ABSTRACT
Human bocavirus (HBoV) has been shown to be a common cause of respiratory infections and gastroenteritis in children. Recently, HBoVs have been detected in sewage and river waters in Italy and worldwide. However, studies on their presence in other water environments and in bivalve mollusks are not yet available. In this study, 316 bivalve shellfish samples collected in three Italian regions over a 6-year period (2012 to 2017) were analyzed by nested PCR and sequencing using broad-range primer pairs targeting the capsid proteins VP1 and VP2 of HBoV. The virus was detected in 27 samples (8.5% of the total samples), and a statistically significant difference was found within the three regions. A further 13 samples, collected in geographic and temporal proximity to positive samples, were included in the study to assess the spread of HBoV in shellfish production areas at the time of contamination. Twelve of these additional samples were found to be positive for HBoV. All positive samples in this study were characterized as HBoV species 2 (17 samples; 8 different sequences) or species 3 (22 samples; 4 different sequences). This study reports the occurrence of HBoV in bivalve shellfish and shows evidence of considerable spatial spread of the virus throughout shellfish production areas. Further studies are needed to elucidate both the role of HBoV as an agent of gastroenteritis and the risk for foodborne transmission of this virus.
IMPORTANCE Human bocavirus is recognized as an important cause of acute respiratory tract infections and has recently been considered an etiological agent of gastroenteritis in the pediatric population. Our findings document that HBoVs are detected in bivalve shellfish with a relevant prevalence and suggest that an assessment of the risk for foodborne transmission of these viruses should be undertaken.
KEYWORDS: bocavirus, PCR, sequencing, mollusk, shellfish-growing areas
INTRODUCTION
Human bocavirus (HBoV) is a member of the Parvoviridae family found worldwide in respiratory samples, mainly from children with acute respiratory infections, and in stool samples from patients with gastroenteritis (1–6).
Four species (HBoV species 1 [HBoV-1], HBoV-2, HBoV-3, and HBoV-4) are currently included in the bocavirus genus. HBoV-1 was first identified in respiratory nasopharyngeal aspirates of children with lower respiratory tract infections (1). It is now recognized as an important cause of acute respiratory tract infections (7), being found globally and throughout the year in about 2 to 20% of airway samples, mainly from children under 5 years of age with respiratory tract illness (8). The other HBoVs, HBoV-2 to -4, are not usually found in airway samples but are detected in several kinds of specimens, including urine, blood/serum, tonsils, saliva, and, primarily, feces (1, 6, 9–12), suggesting that these viruses are transmitted by the fecal-oral route. In particular, in the adult population, HBoV-2 has been detected in 1.5% of stool samples from patients with clinical signs of gastroenteritis (13). Nonetheless, the role of HBoVs as enteropathogens still remains uncertain (14, 15) due to frequent coinfections with other enteric viruses in patients with gastroenteritis (2, 16, 17) and their detection in both symptomatic and asymptomatic individuals (18–20). However, recent studies showing the detection of HBoV alone in stool samples from gastroenteritis cases (21–24) and a meta-analysis of case-control studies (25) hint at a possible role, particularly for HBoV-2, as an etiological agent of gastroenteritis in the pediatric population.
Recently, the presence of HBoV in environmental samples, including wastewaters, sewage sludge, and water impacted by sewage, has also been reported (26–33), therefore suggesting a potential role of water in the transmission of this virus. To date, no information on the presence of HBoV in food matrices is available.
In the present study, we addressed the occurrence and genetic variability of HBoV in bivalve shellfish samples collected over a 6-year period (2012 to 2017) from harvesting areas and retail points in three Italian regions (Campania, Sardinia, and Sicily), in order to provide a clearer picture of the circulation of this virus within the human-water-food interface and of the potential for foodborne transmission of these viruses.
