within 24 hours, and has a high case-fatality rate of 10–60%.6,7 Concomitant viral-bacterial pneumonia is at least three times more common than primary viral pneumonia, and this type of pneumonia tends to develop approximately 6 days following influenza infection.6,8,9 Among bacterial co-pathogens, Streptococcus pneumoniae and Staphylococcus aureus are the most common, followed by Klebsiella pneumoniae and Haemophilus influenzae.8 The case-fatality rate of concomitant viral-bacterial pneumonia is reported to be about 10%.6 It can be difficult to clinically distinguish concomitant viral-bacterial pneumonia from primary viral pneumonia, but it is important to administer antibiotic agents against bacterial co-infection at optimal times in terms of clinical outcomes. Although levels of C-reactive protein and procalcitonin are useful for differential diagnosis accompanying bacterial infections, current influenza guidelines recommend that clinicians should treat all influenza-related pneumonia cases with antibiotics.10,11 Secondary bacterial pneumonia usually develops 1–2 weeks after influenza-like illness following a brief period of improvement. Patients with secondary bacterial pneumonia show typical symptoms and signs of bacterial pneumonia (fever, chill, cough, purulent sputum, dyspnea) with a case fatality rate of about 7%.6
Figure 1.
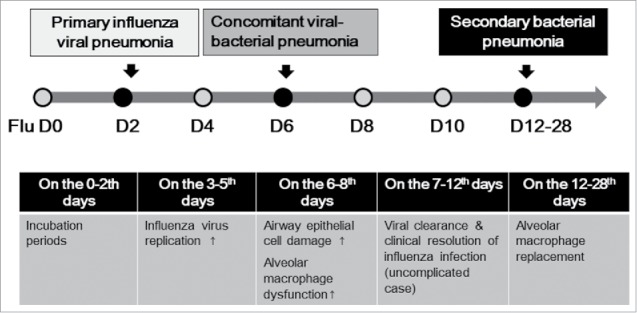
Schematic description of pathophysiological changes and development of pneumonia after influenza infection.
Pneumonia is the leading cause of influenza-related morbidity and mortality during both pandemic and seasonal epidemic periods. Although influenza vaccine is effective for preventing respiratory illness and reducing influenza-related hospitalization, it remains unclear whether influenza vaccine can decrease the risk of influenza-related pneumonia. This review focuses on the pathogenesis of influenza pneumonia and the effectiveness of influenza vaccine against pneumonia.
Pathogenesis of influenza-related pneumonia
Influenza viral proteins and host immune responses both play pivotal roles in the development of pneumonia. Influenza virus is composed of eight RNA gene segments, encoding hemagglutinin (HA), neuraminidase (NA), nucleoprotein (NP), M1, M2, nonstructural (NS) 1 protein, NS2 protein, polymerase acidic (PA) protein, polymerase basic (PB) 1 protein, PB1-F2 and PB2 protein. Among these proteins, influenza virus NS1 antagonizes type I IFN expression from infected cells during early stages. Type I IFNs play an important role inducing innate resistance to influenza viral infection.2 HA is associated with tropism for the target cells in the respiratory tract. Seasonal influenza viruses usually bind to α2,6 sialic acid residues on the epithelial cells of the upper respiratory tract in humans, whereas avian influenza viruses (A/H5N1 and A/H7N9) preferentially bind to α2,3 sialic acid residues of the lower respiratory tract, thereby infecting type 2 pneumocytes.12 Thus, avian influenza viruses can cause severe primary viral pneumonia with limited transmission. In comparison, seasonal influenza viruses can spread widely, but severe viral pneumonia is rare. Of note, the 2009 pandemic influenza A/H1N1 virus had a D222G mutation, which increased its affinity to α2,3 sialic acid receptors, and was thereby more likely to cause pneumonia.12 On the other hand, PB1-F2 is a well-known virulence factor that can cause apoptosis and cytokine storm.2,8 Mutations on PB1-F2 encoding genes might affect virulence. The N66S substitution in PB1-F2 increased virulence in the 1918 Spanish pandemic influenza A/H1N1 and H5N1 influenza viruses, while PB1-F2 truncation in the 2009 pandemic influenza A/H1N1 virus might be associated with the low case-fatality rate observed that year.2,8,10,12
