ABSTRACT
Influenza severity increases and vaccine effectiveness decreases with age. High-dose influenza vaccine (HD) with quadruple the antigen of standard-dose (SD) vaccine is more efficacious in community-dwelling persons 65 years and older. We evaluated the feasibility of recruiting and randomizing Medicare certified nursing homes (NHs) for a pragmatic cluster-randomized trial comparing HD vs. SD (NCT1720277). Residents were long-stay and at least 65 years old. NH leadership agreed to standard of care random assignment with HD (Fluzone® High-Dose) or SD (Fluzone®) influenza vaccine for their facility for the 2012–2013 influenza season. We used Minimum Data Set (MDS) 3.0 and Vital Status records for pre-specified clinical outcomes: 1) all-cause hospitalization, 2) NH mortality, and 3) functional decline. Intent-to-treat analyses were performed at the resident-level using Cox proportional hazards, multivariable Poisson, and logistic regression models accounting for clustering by facility. We randomized 39 NHs (19 SD and 20 HD), coordinated vaccine delivery, implemented web-based data collection, and accessed MDS data, demonstrating feasibility. There were 2,957 eligible residents (SD 1496; HD 1461); characteristics were similar between groups. A total of 301 (20.1%) of SD and 197 (13.5%) of HD allocated residents were ever hospitalized, (adjusted relative risk 0.680; 95% CI: 0.537, 0.862; p = 0.001). NH mortality was 274 (18.3%) SD vs. 249 (17.1%) HD, adjusted relative risk 0.834; 95% CI: 0.678, 1.027; p = 0.087). There were no differences in decline in functional status (13.4 vs. 13.8%, adjusted relative risk 0.994; 95% CI: 0.774,1.278; p = 0.965). We demonstrate that a pragmatic large-scale trial is feasible in a NH setting.
KEYWORDS: Cluster-randomized trials, feasibility, nursing home, influenza, influenza vaccine
Introduction
Influenza remains the most common preventable respiratory viral infection of older adults.1–5 Older adults incur more than 90% of the disease burden,4 and those residing in nursing homes are the most affected subset given their immune senescence, multi-morbidity, and close living quarters.6
Influenza vaccination reduces influenza-related morbidity and mortality7 and is associated with reduced hospitalization, strokes, heart attacks, and death in non-institutional older adult populations.8–11 Nonetheless, the benefit of influenza vaccine for the oldest population has been questioned, citing evidence that the vaccine produces less antibody12 and offers less protection for older individuals.13 In healthy elderly populations, high-dose influenza vaccine results in both higher antibody levels14 and reduced rates of laboratory-confirmed illness.15 Since the high-dose influenza vaccine also generates more antibody in frail elderly individuals residing in a long-term care setting,11 there is hope it will also be clinically more effective for this population.
Estimating influenza vaccination benefit among older adults in long-term care settings using randomized controlled trials (RCTs) requires extensive effort and is costly. A pragmatic RCT in a nursing home population has several advantages as a model for comparing therapeutic approaches. Frail, older individuals have a much higher rate of influenza-related events than do healthier populations.16 U.S. nursing homes are required to submit comprehensive clinical and administrative data on each resident at least every 3 months to the Centers for Medicare & Medicaid Services (CMS). This Minimum Data Set (MDS) provides a continuous record of detailed clinical, morbidity, and mortality data for all residents of CMS-certified facilities nationwide. For this reason, data on individual residents can be used to identify and report on them over time and across settings, limiting loss to follow-up and enhancing the intent-to-treat approach.
The conceptual design of an intent-to-treat pragmatic, cluster-RCT to compare two vaccines head-to-head in an intent-to-treat format is relatively straightforward. Facilities (the “clusters”) are randomized to a treatment that is the recommended standard of care, (i.e., assigned to offer all residents a specific recommended vaccine as the facility's standard of care–either high-dose or standard-dose vaccine) rather than randomizing individual residents within facilities. Residents are analyzed as if they received the assigned treatment, whether or not they accepted the care standard vaccine, as they can refuse treatment. However, execution of such a trial offers many challenges.
Here, we report on the feasibility to recruit, screen, and enroll facilities; randomly assign and coordinate vaccine delivery; collect vaccine administration data; conduct site audits for data validation; and collect outcome variables from various data sources. We also tested the feasibility of using existing administrative data to test the hypothesis that HD reduces hospitalization, functional decline, and mortality in the context of a pragmatic, cluster-randomized approach in the NH setting.
Methods
Study design
We conducted a feasibility study for a pragmatic cluster-randomized controlled trial to test the effect of high-dose (HD) influenza vaccine vs. standard-dose (SD) vaccine on hospitalization, mortality, and functional decline rates of long-stay NH residents. We did not blind residents or staff for practical reasons. The researchers were blinded to the allocation of the NHs during analysis. The ClinicalTrials.gov identifier is NCT01720277. This study was approved by the human research ethics committees of New England Independent, Brown and Case Western Reserve University Institutional Review Boards through an expedited process as it met the criteria for minimal risk with a waiver of consent.
