ABSTRACT
DNA damage kills dry-heated spores of Bacillus subtilis, but dry-heat-treatment effects on spore germination and outgrowth have not been studied. This is important, since if dry-heat-killed spores germinate and undergo outgrowth, toxic proteins could be synthesized. Here, Raman spectroscopy and differential interference contrast microscopy were used to study germination and outgrowth of individual dry-heat-treated B. subtilis and Bacillus megaterium spores. The major findings in this work were as follows: (i) spores dry-heat-treated at 140°C for 20 min lost nearly all viability but retained their Ca2+-dipicolinic acid (CaDPA) depot; (ii) in most cases, dry-heat treatment increased the average times and variability of all major germination events in B. subtilis spore germination with nutrient germinants or CaDPA, and in one nutrient germination event with B. megaterium spores; (iii) B. subtilis spore germination with dodecylamine, which activates the spore CaDPA release channel, was unaffected by dry-heat treatment; (iv) these results indicate that dry-heat treatment likely damages spore proteins important in nutrient germinant recognition and cortex peptidoglycan hydrolysis, but not CaDPA release itself; and (v) analysis of single spores incubated on nutrient-rich agar showed that while dry-heat-treated spores that are dead can complete germination, they cannot proceed into outgrowth and thus not to vegetative growth. The results of this study provide new information on the effects of dry heat on bacterial spores and indicate that dry-heat sterilization regimens should produce spores that cannot outgrow and thus cannot synthesize potentially dangerous proteins.
IMPORTANCE Much research has shown that high-temperature dry heat is a promising means for the inactivation of spores on medical devices and spacecraft decontamination. Dry heat is known to kill Bacillus subtilis spores by DNA damage. However, knowledge about the effects of dry-heat treatment on spore germination and outgrowth is limited, especially at the single spore level. In the current work, Raman spectroscopy and differential interference contrast microscopy were used to analyze CaDPA levels in and kinetics of nutrient- and non-nutrient germination of multiple individual dry-heat-treated B. subtilis and Bacillus megaterium spores that were largely dead. The outgrowth and subsequent cell division of these germinated but dead dry-heat-treated spores were also examined. The knowledge obtained in this study will help understand the effects of dry heat on spores both on Earth and in space, and indicates that dry heat can be safely used for sterilization purposes.
KEYWORDS: Bacillus, spores, dry-heat treatment, germination, outgrowth, vegetative growth
INTRODUCTION
Spores are resting states of some microorganisms and are formed under conditions unfavorable for the survival of growing cells. In bacteria of Bacillus species, spores formed in sporulation are metabolically dormant and extremely resistant to heat, desiccation, radiation, and many toxic chemicals (1). Spores of a number of these species are common in foodstuffs, and the growing organisms of some of these species can cause food spoilage or foodborne disease (1–3). Consequently, there has been much work directed toward understanding the mechanism(s) of extreme resistance of spores, in particular, wet-heat resistance, because wet-heat treatment is the most commonly used treatment to destroy spores in foodstuffs and medical devices. Since many studies have been done on wet-heat sterilization, the mechanism of spore resistance to wet heat and factors responsible for spore wet-heat resistance are relatively well understood (1, 3, 4).
Dry heat, as the name indicates, utilizes hot air that either is free from water vapor or has very little of it, and thus moisture plays a minimal or no role in dry-heat sterilization. Notably, dry-heat sterilization is one of the earliest methods for sterilization that was developed. At present, to avoid microbial contamination during space exploration, one of the provisions set forth by the National Aeronautics and Space Administration (NASA) for unmanned planetary capsules designed to land on the Martian surface is that they be dry-heat-sterilized in an inert gas environment before launch (NASA).
Recently, Bacillus subtilis spores were used in the European Space Agency's EXPOSE-R mission (5, 6), in which spores were exposed to selected parameters of outer space, including space vacuum. Temperature fluctuations during a long journey in space were measured, thereby mimicking the interplanetary transfer of life via impact-ejected rocks, and spore survival was measured. In addition to the effects of microgravity and space vacuum, the temperature in outer space may change from very low (−120°C) to very high (+120°C), depending on the orientation to the sun and albedo of the spacecraft (5). Thus, during periods of high space temperature, spores are exposed to dry heat. Notably, previous work has shown that at least B. subtilis spores are killed by dry heat, largely if not completely by damage to spore DNA (7–12). The evidence leading to this conclusion includes the (i) high levels of mutations in survivors of spores treated with dry heat; (ii) much more rapid dry-heat killing of spores of a recA mutant lacking a major DNA repair protein compared to the rate of dry-heat killing of wild-type spores; and (iii) identification of DNA damage, specifically single strand breaks, in dry-heat-treated spores, although these DNA breaks may not be the primary DNA lesion, but rather, the primary DNA lesions were rapidly converted to strand breaks either in vivo or in vitro.
Given that dry heat kills B. subtilis spores by DNA damage, and that DNA damage alone does not affect spore germination (1), it would be expected that dry-heat-treated spores would germinate even if they are dead as assessed by colony formation assays, especially if dry-heat-killed spores retain the spores' huge depot of Ca2+-dipicolinic acid (CaDPA). Indeed, wet-heat-killed spores of a number of Bacillus species that retain CaDPA do germinate (4, 13, 14). However, the germinated spores from wet-heat-killed dormant spores do not progress into spore outgrowth. The obvious questions about dry-heat-killed B. subtilis spores are as follows. (i) Do these spores retain CaDPA? (ii) If they do retain CaDPA, do these nominally dead spores germinate as assessed by release of CaDPA and full hydration of the spore core? (iii) Do any germinated dry-heat-killed spores progress into outgrowth or even through one or more cell divisions? Finally, (iv) does the germination, outgrowth, and vegetative growth of dry-heat-killed spores decrease as dry-heat treatment is extended? The last two questions are particularly important, since if dry-heat-killed spores can germinate and go through outgrowth and even a few cell divisions, it is possible that spores of pathogens could synthesize low levels of protein toxins prior to eventual cessation of further growth due to their DNA damage. Thus, examining not only the germination of dry-heat-treated spores is important, but also examining what happens to these spores in a nutrient-rich environment.
Consequently, in the current work, the germination kinetics of hundreds of individual dry-heat-killed spores that retain CaDPA, as well as the fate of these germinated spores in a nutrient-rich environment, was investigated using Raman spectroscopy and differential interference contrast (DIC) microscopy. The results of this study have significant implications for the use of dry heat as a routine sterilization process.
RESULTS
Spore viability and CaDPA content during dry-heat treatment.
In our experiments, ∼99% of wild-type B. subtilis spores were killed by dry-heat treatment at 140°C for 15 min, with greater killing at longer times (Fig. 1A). However, most of the killed spores retained CaDPA, even after dry-heat treatment at 140°C for 60 min (Fig. 1A). Similar results were obtained with B. megaterium spores (data not shown).