RESULTS AND DISCUSSION
Human bocaviruses are often detected in different clinical specimens, including stool samples, from patients with gastroenteritis (1–6). In the absence of an animal model or an in vitro replication system, the virus cannot yet be confirmed as a causative agent of disease under Koch's revised postulates (14, 34). However, increasing data suggest a role for this virus, at least for HBoV-2, as an etiological agent of gastroenteritis in the pediatric population (25). HBoV can be discharged in untreated wastewaters through virus shedding and, consequently, disperse throughout water environments, as shown recently in Italy (30, 31) and elsewhere (26, 28, 32). Bivalve mollusks concentrate several human pathogens transmitted by the fecal-oral route, including a wide range of enteric viruses (35–37), whose presence in these aquatic organisms may be evaluated to assess the safety of the product for human consumption (38) or for biomonitoring purposes (39, 40).
In the present study, we aimed to estimate the prevalence and genetic diversity of HBoV in bivalve shellfish collected over a 6-year period in three Italian regions (Campania, Sardinia, and Sicily) of the Central Mediterranean Sea. No PCR inhibition was detected in any of the tested samples, and 27 of 316 samples, equivalent to 8.5% of the total (95% confidence interval [CI], 5.7% to 12.2%), were found to be positive for HBoV (Table 1). This prevalence is lower than the frequencies generally reported for norovirus, the major causative agent of viral gastroenteritis detected in shellfish (41, 42), but is comparable to the prevalence described in previous studies undertaken in Italy and in Spain for other enteric viruses such as astrovirus (12.2% to 28.7%), Aichi virus (6.0% to 12.0%), enterovirus (8.0% to 12.2%), or rotavirus (4.9% to 13.0%) (43, 44).
TABLE 1.
Detection of human bocavirus in tested bivalve shellfish per year and region
| Yr | Region |
Total |
||||||
|---|---|---|---|---|---|---|---|---|
| Campania |
Sardinia |
Sicily |
No. of samples | No. of positive samples (% [95% CI]) | ||||
| No. of samples | No. of positive samples (% [95% CI]) | No. of samples | No. of positive samples (% [95% CI]) | No. of samples | No. of positive samples (% [95% CI]) | |||
| 2012 | 46 | 1 | 15 | 1 | 9 | 0 | 70 | 2 (2.9) |
| 2013 | 14a | 2 | 27 | 2 | 41 | 4 (9.8) | ||
| 2014 | 31 | 6 | 22 | 1 | 53 | 7 (13.2) | ||
| 2015 | 52 | 2 | 1a | 1 | 53 | 3 (5.7) | ||
| 2016 | 44 | 5 | 7 | 0 | 51 | 5 (9.8) | ||
| 2017 | 21 | 5 | 27 | 1 | 48 | 6 (12.5) | ||
| Total | 163 | 13 (8.0 [4.3–13.3]) | 61 | 10 (16.4 [8.2–28.1]) | 92 | 4 (4.3 [1.2–10.8]) | 316 | 27 (8.5 [5.7–12.2]) |
Additional samples collected in Sardinia in 2013 and 2015 are not included in this table.
Of the positive samples, 13 were collected in Campania, 10 were collected in Sardinia, and 4 were collected in Sicily. The prevalences of HBoV-positive samples were 8.0% (CI, 4.3% to 13.3%), 16.4% (CI, 8.2% to 28.1%), and 4.3% (CI, 1.2% to 10.8%) in Campania, Sardinia, and Sicily, respectively, with a statistically significant difference (P value of 0.031 by a chi-square test) among the three geographic areas, which may suggest a more intense circulation of HBoV in the populations of certain regions. With regard to the temporal distribution, the prevalence of the virus ranged from 2.9% in 2012 to 13.2% in 2014, with no statistically significant difference among years (P value of 0.299 by a chi-square test) nor with any clear trend in detection. Interestingly, although geographic and temporal fluctuations in the prevalence of HBoV may partially be related to sampling, such variations have also been reported for other enteric viruses such as norovirus, for which previous studies undertaken in Italy showed that the prevalences varied from ∼50% to ∼80%, depending on shellfish origin (37), and from less than 1% to almost 10%, depending on the sampling year, in seafood products collected at the retail level (45). It is noteworthy that in the present study, no statistically significant difference was detectable between products ready for consumption (on the market and collected from class A areas) and shellfish harvested from class B areas (P value of 0.321 by Fisher's exact test) or between different shellfish species (P value of 0.834 for mussels versus clams by Fisher's exact test), although the limited number of positive samples for each group may have affected the significance of the comparison.
