ABSTRACT
We aimed at identifying potential bacterial factors linking clostridia with necrotizing enterocolitis (NEC). We compared the phenotypic traits, stress responses, cellular cytotoxicity, and inflammatory capabilities of the largest collection of Clostridium butyricum and Clostridium neonatale strains isolated from fecal samples of NEC preterm neonates (PN) and control PNs. When strain characteristics were used as explanatory variables, a statistical discriminant analysis allowed the separation of NEC and control strains into separate groups. Strains isolated from NEC PN were characterized by a higher viability at 30°C (P = 0.03) and higher aerotolerance (P = 0.01), suggesting that NEC strains may have a competitive and/or survival advantage in the environmental gastrointestinal tract conditions of NEC PN. Heat-treated NEC bacteria induced higher production of interleukin-8 in Caco-2 cells (P = 0.03), suggesting proinflammatory activity. In vitro, bacteria, bacterial components, and fecal filtrates showed variable cytotoxic effects affecting the cellular network and/or cell viability, without specific association with NEC or control samples. Altogether, our data support the existence of a specific clostridial strain signature associated with NEC.
IMPORTANCE Clostridia are part of the commensal microbiota in preterm neonates (PN). However, microbiota analyses by culture and metagenomics have linked necrotizing enterocolitis (NEC) and intestinal colonization with clostridial species. Nevertheless, little is known about the specific characteristics that may be shared by clostridia associated with NEC compared to commensal clostridia. Therefore, our goal was to identify specific bacterial factors linking clostridial strains with NEC. We report the existence of a specific bacterial signature associated with NEC and propose that activation of the innate immune response may be a unifying causative mechanism for the development of NEC independent of a specific pathogenic organism. The present study provides new insights into NEC pathophysiology that are needed for better diagnostics and strategies for implementing prevention of the disease.
KEYWORDS: clostridia, necrotizing enterocolitis, preterm neonates, Clostridium butyricum, Clostridium neonatale
INTRODUCTION
The incidence of prematurity is increasing worldwide, and the survival of very preterm neonates (PN) has improved over the last decades (1). Necrotizing enterocolitis (NEC) is the most severe and life-threatening gastrointestinal disease among PN and continues to account for substantial morbidity and mortality in neonatal intensive care units (2). In developed countries, NEC incidence can represent up to 13% of PN (≤33 weeks of gestation or birth weight of ≤2,500 g) (2). Gut bacterial colonization (3), unbalanced inflammatory responses (4), and feeding strategies (5) are major factors implicated in NEC development. However, NEC pathophysiology still remains unclear, impairing the development of novel and effective strategies for disease prevention and treatment.
The role of microbiota in NEC is supported by clinical outbreaks, the fact that early antibiotic use is associated with an increased risk of NEC (6), and the fact that NEC cannot be produced in germfree animals (7). The use of new molecular and analytical technologies linking alterations of the intestinal microbiota with the development of NEC has received tremendous interest and led to proposal of some microbial colonization patterns (2, 3). The microbial dysbiosis preceding NEC was characterized by an increase of Proteobacteria and a decrease in abundances of Firmicutes and Bacteroidetes (3). Other studies linked NEC to Clostridium spp. (5, 8). However, to date, no single infectious agent could be consistently linked with NEC (9).
Although clostridia are part of the commensal microbiota in PN (10), some species have been involved with NEC epidemiological studies (11, 12) and were identified from biological samples of NEC cases (5, 8, 11, 13–18). Based on molecular studies, we very recently linked Clostridium neonatale and Clostridium butyricum with NEC (5). Cassir et al. (8) also associated C. butyricum with the disease. Meanwhile, Clostridium perfringens (14) or a C. perfringens-like group was also associated with NEC (13).