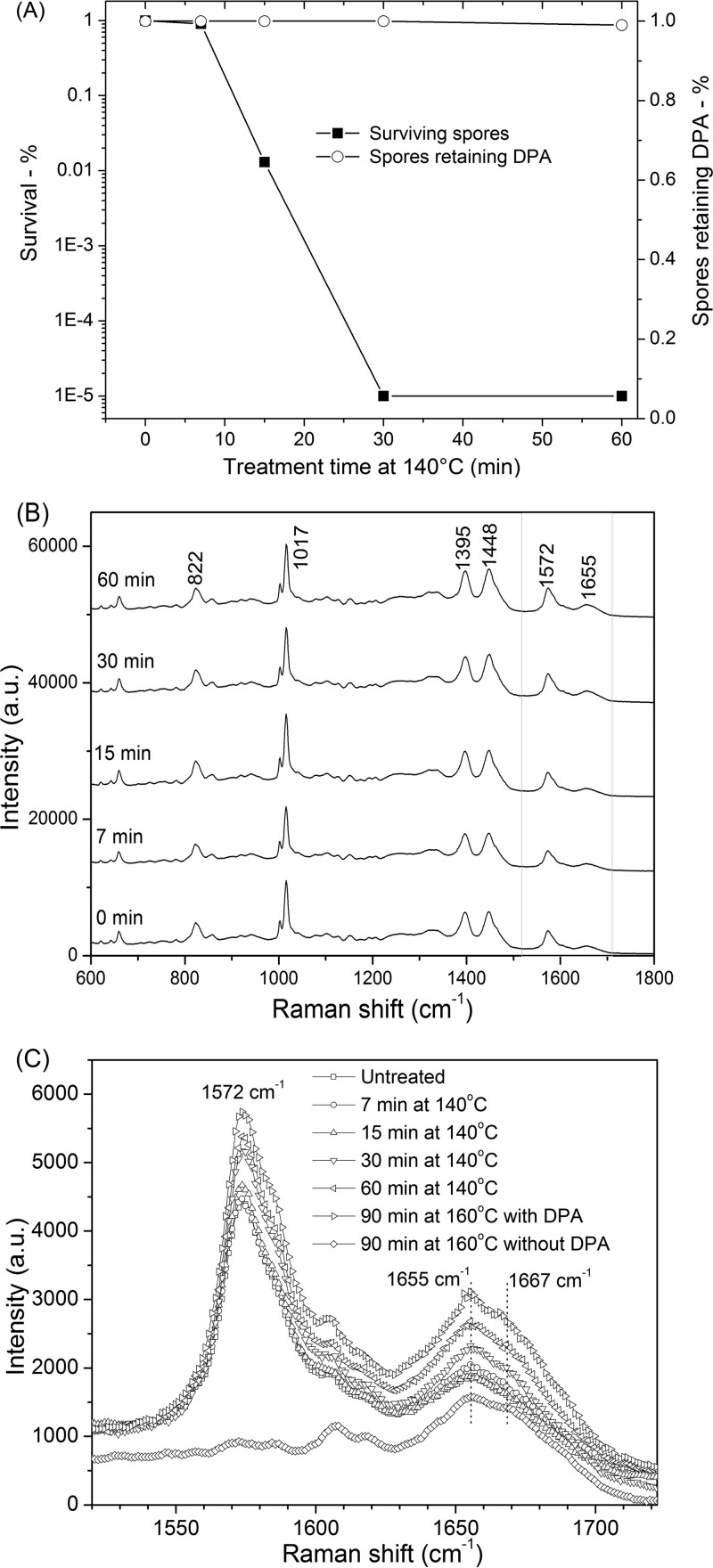
FIG 1.
Effects of dry-heat treatment on B. subtilis spores. (A) Percentages of spores that survived and retained CaDPA as a function of treatment time at 140°C were determined as described in Materials and Methods. (B) Raman spectra of single spores of B. subtilis that retained CaDPA after various dry-heat treatment times. (C) Raman spectra of single spores of B. subtilis in the protein amide band regions. Band intensities are given in arbitrary units (AU).
In the Raman spectrum of an individual unheated wild-type B. subtilis spore (Fig. 1B), Raman bands at 662, 825, 1,017, 1,397, and 1,572 cm−1 are due to CaDPA (15, 16), the band at 1,004 cm−1 is due to phenylalanine in spore proteins, and the bands at 1,655 and 1,667 cm−1 are associated with α-helical and denatured protein, respectively (13, 15, 16). Raman spectra of dry-heat-treated spores that retained CaDPA are shown in Fig. 1B, curves b to f, and these spectra were obtained by averaging the Raman spectra of 30 individual spores which retained CaDPA. In order to analyze the precise state of the core region of dry-heat-treated spores where CaDPA is located, the average spectra in the 1,520 to 1,720 cm−1 region for spores treated at 140°C or 160°C for various times that retained CaDPA were also determined (Fig. 1C). Analyses of these average spectra showed that longer dry-heat treatment increased the Raman signal intensity (I) of peaks at 1,572, 1,655, and 1,667 cm−1. In addition, the I1572/I1017 ratio increased and the I1667/I1655 ratio decreased with longer dry-heat treatment times (Fig. 1C and Table 1), indicating that the spore core environment around CaDPA and protein changes in dry-heat-treated spores, perhaps because of removal of small amounts of bound water (17–19). Dry-heat treatment at 160°C for 90 min also led to the appearance of a more distinctive Raman peak at 1,667 cm−1 in spores that retained CaDPA, indicating the presence of more denatured protein in these spores. This was also seen with spores treated at 160°C for 90 min that had lost CaDPA (Fig. 1C), but the I1667/I1655 ratio (0.990 ± 0.011) of peak intensities for these spores (Table 1) was lower than with wet-heat-treated spores that had lost CaDPA (1.08 ± 0.011) (13, 14, 18, 19). This indicates that there is less protein denaturation in dry-heat-treated spores that have lost CaDPA than in comparable wet-heat-treated spores.
TABLE 1.
Ratios of intensities of the Raman peaks at 1,572, 1,655, 1,667, and 1,017 cm−1 in multiple individual unheated and dry-heat-treated B. subtilis sporesa
| Raman peaks | Intensity ratio (AUb) for treatment parameters: |
|||||
|---|---|---|---|---|---|---|
| Untreated | 140°C, 7 min | 140°C, 15 min | 140°C, 30 min | 140°C, 60 min | 160°C, 90 min | |
| I1572/I1017 | 0.400 ± 0.015 | 0.423 ± 0.014 | 0.430 ± 0.015 | 0.476 ± 0.015 | 0.513 ± 0.017 | 0.575 ± 0.054 |
| I1667/I1655 | 0.883 ± 0.009 | 0.876 ± 0.008 | 0.868 ± 0.010 | 0.866 ± 0.011 | 0.856 ± 0.012 | 0.886 ± 0.007 |
| (0.990 ± 0.011)c | ||||||
I1572, I1655, I1667, and I1017 values were determined by LTRS for 30 individual untreated and dry-heat-treated spores and averaged. Standard deviations are indicated.
AU, arbitrary units.
This value of the I1667/I1655 ratio is from spores that had lost CaDPA.
Kinetics of l-valine germination of dry-heat-treated B. subtilis spores.