Of the 13 additional samples collected in a gulf area of Sardinia, 12 (8 in 2013 and 4 in 2015) were found to be positive, showing that at the time of contamination, HBoV was consistently detected in samples taken from distant points, up to 3 km from each other (see the supplemental material). These additional positive samples were not included in Table 1 so as not to overestimate the prevalence of HBoV.
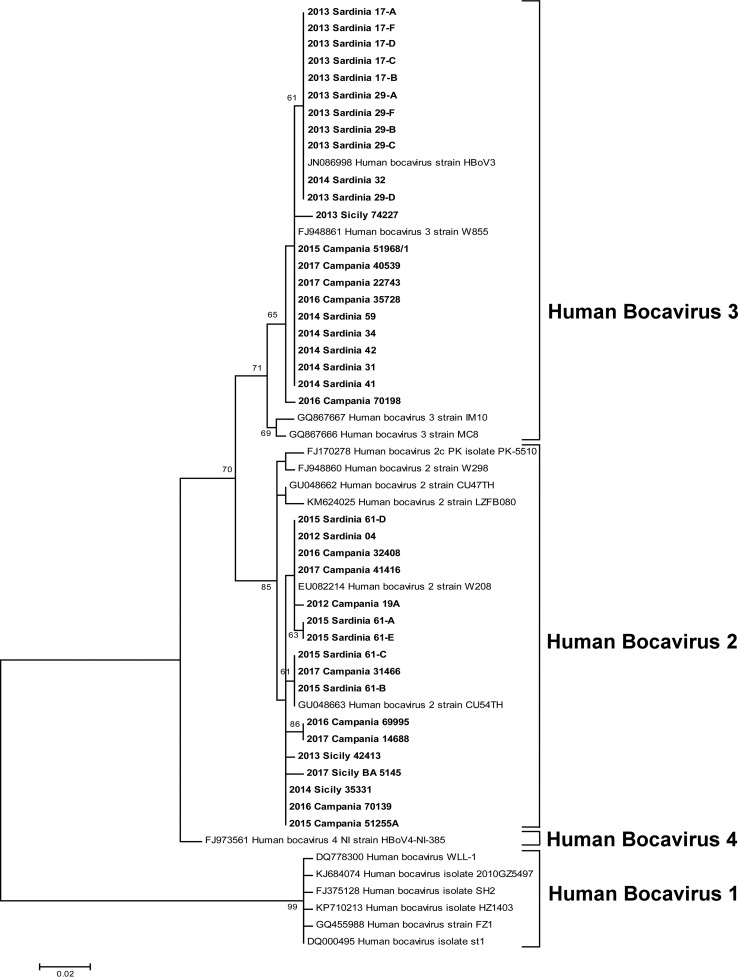
The 39 positive samples (27 positive samples plus 12 sequences from the additional samples) were characterized as HBoV species 2 (17 samples) and species 3 (22 samples) by BLAST searches. No HBoV-4 was detected in the tested samples. Both HBoV-2 and HBoV-3 were detected in samples from all areas and sample years, with the exception of 2012, when HBoV-3 was not detected. The result of the phylogenetic tree constructed with the partial HBoV VP1/2 sequences detected in this study is shown in Fig. 1. The 39 study sequences were compared with 18 genomic sequences available from GenBank, representing HBoV species 1 to 4 (see the legend of Fig. 1). Phylogenetic analysis, in agreement with BLAST searches, showed that 17 sequences (4 of which were obtained from the additional samples) clustered into the HBoV-2 group, whereas 22 (8 from the additional samples) clustered with the HBoV-3 group. Eight and four different sequence variants were detected in the samples for HBoV-2 and HBoV-3, respectively. Nucleotide identities ranged from 98.6% to 100% for HBoV-2 sequences and from 98.3% to 100% for HBoV-3. The mean diversities within the two species, calculated by using MEGA7 (maximum composite likelihood model; 299 positions in a final data set with 39 nucleotide sequences), were 0.007 and 0.003 base substitutions per site for HBoV-2 and HBoV-3, respectively.
FIG 1.