Site recruitment
We recruited facilities with assistance of stakeholder organizations, including AMDA, the Society for Post-Acute and Long-Term Care Medicine, pharmacy provider and consulting companies, and NH chain organizations. The organizations used a variety of strategies to communicate the opportunity of study participation including e-newsletters, email blasts, and website posting. Study investigators personally contacted individuals by mail, email, and telephone at different organizations to invite their participation.
Study sample and random assignment
We enrolled a sample from volunteering Medicare-certified NHs within 50 miles of a CDC FLU reporting city. Nursing homes were excluded if the facility was hospital-based or not submitting MDS data. Facilities where HD influenza vaccine was ordered for all facility residents over 65 years of age, had fewer than 50 long-stay residents or more than 20% of the population under age 65, or where over half of the residents were short stay were also excluded.
Qualifying facilities were offered the opportunity to participate and all facilities accepting and meeting enrollment criteria were enrolled through October 2012. An independent statistician used a randomly permuted blocked design to randomly allocate NHs to either HD or SD vaccine.
Intervention and implementation
Each NH's leadership agreed to random assignment to either HD (Fluzone® High-Dose vaccine, Sanofi Pasteur, Swiftwater, PA) or SD (Fluzone® vaccine, Sanofi Pasteur, Swiftwater, PA) as their standard of care for all residents. All participating facilities received free vaccine sufficient to meet the estimated number of NH residents. NHs were also randomized to receive 1) a free supply of SD vaccine for staff or, 2) no free supply. All NHs were instructed to follow their routine practice for staff vaccination. Vaccines were administered September through December 2012. All facilities were offered influenza and vaccine education for residents and staff.
Site visits and audits
Each participating facility recorded resident and staff census and number of vaccinated residents and staff monthly from November 2012 to March 2013. We conducted 10 site visits to test methods of determining vaccination rates from facility source documents and record keeping, including proof of vaccine supply receipt from the manufacturer. We confirmed vaccine shipment delivery to all facilities.
Data sources
We obtained data from: 1) Online Survey Certification and Reporting (OSCAR) annual surveys of U.S. Medicare/Medicaid certified NHs; 2) Medicare enrollment and vital status files; 3) the NH resident assessment, MDS 3.0; and 4) monthly reports from participating NHs with resident and staff vaccination rates.
OSCAR, a publicly available record system of Centers for Medicare & Medicaid Services reimbursed NHs, provides data on ownership, size, staffing, services, resident acuity, and quality inspection results. These data determined facility eligibility. Vital status records are available for all Medicare beneficiaries, indicating age, sex and race, dual eligibility, managed care membership, and up-to-date vital status information. The MDS, with nearly 400 data elements, reports cognition, communication problems, physical functioning, continence, mood, activity and recreation, diagnoses, nutritional status, oral/dental status, skin conditions, special treatments, and medication use. The MDS is completed upon resident admission to a certified NH and at least quarterly thereafter.17 These data have good reliability and a low rate of missing elements in both research and real-world applications.18
Data collection and merging
We collected census and resident and staff vaccine administration data for five months. We combined data from OSCAR, Medicare enrollment files, and MDS data to report on characteristics at facility and resident-levels and to compare study outcomes experienced by residents in HD vs. SD NHs.
Outcomes
Feasibility of conducting a pragmatic, cluster RCT was based on the ability to recruit, screen, enroll, and randomly assign eligible NHs to the appropriate vaccine group, and to deliver the designated vaccine to the correct NH. Further, we needed to evaluate our ability to successfully engage NHs, to communicate with NH staff, collect vaccination rates for residents and staff and vaccine lot numbers, and to verify vaccine administration. For clinical outcomes, using the MDS, we calculated all-cause hospitalization occurrence during the influenza season and the incidence of declining physical functioning by at least 4 points on the 28-point Activities of Daily Living Scale.19 Mortality occurring in the NH was determined from the MDS file, and outside of the NH from the Vital Status file.
Statistical analyses
Descriptive statistics were used to report our feasibility outcomes. Our target sample size was 100 NHs per arm, which was considered adequate to demonstrate the ability to screen, recruit, and enroll eligible NHs. Data were recorded as “missing” when no vaccine was reported to be administered for staff, and similarly for residents, and these cases were not included in the average facility-level vaccination rates. Facility reported vaccination rates were summarized in each arm using means and standard deviations and compared using two-sample student t-tests with unequal variances and the Satterthwaite degrees of freedom. All clinical outcomes were analyzed under the principle of intent-to-treat using the robust marginal Poisson regression approach.20 We assumed an exchangeable correlation structure (or independence structure in case of non-convergence) with robust standard errors. The models were clustered on NH, and included the natural log of person-time as an off-set term to account for differences in observed follow-up times. Person-time was censored at death, last MDS assessment (loss to follow-up), or March 31, 2013. The analyses were adjusted for pre-specified covariates believed to be associated with resident- and NH-level hospitalization risk, including patients’ prior year hospitalization rate, resident age, mean age of residents in home, individual activities of daily living (ADL) score, mean ADL score in home, Cognitive Function Score (CFS), mean CFS in home, history of heart failure (HF), and prevalence of HF risk-group in home. Robust Poisson regression was used because the resulting covariate-adjusted risk ratios are easier to interpret than adjusted odds ratios from logistic regression.20 All analyses were carried out in SAS v. 9.4 (SAS Institute, Inc., Cary, NC).