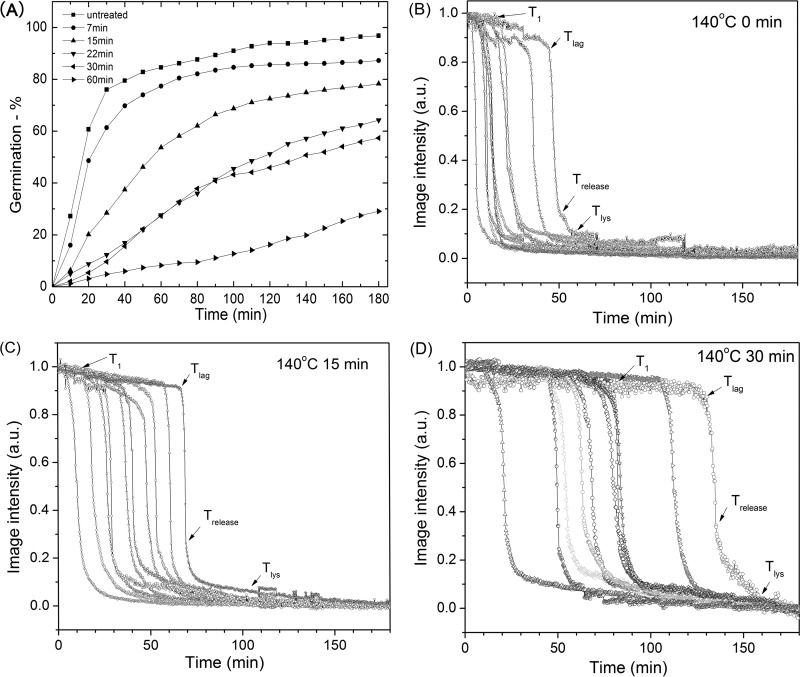
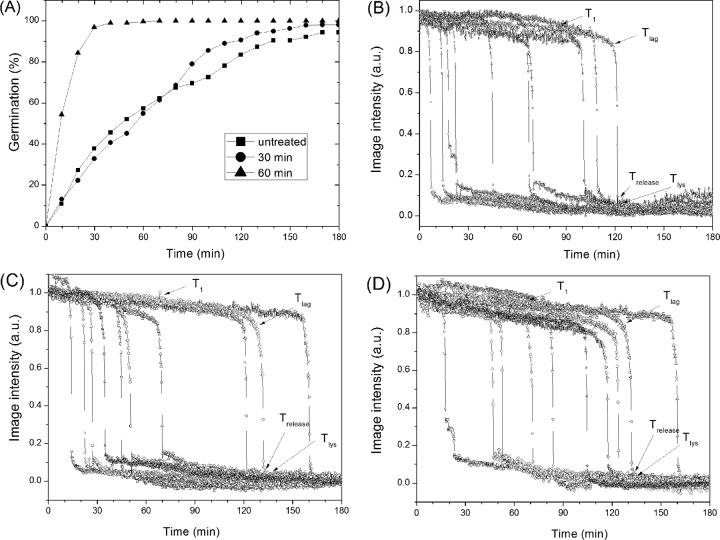
Analysis of the l-valine germination via the GerA germinant receptor (GR) of multiple individual dry-heat-treated B. subtilis spores that retained CaDPA showed that germination decreased as heat treatment time increased (Fig. 2A). While this analysis of spore populations provided some information on the germination behavior of dry-heat-treated spores, these germination curves were only the average behavior of many different spores, whose individual behavior could differ significantly. Therefore, we turned to examination of the l-valine germination of individual untreated and dry-heat-treated B. subtilis spores (Fig. 2B and C). Individual untreated spores germinated rapidly, most by ∼30 min (Fig. 2A), and the rapid CaDPA release for individual spores took place almost in parallel (Fig. 2B and C and data not shown). However, the l-valine germination kinetics of dry-heat-treated spores differed significantly from those of untreated spores (Fig. 2B and C and Table 2). In particular, the dry-heat-treated spores germinated more slowly, and T1, Tlag, ΔTleakage, and ΔTlys values all increased as dry-heat treatment time increased. This was especially notable with spores that were dry-heat treated at 140°C for 60 min, as 70% of these spores did not germinate in 3 h. Even for the 30% of these spores that germinated in 3 h, T1, Tlag, ΔTleakage, and ΔTlys values increased 6-, 5-, 3-, and 2-fold, respectively. However, ΔTrelease times increased only slightly. The kinetic parameters of germinating dry-heat-treated spores were also more heterogeneous than for untreated spores, as dry-heat-treated spores showed increased standard deviations for the values of T1, Tlag, ΔTleakage, and ΔTlys (Table 1).
FIG 2.
l-Valine germination of B. subtilis spores after dry-heat treatment. (A) The percentage of the germination as a function of incubation time of untreated and dry-heat-treated spores with l-valine was determined as described in Materials and Methods. Approximately 300 spores were monitored to obtain the data shown. (B, C, D) Kinetics of the l-valine germination of individual untreated (B) and dry-heat-treated (C and D) B. subtilis spores. Spores were dry-heat treated and germinated with l-valine, and the germination of 10 individual spores was followed, all as described in Materials and Methods. Image intensities are given in arbitrary units (AU).
TABLE 2.
T1, Tlag, ΔTleakage, ΔTrelease, and ΔTlys for germination of B. subtilis spores with various germinants and with or without dry-heat treatmenta
| Germinant | Treatment condition(s) | No. of spores examined (% spore germination) | T1 (mean ± SD) (min) | Tlag (mean ± SD) (min) | ΔTleakage (mean ± SD) (min) | ΔTrelease (mean ± SD) (min) | ΔTlys (mean ± SD) (min) |
|---|---|---|---|---|---|---|---|
| l-Valine | Control (untreated) | 446 (98.0) | 10.3 ± 9.5 | 16.4 ± 11.9 | 6.1 ± 5.6 | 3.2 ± 1.3 | 15.3 ± 7.2 |
| 140°C, 7 min | 800 (87.3) | 15.2 ± 10.2 | 25.8 ± 27.5 | 10.6 ± 8.5 | 3.3 ± 0.9 | 15.8 ± 8.3 | |
| 140°C, 15 min | 793 (78.3) | 49.2 ± 31.7 | 45.5 ± 32.6 | 13.7 ± 9.2 | 3.2 ± 0.8 | 27.2 ± 10.1 | |
| 140°C, 22 min | 699 (64.2) | 49.8 ± 42.0 | 64.3 ± 41.7 | 15.3 ± 10.7 | 3.5 ± 1.5 | 36.9 ± 13.6 | |
| 140°C, 30 min | 333 (57.3) | 71.2 ± 41.4 | 75.4 ± 42.8 | 20.7 ± 15.7 | 4.1 ± 0.7 | 43.5 ± 16.7 | |
| 140°C, 60 min | 801 (29.1) | 74.9 ± 52.3 | 98.3 ± 52.4 | 23.4 ± 17.8 | 5.1 ± 2.1 | 52.0 ± 35.0 | |
| AGFK | Control (untreated) | 1,082 (98.0) | 11.2 ± 10.3 | 17.0 ± 20.0 | 5.8 ± 5.2 | 2.4 ± 0.5 | 7.9 ± 4.0 |
| 140°C, 30 min | 626 (92.0) | 13.2 ± 27.6 | 25.5 ± 28.3 | 12.3 ± 9.4 | 2.6 ± 0.6 | 21.3 ± 6.1 | |
| 140°C, 60 min | 1,020 (86.7) | 39.6 ± 43.4 | 52.9 ± 41.9 | 17.3 ± 15.2 | 2.6 ± 0.7 | 25.6 ± 11.6 | |
| CaDPA | Control (untreated) | 970 (94.8) | 35.4 ± 28.4 | 48.7 ± 19.6 | 14.3 ± 9.6 | 5.7 ± 2.2 | 32.1 ± 10.6 |
| 140°C, 30 min | 395 (91.4) | 36.2 ± 36.1 | 55.5 ± 26.9 | 19.3 ± 16.7 | 6.5 ± 2.7 | 42.3 ± 19.2 | |
| 140°C, 60 min | 537 (25.1) | 40.8 ± 35.3 | 67.2 ± 38.5 | 26.4 ± 17.8 | 9.5 ± 7.6 | 54.2 ± 23.6 | |
| Dodecylamine | Control (untreated) | 230 (94.3) | 37.1 ± 44.8 | 62.3 ± 46.7 | 25.2 ± 18.2 | 2.8 ± 1.0 | 27.0 ± 15.9 |
| 140°C, 30 min | 319 (98.1) | 38.0 ± 41.3 | 59.0 ± 41.6 | 21.0 ± 17.6 | 4.2 ± 2.5 | 32.3 ± 19.9 | |
| 140°C, 60 min | 287 (100.0) | 31.3 ± 46.1 | 60.4 ± 45.6 | 29.1 ± 23.8 | 3.0 ± 1.0 | 42.9 ± 20.9 |
Spores with or without various dry-heat treatments were germinated for 3 h with various germinants, and kinetic parameters of spore germination were determined as described in Materials and Methods. Data from approximately 100 individual spores that contained CaDPA and germinated were used to calculate the kinetic parameters of germination.
