ABSTRACT
The type strain of the mineral-oxidizing acidophilic bacterium Acidithiobacillus ferridurans was grown in liquid medium containing elevated concentrations of sodium chloride with hydrogen as electron donor. While it became more tolerant to chloride, after about 1 year, the salt-stressed acidophile was found to have lost its ability to oxidize iron, though not sulfur or hydrogen. Detailed molecular examination revealed that this was due to an insertion sequence, ISAfd1, which belongs to the ISPepr1 subgroup of the IS4 family, having been inserted downstream of the two promoters PI and PII of the rus operon (which codes for the iron oxidation pathway in this acidophile), thereby preventing its transcription. The ability to oxidize iron was regained on protracted incubation of the culture inoculated onto salt-free solid medium containing ferrous iron and incubated under hydrogen. Two revertant strains were obtained. In one, the insertion sequence ISAfd1 had been excised, leaving an 11-bp signature, while in the other an ∼2,500-bp insertion sequence (belonging to the IS66 family) was detected in the downstream inverted repeat of ISAfd1. The transcriptional start site of the rus operon in the second revertant strain was downstream of the two ISs, due to the creation of a new “hybrid” promoter. The loss and subsequent regaining of the ability of A. ferriduransT to reduce ferric iron were concurrent with those observed for ferrous iron oxidation, suggesting that these two traits are closely linked in this acidophile.
IMPORTANCE Iron-oxidizing acidophilic bacteria have primary roles in the oxidative dissolution of sulfide minerals, a process that underpins commercial mineral-processing biotechnologies (“biomining”). Most of these prokaryotes have relatively low tolerance to chloride, which limits their activities when only saline or brackish waters are available. The study showed that it was possible to adapt a typical iron-oxidizing acidophile to grow in the presence of salt concentrations similar to those in seawater, but in so doing they lost their ability to oxidize iron, though not sulfur or hydrogen. The bacterium regained its capacity for oxidizing iron when the salt stress was removed but simultaneously reverted to tolerating lower concentrations of salt. These results suggest that the bacteria that have the main roles in biomining operations could survive but become ineffective in cases where saline or brackish waters are used for irrigation.
KEYWORDS: Acidithiobacillus, insertion sequence, iron reduction, iron oxidation, salt stress
INTRODUCTION
Chemolithotrophy, the ability to grow using inorganic electron donors, is particularly widespread among acidophilic prokaryotes (1). Ferrous iron, elemental sulfur, and reduced inorganic sulfur compounds and hydrogen have all been reported to be used as electron donors, sometimes by a single bacterial species (some Acidithiobacillus and Sulfobacillus spp.). Of these electron donors, ferrous iron has been invariably found to be the first to be utilized when others are also present, even though the free energy available from oxidizing iron is less than that from reduced or elemental sulfur or from hydrogen (2–4). The most universal electron acceptor used by acidophilic bacteria, and the only thermodynamically feasible option for coupling ferrous iron oxidation, is molecular oxygen, though many species are known to respire on ferric iron, which has a high redox potential and is abundant and often soluble in extremely acidic environments (1).
Soluble ferric iron, generated by iron-oxidizing acidophiles, is a powerful oxidant and can greatly accelerate the dissolution of minerals, including sulfide minerals that contain economically important metals. This is the basis of biomining, a global biotechnology used to recover gold, copper, and other base metals (5). In many parts of the world where biomining is or can be carried out, water quality is sometimes a major constraint, as saline or brackish waters have been widely reported to inhibit the activities of iron-oxidizing acidophiles, the primary catalysts of oxidative sulfide mineral dissolution. Concentrations of 6 g liter−1 (∼100 mM) have been reported to inhibit iron oxidation by Acidithiobacillus ferrooxidans (6), the most widely studied of all biomining prokaryotes. Salt (NaCl) sensitivity is not, however, universal among acidophilic bacteria, with the type strain of A. thiooxidans (a sulfur oxidizer that does not oxidize ferrous iron) reported to grow in 500 mM NaCl (i.e., a concentration similar to that of seawater) (7) and the iron/sulfur oxidizer Acidihalobacter prosperus to grow optimally in the presence of 20 g liter−1 (∼350 mM) NaCl (6). It is, however, not always clear to what extent growth conditions and most notably culture pH are responsible for the variations in salt tolerance that have been reported for acidophilic prokaryotes.
The genus Acidithiobacillus currently contains seven validated species, four of which (A. ferrooxidans, A. ferrivorans, A. ferridurans, and A. ferriphilus) can use ferrous iron as well as reduced sulfur (and, in some cases, hydrogen) as electron donors (8, 9). One strain of A. ferriphilus was isolated from the Rio Tinto (Spain) on a solid medium that contained 25 mM ferrous iron as the electron donor and 500 mM NaCl (7). This isolate could grow in liquid medium containing up to 800 mM NaCl with elemental sulfur as the electron donor but not on ferrous iron in liquid medium that contained >350 mM salt. Here we report the salt tolerance of the type strain of A. ferridurans, which was selected for this study, since unlike A. ferriphilus, it grows on hydrogen, which circumvents the problems (pH variations, formations of precipitates, etc.) encountered with other electron donors. Long-term exposure to elevated salt concentrations resulted in the loss of the ability of A. ferriduransT to oxidize and reduce iron but not sulfur, though this was demonstrated to be a recoverable trait among the population as a whole.
RESULTS
Adaptative laboratory evolution of Acidithiobacillus ferridurans to salt tolerance.
A. ferriduransT grew readily using hydrogen as an electron donor in the presence of 500 mM NaCl at both pH 2.5 and pH 3.0. At pH 2.0, growth was observed in 250 mM NaCl but not in 500 mM NaCl cultures, and cell numbers were smaller than in the higher-pH salt-containing cultures. It was, however, found to be possible to increase the salt tolerance of A. ferriduransT at pH 2.2 to a maximum of 500 mM NaCl, though cells appeared elongated, indicative of stress. This “salt pressure” was maintained by regular subculturing of the acidophile in a liquid medium of the same composition. A single colony isolate obtained from this culture after about 1 year was referred to as derivative clone 33-X. A corresponding colony of A. ferriduransT that had been subcultured in salt-free medium throughout this time was referred to as “33-WT” (wild type).
Physiological analysis of the Acidithiobacillus ferridurans salt-tolerant derivative clone.