Results
Aligning vaccine orders and recruitment timing
In influenza trials, it is important to consider timing for site enrollment. Most NHs order vaccine in the spring before the influenza season. For this study, we recruited in September and began enrolling in October, by which time many facilities had already ordered vaccine they intended to administer. Consequently, most NHs had started vaccinating their staff and residents by the time we were in the position to recruit and ship vaccine, making them ineligible, so we were unable to meet recruitment goals as planned.
Once we determined the number of vaccines required for each enrolled NH based on NH-provided counts, we ordered and monitored vaccine delivery. Nursing homes were instructed to keep the vaccine shipping receipt for the site monitoring visit, which occurred between January and May 2013.
Nursing home characteristics
Our study included NHs in 8 states (CO, NJ, OH, CT, KS, PA, WA, WI), with 14 in New Jersey and 17 in Colorado. We randomly allocated 20 NHs to receive HD vaccine and 19 NHs to SD vaccine for their residents. Forty-one percent of facilities were part of a NH chain and 72% were for-profit. The mean resident census was 116, with 87% long-stay residents. There were no important differences in NH characteristics between groups (Table 1) nor any important differences in staff characteristics: ratio of RN to RN+LPN (mean 0.35, sd 0.12 vs. mean 0.35, sd 0.14), total RN hours/day/resident (mean 0.72, sd 1.32 vs. mean 0.44, sd 0.23), total direct care (RN/CNA/LPN) hours/resident/day (mean 3.89, sd 2.70 vs. mean 3.34, sd 1.00), and percent of NHs with any physician extenders (mean 0.40, sd 0.50 vs. mean 0.32, sd 0.48).
Table 1.
Baseline Characteristics of Nursing Homes for Influenza Season 2012–2013.
| High-Dose Vaccine for Residents | Standard-Dose Vaccine for Residents | |
|---|---|---|
| Characteristics | mean (SD) | mean (SD) |
| Nursing homes randomized (N) | 20 | 19 |
| Number of residents per home | 107.9 (48.6) | 124.2 (53.9) |
| Percent of residents under 65 years | 14.2 (14.7) | 18.9 (18.1) |
| Percent of residents who are long-stay | 88.0 (6.5) | 86.5 (12.1) |
| Number of long-stay residents over 65 years | 78.1 (37.1) | 77.8 (44.4) |
| Percent of residents with Medicaid as payer | 62.5 (12.8) | 62.9 (17.4) |
| Ratio of RN/RN+LPN | 0.35 (0.12) | 0.35 (0.14) |
| Average ADL score on admission (0-28) | 16.8 (1.92) | 16.1 (1.67) |
ADL, activities of daily living; LPN, licensed practical nurse; LTC, long-term care residents are dual-eligible and private-pay, non-Medicare beneficiaries; RN, registered nurse, SD, standard deviation.
High-dose refers to Fluzone High-Dose vaccine. Standard-dose refers to Fluzone vaccine.
Data Sources: Minimum Data Set Assessments OSCAR, HD Flu study.
Long-stay NH resident population and baseline characteristics
A total of 4,438 residents were living in enrolled NHs on October 1, 2012. Our study sample includes 2,957 long-stay residents (i.e., residing in the NH for more than 90 days), which represents 66.6% of the NH population of participating facilities (Fig. 1). The mean resident age was 84 years, 75% were female and 79% white (Table 2) with slight differences in active health diagnoses among residents between arms. Census data were compared with OSCAR and found to be similar.
Figure 1.
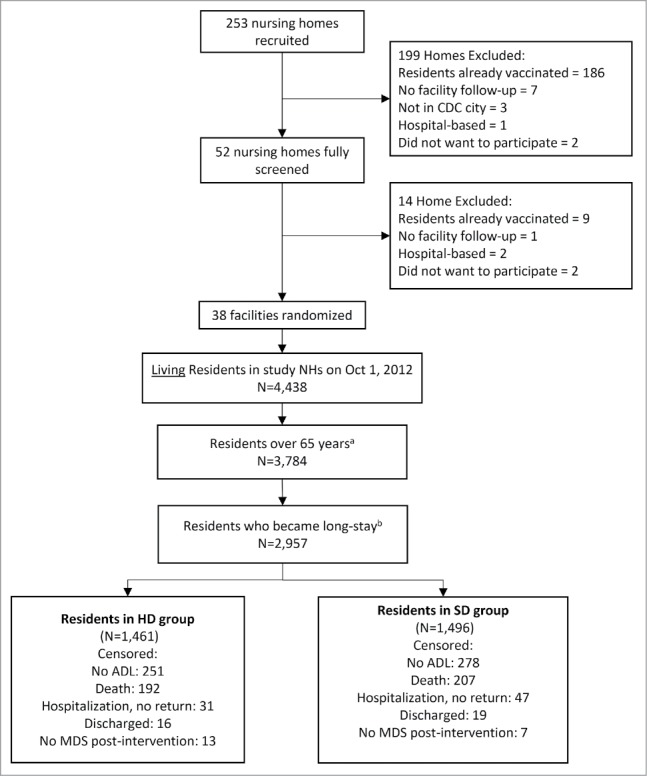
Long-Stay Nursing Home Resident Selection Flowchart. a Residents who were 65 years old on October 1, 2012. b Long-stay residents are NH residents with quarterly and/or annual MDS assessments (June 1-September 30 2012) and still residing in study NHs on October 1, 2012. Residents who were discharged from the nursing home to the community, inpatient rehabilitation facility, hospice, other location, or as dead are excluded from the analytical sample. Residents were included if they were discharged to another nursing home, acute hospital, psychiatric hospital, or mental retardation/developmental disabilities facility. Abbreviations: NH, nursing home; HD, high-dose; MDS, minimum data set (nursing home resident assessment); SD, standard-dose. HD vaccine refers to Fluzone High-Dose vaccine. SD vaccine refers to standard-dose Fluzone vaccine.