In addition to causing primary viral pneumonia, influenza viruses contribute to the development of bacterial coinfections due to enhanced bacterial adherence in the respiratory tract and paradoxical suppression of the host immune system. There are several mechanisms by which bacterial adherence may increase.2,6,8,9 First, influenza virus may induce epithelial cell death, exposing the basal cell layer and basement membrane, which in turn increases bacterial binding to fibronectin and glycoproteins of basal progenitor cells.2,13 Second, influenza virus also up-regulates bacterial receptors including polymeric immunoglobulin receptor (pIgR) and platelet-activating factor receptor (PAFR).2,8,9 Third, influenza viral NA cleaves respiratory epithelial cell sialic acids, leading to increased expressions of bacterial binding receptors.2,8,9 With respect to paradoxical immune suppression, alveolar macrophage function is impaired by dysregulated cytokine responses after influenza infection.2,9 Alveolar macrophage dysfunction is associated with IFN-γ-mediated downregulation of the scavenger receptor MARCO, reduced production of TNF-α by natural killer (NK) cells, activation of transforming growth factor (TGF)-β by NA and disproportionate release of immunosuppressive cytokine (IL-10) from respiratory epithelial cells.2,9 Type I IFN also paradoxically suppresses alveolar macrophages.2 As a consequence, impaired alveolar macrophages release diminished amounts of neutrophil-activating chemokines such as macrophage inflammatory protein 2 (MIP-2) and keratinocyte-derived chemokine (KC).2 Besides impaired recruitment in alveolar spaces, influenza-affected neutrophils have functional defects in phagocytic activity, myeloperoxidase production, respiratory burst, and lysozyme secretion.2 Airway tissue damage is most severe around 6 days after influenza infection, and alveolar macrophage dysfunction is greatest 7–8 days post-infection (Fig. 1).2,6,8,9 The altered alveolar macrophages are then replaced over the next two weeks.8 Thus, patients with influenza are most susceptible to bacterial co-infection 6–8 days after infection, and remain at high risk for secondary bacterial pneumonia up to 3–4 weeks post-infection.2,6,8,9
Increased risk of pneumonia development after influenza infection in chronically ill patients
Elderly people and chronically ill patients are at elevated risk for post-influenza pneumonia.14,15 According to a prospective cohort study based on hospital-based influenza surveillance, most patients with post-influenza pneumonia (72.9%) are ≥ 65 years of age.14 Among co-morbidities, chronic lung disease resulted in the highest risk for pneumonia development, with an odds ratio (OR) of 4.16 (1.98–8.75), followed by chronic renal disease (OR 2.96, 95% CI 1.12–7.87) and cerebrovascular disease (OR 1.61, 95% CI 0.70–3.69).14 Moreover, the risk of post-influenza pneumonia was significantly increased according to the number of concurrent chronic medical conditions: two co-morbidities (OR, 6.9), three co-morbidities (OR, 7.1), or four co-morbidities (OR, 16.3).15
Effectiveness of influenza vaccine against pneumonia
Six meta-analyses were previously conducted to evaluate the effectiveness of influenza vaccine against pneumonia- or influenza-related hospitalization in the elderly (Table 1).16–21 A meta-analysis by Dominich et al. assessed the effectiveness of MF59-adjuvanted influenza vaccine in the elderly.16 All meta-analyses demonstrated that administering influenza vaccine was effective for preventing hospitalization due to pneumonia or influenza, although the estimates of effectiveness ranged from 25 to 53%. Some meta-analyses included only community-dwelling elderly individuals, while others also included institutionalized elderly individuals. The results of previous reviews suggest that the effectiveness of influenza vaccine against pneumonia- or influenza-related hospitalization is higher in the institutionalized elderly (36–47%) than in the community-dwelling elderly (25–33%).17–19,21 Thus, the inclusion of heterogeneous study samples might affect results, resulting in variable vaccine effectiveness assessments between meta-analyses.
Table 1.