Phylogenetic tree constructed with the partial HBoV VP1/2 sequences. The tree includes 39 study sequences and 18 genomic sequences available from GenBank, representing species 1 (accession numbers DQ778300, KJ684074, FJ375128, GQ455988, KP710213, and DQ000495), species 2 (accession numbers EU082214, GU048663, FJ170278, FJ948860, GU048662, and KM624025), species 3 (GQ867667, GQ867666, FJ948861, and JN086998), and species 4 (FJ973561, KC461233, and KM624027). Evolutionary analyses were conducted with MEGA7. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura 3-parameter model. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Only bootstrap values of >60% are represented. The tree is drawn to scale, with branch lengths measured as the number of substitutions per site. There were a total of 300 positions in the final data set.
The results of this study show that the presence of HBoV is a common and steady feature in the water environment where shellfish are grown and harvested and corroborate data from an environmental study conducted in 2016 in Italy, in which HBoVs were detected in 79% of urban wastewaters (30), with a predominance of species 2 and 3. Moreover, HBoV species 2 and 3 were found to be the most frequently detected viruses in river waters in Italy (31), outnumbering even adenovirus, known to be widespread in water environments (46). The high rate of detection of HBoV in sewage and river samples, coupled with its constant detection in shellfish, is evidence of the high incidence of HBoV in the human population in Italy, possibly associated with undiagnosed gastroenteritis or respiratory disease.
In conclusion, this study reports the detection of HBoV in bivalve mollusks and estimates its prevalence in this food matrix. Although the role of HBoV in gastrointestinal infections still remains unclear and no definitive evidence has yet been provided to support a causative role of HBoV in gastroenteritis, it is important to investigate virus circulation among human hosts, the environment, and animals for human consumption. Further studies are required to determine the significance of HBoV in shellfish and in water environments and to elucidate the risk for foodborne or waterborne transmission of these viruses.
MATERIALS AND METHODS
Sampling.
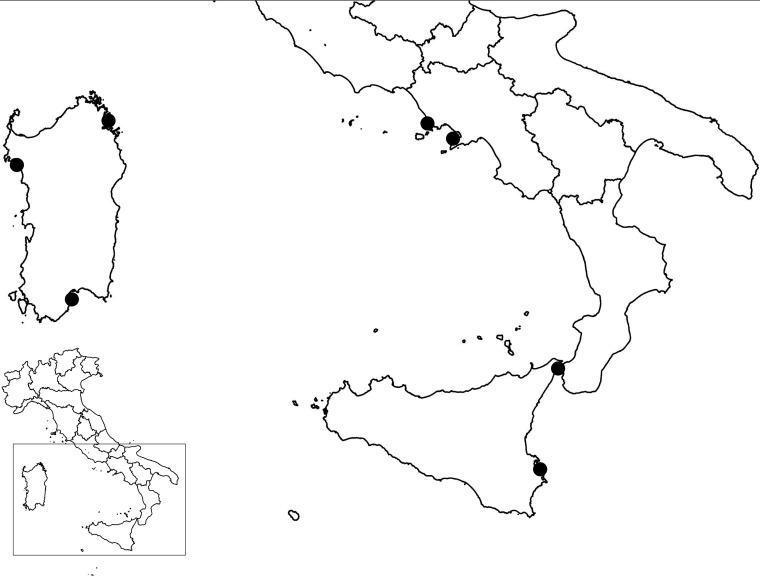
A total of 316 bivalve mollusk samples (264 mussels, 24 carpet shell clams, 12 razor clams, 9 Venus clams, 5 wedge clams, and 2 oysters) collected in three Italian regions on the Central Mediterranean Sea, Campania (n = 163), Sardinia (n = 61), and Sicily (n = 92), were selected for the study (Fig. 2). The samples, collected between 2012 and 2017 (70 samples in 2012, 41 in 2013, 53 in 2014, 53 in 2015, 51 in 2016, and 48 in 2017), were prepared as described below and stored at −70°C until analysis. Samples were collected during official monitoring programs from approved shellfish-harvesting areas (n = 282) of class A (2 areas; n = 8) and class B (42 areas; n = 274) and from dispatch centers, restaurants, fish markets, and supermarkets (n = 35).