Theories about NEC pathogenesis have centered on the relationship between gut microbiota and mucosal immunity (4). In contrast, little is known about the specific characteristics that may be shared by clostridial strains associated with NEC compared to commensal clostridia. Hence, we aimed at challenging the hypothesis that clostridia isolated from NEC cases would have specific signatures compared to those isolated from healthy PN. In the present study, we focused our work on C. butyricum and C. neonatale, the two species that we associated with NEC (5). We took advantage of two multicenter population-based cohorts to compare the characteristics of the largest collection of clostridial strains isolated from fecal samples of NEC and control PN. Strain comparison included phenotypic traits, stress responses, cellular cytotoxicity, and inflammatory capabilities.
RESULTS
Population samples.
A total of 30 C. butyricum and 35 C. neonatale strains were isolated from 60 fecal samples from 29 NEC PN and 31 control PN. The clinical characteristics of the PN are presented in Table 1. Among the 29 NEC PN, 13 (45%) presented specific radiological signs (pneumatosis intestinalis or portal venous air); 5 (17%) of them were treated by surgery.
TABLE 1.
Clinical characteristics of preterm neonates
| Characteristic | Value for indicated patient group |
|
|---|---|---|
| Necrotizing enterocolitis | Control | |
| No. in group | 29 | 31 |
| Gestational age, wks (mean ± SD) | 28.0 ± 1.8 | 28.7 ± 1.6 |
| Birth wt, g (mean ± SD) | 1,087 ± 245 | 1,110 ± 229 |
| Enteral feeding [no. (%)] | 24 (83) | 30 (97) |
| Breastfed [no. (%)] | 15 (52) | 22 (71) |
| Cesarean [no. (%)] | 17 (59) | 20 (65) |
| Antibiotics [no. (%)] | 15 (52) | 16 (52) |
Comparison of NEC versus control strains.
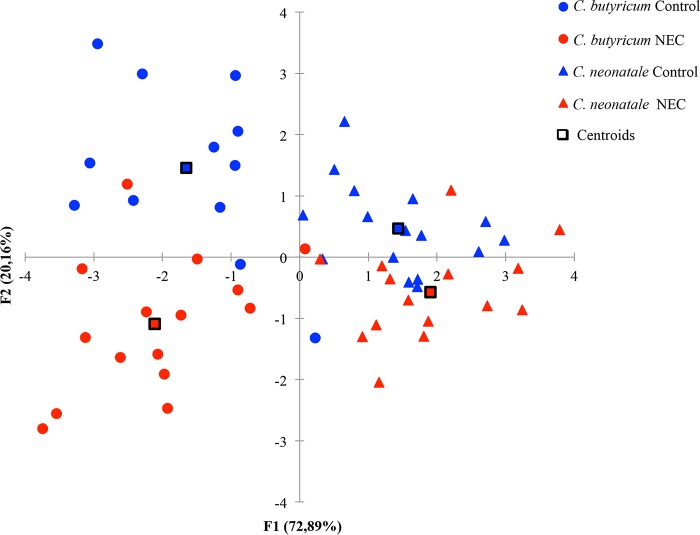
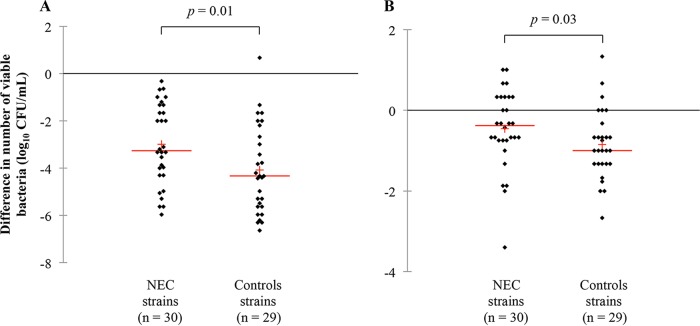
When phenotypic and inflammatory data of strains were used as explanatory variables, a statistical quadratic discriminant analysis allowed separation of strains in NEC and control groups (Fig. 1). Discrimination of strains into their respective species was possible. Strains isolated from NEC PN showed higher aerotolerance (P = 0.01) and viability at 30°C (P = 0.03) (Table 2 and Fig. 2). In terms of aerotolerance, 20 NEC (67%) and 25 control (86%) strains exhibited a greater than 2-log10-CFU/ml reduction in cell numbers. As for temperature viability, the reduction in cell numbers at 30°C was exhibited by 50% (n = 15) and 76% (n = 22) of the NEC and control strains, respectively. No significant differences were observed for bacterial motility, cell surface hydrophobicity, or tolerance to bile, H2O2, acid (pH 4.5), or NaCl (see Tables S1 and S2 in the supplemental material).