Meta-analyses of influenza vaccine effectiveness against pneumonia- or influenza-related hospitalization in the elderly.
| Author | Published year | Setting | Study design (No. of studies) | Subject age (years) | Pooled vaccine effectiveness, percent (95% CI) |
|---|---|---|---|---|---|
| Domnich et al.16 | 2017 | Community and nursing home | Case-control (4) | ≥ 65 | 49 (39 to 61)* |
| Darvishian et at21 | 2014 | Community | Cohort (8) | ≥ 60 | 33 (23 to 43)† |
| 25 (6 to 40)‡ | |||||
| Chan et al.17 | 2014 | Nursing home | Cohort (3) | ≥ 60 | 37 (18 to 53) |
| Case-control (4) | |||||
| Jefferson et al.18 | 2010 | Nursing home | Cohort (17) | ≥ 65 | 46 (33 to 56) |
| Community | Cohort (8) | 27 (21 to 33) | |||
| Vu et al.19 | 2002 | Community | Cohort (2) | ≥ 65 | 33 (27 to 38) |
| Case-control (7) | |||||
| Gross et al.20 | 1995 | Community and nursing home§ | Cohort (9) | ≥ 65 | 53 (35 to 66) |
Vaccine effectiveness of MF59-adjuvanted influenza vaccine.
Vaccine effectiveness from conventional meta-analyses.
Vaccine effectiveness from meta-analyses adjusted for internal and external bias.
Most studies consisted of institutionalized elderly except for one study.
When we examined individual studies of the community-dwelling elderly, we found that the studies were conducted in geographically diverse areas during different influenza seasons (Tables 2 and 3).14,22–38 The size of the influenza epidemic and the degree of vaccine mismatch varied widely by study setting. Thus, some studies showed good effectiveness of influenza vaccine against pneumonia, while others showed unfavorable results. In cohort studies, five among nine (55.6%) showed significant effectiveness of influenza vaccine for preventing pneumonia- or influenza-related hospitalization (Table 2). The case-control studies also showed results similar to cohort studies; influenza vaccine was effective in six of nine studies (66.7%) and the effect was statistically significant (Table 3). Although meta-analyses and many observational studies have shown significant effectiveness for preventing pneumonia- or influenza-related hospitalization among vaccinated elderly people, most studies included severe acute respiratory infections (SARIs) and were not confined to pneumonia cases. In addition, most were conducted during past periods when influenza vaccine uptake rates were low and available anti-viral agents were limited. In a modern context, in which vaccine uptake rates are high, the herd effect may be more significant, and early antiviral treatment may therefore reduce the chance of progression to pneumonia among influenza-infected patients.6,39 Further studies are required to better understand influenza vaccine effectiveness against pneumonia in a modern context, characterized by high vaccine uptake and antiviral use.
Table 2.
Cohort studies of influenza vaccine effectiveness (VE) for preventing pneumonia- or influenza-related hospitalization among the community-dwelling elderly.
| Author | Publication year | Country | Study periods | Subject age (years) | Sample size | VE (95% CI) |
|---|---|---|---|---|---|---|
| Arriola et al.22 | 2017 | United States | 2013–2014 | ≥65 | 1,588 | −2 (−27 to 19) |
| Arriola et al.25 | 2015 | United States | 2012–2013 | 65–74 | 1,296 | 20 (−6 to 39) |
| ≥75 | 2,680 | −2 (−23 to 16) | ||||
| Song et al.14 | 2015 | South Korea | 2013–2014 | ≥65 | 573 | 55 (29 to 71) |
| Simpson et al.26 | 2013 | United Kingdom | 2000–2009 | ≥65 | 1,358 | 12 (−2 to 24) |
| Baxter et al.27 | 2010 | United States | 1997–2008 | ≥65 | 4,018,380 | 9 (3 to 14) |
| Nichol et al.29 | 2007 | United States | 1990–2000 | ≥65 | 713,812 | 27 (23 to 32) |
| Nordin et al.30 | 2001 | United States | 1996–1997 | ≥65 | 124,582 | 20 (5 to 31) |
| 1997–1998 | 158,454 | 24 (14 to 34) | ||||
| Nichol e al.32 | 1999 | United States | 1990–1996 | ≥65 | 147,551 | 39 (26 to 52) |
| Baker et al.38 | 1980 | United States | 1968–1969 | ≥65 | 9,760 | 2 (−23 to 13) |
| 1972–1973 | 10,740 | 74 (−8 to 94) |
Table 3.