FIG 2.
Geographic origin of the shellfish samples included in the study. (Template maps are from the Italian National Institute for Statistics and were modified by the use of QGIS.)
In a later stage of the study, the spatial distribution of HBoV in shellfish production areas during a contamination event was assessed by examining additional samples collected at different points in the areas, in close temporal proximity (within 24 h) to HBoV-positive samples. Thirteen additional mussel samples, harvested in a gulf area of the Sardinia region in 2013 (n = 8) and 2015 (n = 5), were analyzed in this second screening (see the supplemental material).
Sample preparation.
Virus extraction from shellfish samples was performed according to the procedure described in ISO/TS 15216-2:2013 (48). Briefly, depending on the species size, 10 to 60 individuals were randomly selected, and digestive glands were dissected, cleaned, and finely chopped. Aliquots of 2.0 g, spiked with 10 μl of titrated mengovirus process control strain MC0 (1.6 × 104 50% tissue culture infective doses [TCID50]/ml), were digested with 2 ml of proteinase K (0.1 mg/ml) at 37°C for 60 min with shaking and then placed at 60°C for 15 min. Finally, the samples were centrifuged at 3,000 ×g for 5 min, the supernatant was collected, and its volume was recorded. Nucleic acid extraction and purification were performed on 500 μl of the supernatant by using the NucliSens MiniMag system (bioMérieux, France) according to the manufacturer's instructions, and nucleic acids were eluted in 100 μl. The efficiency of virus recovery was assessed as described previously by Costafreda et al. (47), with a 1% acceptability criterion.
Nested PCR for detection of HBoV.
The presence of HBoV genomes was investigated by nested PCR and sequencing using broad-range primer pairs targeting the capsid proteins VP1 and VP2 of HBoV, successfully used previously with both clinical and environmental samples (16, 30, 31).
PCR was performed using 5 μl of DNA and 1 μl (10 pmol) of each primer (2018fw [5′-GAAATGCTTTCTGCTGYTGAAA-3′] and 2029rev [5′-GTGGATATACCCACAYCAGAA-3′] for the first PCR and 2030fw [5′-GGTGGGTGCTTCCTGGTTA-3′] and 2031rev [5′-TCTTGRATTTCATTTTCAGACAT-3′] for nested PCR) in a 25-μl reaction mixture, using the MyTaq Red Mix PCR kit (Bioline, UK). After the initial denaturation step at 94°C (10 min), amplification was performed with 35 cycles of denaturation at 94°C (30 s), annealing at 51°C (30 s), and extension of 72°C (1 min), followed by a final extension step at 72°C (5 min). Two microliters of the PCR product was used as a template in the nested PCR assay, using an annealing temperature of 50°C. As a positive PCR control, HBoV DNA characterized in stool samples from a patient with gastroenteritis (16) was used. Amplifications were carried out with a T100 thermal cycler (Bio-Rad), and standard precautions were followed to prevent PCR contamination. All positive results were confirmed by replicating the analysis in a second, independent PCR amplification. Results were visualized by gel electrophoresis (1.5% agarose gel) using gel red staining (Biotium, CA, USA).
Sequencing and phylogenetic analysis.
PCR products were purified by using a Montage PCRm96 Microwell filter plate (Millipore, MA, USA) and subjected to direct automated sequencing on both strands (Bio-Fab Research, Italy). The raw forward and reverse ABI files obtained by sequencing were aligned and assembled into a consensus sequence by using MEGA7 software, and sequences were submitted for BLAST analysis for genotyping (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Statistical analysis.
Confidence intervals (95%) were calculated for proportions. A chi-square test was performed to compare differences among prevalence values in distinct regions and years. Fisher's exact test was used to compare prevalences between shellfish species and on the basis of collection source (samples taken at the market level and from class A areas, considered a single group, versus samples harvested from class B areas). For the latter comparison, only samples from geographic regions showing no statistically significant differences (i.e., Campania and Sicily) were included. All calculations were performed by using MedCalc software version 17.9.2 (MedCalc Software BVBA, Ostend, Belgium).
Accession number(s).