The derivative clone 33-X grew at pH 2.2 using hydrogen as an electron donor in the presence of 0 or 500 mM NaCl. It also grew in the presence of 500 mM NaCl in both tetrathionate and elemental sulfur liquid media. However, attempts to grow 33-X on ferrous iron as the electron donor (at pH 2.0 or 2.5) in both salt-containing and salt-free liquid media failed. In ferrous-iron-supplemented liquid media incubated in the presence of hydrogen (with or without salt), the bacteria grew but again did not oxidize iron. In contrast, the non-salt-stressed strain (33-WT) oxidized iron immediately when subcultured in salt-free 10 mM ferrous medium; no lag phase was observed even when cultures were incubated in the presence of hydrogen. A. ferridurans 33-WT also grew in tetrathionate and sulfur liquid media in the presence of 500 mM NaCl, as did 33-X. It was also found that the derivative clone 33-X did not grow or reduce ferric iron when incubated anaerobically in the presence of hydrogen, in contrast to 33-WT, which catalyzed the dissimilatory reduction of ferric iron as reported previously (10).
Molecular characterization of the Acidithiobacillus ferridurans salt-tolerant derivative clone 33-X.
To confirm that the salt-tolerant derivative clone 33-X was not a contaminant, an ∼1,390-bp region of its 16S rRNA gene sequence was amplified by PCR (Table 1) and sequenced. The sequence obtained was 100% identical to that of A. ferridurans ATCC 33030T (data not shown). In addition, PCR was performed with primers specific to alaS and nifH genes of different species of iron-oxidizing acidithiobacilli (11) (Table 1). Amplicons of the expected size were obtained (data not shown), indicating that the salt-tolerant derivative clone 33-X was indeed A. ferriduransT.
TABLE 1.
Primers used in this study
| Target region | Primera | Primer sequence (5′–3′) | Amplicon size (bp)b | Reference |
|---|---|---|---|---|
| 16S rRNA gene | 27F | AGAGTTTGATCMTGGCTCAG | ~1,390 | 37 |
| 1387R | GGGCGGWGTGTACAAGGC | 38 | ||
| PCR analysis | ||||
| rrs (16S rRNA) | 16S-D | ACCGCCTACGCACCCTTTAC | 277 | 4 |
| 16S-G | ACACTGGGACTGAGACACGG | |||
| atpD | atpD-F | CCATATCGTCCAGGTGATCG | 1,341 | 11 |
| atpD-R | GCTTCATCTATGGTGCCGAC | |||
| nifH | nifH19F | GCIWTYYAYGGIAARGGIGG | 426 | 11 |
| nifH407R | AAICCRCCRCAIACIACRTC | |||
| cyc2 | inv4 (f) | GGTTATGGTGTCATCGTCCGTTGG | 386 | 39 |
| ainv2 (n) | GAACATTATTGTTGGGAGAAGC | |||
| cup (acoP) | 3006L (g) | ATTGCGCATCAGTTCTCCAT | 200 | 40 |
| 3006R (o) | GGCACCACAAAACTGAAGGT | |||
| rus | rusA-F (h) | ACTGGTATGTAACTGTTGGTGCG | 11 | |
| 3010R (p) | ATCTCCAAGGTCGGGTTCTT | 265 with h | 40 | |
| rusA-R (q) | GTGTATCCGAACTTGCCATCT | 436 with h | 11 | |
| rus regulatory region | inv0 (a) | TTGGCATGTCGATTTTTGGACC | 469 with k; 508 with l | 39 |
| inv1 (b) | ATGGTTAACATGATAAAATAACG | 456 with l; 345 with j | 39 | |
| inv3 (c) | GTTTGTATTAAATAGAACGTGTGG | 240 with l | 39 | |
| cyc2N-BamH1 (d) | AAGGATCCGCGTATTTTGTTTATCTAATATGCC | 444 with n | 4 | |
| ced1(e) | ATTTTGTTTATCTAATATGCC | 433 with n | ||
| inv4 (f) | GGTTATGGTGTCATCGTCCGTTGG | 386 with n | 39 | |
| ainv3 (i) | ACACGTTCTATTTAATACAAACCG | 290 with a; 238 with b | 39 | |
| ainv6 (j) | GCTCATGCGCCCGGTCTTCCTGCC | 397 with a; 129 with c | 39 | |
| ainv4-2 (k) | CCAACGGACGATGACACCATAACC | 417 with b; 201 with c | 39 | |
| R-PII-cyc2 (l) | ACTGCTGCTAATGCTACGA | |||
| ainv5 (m) | CGCAAAGGATGGCAGTGCCCAGG | 289 with c; 505 with b | 39 | |
| ainv2 (n) | GAACATTATTGTTGGGAGAAGC | 563 with c; 779 with b | 39 | |
| ISAfd1 | IS-F2 (r) | ACCCCACATCCCCGTACC | 1,083 (M) with l; 1,406 (M) with n; 972 (M) with j; 1,044 (M) with k | |
| IS-R (s) | CGTTTCCCATAGAGATCC | 1,186 (M) with b; 261 (M) with r | ||
| 5′ RACE—rus regulatory region | R-PII-cyc2 (l) | ACTGCTGCTAATGCTACGA | ||
| ainv4-2 (k) | CCAACGGACGATGACACCATAACC | 39 | ||
| RT-PCR | ||||
| rrs (16S rRNA) | 16S-D | ACCGCCTACGCACCCTTTAC | 277 | 4 |
| 16S-G | ACACTGGGACTGAGACACGG | |||
| cyc2 | inv4 (f) | GGTTATGGTGTCATCGTCCGTTGG | 386 | 39 |
| ainv2 (n) | GAACATTATTGTTGGGAGAAGC | |||
| rus | rusA-F (h) | ACTGGTATGTAACTGTTGGTGCG | 436 | 11 |
| rusA-R (q) | GTGTATCCGAACTTGCCATCT |
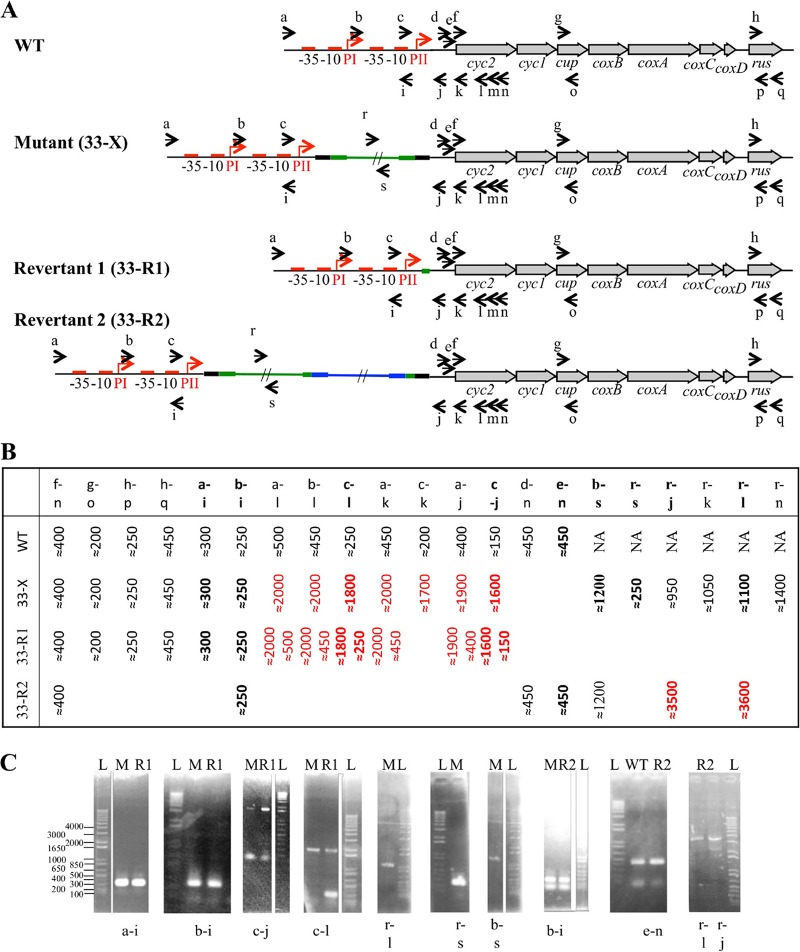
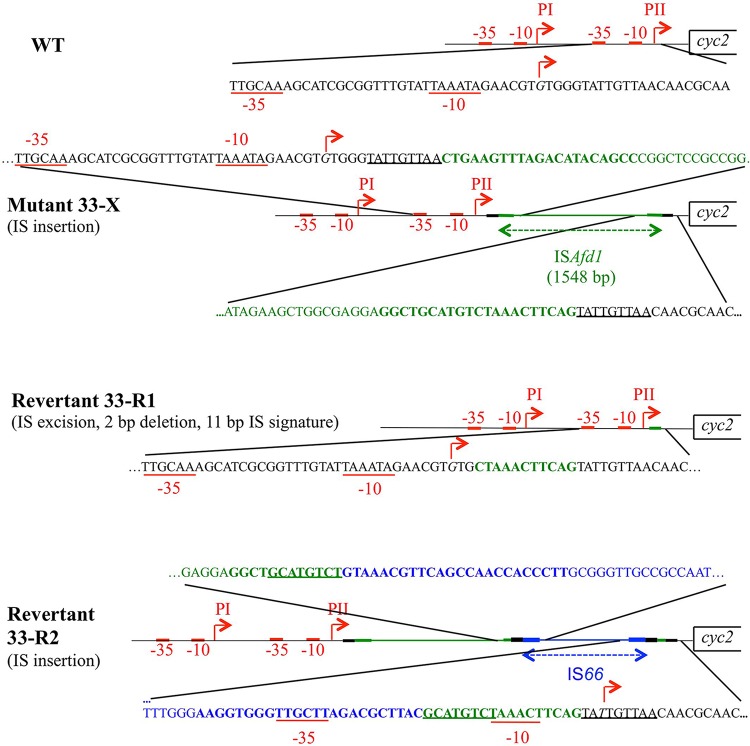