Table 2.
Baseline Demographic and Clinical Characteristics of Eligible Nursing Home Residents (n = 2,957).
| High-Dose Vaccine for Residents | Standard-Dose Vaccine for Residents | |
|---|---|---|
| Characteristics | n (%) | n (%) |
| Long-stay residents over 65 years (N) | 1,461 | 1,496 |
| Age, mean (sd) | 84.5 (8.4) | 83.4 (8.7) |
| Female | 1094 (74.9) | 1113 (74.4) |
| Married | 266 (18.3) | 262 (17.5) |
| Race/Ethnicity | ||
| African American | 194 (13.3) | 174 (11.6) |
| White | 1157 (79.2) | 1184 (79.1) |
| Hispanic | 88 (6.0) | 87 (5.8) |
| Othera | 22 (1.5) | 42 (2.8) |
| Active Health Diagnosis | ||
| Heart Failure | 324 (22.2) | 253 (16.9) |
| Stroke/CVA/TIA | 192 (13.1) | 210 (14.0) |
| Hypertension | 1135 (77.7) | 1078 (72.1) |
| Pneumonia | 13 (0.9) | 25 (1.8) |
| Diabetes Mellitus | 427 (29.2) | 440 (29.4) |
| Asthma/COPD/CLD | 326 (22.3) | 256 (17.1) |
| Cancerb | 45 (3.1) | 48 (3.2) |
| ESRD | 112 (7.7) | 86 (5.8) |
| Any hospitalization in baseline periodc | 122 (8.4) | 156 (10.4) |
CLD, chronic lung disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ESRD, end stage renal disease; SD, standard deviation; TIA, transient ischemic attack.
High-dose refers to Fluzone High-Dose vaccine. Standard-dose refers to Fluzone vaccine.
Race/ethnicity Other includes American Indians, Alaskan Natives, Native Americans, Pacific Islanders, and Asians.
This health diagnosis is not populated well; 31.6% missing in both groups.
Residents had at least one hospitalization during the baseline (June-September 2012).
Data Source: Minimum Data Set Assessments.
Outcomes
We recruited 39 NHs, short of our goal. Late recruitment impinged on the vaccination season; over 195 NHs we recruited became ineligible to participate. Our screening, recruiting, randomization, and delivery methods were established, and vaccine was correctly delivered to facilities by November 2012. Monitoring effectiveness and other feasibility considerations were confirmed. There was a non-statistical difference in the greater uptake for both residents and staff in facilities randomized to HD (Table 3).
Table 3.
Facility Reported Vaccination Rates.
| High-Dose Vaccine Facilities (n = 20) | Standard-Dose Vaccine Facilities (n = 19) | ||
|---|---|---|---|
| Population | mean (SD) | mean (SD) | p-value |
| Percent of residents vaccinated | 90.8 (8.8) | 88.1 (15.7) | 0.54 |
| Percent of LTC residents vaccinated | 91.7 (8.6) | 89.6 (15.1) | 0.62 |
| Percent of staff vaccinateda,b | 76.0 (28.8) | 61.5 (33.7) | 0.20 |
High-dose refers to Fluzone High-Dose vaccine. Standard-dose refers to Fluzone vaccine.
Facility-reported data. 3 facilities in HD group and 1 facility in SD group did not report data. Some facilities reported zero for their vaccination data.
There are 4 NHs that did not report staff census (1 SD; 3 HD) and are not included in the average reported here. Additionally, 2 SD NHs reported zero for the number of staff vaccinated; these facilities are not included in the facility vaccination averages.
For clinical outcomes, compared to the SD vaccine group, the calculated unadjusted and adjusted all-cause hospitalization and mortality risks were lower in the HD vaccine group (Table 4). The all-cause hospitalization unadjusted rates were lower in the HD vaccine group (Table 4; 13.5% vs. 20.2%, and Fig. 2). The adjusted relative risk for any hospitalization during influenza season was 0.68 (CI: 0.54, 0.86, p = 0.001) and for mortality was 0.83 (CI: 0.68, 1.03, p = 0.087) for residents receiving HD compared to SD vaccine. (Fig. 3). Functional decline outcomes were not different, based on average ADL scores before and after, or by threshold worsening of a clinically meaningful 4 points or more (13.4% HD, 13.8% SD, p = 0.74) (ARR 0.99, CI: 0.77, 1.28, p = 0.964).