AGFK germination of dry-heat-treated B. subtilis spores.
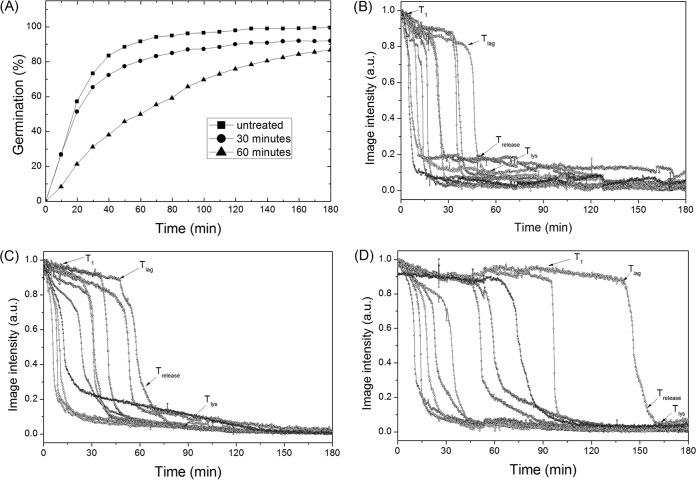
In addition to l-valine, B. subtilis spores are also germinated with the AGFK mixture of germinants (l-asparagine, d-glucose, d-fructose, and K+) that requires the participation of two different GRs, GerB and GerK (20). As found with l-valine germination, dry-heat treatment also significantly slowed wild-type B. subtilis spore germination with AGFK, although the effects were less than with l-valine germination (compare Fig. 2 and 3; Table 2). Comparing the average AGFK germination parameters of multiple untreated and dry-heat-treated B. subtilis spores (Table 2) revealed some of the general findings seen with l-valine germination, such as increased T1, Tlag, ΔTleakage, and ΔTlys times with increasing dry-heat treatment, but with no significant change in ΔTrelease times. The standard deviations of the values of Tlag, ΔTrelease, and ΔTlys indicating heterogeneity in spore germination also all increased. However, as indicated above, the effects of dry-heat treatment on AGFK germination were much smaller than on l-valine germination, as ∼ 87% of spores dry-heat treated at 140°C for 60 min germinated with AGFK, but only 29% germinated with l-valine. Thus, the actual effects of dry-heat treatment were much greater on l-valine germination than on germination with AGFK.
FIG 3.
AGFK germination of B. subtilis spores after dry-heat treatment. (A) Spores with and without various dry-heat treatments at 140°C were germinated with AGFK, and the germination of 400 individual spores was followed as described in Materials and Methods. (B, C, D) AGFK germination of 10 individual spores with and without dry-heat treatment at 140°C for (B) 0, (C) 30, or (D) 60 min was monitored as described in Materials and Methods. Image intensities are given in arbitrary units (AU).
CaDPA and dodecylamine germination of dry-heat-treated B. subtilis spores.
While GRs are essential for spore germination in response to nutrients, they are dispensable for non-nutrient-induced spore germination, as mechanisms of nutrient-induced and non-nutrient-induced spore germinations are different (20). Thus, analysis of the kinetics of non-nutrient germination could provide further insight into the precise damage to the spore germination apparatus caused by dry-heat treatment, since GR damage would not affect non-nutrient germination of spores.
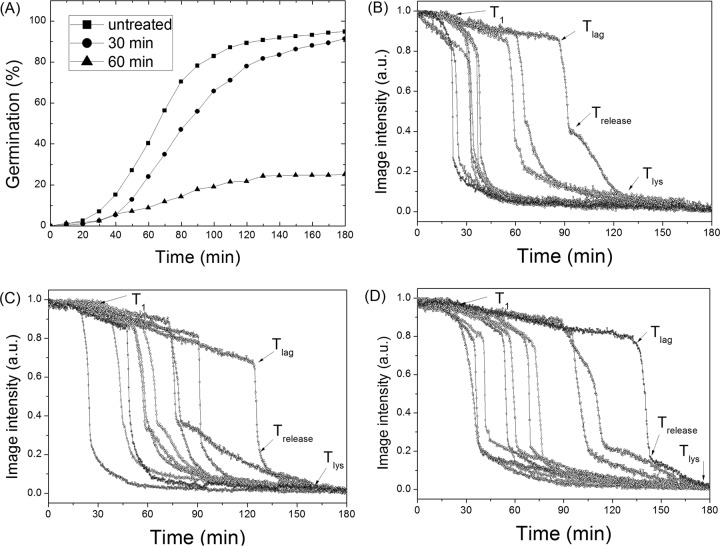
B. subtilis spores are generally germinated by two kinds of nonnutrient agents, CaDPA and the cationic surfactant dodecylamine (20, 21). Exogenous CaDPA directly or indirectly activates the spore cortex peptidoglycan lytic enzyme (CLE) CwlJ, thus triggering spore germination. Consequently, examination of effects of dry-heat treatment on CaDPA germination may indicate whether dry-heat treatment damages the CLE CwlJ. As observed with l-valine germination, dry-heat treatment significantly reduced spore germination with CaDPA, with ∼91% and ∼25% germination with spores dry-heat-treated at 140°C for 30 min and 60 min, respectively (Fig. 4). The analysis of the germination of multiple individual spores with CaDPA indicated that T1, Tlag, ΔTleakage, ΔTrelease, and ΔTlys all increased with the extension of dry-heat treatment time at 140°C. The increase in the standard deviations of the values of Tlag, ΔTleakage, ΔTrelease, and ΔTlys again indicated that there was increased heterogeneity for dry-heat-treated spores germinating with CaDPA (Table 2).
FIG 4.
CaDPA germination of B. subtilis spores with and without dry-heat treatment. (A) CaDPA germination of spores given various treatments at 140°C was followed, and 400 individual spores were examined as described in Materials and Methods. (B, C, D) CaDPA germination of 10 single spores after dry-heat treatment at 140°C for (B) 0, (C) 30, or (D) 60 min, respectively. Germination was followed as described in Materials and Methods. Image intensities are given in arbitrary units (AU).
Germination of B. subtilis spores by the other major nonnutrient germinant, dodecylamine, does not involve the spore GRs, and dodecylamine also does not directly activate CLEs (20, 21). Rather, dodecylamine directly or indirectly opens channels in spore inner membranes that allow CaDPA release (20–23). Interestingly, the effects of dry-heat treatment on spore germination with dodecylamine were very different from those on spore germination with nutrient germinants or CaDPA (Table 2; and compare Fig. 5 with Fig. 2 to 4). Dry-heat treatment for 60 min at 140°C increased the percentage of spore germination with dodecylamine, but the average ΔTlys values increased to ∼1.5-fold (Fig. 5 and Table 2).
FIG 5.