As described above, 33-X was unable to oxidize ferrous iron, suggesting that the prolonged exposure to elevated concentrations of NaCl at pH 2.0 had impacted its ferrous iron oxidation pathway. The redox proteins involved in electron transfer between ferrous iron and oxygen are encoded by the rus operon (see reference 8 and references therein). We therefore checked that this operon was not deleted in 33-X. Using PCR, it was possible to amplify the internal fragment of the first gene (cyc2), a midrange gene (cup), and the last gene (rus) of the operon (primers f to n, g to o, and h to p) (Fig. 1 and Table 1), suggesting that at least the genes encoding the outer membrane cytochrome c, the copper binding protein Cup, and rusticyanin were present in the salt-tolerant derivative clone 33-X. Following this, the regulatory region of the rus operon was analyzed by PCR using some of the primers depicted in Fig. 1A and listed in Table 1. Amplicons of the expected size were obtained upstream of the PII promoter (primers a, b, and c with primer i) (Fig. 1 and data not shown; Table 1). The same results were obtained with primers d, e, and f with primer n (Fig. 1 and Table 1), a region encompassing the cyc2 gene and its Shine and Dalgarno sequence. However, the amplified sequences between these two regions were ∼1.5 kb larger than expected (primers a, b, and c, with primers j, k, or l) (Fig. 1 and Table 1), suggesting the presence of an insertion in the amplicon obtained between primers c and j. The sequence of the amplicons obtained with primers c to l and b to l confirmed the presence of an insertion sequence (IS) 13 bp downstream of the transcriptional start site PII in 33-X (Fig. 2). Two primers (r and s [Fig. 1 and Table 1]) were designed from this insertion sequence to amplify and sequence the fragments between primers b and s, r and l, and r and s. The data obtained confirmed the presence of a 1,548-bp IS. At the insertion site, a 9-bp sequence (TATTGTTAA) was duplicated (Fig. 2). The IS had a 21-bp nearly perfect inverted repeat (CTGAAGTTTAGACATa/gCAGCC, where lowercase letters indicate nucleotide differences) at each end (Fig. 2; see also Fig. S1 in the supplemental material). These characteristics suggest that the IS belongs the ISPepr1 subgroup of the IS4 family (12–14). Interestingly, the nucleotide sequence of this IS element (Fig. S1) has 99% homology with an IS present in pTF5 plasmid previously identified in A. ferriduransT (15, 16). This IS, here named ISAfd1, encodes only one protein of 440 amino acids, the transposase with the characteristic catalytic residues DDE embedded in the amino acid consensus of the ISPepr1 subgroup of the IS4 family (12) (Fig. S1).
FIG 1.
PCR analysis of the rus operon of the salt-tolerant derivative (33-X) and the two revertants (33-R1 and 33-R2). (A) Schematic representation of the rus operon in the mutant and the revertants. The primers used (Table 1) are indicated as small arrows. The ISAfd1 and the IS66 are in green and blue, respectively. Their terminal inverted repeats are represented with a thicker green or blue line. The direct repeats generated on insertion are represented with a thicker black line. (B) Sizes (in base pairs) of the expected (WT) or observed amplicons in the salt-tolerant derivative (33-X) and the revertants (33-R1 and 33-R2). (C) Results of PCR performed on the salt-tolerant derivative (33-X) and the two revertants (33-R1 and 33-R2). L, 1 Kb Plus DNA Ladder (Thermo Fisher Scientific).
FIG 2.
Schematic representation of the sequence of the regulatory region of the rus operon in the wild type (WT), the mutant 33-X, and the two revertants 33-R1 and 33-R2. The ISAfd1 and the IS66 are in green and blue, respectively. Their terminal inverted repeats are represented with a thicker green or blue line and with bold letters in the sequences. The direct repeats generated on insertion are represented with a thicker black line and are underlined in the sequences. The −35 and the −10 boxes are underlined in red. The transcriptional start sites are italicized and indicated by red arrows.
Isolation and characteristics of revertant strains from Acidithiobacillus ferridurans 33-X.