FIG 1.
Discriminant quadratic analysis using NEC and control strain characteristics. Explanatory variables were motility (swimming, swarming, twitching), surface hydrophobicity, tolerance to H2O2 (1%, vol/vol), porcine bile salts (0.3%, wt/vol), acid (pH 4.5), NaCl (5% and 9%, wt/vol), and temperature (30°C and 50°C), aerotolerance, and tolerance to IL-8 stimulation.
TABLE 2.
Aerotolerance, temperature viability, and IL-8 production stimulated by heat-treated bacteria of NEC and control strains in Caco-2 cells
| Strain group (n) | Aerotolerancea (95% CIb) (log10 CFU/ml) | Reduction in viable bacteriaa (95% CI) (log10 CFU/ml) at temp: |
IL-8 production by heat-treated bacteriac (95% CI) (pg/ml) | |
|---|---|---|---|---|
| 30°C | 50°C | |||
| Control (29) | −4.08 (−4.78 to −3.37) | −0.85 (−1.18 to −0.52) | −5.85 (−6.42 to −5.27) | 43.8 (31.7 to 56.0) |
| NEC (30) | −2.99 (−3.62 to −2.35) | −0.46 (−0.82 to −0.10) | −5.02 (−5.84 to −4.21) | 62.4 (46.1 to 78.7) |
| P value | 0.01d | 0.03d | 0.19 | 0.03d |
Average differences in cell count of the tested strains compared to the experimental control strains.
95% CI, 95% confidence interval.
Average IL-8 level of the tested strains.
Significant difference between the NEC and control strains (Wilcoxon-Mann and Whitney U test [P < 0.05]).
FIG 2.
NEC and control strain aerotolerance (A) and viability at 30°C (B). Lines represent the median value; crosses represent the mean value.
Stimulation of interleukin-8 (IL-8) production was assessed in Caco-2 cells exposed to bacteria, bacterial culture supernatants, heat-treated bacteria, bacterial debris, and cytoplasmic contents. We showed that heat-treated NEC bacteria induced higher IL-8 production levels (P = 0.03) (Table 2 and Fig. 3). The values of IL-8 concentrations ranged from 2 to 436 pg/ml (Table 2; see also Table S5 in the supplemental material).
FIG 3.
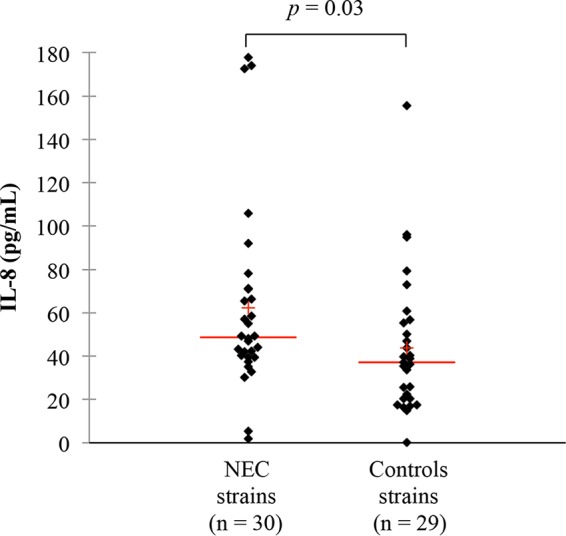
IL-8 secretion in Caco-2 cells after incubation for 24 h with heat-treated bacteria (dilution, 1/20) of NEC and control strains. IL-8 concentration was determined by enzyme-linked immunosorbent assay (ELISA). Lines represent the median value; crosses represent the mean value.