Dodecylamine germination of B. subtilis spores with and without dry-heat treatment. (A) Germination after various dry-heat treatment times with dodecylamine, with >300 spores examined for each condition as described in Materials and Methods. (B, C, D) Dodecylamine germination of 10 single spores after dry-heat treatment at 140°C for (B) 0, (C) 30, or (D) 60 min, respectively. Germination was followed as described in Materials and Methods. Image intensities are given in arbitrary units (AU).
GR-dependent germination of dry-heat-treated B. megaterium spores.
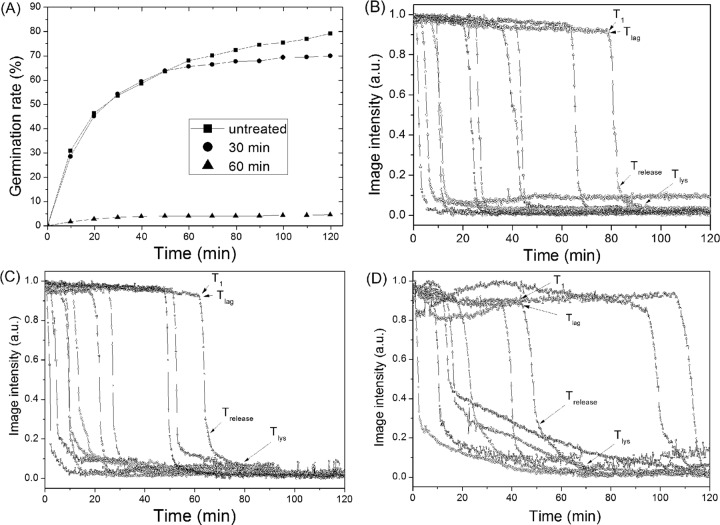
The effects of dry-heat treatment on the germination of B. megaterium spores with the GR-dependent germinant glucose exhibited both similarities to and differences from the effects on GR-dependent germination of B. subtilis spores (Fig. 6, Table 3). As with B. subtilis spores, a large percentage of the dry-heat-killed B. megaterium spores retained CaDPA (data not shown). Glucose germination of B. megaterium spores was also decreased by dry-heat treatment (Fig. 6A), just as with GR-dependent germination of B. subtilis spores. Therefore, it was of obvious interest to examine the kinetics of GR-dependent germination of individual dry-heat-treated B. megaterium spores (Fig. 6 and Table 3). Surprisingly, dry-heat treatment did not increase the B. megaterium spore germination parameter values for T1, Tlag, and ΔTleakage significantly, even when only 5% of spores germinated after treatment at 140°C for 60 min (Table 3). This is in contrast to results with GR-dependent germination of B. subtilis spores (Table 1). However, as seen with B. subtilis spore germination, the glucose germination of maximally dry-heat-treated B. megaterium spores exhibited a slight increase in ΔTrelease times and an ∼3-fold increase in ΔTlys times (Table 3).
FIG 6.
Glucose germination of B. megaterium spores with and without dry-heat treatment. (A) Glucose germination of spores dry-heat treated at 140°C for various times. More than 500 spores were examined for each treatment as described in Materials and Methods. (B, C, D) Glucose germination of 10 single spores after dry-heat treatment at 140°C for (B) 0, (C) 30, or (D) 60 min, respectively, was followed as described in Materials and Materials and Methods. Image intensities are given in arbitrary units (AU).
TABLE 3.
T1, Tlag, ΔTleakage, ΔTrelease, and ΔTlys for glucose germination of B. megaterium spores with or without dry-heat treatmenta
| Treatment condition(s) | No. of spores examined (% spore germination) | T1 (mean ± SD) (min) | Tlag (mean ± SD) (min) | ΔTleakage (mean ± SD) (min) | ΔTrelease (mean ± SD) (min) | ΔTlys (mean ± SD) (min) |
|---|---|---|---|---|---|---|
| Control (untreated) | 582 (79.2) | 26.5 ± 25.4 | 28.9 ± 28.2 | 2.4 ± 1.8 | 3.3 ± 0.8 | 7.7 ± 4.5 |
| 140°C, 30 min | 797 (70.0) | 19.9 ± 17.5 | 22.5 ± 16.5 | 2.6 ± 1.5 | 3.1 ± 1.1 | 11.6 ± 6.1 |
| 140°C, 60 min | 526 (4.6) | 25.3 ± 20.3 | 27.5 ± 32.8 | 2.2 ± 1.9 | 5.2 ± 1.8 | 29.9 ± 16.2 |
B. megaterium spores with or without dry-heat treatment were germinated with glucose for 2 h, and spore germination parameters were determined as described in Materials and Methods. Data from approximately 100 individual spores that contained CaDPA and germinated were used to calculate the kinetic parameters of germination.
Germination, outgrowth, and growth of individual dry-heat-treated B. subtilis spores in nutrient-rich agar.
The effects of dry-heat treatment on the germination, outgrowth, and growth of individual B. subtilis spores in nutrient-rich agar are shown in Fig. 7. Without dry-heat treatment (Fig. 7A), or with dry-heat treatment at 140°C for only 7 min (∼90% survival) (Fig. 7B), nearly all individual spores went through germination and outgrowth, and then formed vegetative cells and underwent some cell division in 4 to 6 h. However, after dry-heat treatment at 140°C for 30 min (∼5 log spores killed) (Fig. 7C), the germination of single spores became much slower, although almost all spores still ultimately completed germination. Most interestingly, none of the germinated spores were observed to proceed into outgrowth within 15 h, even when >1,000 total spores were examined (data not shown). Consequently, no growing cells were even seen. This result is consistent with the results of spore viability assessed by measuring colony formation on LB plates, in which ∼99% of wild-type B. subtilis spores were killed by dry-heat treatment at 140°C for >15 min (Fig. 1A). However, most of these killed spores retained CaDPA, even with dry-heat treatment at 140°C for 30 to 60 min. Single spore measurements indicated that these treated spores can complete germination in LB medium, but cannot proceed to outgrowth and growth, consistent with the great majority of these spores being dead.
FIG 7.