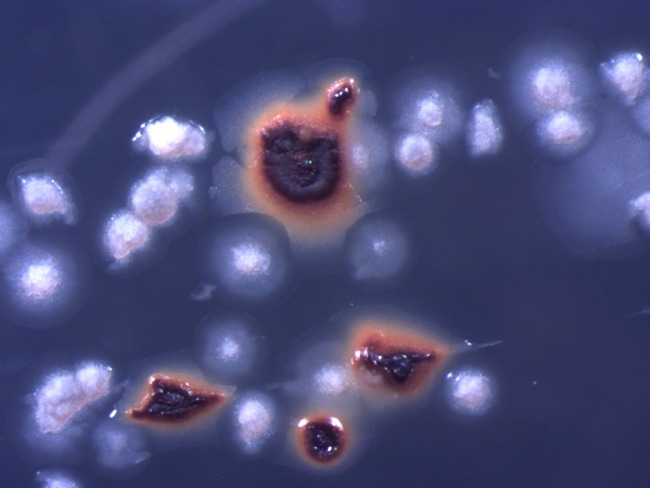
The derivative clone 33-X of A. ferridurans, inoculated onto iron overlay plates incubated in the presence of hydrogen, formed white colonies, indicating that ferrous iron was not being oxidized (ferric iron formed by this reaction causes colonies to be strongly colored orange/bronze). It was noted, however, that protracted incubation of plates for over 1 month in the presence of hydrogen resulted in the appearance of some typical wild-type (iron-encrusted) colonies alongside the white colonies (Fig. 3). No colonies were found on solid medium incubated in the absence of hydrogen. Ferric iron-encrusted colonies were removed and streak inoculated onto fresh iron overlay plates in the presence of hydrogen to obtain single colonies, which were then inoculated into 10 mM ferrous iron liquid medium (pH 2.0). Two such revertant strains (named 33-R1 and 33-R2) were obtained on separate occasions.
FIG 3.
Colonies of the salt-tolerant derivative clone 33-X (white) and the revertant strain 33-R1 (ferric iron-encrusted) of A. ferriduransT grown on ferrous iron overlay solid medium under H2-CO2-enriched air.
Physiological tests carried out with 33-R1 and 33-R2 confirmed, as suggested by their colony morphologies, that both were able to oxidize ferrous iron. Unlike the revertant clone 33-X (but as with A. ferriduransT), both 33-R1 and 33-R2 were able to reduce ferric iron to ferrous iron when incubated anaerobically with hydrogen as the electron donor. Both 33-R1 and 33-R2 were also able to use elemental sulfur and tetrathionate as electron donors. In contrast to the salt-tolerant derivative strain 33-X, both revertant strains (33-R1 and 33-R2) were unable to grow in liquid medium containing 500 mM NaCl (pH 2.2) with either hydrogen or ferrous iron as the electron donor, though they grew on hydrogen in liquid medium containing 250 mM NaCl, a characteristic shared with the wild-type strain, 33-WT.
Molecular characterization of the revertant clones 33-R1 and 33-R2.
Genomic DNA from revertant clone 33-R1 was extracted, and the rus operon was analyzed by PCR as described above for 33-X. As shown in Fig. 1, internal fragments of the cyc2, cup, and rus structural genes (amplicons with primers f to n, g to o, and h to p [Table 1]) as well as the region upstream of the PII promoter (primers a, b, and c with primer l [Table 1]) had the expected sizes. Surprisingly, between the PII promoter and cyc2, two amplicons were detected irrespective of the primers used (a, b, and c with primers j, k, or l) (Fig. 1 and data not shown; Table 1): one amplicon corresponded to the wild type and the other to the salt-tolerant derivative clone 33-X. These data suggest that (i) this culture contained both the derivative clone (33-X) and the revertant strain 33-R1, and (ii) the ISAfd1 had been excised in the revertant strain 33-R1. The sequence of the ∼450-bp amplicon obtained with primers b and l confirmed that the insertion sequence ISAfd1 had been excised and had left an 11-bp signature, the sequence of which corresponded to the end of the downstream inverted repeat of the ISAfd1 (Fig. 2).
The other revertant clone, 33-R2, was studied following the loss of viability of the 33-R1 strain. As shown in Fig. 1, it appears that while the regions upstream of the promoter PII (primers b to i) and downstream of the Shine-Dalgarno of cyc2 (primers d, e, and f with n) were intact in revertant strain 33-R2, another ∼2,500-bp insertion occurred downstream of the ISAfd1 (primer r with j and l) (Table 1). The sequence of the amplicon obtained between primers r and l (Table 1) indicates that this new IS belongs to the IS66 family (13, 14, 17, 18) and was inserted inside the downstream inverted repeat of the first insertion sequence ISAfd1 (Fig. 2). At the insertion site, an 8-bp region was duplicated (GCATGTCT) (Fig. 2). This IS66 has a 24-bp imperfect inverted repeat sequence (GTAAa/gCGTt/cc/tAg/ac/gCAACCa/cc/aCCTT) at each end (Fig. 2).
Expression of the rus operon in the 33-R2 revertant of the Acidithiobacillus ferridurans salt-tolerant derivative.
Since ferrous iron oxidation was restored in the revertant strain 33-R2 while two ISs, ISAfd1 and IS66, were inserted downstream of the rus operon promoters PI and PII, the question of how rus transcription occurred was addressed. A 5′ rapid amplification of cDNA ends (RACE) was carried out to determine the transcriptional start site of the rus operon from sulfur- and ferrous iron-grown 33-R2 cells with two primers (k and l [Table 1]). The results obtained showed that the transcriptional start site of the rus operon in 33-R2 cells was located in the downstream direct repeat of ISAfd1 (Fig. 2), i.e., downstream of PI and PII promoters. Upstream of it were predicted to occur a −10 box (TAaAcT, inside the downstream inverted repeat of ISAfd1) and, 17 bp upstream, a −35 box (TTGctt, inside the downstream inverted repeat of IS66). The bands amplified by 5′ RACE appeared to be as intense in 33-R2 grown on sulfur as on ferrous iron (data not shown). To confirm these results, reverse transcription (RT)-PCR experiments were performed using RNA extracted from sulfur- and ferrous iron-grown 33-R2 cells with primers specific to the 16S RNA (16S-G and 16S-D), cyc2 (f and n), and rus (h and q) genes (Fig. 1 and Table 1). The data obtained indicated that the 16S RNA gene, cyc2, and rus were not differentially transcribed in the revertant strain 33-R2 under sulfur- or ferrous iron-growth conditions (Fig. 4).
FIG 4.