Comparison of NEC versus control strains at the species level.
When considering C. butyricum, NEC strains showed a significantly higher aerotolerance (P = 0.02), tolerance to acid (P = 0.02), and viability at 50°C (P = 0.04) (see Table S3 and Fig. S1 to S3 in the supplemental material). C. neonatale NEC strains showed significantly higher viability at 30°C than control strains (P = 0.02) (see Table S3 and Fig. S4 in the supplemental material). Values corresponding to the phenotypic and stress tests without statistically significant differences are presented in Table S3 and S4 in the supplemental material.
At the species level, NEC C. butyricum bacteria (P = 0.046), heat-treated bacteria (P = 0.03), and bacterial debris (P = 0.02) showed higher stimulation of IL-8 production in Caco-2 cells than control strains (see Table S6 and Fig. S5 to S7 in the supplemental material).
Cytotoxic activity of bacterial and fecal filtrates.
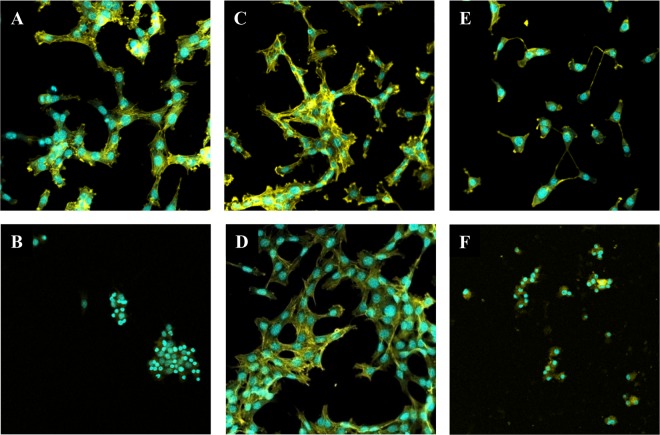
For cytotoxicity assays, 3T3 mouse embryo fibroblasts were exposed to 2-fold serial dilutions of bacteria, their culture supernatants, bacterial debris, or cytoplasmic contents. Results showed either an absence of or a low-titer cytotoxic effect at the highest concentration tested (1/20) (data not shown). Cytotoxicity of filtrates obtained from fecal samples containing C. butyricum (n = 12), C. neonatale (n = 14), C. butyricum and C. neonatale (n = 2), or neither of these 2 species (n = 4) was also tested. A cytotoxic effect was observed for 30 samples out of 32 tested (titer ranging from 1/20 to 1/600) (see Fig. S8 in the supplemental material). Heat or phenylmethylsulfonyl fluoride (PMSF) treatment of the fecal filtrates showed an absence of specific effect on the observed cellular cytotoxicity (P > 0.05). However, when cell viability was assessed by means of ATP quantification, few samples showed a decrease (25%) in cell viability (data not shown). Confocal microscopy confirmed the cytotoxic effect of the fecal filtrates, showing an absence of effect (Fig. 4C and D), a disorganized actin network (Fig. 4E), or a disorganized actin network and affected nucleus suggesting cellular death (Fig. 4F). Although bacteria, their components, and fecal filtrates showed cellular cytotoxic effects, the activity of the observed cytotoxicity was variable and not specific to NEC or control fecal samples.
FIG 4.