Case-control studies of influenza vaccine effectiveness (VE) for preventing pneumonia- or influenza-related hospitalization among community-dwelling elderly.
| Author | Publication year | Country | Study period | Subject age (years) | Sample size | VE (95% CI) |
|---|---|---|---|---|---|---|
| Grijalva et al.24 | 2015 | United States | 2010–2012 | ≥65 | 429 | 48 (−33 to 80) |
| Mahmud et al.23 | 2015 | Canada | 2009–2010 | ≥65 | 5,852 | 10 (−16–30) |
| Jackson et al.28 | 2008 | United States | 2000–2003 | ≥65 | 3,519 | 9 (−10 to 23) |
| Crocetti et al.31 | 2001 | Italy | 1994–1995 | ≥65 | 825 | 33 (5 to 52) |
| Puig-Barbera et al.33 | 1997 | Spain | 1994–1995 | ≥65 | 249 | 79 (45 to 92) |
| Ohmit et al.34 | 1995 | United States | 1990–1991 | ≥65 | 2,197 | 31 (4 to 51) |
| 1991–1992 | 2,761 | 32 (7 to 50) | ||||
| Mullooly et al.35 | 1994 | United States | 1981–1989 | ≥65, non-high-risk group | 150,713 | 40 (1 to 64) |
| ≥65, high-risk group | 100,321 | 30 (17 to 42) | ||||
| Fedson et al.36 | 1993 | United States | 1982–1983 | ≥65 | 10,471 | 37 (15 to 53) |
| 1985–1986 | 9,720 | 39 (19 to 53) | ||||
| Foster et al.37 | 1992 | United States | 1989–1990 | ≥65 | 2,507 | 33 (8 to 52) |
Perspectives
Although vaccine effectiveness varies for seasonal influenza according to antigenic drift, trivalent inactivated influenza vaccine showed 59% efficacy (95% CI, 51–67%) against laboratory-confirmed influenza in randomized clinical trials.40 In addition, trivalent inactivated influenza vaccine significantly reduced pneumonia- or influenza-related hospitalizations, with a vaccine effectiveness of 25–53% across meta-analyses.16,18–21,41 However, there are several limitations of the current influenza vaccines. Vaccine effectiveness is low in the elderly, who are at heightened risk for post-influenza pneumonia. Influenza vaccine lacks effectiveness in the elderly (aged ≥ 65 years) during seasons with antigenic mismatch.42 It remains unclear whether the conventional influenza vaccine is effective for preventing pneumonia even during seasons with marked antigenic drift. Moreover, although the elderly are advised to undergo annual influenza vaccination, there is a concern of blunted antibody response after repeated vaccination, particularly against A/H3N2.43 Further studies are required to clarify whether repeated influenza vaccination reduces antibody response and clinical effectiveness or not.
Considering the low effectiveness of conventional influenza vaccines in the elderly, several highly immunogenic influenza vaccines have been developed and are available in developed countries: MF59-adjuvanted influenza vaccine, ASO3-aduvanted influenza vaccine, intradermal influenza vaccine and high-dose influenza vaccine (60 μg of hemagglutinin per strain). The MF59-adjuvanted vaccine showed superior relative effectiveness (25–63%) to unadjuvanted conventional vaccines for preventing hospitalizations due to pneumonia/influenza.16,44,45 Similarly, patients receiving the high-dose vaccine had significantly lower risk of developing laboratory-confirmed influenza infections (relative risk 0.76, 95% CI 0.65–0.90) compared to those receiving the standard-dose vaccine.46 In addition, quadrivalent influenza vaccines have shown superior immunogenicity against the B lineage not included in trivalent influenza vaccines.47 Quadrivalent influenza vaccines are expected to reduce influenza disease burden and to be cost-effective in the elderly compared with trivalent influenza vaccines.48,49 Thus, further evaluations of the comparative effectiveness of diverse vaccine formulations for preventing influenza infection, post-influenza pneumonia and related hospitalization/mortality are warranted. Based on the desire to maximize cost-effectiveness and budget impact, influenza vaccination strategies should be tailored in the elderly.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Nicholson KG. Human Influenza In: Nicholson KG, Webster RG, Hay AJ, Textbook of Influenza. Oxford: Blackwell Science; 1998. p.219–264. [Google Scholar]