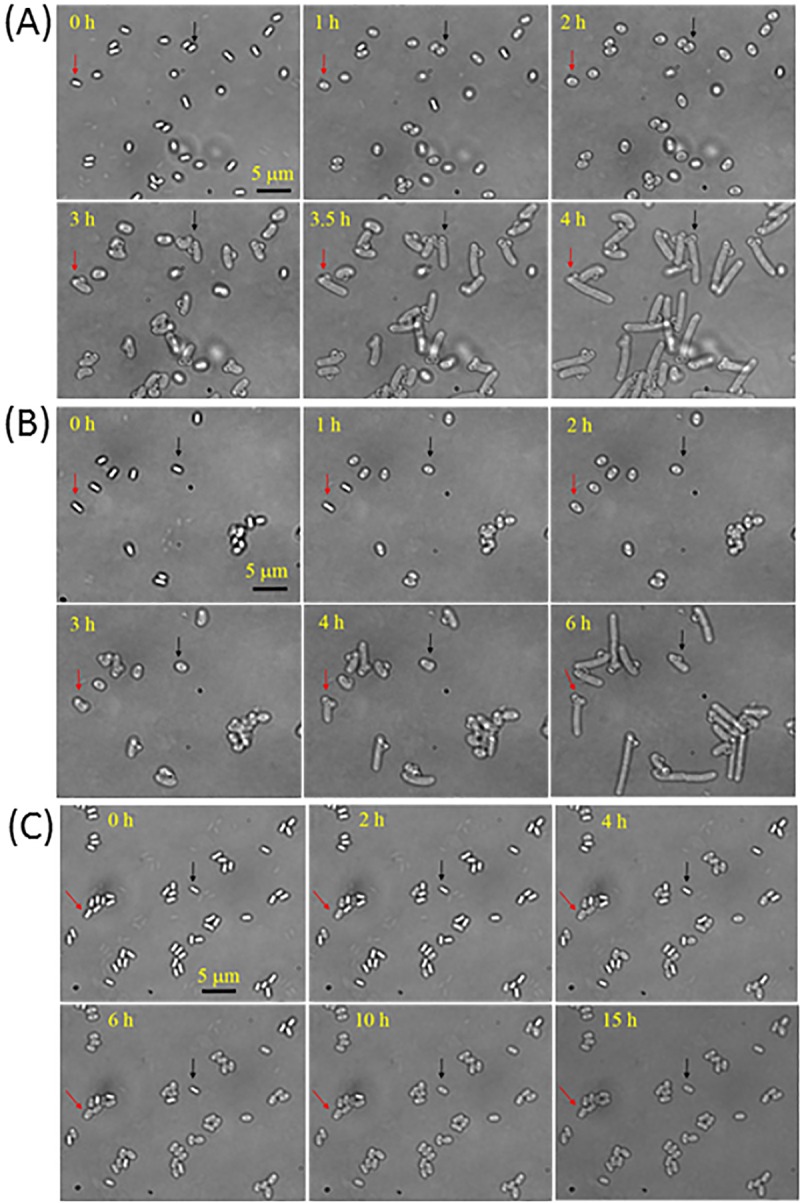
Germination, outgrowth, and growth of individual B. subtilis spores with and without dry-heat treatment. Time-lapse images of single B. subtilis spores given various treatments at 140°C that were then incubated in LB medium agar at 37°C were recorded as described in Materials and Methods. (A) Time-lapse images of single spores without dry-heat treatment. (B, C) Time-lapse images of single spores after dry-heat treatments at 140°C for 7 min and 30 min, respectively. The two arrows in A and B indicate two individual spores that germinated, outgrew, and began vegetative growth. Note that in C, spores that germinated did not go through outgrowth, even after 15 h. Bar, 5 μm.
DISCUSSION
It was perhaps not surprising that dry-heat-killed Bacillus spores retained CaDPA, as this is also true for wet-heat-killed Bacillus spores (13, 14). Indeed, the absence of water in spore killing by dry heat would certainly make CaDPA release during this treatment unlikely. However, the lack of CaDPA release from dry-heat-killed spores upon their suspension in water indicates that either (i) dry heat causes minimal damage to the permeability barrier of dormant spores that retains CaDPA, including at least the spore inner membrane, or (ii) the dry-heat damage to spore permeability barriers is rapidly reversed upon suspension in water. Of the last two alternatives, it seems much more likely that damage to spore permeability barriers is not caused by dry-heat treatment. Indeed, as noted above, there are multiple lines of evidence that dry heat kills spores by DNA damage (7–12).
Given that dry heat kills spores by DNA damage, it is not surprising that dry-heat-killed spores germinated, as a fully functional intact spore chromosome is not essential for spore germination. Indeed, UV-killed B. subtilis spores germinate relatively normally (7). However, it is clear that dry-heat treatment causes damage to spores in addition to DNA damage, as extended dry-heat treatment greatly slowed spore germination. An obvious question then is what spore germination component or components are damaged by dry heat, such that germination is slowed.
Analysis of the effects of dry-heat treatment on spore germination by different nutrient and nonnutrient germinants indicates that there are at least two germination targets, GRs and CLEs, but the inner membrane CaDPA release channel composed of SpoVA proteins is not a target. The evidence for these conclusions is as follows. (i) Germination by dodecylamine, which probably directly activates the CaDPA release channel (21–23), was affected minimally by a dry-heat treatment for 60 min at 160°C that killed ≫5 logs of spores. The only effect on a germination parameter was a slight increase in the ΔTlys value, which could have been due to damage to CLEs (see below). The only proviso against completely ruling out the CaDPA channel as damaged by dry heat is that severe damage to the outer coat layer of B. subtilis spores results in increased rates of dodecylamine germination (21). Thus, it is possible that there are competing effects of dry-heat treatment on dodecylamine germination, decreasing germination by damaging the CaDPA channel and increasing it by damaging the spore coat, and that these effects always balance out. There is indeed a report that dry-heat treatment causes damage to the outer exosporium layer of Bacillus anthracis spores (24). However, it is not clear if this damage also includes damage to the spore coat, nor how severe the damage is; it also appears likely that this damage takes place very long after spores are dead. Given the current state of knowledge, at present we conclude that it is most likely that damage to the CaDPA channel is not especially important in the effects of dry-heat treatment on germination of at least B. subtilis spores. (ii) It appears clear that GRs, or at least one or more GR subunits, are damaged by dry heat, resulting in slowed GR-dependent germination. The strongest evidence for this conclusion is that B. subtilis AGFK germination via the GerB and GerK GRs was much less sensitive to dry-heat treatment than was l-valine germination via the GerA GR. The large difference in the dry-heat sensitivity of these two GR-dependent germinations is also consistent with damage to the CaDPA release channel not being the primary damage to the spore germination apparatus caused by dry heat, and with lack of dry-heat damage to the auxiliary germination protein GerD that is essential for both GerA and GerB/GerK-dependent germination (20). The greater dry-heat sensitivity of GerA germination relative to that of GerB/GerK germination is also consistent with the need for longer high-temperature wet-heat exposure to optimally heat activate B. subtilis spores for AGFK germination (25), consistent with the GerB/GerK GRs being more heat stable than the GerA GR. Indeed, at a wet-heat exposure giving optimal heat activation of AGFK germination, l-valine germination is significantly inactivated (25). (iii) It also appears clear that at least the CLE CwlJ is damaged by dry heat, as the germination of spores by CaDPA, which activates CwlJ and does not involve GRs (20), is also slowed by dry-heat treatment. Damage to CwlJ and the other redundant Bacillus CLE, SleB, would also explain the increased ΔTlys values in germination of dry-heat-treated spores with all germinants.
Despite the tentative identification of GRs and CLEs as important targets for damage by dry heat that decreases rates of spore germination, the precise damage to these germination proteins is not clear. Presumably this is protein denaturation, and there certainly is some protein denaturation in spores given a dry-heat treatment at 160°C for 90 min. However, there are minimal amounts of protein denatured by milder dry-heat treatments, as determined by Raman spectroscopy, even with dry-heat-treated spores that have lost CaDPA. Indeed, denaturation of the small amounts of soluble protein outside the spore inner membrane (IM), such as CLEs and some GR subunit domains, might not be detectable against a likely background of the large amounts of α-helical spore core and coat protein. These coat proteins could be well protected against dry-heat denaturation by their high degree of protein-protein cross-linking (26).
Perhaps one of the most important observations made in this work, at least from an applied perspective, was that there was minimal, if any, outgrowth of dry-heat-killed spores. The applied importance of this observation is that it indicates that spores in dry-heat-sterilized materials will not outgrow and thus will likely not synthesize proteins. Since spores of at least some pathogens, B. anthracis in particular, can synthesize proteins in outgrowth soon after completion of germination (27), the observation above indicates that dry-heat sterilization should be effective against spores of pathogens. However, a major question that remains is why there is no outgrowth of germinated spores from dry-heat-killed dormant spores. These germinated spores will certainly have significant DNA damage, and certainly some of this damage is mutagenic. However, why this DNA damage alone would prevent spore outgrowth is not clear, and clearly is a matter for further study.