Nucleotide sequences of the partial VP1/2 genes of the HBoV isolates reported in this study were deposited in GenBank under accession numbers MG669586 to MG669624.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Italian Ministry of Health Ricerca Corrente grant IZS SI 06/14, Sicurezza Alimentare: Ricerca di Virus Enterici in Alimenti di Origine Animale e Vegetale, and Ricerca Finalizzata grant RF-2011-02349693, Vibrio and Viruses in Shellfish: Old and Emerging Pathogens—Evaluation of Exposure Levels for the Implementation of Prevention Strategies.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02754-17.
REFERENCES
- 1.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. 2009. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog 5:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, Triki H, Bahri O, Oderinde BS, Baba MM, Bukbuk DN, Besser J, Bartkus J, Delwart E. 2010. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis 201:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, Alam MM, Sharif S, Angez M, Zaidi S, Delwart E. 2009. A newly identified bocavirus species in human stool. J Infect Dis 199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peltola V, Soderlund-Venermo M, Jartti T. 2013. Human bocavirus infections. Pediatr Infect Dis J 32:178–179. doi: 10.1097/INF.0b013e31827fef67. [DOI] [PubMed] [Google Scholar]
- 6.Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E. 2007. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis 13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Soderlund-Venermo M. 2012. Human bocavirus—the first 5 years. Rev Med Virol 22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 8.Qiu J, Soderlund-Venermo M, Young NS. 2017. Human parvoviruses. Clin Microbiol Rev 30:43–113. doi: 10.1128/CMR.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campe H, Hartberger C, Sing A. 2008. Role of human bocavirus infections in outbreaks of gastroenteritis. J Clin Virol 43:340–342. doi: 10.1016/j.jcv.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, Anderson LJ, Erdman D, Olsen SJ. 2007. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau SK, Yip CC, Que TL, Lee RA, Au-Yeung RK, Zhou B, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis 196:986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pozo F, Garcia-Garcia ML, Calvo C, Cuesta I, Perez-Brena P, Casas I. 2007. High incidence of human bocavirus infection in children in Spain. J Clin Virol 40:224–228. doi: 10.1016/j.jcv.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow BD, Ou Z, Esper FP. 2010. Newly recognized bocaviruses (HBoV, HBoV2) in children and adults with gastrointestinal illness in the United States. J Clin Virol 47:143–147. doi: 10.1016/j.jcv.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Guido M, Tumolo MR, Verri T, Romano A, Serio F, De Giorgi M, De Donno A, Bagordo F, Zizza A. 2016. Human bocavirus: current knowledge and future challenges. World J Gastroenterol 22:8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smits SL, Osterhaus AD, Koopmans MP. 2016. Newly identified viruses in human gastroenteritis: pathogens or not? Pediatr Infect Dis J 35:104–107. doi: 10.1097/INF.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa G, Della Libera S, Iaconelli M, Donia D, Cenko F, Xhelilaj G, Cozza P, Divizia M. 2016. Human bocavirus in children with acute gastroenteritis in Albania. J Med Virol 88:906–910. doi: 10.1002/jmv.24415. [DOI] [PubMed] [Google Scholar]
- 17.Paloniemi M, Lappalainen S, Salminen M, Katka M, Kantola K, Hedman L, Hedman K, Soderlund-Venermo M, Vesikari T. 2014. Human bocaviruses are commonly found in stools of hospitalized children without causal association to acute gastroenteritis. Eur J Pediatr 173:1051–1057. doi: 10.1007/s00431-014-2290-x. [DOI] [PubMed] [Google Scholar]