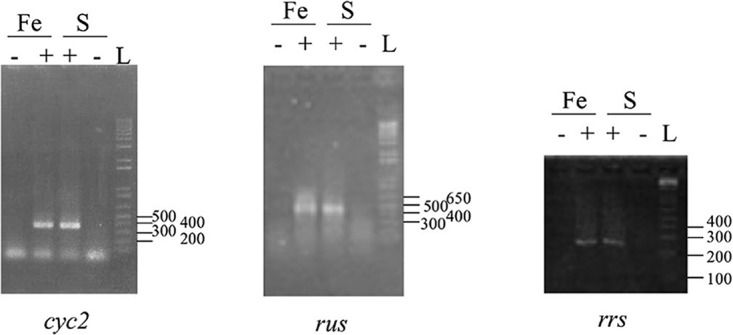
Expression of cyc2, rus, and 16S rRNA (rrs) genes in the revertant 33-R2. RT-PCR experiments were performed on total RNA extracted from the revertant 33-R2 grown on ferrous iron (Fe) or sulfur (S) with (+) or without reverse transcriptase (−). L, 1 Kb Plus DNA Ladder (Thermo Fisher Scientific).
DISCUSSION
The ability of acidophilic bacteria to grow in the presence of elevated concentrations of sodium chloride is determined to a significant extent by the pH of their growth medium. In the present study, this was illustrated by the fact that A. ferriduransT grew readily in the presence of 500 mM NaCl at pH 2.5 and 3.0 but not at pH 2.0, while similar results have been obtained with a phylogenetically diverse range of other acidophilic bacteria (Carmen Falagan and D. Barrie Johnson, Bangor University, United Kingdom, unpublished data). Some other published observations can also be interpreted as being due to differences in culture pH. For example, A. ferriphilus strain ST2 was reported to grow and oxidize ferrous iron on a solid medium (pH ∼2.8) containing 500 mM NaCl, while growth was completely inhibited by 350 mM NaCl in pH 2.0 liquid ferrous iron medium (7). Batch cultures of acidophiles grown on sulfur usually have an initial pH of about 3, as sulfur oxidation generates protons and cultures acidify rapidly. In contrast, oxidation of hydrogen coupled to oxygen is a pH-neutral reaction (2 H2 + O2 → 2 H2O) and pH fluctuations during growth are generally <0.1 of a pH unit. Hydrogen is therefore a very useful electron donor for cultivating chemolithotrophic acidophiles in batch culture.
Most acidophilic bacteria appear to be highly osmotolerant and can grow in liquid media containing sulfate salts in excess of 1 M (for examples, see reference 10). The toxicity of chloride anions (as opposed to the relative nontoxicity of sodium cations) to acidophiles appears to be related to the uncontrolled, or ingress of, negatively charged ions (apart from sulfate), causing a collapse of their normally strongly positive membrane potentials (ΔΨ [19]). However, the magnitude and even polarity of ΔΨ values change in response to both the pH values of external media and the physiological status of cells (i.e., whether resting or energized), and this in turn influences the sensitivity of acidophiles to chloride and other anions, with the notable exception of sulfate.
Protracted exposure of A. ferriduransT to concentrations of NaCl that were approaching inhibitory levels (at low pH) resulted in the loss of its ability to carry out its most characteristic physiological trait, ferrous iron oxidation, but did not cause it to lose the ability to grow on either sulfur or hydrogen. A detailed molecular examination of the rus operon revealed how this had occurred. An insertion sequence, ISAfd1, belonging to the ISPepr1 subgroup of the IS4 family (12–14), had been inserted downstream of the two promoters PI and PII of the rus operon, which has been characterized in both A. ferriduransT (4) and A. ferrooxidansT (20). It is known that stress conditions increase IS hopping into new sites, sometimes enabling the host to relieve this stress, to adapt to new environmental challenges, and to colonize new niches (21). The ISAfd1 encodes only the transposase, and the corresponding gene is transcribed in the same direction as the rus operon. It is therefore very likely that the transcription from PI and PII was ending at the transcriptional termination site of the transposase gene, preventing rus operon transcription. Many cases of inactivation of gene expression by IS insertion disrupting a target gene promoter have previously been described (see reference 21 and references therein).
The ability to oxidize (and reduce) iron was regained by the salt-adapted strain 33-X but only after exposed incubation on salt-free solid medium and in the presence of hydrogen, which supported the growth of the bacteria during this time. No bacterial colonies were obtained on ferrous iron-containing plates that were incubated under atmospheric air. The excision of ISAfd1 (in revertant 33-R1) left an 11-bp signature and restored the characteristic wild-type phenotype for both ferrous iron oxidation and ferric iron reduction abilities. Surprisingly, in the second revertant analyzed (33-R2), a different insertion sequence, an IS66 group member, translocated inside the downstream inverted repeat of ISAfd1, creating a new promoter. This hybrid promoter comprises the outward-directed −35 promoter component located in the downstream inverted repeat of IS66 and the −10 promoter component located in the downstream inverted repeat of the ISAfd1. While it is known that IS elements can create hybrid promoters by the correct placement of a −35 box present within the downstream IS inverted repeat, relative to a suitable −10 box in the flanking genomic region (21), to our knowledge there are no previous reports of a “flanking genomic region” being the inverted repeat of another IS element.
It is noteworthy that in the derivative clone 33-X, inactivation of the rus operon led not only to the loss of the ability to oxidize ferrous iron but also to the inability to reduce ferric iron in the absence of molecular oxygen. These two phenotypes were therefore linked and were due to only one event, the insertion of an IS element in the regulatory region of the rus operon. Both traits were reestablished in the two independent revertants, either by excision of this IS element (33-R1) or by creation of a hybrid promoter by translocation of another insertion element in the previous one (33-R2). Therefore, these data indicate that one or more of the redox proteins encoded by the rus operon are involved both in ferrous iron oxidation and in ferric iron reduction. Two different mechanisms of anaerobic sulfur oxidation coupled to ferric iron reduction have been proposed in A. ferrooxidans, one in the type strain (ATCC 23270T [22]) and another in strain CCM 4253 (23, 24). In the model proposed for A. ferrooxidansT, the electrons flow from sulfur to quinone and onwards to the bc1 complex and then to CycA2, these redox proteins being encoded by the petII operon. The final redox protein of this electron transport chain reducing ferric iron was not identified. The phenotypes of 33-X, 33-R1, and 33-R2 strongly suggest that, at least in A. ferriduransT, the electrons could be transferred from CycA2 to rusticyanin and from there to the outer membrane cytochrome c Cyc2, where ferric iron reduction could occur (Fig. 5), as proposed elsewhere (23).
FIG 5.