- 2.Ballinger MN, Standiford TJ. Postinfluenza bacterial pneumonia: host defenses gone awry. J Interferon Cytokine Res. 2010;30:643–652. doi: 10.1089/jir.2010.0049. PMID:20726789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society , Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. PMID:15699079 [DOI] [PubMed] [Google Scholar]
- 4.Simonsen L, Fukuda K, Schonberger LB, Cox NJ. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181:831–837. doi: 10.1086/315320. PMID:10720501 [DOI] [PubMed] [Google Scholar]
- 5.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi. doi: 10.1001/jama.289.2.179. PMID:12517228 [DOI] [PubMed] [Google Scholar]
- 6.Metersky ML, Masterton RG, Lode H, File TM Jr., Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16:e321–331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bui C, Bethmont A, Chughtai AA, Gardner L, Sarkar S, Hassan S, Seale H, MacIntyre CR. A Systematic Review of the Comparative Epidemiology of Avian and Human Influenza A H5N1 and H7N9 – Lessons and Unanswered Questions. Transbound Emerg Dis. 2016;63:602–620. doi: 10.1111/tbed.12327. PMID:25644240 [DOI] [PubMed] [Google Scholar]
- 8.McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:252–262. doi: 10.1038/nrmicro3231. PMID:24590244 [DOI] [PubMed] [Google Scholar]
- 9.Metzger DW, Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol. 2013;191:2047–2052. doi: 10.4049/jimmunol.1301152. doi: 10.4049/jimmunol.1301152. PMID:23964104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JY, Cheong HJ, Heo JY, Noh JY, Yong HS, Kim YK, Kang EY, Choi WS, Jo YM, Kim WJ. Clinical, laboratory and radiologic characteristics of 2009 pandemic influenza A/H1N1 pneumonia: primary influenza pneumonia versus concomitant/secondary bacterial pneumonia. Influenza Other Respir Viruses. 2011;5:e535–543. doi: 10.1111/j.1750-2659.2011.00269.x. PMID:21682848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi WS, Baek JH, Seo YB, Kee SY, Jeong HW, Lee HY, Eun BW, Choo EJ, Lee J, Kim YK, et al.. Severe influenza treatment guideline. Korean J Intern Med. 2014;29:132–147. doi: 10.3904/kjim.2014.29.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuyama S, Kawaoka Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Curr Opin Immunol. 2011;23:481–486. doi: 10.1016/j.coi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JY, Nahm MH, Cheong HJ, Kim WJ. Impact of preceding flu-like illness on the serotype distribution of pneumococcal pneumonia. PLoS One. 2014;9:e93477. doi: 10.1371/journal.pone.0093477. PMID:24691515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JY, Lee JS, Wie SH, Kim HY, Lee J, Seo YB, Jeong HW, Kim SW, Lee SH, Park KH, et al.. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2015;22:229–234. doi: 10.1128/CVI.00673-14. PMID:25540271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SH, Cheong HJ, Song JY, Noh JY, Jeon JH, Choi MJ, Lee J, Seo YB, Lee JS, Wie SH, et al.. Analysis of Risk Factors for Severe Acute Respiratory Infection and Pneumonia and among Adult Patients with Acute Respiratory Illness during 2011–2014 Influenza Seasons in Korea. Infect Chemother. 2016;48:294–301. doi: 10.3947/ic.2016.48.4.294. PMID:27883375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domnich A, Arata L, Amicizia D, Puig-Barbera J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine. 2017;35:513–520. doi: 10.1016/j.vaccine.2016.12.011. PMID:28024956 [DOI] [PubMed] [Google Scholar]
- 17.Chan TC, Fan-Ngai Hung I, Ka-Hay Luk J, Chu LW, Hon-Wai Chan F. Effectiveness of influenza vaccination in institutionalized older adults: a systematic review. J Am Med Dir Assoc. 2014;15:226.e221–226. doi: 10.1016/j.jamda.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;doi: 10.1002/14651858.CD004876.pub3.Cd004876. [DOI] [PubMed] [Google Scholar]