- 18.Chhabra P, Payne DC, Szilagyi PG, Edwards KM, Staat MA, Shirley SH, Wikswo M, Nix WA, Lu X, Parashar UD, Vinje J. 2013. Etiology of viral gastroenteritis in children <5 years of age in the United States, 2008-2009. J Infect Dis 208:790–800. doi: 10.1093/infdis/jit254. [DOI] [PubMed] [Google Scholar]
- 19.Kim S. 2014. Prevalence of human bocavirus 1 among people without gastroenteritis symptoms in South Korea between 2008 and 2010. Arch Virol 159:2741–2744. doi: 10.1007/s00705-014-2125-0. [DOI] [PubMed] [Google Scholar]
- 20.Nawaz S, Allen DJ, Aladin F, Gallimore C, Iturriza-Gomara M. 2012. Human bocaviruses are not significantly associated with gastroenteritis: results of retesting archive DNA from a case control study in the UK. PLoS One 7:e41346. doi: 10.1371/journal.pone.0041346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasure N, Gopalkrishna V. 2017. Molecular epidemiology and clinical severity of human bocavirus (HBoV) 1-4 in children with acute gastroenteritis from Pune, Western India. J Med Virol 89:17–23. doi: 10.1002/jmv.24593. [DOI] [PubMed] [Google Scholar]
- 22.Tymentsev A, Tikunov A, Zhirakovskaia E, Kurilschikov A, Babkin I, Klemesheva V, Netesov S, Tikunova N. 2016. Human bocavirus in hospitalized children with acute gastroenteritis in Russia from 2010 to 2012. Infect Genet Evol 37:143–149. doi: 10.1016/j.meegid.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang DM, Ma MM, Wen WT, Zhu X, Xu L, He ZJ, He X, Wu JH, Hu YW, Zheng Y, Deng Y, Lin CJ, Lu JH, Li MF, Cao KY. 2015. Clinical epidemiology and molecular profiling of human bocavirus in faecal samples from children with diarrhoea in Guangzhou, China. Epidemiol Infect 143:2315–2329. doi: 10.1017/S0950268814003203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T, Chen Y, Chen J, Hu P, Zheng T, Xu X, Pei X. 2017. Prevalence and clinical profile of human bocavirus in children with acute gastroenteritis in Chengdu, West China, 2012-2013. J Med Virol 89:1743–1748. doi: 10.1002/jmv.24787. [DOI] [PubMed] [Google Scholar]
- 25.De R, Liu L, Qian Y, Zhu R, Deng J, Wang F, Sun Y, Dong H, Jia L, Zhao L. 2017. Risk of acute gastroenteritis associated with human bocavirus infection in children: a systematic review and meta-analysis. PLoS One 12:e0184833. doi: 10.1371/journal.pone.0184833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bibby K, Peccia J. 2013. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ Sci Technol 47:1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blinkova O, Rosario K, Li L, Kapoor A, Slikas B, Bernardin F, Breitbart M, Delwart E. 2009. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol 47:3507–3513. doi: 10.1128/JCM.01062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamza H, Leifels M, Wilhelm M, Hamza IA. 2017. Relative abundance of human bocaviruses in urban sewage in Greater Cairo, Egypt. Food Environ Virol 9:304–313. doi: 10.1007/s12560-017-9287-3. [DOI] [PubMed] [Google Scholar]
- 29.Hamza IA, Jurzik L, Wilhelm M, Uberla K. 5 August 2009. Detection and quantification of human bocavirus in river water. J Gen Virol doi: 10.1099/vir.0.013557-0. [DOI] [PubMed] [Google Scholar]
- 30.Iaconelli M, Divizia M, Della Libera S, Di Bonito P, La Rosa G. 2016. Frequent detection and genetic diversity of human bocavirus in urban sewage samples. Food Environ Virol 8:289–295. doi: 10.1007/s12560-016-9251-7. [DOI] [PubMed] [Google Scholar]
- 31.La Rosa G, Sanseverino I, Della Libera S, Iaconelli M, Ferrero VEV, Barra CA, Lettieri T. 2017. The impact of anthropogenic pressure on the virological quality of water from the Tiber river, Italy. Lett Appl Microbiol 65:298–305. doi: 10.1111/lam.12774. [DOI] [PubMed] [Google Scholar]
- 32.Myrmel M, Lange H, Rimstad E. 2015. A 1-year quantitative survey of noro-, adeno-, human boca-, and hepatitis E viruses in raw and secondarily treated sewage from two plants in Norway. Food Environ Virol 7:213–223. doi: 10.1007/s12560-015-9200-x. [DOI] [PubMed] [Google Scholar]