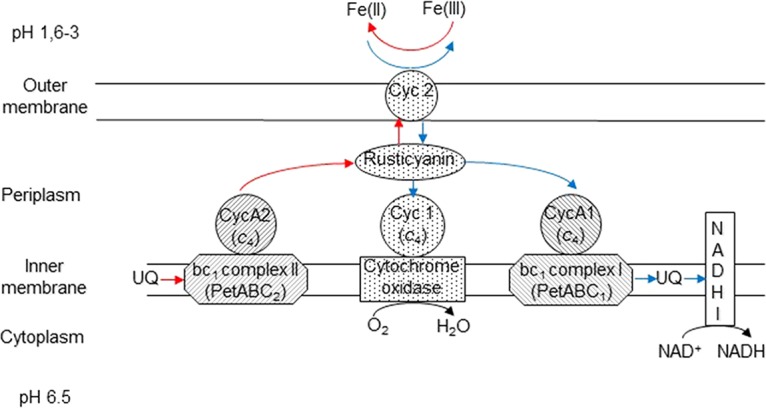
Proposed model for iron energetic metabolism in A. ferridurans. Blue lines represent electron transport from ferrous iron [Fe(II)] under aerobic conditions, while red lines represent electron transport to ferric iron [Fe(III)] in the absence of oxygen. The redox proteins encoded by the same transcriptional unit are represented with the same background pattern.
The apparent relationship between tolerance to elevated concentrations of salt and iron oxidation/reduction in A. ferriduransT is intriguing. Bacteria that had been grown aerobically with hydrogen as the electron donor in medium containing NaCl concentrations close to those that completely inhibited growth at pH 2.2 were unable to oxidize or reduce iron, implying that the modification of the genome of A. ferriduransT that had caused the loss of iron oxidoreduction was paralleled by enhanced tolerance to salt. The loss of the ability to oxidize and reduce iron was the only physiological trait detected, though this does not necessarily imply that this was the only modification that had occurred. However, it is possible to infer that many other core functions were not disabled. The culture was maintained on H2, and salt-induced elimination of the ability to use this electron donor would obviously mean that growth would not have been observed. The same argument holds true for many other key functions, such as uptake and assimilation of phosphate and other inorganic nutrients. Likewise, the ability of the mutant strain to use reduced S as the electron donor was retained, even though it did not have the opportunity to grow on S during the 3-year-long experiment. Comparison of the genomes of the wild-type 33-WT and the mutant 33-X strains would reveal whether other genetic modifications, which did not impact the viability of the bacteria, had occurred, but this was beyond the scope of the current study.
Iron oxidation is the most important function of this acidophile, and many others, in mineral processing operations, and the finding that protracted exposure to elevated salt concentrations resulted in the disabling of this key trait is of particular significance. The greater tolerance to salt exhibited by the mutant strain than the wild-type and both revertant strains could have been due to adaptation during the protracted period during which the original culture had been maintained at 500 mM NaCl and the loss of this ability when the “salt stress” was removed. Both revertant strains 33-R1 and 33-R2 were subcultured several times in salt-free ferrous iron liquid medium before being tested for growth in the presence of 500 mM NaCl with either hydrogen or ferrous iron as an electron donor. While salt stress effectively knocked out iron oxidoreduction in the mutant strain 33-X, the bacterium was able to regain this ability only when this stress was removed, when maintained at pH 2.2. Whether or not the ability to oxidize and reduce iron would have been regained if strain 33-X had been grown at pH 2.5 and above, pHs at which, as noted, wild-type A. ferriduransT grows readily in the presence of 500 mM NaCl (i.e., there is little or no apparent chloride toxicity), is an intriguing possibility that will be investigated in future work, along with experiments that will investigate whether long-term salt exposure can knock out iron oxidoreduction in other species of iron-oxidizing bacteria (A. ferrooxidans, A. ferrivorans, and A. ferriphilus).
Interestingly, the only previously documented study on IS transposition and excision in iron-oxidizing acidophiles that described both loss and recovery of the ability to oxidize ferrous iron involved A. ferrooxidans strain ATCC 19859 (25, 26). This “phenotypic switching” occurred on a solid medium containing both thiosulfate and ferrous iron and was proposed to be due to the reversible transposition of the insertion sequence ISafe1 in the resB gene involved in cytochrome c biogenesis. The mutant strain in those studies had lost the ability to oxidize ferrous iron but retained its ability to oxidize reduced sulfur, as found for the salt-tolerant derivative strain 33-X. The inactivation of resB by the ISafe1 insertion could result in the absence of heme C in cytochromes c and therefore to their inactivation. In A. ferrooxidansT, four cytochromes c are involved in iron oxidation (Cyc2 and CycA1, encoded by the rus operon, and CycA1 and Cyc1, encoded by the petI operon) compared with only two in sulfur oxidation (CycA2 and Cyc1, encoded by the petII operon) involved in a minor and facultative pathway (see reference 8 and references therein). Therefore, cytochromes c are absolutely required for ferrous iron oxidation but are optional for sulfur oxidation. Elsewhere, it has been shown that in the closely related species A. ferriduransT, lower levels of total cytochromes c occur in sulfur- than in ferrous iron-grown cells (27). Accordingly, by inactivating the res operon, ISafe1 insertion prevented ferrous iron oxidation, as illustrated in the present study by the ISAfd1 insertion in the regulatory region of the rus operon. Similar “phenotypic switching” was also observed in A. ferrooxidansT, A. ferriduransT, and other private culture collection strains (25). Therefore, it seems that in iron-oxidizing acidithiobacilli, environmental changes increase IS element transposition in new sites, in particular in genes involved in iron oxidoreduction.
The results of this study have implications for mineral bioprocessing technologies. Commercial bioleaching is carried out in stirred tanks, bioheaps, and rock dumps by consortia of acidophiles, carrying out different roles that include acid production (from oxidation of reduced sulfur) and catabolism of potentially toxic organic metabolites (5). The prima facie reaction is, however, the continuous regeneration of the oxidant ferric iron, by prokaryotic oxidation of ferrous iron. The finding that salt-induced stress caused a typical iron-oxidizer to lose its ability to catalyze the oxidoreduction of iron implies that such bacteria, which are well-known for their activity in bioheaps and rock dumps in particular, could become ineffective in cases where saline or brackish waters are used for irrigation.
MATERIALS AND METHODS
Bacteria, growth conditions, and adaptative laboratory evolution to elevated concentrations of sodium chloride.
The type strain of Acidithiobacillus ferridurans (ATCC 33020T) (10), maintained in active axenic cultures in both laboratories, was used in the experiments described here. The acidophile was routinely maintained in liquid medium containing 20 mM ferrous iron (pH 1.7) supplemented with ∼0.1% (wt/vol) sterile pyrite. Prior to use, a single colony from an iron overlay plate (28) that had been streak inoculated was removed and placed into fresh liquid medium. Next, this was subcultured in a pH 2.0 liquid medium containing 100 μM ferrous iron (20 ml) in a 50-ml conical flask that was incubated (shaken at 30°C) in a sealed jar with a hydrogen- and carbon dioxide-enriched atmosphere (29). The culture was removed periodically and checked for growth. Once the culture had become turbid (and cell numbers exceeded 5 × 108 ml−1), it was subcultured into fresh medium and grown routinely using hydrogen as the electron donor. The hydrogen-adapted A. ferridurans was subcultured in basal salts-trace elements medium (30) containing 100 μM ferrous iron and 0, 250, or 500 mM NaCl. The culture pH values were set initially between pH 2.0, 2.5, or 3.0. Thereafter, the culture was grown on hydrogen at pH 2.2 in liquid medium supplemented with 0 or 500 mM NaCl for up to 1 year.