- 19.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–1836. doi. doi: 10.1016/S0264-410X(02)00041-5. PMID:11906772 [DOI] [PubMed] [Google Scholar]
- 20.Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med 1995;123:518–527. doi. doi: 10.7326/0003-4819-123-7-199510010-00008. PMID:7661497 [DOI] [PubMed] [Google Scholar]
- 21.Darvishian M, Gefenaite G, Turner RM, Pechlivanoglou P, Van der Hoek W, Van den Heuvel ER, Hak E. After adjusting for bias in meta-analysis seasonal influenza vaccine remains effective in community-dwelling elderly. J Clin Epidemiol. 2014;67:734–744. doi: 10.1016/j.jclinepi.2014.02.009. PMID:24768004 [DOI] [PubMed] [Google Scholar]
- 22.Arriola CS, Garg S, Anderson EJ, Ryan PA, George A, Zansky SM, Bennett N, Reingold A, Bargsten M, Miller L, et al.. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis. 2017;65:1289–1297. doi: 10.1093/cid/cix468. PMID:28525597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmud SM, Bozat-Emre S, Hammond G, Elliott L, Van Caeseele P. Did the H1N1 Vaccine Reduce the Risk of Admission with Influenza and Pneumonia during the Pandemic? PLoS One. 2015;10:e0142754. doi: 10.1371/journal.pone.0142754. PMID:26600435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grijalva CG, Zhu Y, Williams DJ, Self WH, Ampofo K, Pavia AT, Stockmann CR, McCullers J, Arnold SR, Wunderink RG, et al.. Association Between Hospitalization With Community-Acquired Laboratory-Confirmed Influenza Pneumonia and Prior Receipt of Influenza Vaccination. JAMA. 2015;314:1488–1497. doi: 10.1001/jama.2015.12160. PMID:26436611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arriola CS, Anderson EJ, Baumbach J, Bennett N, Bohm S, Hill M, Lindegren ML, Lung K, Meek J, Mermel E, et al.. Does Influenza Vaccination Modify Influenza Severity? Data on Older Adults Hospitalized With Influenza During the 2012–2013 Season in the United States. J Infect Dis. 2015;212:1200–1208. doi: 10.1093/infdis/jiv200. PMID:25821227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson CR, Lone N, Kavanagh K, Ritchie LD, Robertson C, Sheikh A, McMenamin J. Seasonal Influenza Vaccine Effectiveness (SIVE): an observational retrospective cohort study – exploitation of a unique community-based national-linked database to determine the effectiveness of the seasonal trivalent influenza vaccine. Health Services and Delivery Research. 2013;1:1–45. doi: 10.3310/hsdr01100. [DOI] [PubMed] [Google Scholar]
- 27.Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010;28:7267–7272. doi: 10.1016/j.vaccine.2010.08.088. PMID:20832494 [DOI] [PubMed] [Google Scholar]
- 28.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. PMID:18675690 [DOI] [PubMed] [Google Scholar]
- 29.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. PMID:17914038 [DOI] [PubMed] [Google Scholar]
- 30.Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, Rush B, Safirstein B, Wheeler D, Nichol KL. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184:665–670. doi: 10.1086/323085. PMID:11517426 [DOI] [PubMed] [Google Scholar]
- 31.Crocetti E, Arniani S, Bordoni F, Maciocco G, Zappa M, Buiatti E. Effectiveness of influenza vaccination in the elderly in a community in Italy. Eur J Epidemiol. 2001;17:163–168. doi. doi: 10.1023/A:1017978420601. PMID:11599691 [DOI] [PubMed] [Google Scholar]
- 32.Nichol KL. Complications of influenza and benefits of vaccination. Vaccine 1999;17(Suppl 1):S47–52. doi. doi: 10.1016/S0264-410X(99)00105-X. PMID:10471180 [DOI] [PubMed] [Google Scholar]
- 33.Puig-Barberà J, Márquez-Calderón S, Masoliver-Fores A, Lloria-Paes F, Ortega-Dicha A, Gil-Martín M, Calero-Martínez MJ. Reduction in hospital admissions for pneumonia in non-institutionalised elderly people as a result of influenza vaccination: a case-control study in Spain. J Epidemiol Community Health 1997;51:526–530. doi. doi: 10.1136/jech.51.5.526. PMID:9425463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmit SE, Monto AS. Influenza vaccine effectiveness in preventing hospitalization among the elderly during influenza type A and type B seasons. Int J Epidemiol 1995;24:1240–1248. doi. doi: 10.1093/ije/24.6.1240. PMID:8824869 [DOI] [PubMed] [Google Scholar]