MATERIALS AND METHODS
Bacillus species and strains used and spore preparation and purification.
The Bacillus strains used in this work were B. subtilis PS533 (wild-type), an isogenic derivative of B. subtilis strain 168 (7), and Bacillus megaterium QM B1551 (wild-type) (originally obtained from H. S. Levinson).
B. subtilis spores were prepared on 2xSG medium agar plates at 37°C without antibiotics (28–30). After incubation for 2 to 3 days, plates were incubated for several additional days at 23°C to allow lysis of any growing and sporulating cells. Spores were then scraped from the plates and suspended in 4°C water. B. megaterium spores were prepared at 30°C in supplemented nutrient broth (14), and these cultures were harvested by centrifugation after 36 to 48 h of incubation. Harvested spores were purified by repeated centrifugation and washing with cold water, as well as by several sonication treatments (14, 28–30). Purified spores were stored at 4°C in water protected from light and were free (98%) of growing and sporulating cells, germinated spores, and cell debris as observed by phase-contrast microscopy.
Spore dry-heat treatment and measurement of spore viability and CaDPA content.
Fifty microliters of stored spores (∼108 spores/ml) was placed in small glass tubes that were centrifuged (10,000 × g for 2 min), the supernatant fluid discarded, and the pellet frozen in dry ice-ethanol. The tubes were then placed in a vacuum chamber and held overnight to dry the spores. For dry-heat treatment, the tubes were placed in a temperature-controlled oven to treat samples at a desired temperature for various times. Generally, the industrial standard for dry-heat sterilization is 160°C for 2 h or 170°C for 1 h. Our experimental results indicated that treating spores at 140°C for ≥20 min was sufficient to kill the great majority of spores. Hence, 140°C was chosen as the routine dry-heat treatment temperature. After dry-heat treatment, spores were suspended in distilled water to give ∼108 spores/ml and stored on ice.
The viability of dry-heat-treated spores was measured by serial dilution in water, spreading aliquots on LB medium plates (29), incubating plates for 24 to 36 h at 37°C, and counting colonies. Incubation for longer times gave no increase in the numbers of colonies. The CaDPA content of spores was determined by Raman spectroscopy using laser tweezers Raman spectroscopy (LTRS) as described previously (15, 31). Individual spores suspended in water were randomly trapped by a 780-nm laser beam, and Raman scattering excited by the same laser beam was acquired by a charge-coupled device (CCD). The CaDPA content of spores was determined from the intensity of the CaDPA-specific Raman band at 1,017 cm−1 relative to that of CaDPA standard (15, 31).
Spore germination.
B. subtilis spores were germinated in (i) 10 mM l-valine in 25 mM K-HEPES buffer (pH 7.4) at 37°C; (ii) 10 mM AGFK (10 mM [each] l-asparagine, d-glucose, d-fructose, and K+) in 25 mM K-HEPES buffer (pH 7.4) at 37°C; (iii) 50 mM CaDPA (pH 7.5) at room temperature (25°C); or (iv) 1.2 mM dodecylamine in 25 mM K-HEPES buffer (pH 7.4) at 45°C. B. megaterium spores were germinated with 10 mM glucose in 25 mM KPO4 buffer (pH 7.4) at 37°C. Although spores had been exposed to dry heat, heat activation was still used, as spores that are not dry-heat treated need this for optimal germination. Except for CaDPA and dodecylamine germination, spores were heat-activated prior to germination by incubation in a water bath at 70°C for 30 min. In previous experiments, B. megaterium spores were usually heat-activated at 60°C for 15 min. However, we found that 70°C for 30 min was more effective for heat activation of these spores (data not shown). After heat activation, spores were cooled on ice for 15 to 30 min prior to germination experiments.
Monitoring spore germination.
Analysis of the germination of multiple individual spores by DIC microscopy was as previously described (32, 33). Briefly, heat-activated spores (∼0.5 to 1 μl of ∼108 spores/ml in water) were spread on the surface of a microscope coverslip that was then dried in a vacuum desiccator for 5 to 10 min. The coverslip was then mounted on and sealed to a microscope sample holder kept at a constant temperature. After adding preheated germinant/buffer solution to spores on the coverslips, a digital CCD camera (12 bits, 1,392 by 1,040 pixels) was used to record the DIC images at a rate of 1 frame every 15 s for 120 to 180 min. The DIC microscope was set such that the polarizer and analyzer were crossed, and thus the DIC bias phase was zero (33). The DIC images were analyzed with a program written in Matlab to locate each spore's position and to calculate the averaged pixel intensity of an area with a 20-pixel diameter that covered the whole individual spore on a DIC image. The DIC image intensity of each individual spore was plotted as a function of the incubation time, with a resolution of 15 s. The initial intensity at T0 (the first DIC image recorded after the addition of the germinant) was normalized to 1, and the intensity at the end of measurements was normalized to zero. The latter value had to have been constant for at least 10 min at the end of measurements. From the time-lapse DIC image intensity, we can determine the time of completion of the rapid fall of ∼75% in a spore's DIC image intensity, which coincides with the period of release of almost all of a spore's CaDPA; the end of this period is defined as Trelease, as confirmed by LTRS (33).
CaDPA release and spore cortex peptidoglycan hydrolysis kinetics during the germination of individual spores were described by a number of parameters as described previously (20), including (i) T1 (also termed Tleak and Tc), the time when a spore begins slow leakage of a small fraction of its CaDPA; (ii) Tlag, when a spore initiates rapid release of the great majority of its CaDPA (ΔTleakage = Tlag − T1); (iii) ΔTrelease = Trelease − Tlag; (iv) Tlys, the time when spore cortex hydrolysis is completed as determined by the completion of the fall in a spore's DIC image intensity (20, 33, 34); and (v) ΔTlys = Tlys − Trelease.
Curves for the germination of multiple untreated and dry-heat-treated spores of different species were obtained by determining the germinated spores at various times and dividing by the number of dormant spores at the beginning of experiments. In these measurements, ≥300 individual dry-heat-treated spores that retained CaDPA were analyzed. A germinated spore was defined as one that (i) released CaDPA and (ii) had hydrolyzed the cortex. Analysis of ∼300 spores dry-heat treated for various times and incubated in germination media never found one that released CaDPA but did not degrade the cortex (data not shown). For dry-heat-treated spores, only the initial spores retaining CaDPA were treated as dormant spores and selected by the program, since (i) no dry-heat-treated spores that had lost CaDPA exhibited any change in their DIC image intensity when incubated in germination medium (data not shown) and (ii) wet-heat-treated spores that have lost their CaDPA also do not germinate (13, 14).
Monitoring germination, outgrowth of, and growth from single spores incubated in an LB medium agar pad.
A small drop (∼1 μl of ∼108 spores/ml in water) of B. subtilis spores was spread on the surface of a glass coverslip mounted on a homemade sample holder that was dried in a vacuum desiccator for >5 min. The spores on the coverslip were then given a dry-heat treatment in an oven at 140°C for 7 or 30 min. After dry-heat treatment, the sample was cooled to room temperature and then mounted on a microscope sample holder kept at 37°C. Approximately 250 μl of melted LB medium agar (29) at ∼60°C was added on top of the spores on the coverslip to form an agar pad with a thickness of ∼3 mm. Some agar in the middle of the pad was removed to form a small hole to store air, and then a second coverslip was used to seal the top of the agar pad, thus preparing the spores for DIC microscopy as described above. A digital CCD camera (12 bits, 1,392 by 1,040 pixels) was used to record the bright-field or DIC images on the spores on the coverslip at a rate of 1 frame every 15 s or 60 s for 4 to 15 h. These images were analyzed with a program written in Matlab as described above. In these measurements, >200 individual spores were monitored.