Table 4.
Results of Robust Poisson Regression Analysis Comparing High-Dose and Standard-Dose Arms.
| HD | SD | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|
| Outcome |
N (%) |
N (%) |
Relative Risk (LCL – UCL) |
p-value |
Relative Risk (LCL – UCL) |
p-value |
| Ever Hospitalized | 197 (13.5%) | 301 (20.1%) | 0.669 (0.512-0.873) | 0.003 | 0.680 (0.537-0.862) | 0.001 |
| Death in NH | 249 (17.1%) | 274 (18.3%) | 0.945 (0.756-1.171) | 0.586 | 0.834 (0.678-1.027) | 0.087 |
| ADL: 4 point decline in score | 196 (13.4%) | 206 (13.8%) | 0.945 (0.731-1.221) | 0.667 | 0.994 (0.774-1.278) | 0.965 |
Abbreviation: NH = nursing home, LCL = lower control limit, UCL = upper control limit.
Adjusted for prior year hospitalization rate, age of resident, mean age of residents in home, individual ADL score, mean ADL score in home, Cognitive Function Score (CFS), Mean CFS in home, history of heart failure risk-group, prevalence of heart failure risk-group in home.
Figure 2.
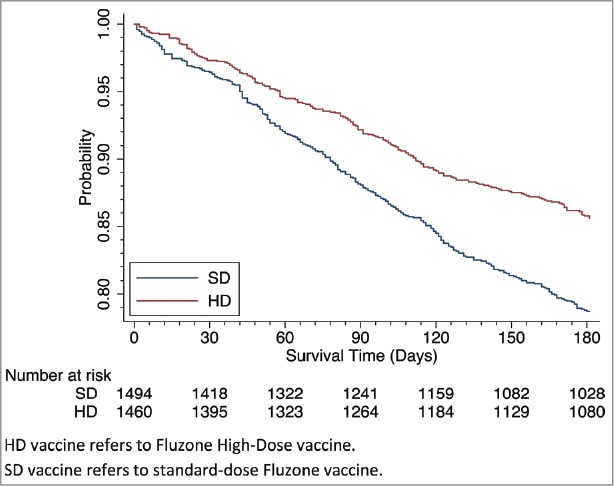
Survival Analysis: Time to First Hospitalization by HD vs. SD. HD vaccine refers to Fluzone High-Dose vaccine. SD vaccine refers to standard-dose Fluzone vaccine.
Figure 3.
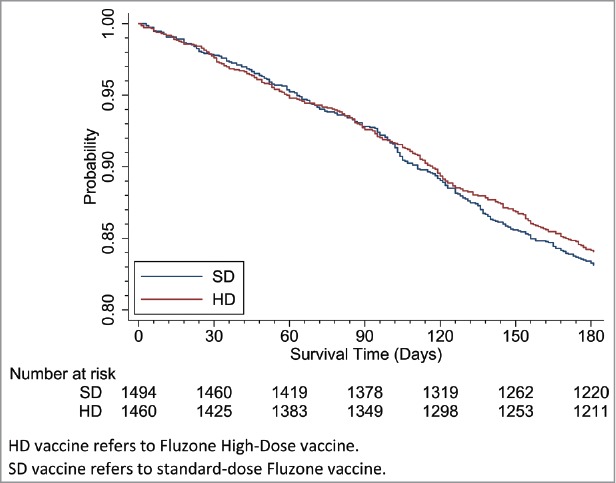
Survival Analysis: Time to Death by HD vs. SD. HD vaccine refers to Fluzone High-Dose vaccine. SD vaccine refers to standard-dose Fluzone vaccine.
Discussion
Long-term care facilities provide significant challenges for researchers, most notably related to recruiting a large, frail, vulnerable population with high clinical event rates, such as hospitalization. Trials in NHs are often deemed impractical, limiting the size and extent of scientific studies conducted. Differences between facilities are a challenge, related to either facility policies, care practices, staffing, or geographic distribution. A cluster-randomized trial of a sufficiently large number of NHs helps overcome many constraints, but does not solve facility-level challenges around finding and recruiting facility leadership, following and validating facility data, or engaging staff to improve study integrity and patient care. We showed that many of these concerns can be adequately addressed.
Our study's main question involved comparing two standards of care deemed equivalent by authoritative bodies, including CDC's ACIP, a factor essential to effective implementation.21 We recruited facilities to a care standard they reasonably could adopt, without changing underlying practices of how and when vaccine is administered, an individual's decision to accept or reject vaccine or demand an alternative, or facilities’ underlying standing order policies for implied consent or individual consent to the care standard. We incentivized facility-level participation by providing free vaccine to staff and residents. In the construct of this feasibility study, free vaccine did not offer much of a financial incentive because of our late recruitment start. Most vaccine orders to supply staff and residents with vaccine are submitted in the spring, and our enrollment in the fall was hampered because not only had facilities ordered vaccine, the majority had begun administering vaccine before enrollment could be completed. However, we believe vaccine for NH staff usually is costed to and written off by operations, free vaccine for staff can incentivize participation at the facility level.