Isolation and characterization of a salt-tolerant derivative clone of A. ferriduransT.
The two cultures of A. ferriduransT, grown either with or without 500 mM NaCl at pH 2.2, were streak inoculated onto both standard (containing 25 mM ferrous iron) and modified (100 μM ferrous iron) overlay media, both without NaCl. The plates were again incubated in aerobic H2-CO2-enhanced atmosphere jars. Individual colonies were removed and used to inoculate NaCl-free liquid medium containing either 100 μM or 10 mM ferrous iron (pH 2.0), 2.5 mM tetrathionate (pH 3.0), or 0.5% (wt/vol) elemental sulfur (also at pH 3.0). These were incubated either under atmospheric- or H2-CO2-supplemented air.
The abilities of both the salt-tolerant derivative clone and nonadapted A. ferriduransT to couple the oxidation of hydrogen to the reduction of ferric iron in the absence of oxygen were tested by subculturing both variants in basal salts-trace elements liquid medium containing 25 mM ferric sulfate and incubating cultures in sealed jars with H2-CO2-supplemented atmospheres that were deoxygenated using AnaeroGen sachets (Fisher Scientific, UK). Samples were removed at intervals and concentrations of ferrous iron determined using the 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine (ferrozine) assay (31).
DNA manipulations.
A. ferriduransT genomic DNA was prepared from washed bacterial cell pellets using a cetyltrimethylammonium bromide (CTAB)-high-salt extraction method. Cells were lysed by incubating in 0.25% SDS containing proteinase K and RNase A. For routine PCR amplification, GoTaq G2 Flexi (Promega) DNA polymerase was used, but for amplification of fragments longer than 2 kb, PrimeSTAR Max (TaKaRa Bio) was preferred. The PCR conditions followed the manufacturers' recommendations. The primers used for PCR amplification and the sizes of the expected amplicons are listed in Table 1. When sequenced, amplicons were first purified and concentrated with Amicon Ultra-0.5 centrifugal filter units (Millipore) according to the manufacturer's guidelines. The nucleotide sequences were determined by GATC Biotech (Germany).
RNA manipulations.
Total RNA was prepared by using acid-phenol extraction (22) with an additional DNase I treatment with the reagents from a Turbo DNA-free kit (Ambion). The lack of DNA contamination was checked by PCR on each RNA sample using primers rusA-F and rusA-R. The absence of RNA degradation was confirmed by agarose gel electrophoresis.
Coupled RT-PCR experiments were performed with the Access RT-PCR system (Promega) using total RNA extracted from ferrous iron- and sulfur-grown cells and the primers listed in Table 1. For each RT-PCR experiment, at least two replicates with 30 cycles were performed. Three controls were used: one without template to detect potential contaminations, one with genomic DNA as a positive control for PCR amplification, and one with RNA not treated with reverse transcriptase to check for DNA contamination during RNA preparation.
The transcriptional start site of the rus operon in the revertant strain 33-R2 was determined with the 5′ rapid amplification of cDNA ends (RACE) system (Invitrogen) with the primers listed in Table 1 from sulfur- and ferrous iron-grown cells, as described in reference 32.
Bioinformatic analysis.
DNA sequences were visualized and edited with 4Peaks (A. Griekspoor and T. Groothuis, Nucleobytes, Amsterdam, Netherlands), aligned with Kalign, assembled with CAP3 (33) and Clustal Omega (34), and compared with the data bank BLAST (35) through the World Wide Web Netscape facilities.
Data availability.
The sequence of the IS identified in the mutant was deposited in ISfinder (36) (http://www-is.biotoul.fr) under the name ISAfd1.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to David S. Holmes (Fundación Ciencia & Vida, Santiago, Chile) and Mick Chandler (C.N.R.S., Toulouse, France, and Georgetown University Medical Center, Washington, DC, USA) for their helpful discussions of this work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02795-17.
REFERENCES
- 1.Quatrini R, Johnson DB (ed). 2016. Acidophiles: life in extremely acidic environments. Caister Academic Press, Haverhill, UK. [Google Scholar]
- 2.Liljeqvist M, Rzhepishevska OI, Dopson M. 2013. Gene identification and substrate regulation provide insights into sulfur accumulation during bioleaching with the psychrotolerant acidophile Acidithiobacillus ferrivorans. Appl Environ Microbiol 79:951–957. doi: 10.1128/AEM.02989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandoval Ponce J, Moinier D, Byrne D, Amouric A, Bonnefoy V.. 2012. Acidithiobacillus ferrooxidans oxidizes ferrous iron before sulfur likely through transcriptional regulation by the global redox responding RegBA signal transducing system. Hydrometallurgy 127-128: 187–194. [Google Scholar]
- 4.Yarzabal A, Appia-Ayme C, Ratouchniak J, Bonnefoy V. 2004. Regulation of the expression of the Acidithiobacillus ferrooxidans rus operon encoding two cytochromes c, a cytochrome oxidase and rusticyanin. Microbiology 150:2113–2123. doi: 10.1099/mic.0.26966-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DB. 2014. Biomining—biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol 30:24–31. doi: 10.1016/j.copbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Dopson M, Holmes DS, Lazcano M, McCredden TJ, Bryan CG, Mulroney KT, Steuart R, Jackaman C, Watkin EL. 2016. Multiple osmotic stress responses in Acidihalobacter prosperus result in tolerance to chloride ions. Front Microbiol 7:2132. doi: 10.3389/fmicb.2016.02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DB, Grail BM, Bonnefoy V. 2015. New insights into salt-tolerance in acidophilic iron-oxidising bacteria. Adv Mat Res 1130:3–6. [Google Scholar]
- 8.Nitschke W, Bonnefoy V. 2016. Energy acquisition in low-pH environments, p 19–47. In Quatrini R, Johnson DB (ed), Acidophiles: life in extremely acidic environments. Caister Academic Press, Norfolk, UK. [Google Scholar]
- 9.Nunez H, Moya-Beltran A, Covarrubias PC, Issotta F, Cardenas JP, Gonzalez M, Atavales J, Acuna LG, Johnson DB, Quatrini R. 2017. Molecular systematics of the genus Acidithiobacillus: insights into the phylogenetic structure and diversification of the taxon. Front Microbiol 8:30. doi: 10.3389/fmicb.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedrich S, Johnson DB. 2013. Acidithiobacillus ferridurans sp. nov., an acidophilic iron-, sulfur- and hydrogen-metabolizing chemolithotrophic gammaproteobacterium. Int J Syst Evol Microbiol 63:4018–4025. doi: 10.1099/ijs.0.049759-0. [DOI] [PubMed] [Google Scholar]