- 33.Rasanen S, Lappalainen S, Kaikkonen S, Hamalainen M, Salminen M, Vesikari T. 2010. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol Infect 138:1227–1234. doi: 10.1017/S0950268809991671. [DOI] [PubMed] [Google Scholar]
- 34.Fredricks DN, Relman DA. 1996. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev 9:18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.La Rosa G, Della Libera S, Iaconelli M, Proroga YT, De Medici D, Martella V, Suffredini E. 2017. Detection of norovirus GII.17 Kawasaki 2014 in shellfish, marine water and underwater sewage discharges in Italy. Food Environ Virol 9:326–333. doi: 10.1007/s12560-017-9290-8. [DOI] [PubMed] [Google Scholar]
- 36.Romalde JL, Rivadulla E, Varela MF, Barja JL. 2 November 2017. An overview of 20 years of studies on the prevalence of human enteric viruses in shellfish from Galicia, Spain. J Appl Microbiol doi: 10.1111/jam.13614. [DOI] [PubMed] [Google Scholar]
- 37.Suffredini E, Lanni L, Arcangeli G, Pepe T, Mazzette R, Ciccaglioni G, Croci L. 2014. Qualitative and quantitative assessment of viral contamination in bivalve molluscs harvested in Italy. Int J Food Microbiol 184:21–26. doi: 10.1016/j.ijfoodmicro.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Bellou M, Kokkinos P, Vantarakis A. 2013. Shellfish-borne viral outbreaks: a systematic review. Food Environ Virol 5:13–23. doi: 10.1007/s12560-012-9097-6. [DOI] [PubMed] [Google Scholar]
- 39.De Donno A, Grassi T, Bagordo F, Idolo A, Serio F, Gabutti G. 2012. Detection of viruses in coastal seawater using Mytilus galloprovincialis as an accumulation matrix. Food Environ Virol 4:81–88. doi: 10.1007/s12560-012-9079-8. [DOI] [PubMed] [Google Scholar]
- 40.Graczyk TK, Conn DB. 2008. Molecular markers and sentinel organisms for environmental monitoring. Parasite 15:458–462. doi: 10.1051/parasite/2008153458. [DOI] [PubMed] [Google Scholar]
- 41.EFSA Panel on Biological Hazards. 2012. Norovirus (NoV) in oysters: methods, limits and control options. EFSA J 10:2500. doi: 10.2903/j.efsa.2012.2500. [DOI] [Google Scholar]
- 42.Lowther JA, Gustar NE, Powell AL, Hartnell RE, Lees DN. 2012. Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Appl Environ Microbiol 78:5812–5817. doi: 10.1128/AEM.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusco G, Di Bartolo I, Cioffi B, Ianiro G, Palermo P, Monini M, Amoroso MG. 2017. Prevalence of foodborne viruses in mussels in southern Italy. Food Environ Virol 9:187–194. doi: 10.1007/s12560-016-9277-x. [DOI] [PubMed] [Google Scholar]
- 44.Gabrieli R, Macaluso A, Lanni L, Saccares S, Di Giamberardino F, Cencioni B, Petrinca AR, Divizia M. 2007. Enteric viruses in molluscan shellfish. New Microbiol 30:471–475. [PubMed] [Google Scholar]
- 45.Pavoni E, Consoli M, Suffredini E, Arcangeli G, Serracca L, Battistini R, Rossini I, Croci L, Losio MN. 2013. Noroviruses in seafood: a 9-year monitoring in Italy. Foodborne Pathog Dis 10:533–539. doi: 10.1089/fpd.2012.1399. [DOI] [PubMed] [Google Scholar]
- 46.Iaconelli M, Valdazo-Gonzalez B, Equestre M, Ciccaglione AR, Marcantonio C, Della Libera S, La Rosa G. 2017. Molecular characterization of human adenoviruses in urban wastewaters using next generation and Sanger sequencing. Water Res 121:240–247. doi: 10.1016/j.watres.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 47.Costafreda MI, Bosch A, Pinto RM. 2006. Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Appl Environ Microbiol 72:3846–3855. doi: 10.1128/AEM.02660-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ISO 2013. ISO/TS 15216-2 Microbiology of food and animal feed—horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR—part 2: method for qualitative detection. ISO, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.