Randomization by residents rather than NHs can control for NH-level differences but has pragmatic limitations due to recruitment cost inherent in the NH setting and cognitive capacity of individual residents. Pragmatically randomizing many NHs rather than residents allows for efficient enrollment (e.g., each NH houses many evaluable residents) and comparisons of standards of care at the NH level. Other differences can be assessed and controlled for statistically using NH-level characteristics data (e.g., OSCAR).
Nursing homes are the most regulated healthcare environment in the U.S., and NH staff complain of inadequate staffing levels, pay, and resulting in high staff turnover. Recruiting NHs requires developing a relationship with NH leadership, providing a value-based argument of how participation improves care and staff resources, informs staff around best practices and regulations, and positions research as an opportunity to demonstrate leadership in their community for advancing care. Staff turnover includes not only front-line staff, but also leadership who agree to participate, share data, and keep logs of medications dispensed. For these reasons, researchers need contingency plans to track and engage facilities to ensure continued facility participation and improved data capture.
The primary challenge in this study was recruiting facilities ahead of their routine vaccine ordering and administration activities. Facilities had already ordered their vaccine at the time of recruitment, putting them at potential financial risk for vaccine already purchased. Furthermore, facilities often begin vaccine administration as soon as it is available. Unfortunately, if vaccinations began in August or September, these facilities were automatically excluded from participation. Our September start limited the facility pool from which to recruit, however we were still able to enroll NHs. Our experience from this feasibility study confirms that stakeholder collaboration is important for efficient identification of facilities eligible for participation, offering a central point of communication, and providing contracting and follow-up support. Electronic communication via email was critical to study conduct.
There were additional challenges to consider when working with NHs and their staff. We used a web-based data collection and information distribution system, so limited computer access, equipment, and skill should be kept in mind. As mentioned previously, high staff turnover is always an issue, which we experienced, and led to educating new individuals and getting buy-in on the study's conduct. Similarly, frequent point-of-contact changes presented ongoing communication challenges. Nursing home staff are very busy, and there is resistance to adding any task, no matter how small or simple it seems. We designed the study activities at the site level to be as consistent with the usual practice as possible and made our study staff available as much as needed for “hand-holding.” Getting buy-in from NH leadership and from those doing the actual tasks is critical.
Staff vaccination rates have been reported to influence resident influenza, hospitalization, and mortality in several nursing home observational studies,22–24 and two RCTs in hospitalized25 and older adult home26 settings in the U.S. and UK. In our feasibility study, vaccine uptake appears to be greater among staff of facilities randomized to HD over SD (Table 3). For our study, setting aside the limitations of self-reported data as we have for staff vaccination rates, we also would not expect any facility, perhaps especially facilities participating in an influenza vaccine study, to have no staff vaccinated. In any case, the relative impact of staff vaccination on hospitalization is difficult to ascertain for our sample, but may have favored reduced hospitalization for the nursing home residents in the HD group.
Also, the important morbidities that increase risk for hospitalization (e.g. heart failure, COPD and ESRD; Table 2) appear to be greater among the group randomized to HD vaccine. Meanwhile, the baseline higher hospitalization rate for SD over HD may have favored SD vaccine. Our statistical adjustment for these confounders still indicates significantly and clinically important lower hospitalization risk for those residents in those facilities assigned to HD over those assigned to SD vaccine.
Our clinical finding favoring HD influenza vaccine over SD vaccine for reduced hospitalization risk or the less common outcome of death shows the value of the pragmatic RCT approach even when based solely on MDS data for both NH and resident-level metrics for adequately powering these studies. That study reports reductions in hospitalizations in HD compared to SD for the NH setting, consistent with the findings of this feasibility study.27 The relative benefit of HD over SD observed in this pilot was much greater than that observed in our large-scale RCT. Several possible explanations for this difference including the virulence of the circulating strain differences between the two study seasons, the quality of the vaccine match to the circulating strain and the sampling error related to sample size. We suspect that most of the observed difference between the hospitalization rates and mortality reported for this feasibility study and our large RCT relates to differences in the predominantly circulating influenza strain, A/H3N2 for the 2012–2013 season vs. A/H1N1 for the following 2013–2104 season, as a driver for hospitalization. Hospitalization rates nationally were several-fold higher during the 2012–2013 season, the year of this feasibility study.28 Contrasting these two seasons may offer a glimpse of a potential range for effectiveness in the relative risk reduction for hospitalization across influenza seasons of varying severity, if not also due to differences in vaccine uptake.
In any case, the feasibility study confirmed the potential for the intervention to affect hospitalization outcomes in the follow-up definitive RCT. Its successful conduct resulted in the funding and execution of that larger study.