ACKNOWLEDGMENTS
L.H. and S.W. received support through award XDA15007808 from the National Space Science Center of the Chinese Academy of Sciences. S.W. and Y.L. received support from the National Natural Science Foundation of China (grant 91751110 to S.W. and Y.L. and grant 31770152 to S.W.). Y.L. received support from the High-Level Talent Program of Dongguan University of Technology (grant KCYCXPT2017003). Y.L. and P.S. received funding support through award R21AI26067 from the National Institutes of Allergy and Infectious Diseases (NIAID).
REFERENCES
- 1.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 2.Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food microbiology: fundamentals and frontiers, 4th ed ASM Press, Washington, DC. [Google Scholar]
- 3.Gerhardt P, Marquis RE. 1989. Spore thermoresistance mechanisms, p 43–63. In Smith I, Slepecky RA, Setlow P (ed), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC. [Google Scholar]
- 4.Wang G, Zhang P, Setlow P, Li Y. 2011. Kinetics of germination of wet-heat-treated individual spores of Bacillus. Appl Environ Microbiol 77:3368–3379. doi: 10.1128/AEM.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horneck G, Klaus DM, Mancinelli RL. 2010. Space microbiology. Microbiol Mol Biol Rev 74:121–156. doi: 10.1128/MMBR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panitz C, Horneck G, Rabbow E, Rettberg P, Moeller R, Cadet J, Douki T, Reitz G. 2014. The SPORES experiment of the EXPOSE-R mission: Bacillus subtilis spores in artificial meteorites. Int J Astrobiol 14:105–114. doi: 10.1017/S1473550414000251. [DOI] [Google Scholar]
- 7.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol 178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setlow P. 2016. Spore resistance properties, p 201–215. In Eichenberger P, Driks A (ed), The bacterial spore: from molecules to systems. ASM Press, Washington, DC. [Google Scholar]
- 9.Setlow B, Atluri S, Kitchel R, Koziol-Dube K, Setlow P. 2006. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β-type small, acid-soluble proteins. J Bacteriol 188:3740–3747. doi: 10.1128/JB.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setlow B, Setlow P. 1995. Small, acid-soluble proteins bind to DNA and protect Bacillus subtilis spores from killing by dry heat. Appl Environ Microbiol 61:2787–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamenhof S. 1960. Effects of heating dry bacteria and spores on their phenotype and genotype. Proc Natl Acad Sci U S A 46:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Carmen Huesca Espitia L, Caley C, Bagyan I, Setlow P. 2002. Base-change mutations induced by various treatments of Bacillus subtilis spores with and without DNA protective small, acid-soluble spore proteins. Mutat Res 503:77–84. doi: 10.1016/S0027-5107(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 13.Coleman WH, Chen D, Li YQ, Cowan AE, Setlow P. 2007. How moist heat kills spores of Bacillus subtilis. J Bacteriol 189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman WH, Zhang P, Li YQ, Setlow P. 2010. Mechanism of killing of spores of Bacillus cereus and Bacillus megaterium by wet heat. Lett Appl Microbiol 50:507–514. doi: 10.1111/j.1472-765X.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang S-S, Chen D, Pelczar PL, Vepachedu VR, Setlow P, Li Y-Q. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J Bacteriol 189:4681–4687. doi: 10.1128/JB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magge A, Granger AC, Wahome PG, Setlow B, Vepachedu VR, Loshon CA, Peng L, Chen D, Li Y-Q, Setlow P. 2008. Role of dipicolinic acid in the germination, stability, and viability of spores of Bacillus subtilis. J Bacteriol 190:4798–4807. doi: 10.1128/JB.00477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong LB, Setlow P, Li YQ. 2012. Analysis of the Raman spectra of Ca2+-dipicolinic acid alone and in the bacterial spore core in both aqueous and dehydrated environments. Analyst 137:3683–3689. doi: 10.1039/c2an35468c. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Setlow P, Li Y. 2009. Characterization of single heat-activated Bacillus spores using laser tweezers Raman spectroscopy. Optics Express 17:16480–16491. doi: 10.1364/OE.17.016480. [DOI] [PubMed] [Google Scholar]
- 19.Zhang PF, Kong LB, Wang GW, Setlow P, Li YQ. 2011. Monitoring the wet-heat inactivation dynamics of single spores of Bacillus species by using Raman tweezers, differential interference contrast microscopy, and nucleic acid dye fluorescence microscopy. Appl Environ Microbiol 77:4754–4769. doi: 10.1128/AEM.00194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setlow P, Wang S, Li Y-Q. 2017. Germination of spores of the orders Bacillales and Clostridiales. Annu Rev Microbiol 71:459–477. doi: 10.1146/annurev-micro-090816-093558. [DOI] [PubMed] [Google Scholar]
- 21.Setlow B, Cowan AE, Setlow P. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J Appl Microbiol 95:637–648. doi: 10.1046/j.1365-2672.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- 22.Vepachedu VR, Setlow P. 2007. Role of SpoVA proteins in release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J Bacteriol 189:1565–1572. doi: 10.1128/JB.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velásquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. 2014. Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol 92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Li A, Felker DL, Burggraf LW. 2014. Nanoscale structural and mechanical analysis of Bacillus anthracis spores inactivated by rapid dry heating. Appl Environ Microbiol 80:1739–1749. doi: 10.1128/AEM.03483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luu S, Cruz-Mora J, Setlow B, Feeherry FE, Doona CJ, Setlow P. 2015. The effects of heat activation on nutrient and high-pressure germination of spores of Bacillus species with and without germination proteins. Appl Environ Microbiol 81:2927–2938. doi: 10.1128/AEM.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driks A, Eichenberger P. 2016. The spore coat, p 179–200. In Driks A, Eichenberger P (ed), The bacterial spore: from molecules to systems. ASM Press, Washington, DC. [Google Scholar]
- 27.Cote C, Welkos S. 2015. Anthrax toxins in context of Bacillus anthracis spores and spore germination. Toxins 7:3167–3178. doi: 10.3390/toxins7083167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson WL, Setlow P. 1990. Sporulation, germination and outgrowth, p 391–450. In Harwood C, Cutting A (ed), Molecular biological methods for Bacillus. John Wiley, Chichester, United Kingdom. [Google Scholar]
- 29.Paidhungat M, Setlow P. 2000. Role of GerP proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J Bacteriol 182:2513–2519. doi: 10.1128/JB.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol 182:5505–5512. doi: 10.1128/JB.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen D, Huang S-S, Li Y-Q. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal Chem 78:6936–6941. doi: 10.1021/ac061090e. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P, Garner W, Yi X, Yu J, Li Y-Q, Setlow P. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J Bacteriol 192:3608–3619. doi: 10.1128/JB.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P, Kong L, Wang G, Setlow P, Li Y-Q. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J Biomed Opt 15:056010–056019. doi: 10.1117/1.3494567. [DOI] [PubMed] [Google Scholar]
- 34.Kong L, Zhang P, Yu J, Setlow P, Li YQ. 2010. Monitoring the kinetics of uptake of a nucleic acid dye during the germination of single spores of Bacillus species. Anal Chem 82:8717–8724. doi: 10.1021/ac1022327. [DOI] [PubMed] [Google Scholar]