- 11.Amouric A, Brochier-Armanet C, Johnson DB, Bonnefoy V, Hallberg KB. 2011. Phylogenetic and genetic variation among Fe(II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways. Microbiology 157:111–122. doi: 10.1099/mic.0.044537-0. [DOI] [PubMed] [Google Scholar]
- 12.De Palmenaer D, Siguier P, Mahillon J. 2008. IS4 family goes genomic. BMC Evol Biol 8:18. doi: 10.1186/1471-2148-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siguier P, Gourbeyre E, Chandler M. 2014. Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol Rev 38:865–891. doi: 10.1111/1574-6976.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siguier P, Gourbeyre E, Varani A, Ton-Hoang B, Chandler M. 2015. Everyman's guide to bacterial insertion sequences. Microbiol Spectr 3:MDNA3-0030–2014. doi: 10.1128/microbiolspec.MDNA3-0030-2014. [DOI] [PubMed] [Google Scholar]
- 15.Dominy CN, Coram NJ, Rawlings DE. 1998. Sequence analyzis of plasmid pTF5, a 19.8-kb geographically widespread member of the Thiobacillus ferrooxidans pTFI91-like plasmid family. Plasmid 40:50–57. doi: 10.1006/plas.1998.1344. [DOI] [PubMed] [Google Scholar]
- 16.Dominy CN, Deane SM, Rawlings DE. 1997. A geographically widespread plasmid from Thiobacillus ferrooxidans has genes for ferredoxin-, FNR-, prismane- and NADH-oxidoreductase-like proteins which are also located on the chromosome. Microbiology 143:3123–3136. doi: 10.1099/00221287-143-10-3123. [DOI] [PubMed] [Google Scholar]
- 17.Han CG, Shiga Y, Tobe T, Sasakawa C, Ohtsubo E. 2001. Structural and functional characterization of IS679 and IS66-family elements. J Bacteriol 183:4296–4304. doi: 10.1128/JB.183.14.4296-4304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gourbeyre E, Siguier P, Chandler M. 2010. Route 66: investigations into the organisation and distribution of the IS66 family of prokaryotic insertion sequences. Res Microbiol 161:136–143. doi: 10.1016/j.resmic.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Norris PR, Ingledew WJ. 1992. Acidophilic bacteria: adaptations and applications. In Herbert RA, Sharp RJ (ed), Molecular biology and biotechnology of extremophiles. Blackie, Glasgow, UK. [Google Scholar]
- 20.Moinier D, Byrne D, Amouric A, Bonnefoy V. 2017. The global redox responding RegB/RegA signal transduction system regulates the genes involved in ferrous iron and inorganic sulfur compound oxidation of the acidophilic Acidithiobacillus ferrooxidans. Front Microbiol 8:1277. doi: 10.3389/fmicb.2017.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandecraen J, Chandler M, Aertsen A, Van Houdt R. 2017. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit Rev Microbiol 43:709–730. doi: 10.1080/1040841X.2017.1303661. [DOI] [PubMed] [Google Scholar]
- 22.Osorio H, Mangold S, Denis Y, Ňancucheo I, Esparza M, Johnson DB, Bonnefoy V, Dopson M, Holmes DS. 2013. Anaerobic sulfur metabolism coupled to dissimilatory iron reduction in the extremophile Acidithiobacillus ferrooxidans. Appl Environ Microbiol 79:2172–2181. doi: 10.1128/AEM.03057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucera J, Pakostova E, Lochman J, Janiczek O, Mandl M. 2016. Are there multiple mechanisms of anaerobic sulfur oxidation with ferric iron in Acidithiobacillus ferrooxidans? Res Microbiol 167:357–366. doi: 10.1016/j.resmic.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Kucera J, Sedo O, Potesil D, Janiczek O, Zdrahal Z, Mandl M. 2016. Comparative proteomic analyzis of sulfur-oxidizing Acidithiobacillus ferrooxidans CCM 4253 cultures having lost the ability to couple anaerobic elemental sulfur oxidation with ferric iron reduction. Res Microbiol 167:587–594. doi: 10.1016/j.resmic.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Schrader JA, Holmes DS. 1988. Phenotypic switching of Thiobacillus ferrooxidans. J Bacteriol 170:3915–3923. doi: 10.1128/jb.170.9.3915-3923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabrejos ME, Zhao HL, Guacucano M, Bueno S, Levican G, Garcia E, Jedlicki E, Holmes DS. 1999. IST1 insertional inactivation of the resB gene: implications for phenotypic switching in Thiobacillus ferrooxidans. FEMS Microbiol Lett 175:223–229. doi: 10.1111/j.1574-6968.1999.tb13624.x. [DOI] [PubMed] [Google Scholar]
- 27.Yarzabal A, Brasseur G, Bonnefoy V. 2002. Cytochromes c of Acidithiobacillus ferrooxidans. FEMS Microbiol Lett 209:189–195. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DB, Hallberg KB. 2007. Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms, p 237–261. In Rawlings DE, Johnson DB (ed), Biomining. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 29.Hedrich S, Johnson DB. 2013. Aerobic and anaerobic oxidation of hydrogen by acidophilic bacteria. FEMS Microbiol Lett 349:40–45. doi: 10.1111/1574-6968.12290. [DOI] [PubMed] [Google Scholar]
- 30.Ňancucheo I, Rowe OF, Hedrich S, Johnson DB. 2016. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol Lett 363(10):fnw083. doi: 10.1093/femsle/fnw083. [DOI] [PubMed] [Google Scholar]
- 31.Stookey L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781. doi: 10.1021/ac60289a016. [DOI] [Google Scholar]
- 32.Moinier D, Slyemi D, Byrne D, Lignon S, Lebrun R, Talla E, Bonnefoy V. 2014. An ArsR/SmtB family member is involved in the regulation by arsenic of the arsenite oxidase operon in Thiomonas arsenitoxydans. Appl Environ Microbiol 80:6413–6426. doi: 10.1128/AEM.01771-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Madan A. 1999. CAP3: a DNA sequence assembly program. Genome Res 9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane DJ. 1991. 16S/23S rRNA sequencing, p115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 38.Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Appia-Ayme C, Guiliani N, Ratouchniak J, Bonnefoy V. 1999. Characterization of an operon encoding two c-type cytochromes, an aa(3)-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl Environ Microbiol 65:4781–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quatrini R, Appia-Ayme C, Denis Y, Jedlicki E, Holmes DS, Bonnefoy V. 2009. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genomics 10:394. doi: 10.1186/1471-2164-10-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence of the IS identified in the mutant was deposited in ISfinder (36) (http://www-is.biotoul.fr) under the name ISAfd1.