Limitations
In this study designed to demonstrate feasibility, we had a small sample size. Randomized NHs were primarily in two states (Colorado and New Jersey), due to late recruitment. The NHs are similar in most respects, including staffing, resident age, Medicaid insurance, and for-profit status, but the small sample size does not adequately allow us to determine, for example, if differences in being a member of a large NH chain may have affected our outcome. Although not significantly so, the subjects and staff within the HD facilities had greater vaccine uptake, potentially reducing influenza exposure risk and subsequent hospitalization of the HD population through herd immunity. We did not determine how their staff provide care, how they conduct influenza surveillance, presence or effectiveness of policies to limit work for staff presenting with respiratory illness during influenza season, or strategies to detect and limit ill visitors from the community from mingling or infecting the residents. The small sample size limits generalizability and the ability to control for differences between facilities that might affect resident outcomes. However, facility randomization can address these concerns, and such concerns would be more fully mitigated with a larger sample size.
The results of our feasibility study provide a framework to determine if HD vaccine performs better in a frail older population in reducing the rate of hospitalization during the influenza season. Our study does not consider insurance claims, and therefore cannot weigh in on cost-effectiveness. A large-scale pragmatic cluster-randomized controlled trial can confirm these preliminary findings for different influenza strains, and allow for more precise extrapolation of these results to a chronically ill and frail population nationally. Such a study is feasible, and was conducted for the 2013–14 influenza season.
Conclusion
Nursing homes provide an important, if challenging, and potentially rewarding setting for conducting research. NH cluster-randomized controlled trials are feasible and offer an efficient way to gather data comparing non-preferentially recommended care standards, such as two different influenza vaccines. Researchers can leverage NH administrative datasets from MDS on quality and insurance claims for care delivery. A large-scale study can confirm if HD influenza vaccine reduces hospitalization better than SD vaccine for A/H3N2 and other influenza strains, and could efficiently be conducted as a pragmatic cluster-randomized controlled trial.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Joanne Stewart, Insight Therapeutics, LLC, for review of the final manuscript.
Funding
Funded by an investigator-initiated (SG) grant from Sanofi Pasteur, Swiftwater, PA.
| *Author 1 Stefan Gravenstein |
Author 2 H. Edward Davidson |
Author 3 Lisa F. Han |
Author 4 Jessica Ogarek |
Author 5 Roshani Dahal |
Author 6 Pedro L. Gozalo |
Author 7 Monica Taljaard |
Author 8 Vincent Mor |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
|
| Employment or Affiliation | X | X | X | X | X | X | X | X | |||||||||
| Grants/ Funds | X | X | X | X | X | X | X | X | |||||||||
| Honoraria | X | X | X | X | X | X | X | X | |||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | |||||||||
| Consultant | X | X | X | X | X | X | X | X | |||||||||
| Stocks | X | X | X | X | X | X | X | X | |||||||||
| Royalties | X | X | X | X | X | X | X | X | |||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | |||||||||
| Board Member | X | X | X | X | X | X | X | X | |||||||||
| Patents | X | X | X | X | X | X | X | X | |||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | |||||||||
Authors can be listed by abbreviations of their names.
For “yes” x mark(s): give brief explanation below:
HED – research funding from Sanofi Pasteur; LFH – research funding from Sanofi Pasteur
Author contributions
Study concept and design: S. Gravenstein, V. Mor
Acquisition of data: L. F. Han, H. E. Davidson, J. A. Ogarek, P. L. Gozalo
Analysis and interpretation of data: All authors.
Drafting of the manuscript: R. Dahal, S. Gravenstein
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: R. Dahal, J. A. Ogarek, P. L. Gozalo, M. Taljaard, V. Mor, S. Gravenstein
Obtaining funding: H. E. Davidson, S. Gravenstein, V. Mor
Study supervision: H. E. Davidson, L. Han, S. Gravenstein, V. Mor
Sponsor's role
The study was supported by an investigator-initiated grant from Sanofi Pasteur to Insight Therapeutics, LLC. Sanofi Pasteur had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study.
Data results presented as a Late Breaker Oral Abstract Session, Saturday, October 10, 2015, at the Infectious Disease Society of America's ID Week 2015, San Diego, CA.