- 35.Mullooly JP, Bennett MD, Hornbrook MC, Barker WH, Williams WW, Patriarca PA, Rhodes PH. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med 1994;121:947–952. doi. doi: 10.7326/0003-4819-121-12-199412150-00008. PMID:7978721 [DOI] [PubMed] [Google Scholar]
- 36.Fedson DS, Wajda A, Nicol JP, Hammond GW, Kaiser DL, Roos LL. Clinical effectiveness of influenza vaccination in Manitoba. JAMA 1993;270:1956–1961. doi. doi: 10.1001/jama.1993.03510160074032. PMID:8411553 [DOI] [PubMed] [Google Scholar]
- 37.Foster DA, Talsma A, Furumoto-Dawson A, Ohmit SE, Margulies JR, Arden NH, Monto AS. Influenza vaccine effectiveness in preventing hospitalization for pneumonia in the elderly. Am J Epidemiol 1992;136:296–307. doi. doi: 10.1093/oxfordjournals.aje.a116495. PMID:1415151 [DOI] [PubMed] [Google Scholar]
- 38.Barker WH, Mullooly JP. Influenza vaccination of elderly persons. Reduction in pneumonia and influenza hospitalizations and deaths. JAMA 1980;244:2547–2549. doi. doi: 10.1001/jama.1980.03310220045026. PMID:7431593 [DOI] [PubMed] [Google Scholar]
- 39.Song JY, Noh JY, Choi WS, Cheong HJ, Kim WJ. Antiviral therapy in seasonal influenza and 2009 H1N1 pandemic influenza: Korean experiences and perspectives. Expert Rev Anti Infect Ther. 2015;13:1361–1372. doi: 10.1586/14787210.2015.1076334. PMID:26256778 [DOI] [PubMed] [Google Scholar]
- 40.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. PMID:22032844 [DOI] [PubMed] [Google Scholar]
- 41.Hong KW, Cheong HJ, Choi WS, Lee J, Wie SH, Baek JH, Kim HY, Jeong HW, Kim WJ. Clinical courses and outcomes of hospitalized adult patients with seasonal influenza in Korea, 2011–2012: Hospital-based Influenza Morbidity & Mortality (HIMM) surveillance. J Infect Chemother. 2014; 20:9–14. doi: 10.1016/j.jiac.2013.07.001. PMID:24462445 [DOI] [PubMed] [Google Scholar]
- 42.Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine. 2006;24:6605–6611. doi: 10.1016/j.vaccine.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 43.Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16:1–14. doi: 10.1080/14760584.2017.1334554. PMID:28562111 [DOI] [PubMed] [Google Scholar]
- 44.Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, Boldori L, Caramaschi F, Gattinoni A, Malchiodi G, et al.. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176:527–533. doi: 10.1093/aje/kws313. PMID:22940713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, Bigham M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013;31:6122–6128. doi: 10.1016/j.vaccine.2013.07.059. PMID:23933368 [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson K, Wei Y, Szwajcer A, Rabbani R, Zarychanski R, Abou-Setta AM, Mahmud SM. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine. 2017;35:2775–2780. doi: 10.1016/j.vaccine.2017.03.092. PMID:28431815 [DOI] [PubMed] [Google Scholar]
- 47.Moa AM, Chughtai AA, Muscatello DJ, Turner RM, MacIntyre CR. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials. Vaccine. 2016;34:4092–4102. doi: 10.1016/j.vaccine.2016.06.064. PMID:27381642 [DOI] [PubMed] [Google Scholar]
- 48.You JH, Ming WK, Chan PK. Cost-effectiveness analysis of quadrivalent influenza vaccine versus trivalent influenza vaccine for elderly in Hong Kong. BMC Infect Dis. 2014;14:618. doi: 10.1186/s12879-014-0618-9. PMID:25420713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Bellinghen LA, Meier G, Van Vlaenderen I. The potential cost-effectiveness of quadrivalent versus trivalent influenza vaccine in elderly people and clinical risk groups in the UK: a lifetime multi-cohort model. PLoS One. 2014;9:e98437. doi: 10.1371/journal.pone.0098437. PMID:24905235 [DOI] [PMC free article] [PubMed] [Google Scholar]