References
- 1.Nicholson KG. Impact of influenza and respiratory syncytial virus on mortality in England and Wales from January 1975 to December 1990. Epidemiol Infect. 1996;116(1):51–63. doi: 10.1017/S0950268800058957. PMID:8626004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. PMID:12517228. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40. doi: 10.1001/jama.292.11.1333. PMID:15367555. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59(33):1057–62. PMID:20798667. [PubMed] [Google Scholar]
- 5.Dao CN, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, Arnold KE, Farley M, Ryan P, Lynfield R, et al. . Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202(6):881–8. doi: 10.1086/655904. PMID:20677944. [DOI] [PubMed] [Google Scholar]
- 6.Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36(7):870–6. doi: 10.1086/368197. PMID:12652388. [DOI] [PubMed] [Google Scholar]
- 7.Kostova D, Reed C, Finelli L, Cheng PY, Gargiullo PM, Shay DK, Singleton JA, Meltzer MI, Lu PJ, Bresee JS. Influenza illness and hospitalizations averted by influenza vaccination in the United States, 2005–2011. PloS One. 2013;8(6):e66312. doi: 10.1371/journal.pone.0066312. PMID:23840439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravenstein S. High dose influenza vaccination and morbidity and mortality in U.S. Nursing Homes – A Pilot Evaluation. In: ClinicalTrials.gov [Internet]. Bethesda: (MD: ): National Library of Medicine (US) 20160824 Available from https://clinicaltrials.gov/ct2/show/NCT01720277?term = gravenstein&rank = 3: NCT01720277. [Google Scholar]
- 9.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32(14):1730–5. doi: 10.1093/eurheartj/ehr004. PMID:21289042. [DOI] [PubMed] [Google Scholar]
- 10.Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015;5(5):CD005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nace DA, Lin CJ, Ross TM, Saracco S, Churilla RM, Zimmerman RK. Randomized, controlled trial of high-dose influenza vaccine among frail residents of long-term care facilities. J Infect Dis. 2015;211(12):1915–24. doi: 10.1093/infdis/jiu622. PMID:25525051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. doi: 10.1016/j.vaccine.2005.08.105. PMID:16213065. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010;(7):CD001269. doi: 10.1002/14651858.CD001269.pub4. PMID:20166072. [DOI] [PubMed] [Google Scholar]
- 14.Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, Couch RB. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166(10):1121–7. doi: 10.1001/archinte.166.10.1121. PMID:16717175. [DOI] [PubMed] [Google Scholar]
- 15.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–45. doi: 10.1056/NEJMoa1315727. PMID:25119609. [DOI] [PubMed] [Google Scholar]
- 16.Appiah GD, Blanton L, D'Mello T, Kniss K, Smith S, Mustaquim D, Steffens C, Dhara R, Cohen J, Chaves SS. Influenza activity – United States, 2014–15 season and composition of the 2015–16 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2015;64(2):583–90. PMID:26042650. [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services Long term care Minimum Data Set (MDS). Baltimore: (MD: ): Centers for Medicare & Medicaid Services; 2012. [Google Scholar]
- 18.Thomas KS, Wysocki A, Intrator O, Mor V. Finding Gertrude: the resident's voice in Minimum Data Set 3.0. J Am Med Dir Assoc. 2014;15:802–6. doi: 10.1016/j.jamda.2014.01.012. PMID:24630068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gozalo PL, Pop-Vicas A, Feng Z, Gravenstein S, Mor V. Effect of influenza on functional decline. J Am Geriatr Soc. 2012;60:1260–7. doi: 10.1111/j.1532-5415.2012.04048.x. PMID:22724499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou GY, Donner A. Extension of the modified poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–70. doi: 10.1177/0962280211427759. PMID:22072596. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Fluzone high-dose seasonal influenza vaccine. Available at www.cdc.gov/flu/protect/vaccine/qa_fluzone.htm Accessed September 28, 2012.
- 22.Sugarman LR, Hales C, Setodji CM, Bardenheier B, Lynn J. The influence of staff and resident immunization rates on influenza-like illness outbreaks in nursing homes. J Am Med Dire Assoc. 2006;7:562–67. doi: 10.1016/j.jamda.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Potter J, Stott DJ, Roberts MA, Elder AG, O'Donnell B, Knight PV, Carman WF. Influenza vaccination of health care workers in long-term-care hospitals reduces the mortality of elderly patients. J Infect Dis. 1997;175:1–6. doi: 10.1093/infdis/175.1.1. PMID:8985189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre M, Meret T, Rothan-Tondeur M, Belmin J, Lejonc JL, Luquel L, Piette F, Salom M, Verny M, Vetel JM, et al. . Effect of influenza vaccination of nursing home staff on mortality of residents; a cluster-randomized trial. J Am Geriatr Soc. 2009;57:1580–6. doi: 10.1111/j.1532-5415.2009.02402.x. PMID:19682118. [DOI] [PubMed] [Google Scholar]
- 25.Carmen WF, Elder AG, Wallace LA, McAulay K, Walker A, Murray GD, Stott DJ.. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: a randomized controlled trial. Lancet. 2000;355(9198):93–7. doi: 10.1016/S0140-6736(99)05190-9. PMID:10675165. [DOI] [PubMed] [Google Scholar]
- 26.Hayward AC, Harling R, Wetten S, Johnson AM, Munro S, Smedley J, Murad S, Watson JM. Effectiveness of an influenza vaccine programme for care home staff to prevent death, morbidity, and health service use among residents: cluster randomized controlled trial. BMJ. 2006;333(7581):1241. doi: 10.1136/bmj.39010.581354.55. PMID:17142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, Mor V. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5(9):738–46. doi: 10.1016/S2213-2600(17)30235-7. PMID:28736045. [DOI] [PubMed] [Google Scholar]
- 28.Laboratory-confirmed influenza hospitalizations Flu-View, centers for disease control and prevention. U.S. Department of Health and Human Services. https://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed September 28, 2017. [Google Scholar]