Confocal microscopy observation of examples of cytotoxic effects of fecal filtrates on 3T3 cells (2 × 104 cells/well) in a Lab-Tek 8-chamber slide system after 24 h of exposure. Actin and cell nuclei were stained with phalloidin (yellow) and TO-PRO-3 (blue), respectively. (A) Negative control (PBS); (B) positive control (toxigenic C. difficile fecal filtrate at a dilution of 1/2,000); (C and D) absence of cytotoxic effect (dilution, 1/100); (E) positive cytotoxic effect; and (F) fecal filtrate with a positive cytotoxic effect and decreased cell viability (dilution, 1/100). Magnification, 400×.
DISCUSSION
The abnormal pattern of the gut microbiota in PN is recognized as a key factor in NEC pathogenesis. Bacteria very likely participate in NEC by increasing disease progression and severity of lesions. Nevertheless, data concerning the role of potential pathogenic bacterial factors that may participate in NEC development are scarce. In this study, we chose to work with clostridia because epidemiological studies, clinical signs, and animal models repeatedly support participation of clostridia in NEC development.
When phenotypic and immune inflammatory properties were used as explanatory variables, a statistical discriminant analysis allowed separation of strains into NEC and control groups. This suggests the existence of a specific signature characterizing clostridial strains isolated from NEC PN. In our experimental conditions, NEC strains showed higher viability at 30°C and higher aerotolerance. Tolerance to oxidative stress conditions involves chaperone protein expression to optimize cell viability (19). Besides, as anaerobic bacteria, clostridia maintain their cellular redox balance through oxidative stress response and modulation of the fermentation end products (20, 21). In PN with NEC, intestinal mucosal damage is proposed to be a consequence of the inflammatory process (4). During the inflammatory cascade, oxidative stress is induced by reactive oxygen species that may result in a disadvantageous environment for Clostridium spp. (22). Temperature can influence Gram-positive cell walls (23). Therefore, we suggest the possible adaptation of some strains to the environmental gastrointestinal tract conditions of NEC PN.
In the current study, heat-treated bacteria from NEC cases induced higher production of IL-8 by Caco-2 cells. We suggest that clostridial NEC strains may activate signaling pathways, resulting in a cellular response, i.e., IL-8 production. Interestingly, increased expression levels of proinflammatory cytokines, including IL-8, have been reported in NEC (4). Among the family of pattern recognition receptors (PRR), the Toll-like receptor TLR-2 and NOD2-like receptors have been implicated in the pathogenesis of NEC in human and animal studies (4). These receptors sense bacterial cell wall components, such as peptidoglycan, and trigger an innate immune response (24). To date, no data are available about involvement of clostridia in the inflammatory process during NEC. Our results indirectly link Gram-positive bacterial cell wall components as potential microbe-associated molecular patterns participating in the local inflammation process. Of note, Gram-negative bacterial cell wall lipopolysaccharide activation of epithelial cell TLR-4 plays a major role in the inflammatory signaling and development of lesions in NEC (4). We showed a higher viability at 30°C of NEC strains, suggesting potential cell wall differences (23). Indirectly, our data support the hypothesis that the development of NEC may occur by activation of the innate immune response independently of a specific pathogenic organism. This hypothesis may be one explanation as to why no specific bacteria or bacterial colonization pattern has been causally associated with the development of NEC.
Some reports have suggested a potential role for clostridial toxigenic factors in the pathogenesis of NEC. Although toxin production (8, 16, 25) or metabolic fermentation end products (26, 27) have been proposed to participate in NEC lesion development, evidence is still limited and no clear positive correlation has been found. We report that bacteria and their components have no or low-titer cytotoxic effects in vitro. Because in vitro assays do not necessarily inform about in vivo production of toxigenic factors, we tested the cytotoxicity activity of PN fecal filtrates. Our data showed an absence of specific associations between the observed cytotoxic effects and the tested NEC or control fecal samples.
Differences in biological behavior reflecting species specificity were reported in C. butyricum and C. neonatale strains isolated from fecal samples of healthy PN (28). In the present study, we confirmed strain discrimination into different categories corresponding to their respective species by a statistical discriminant analysis.
A systematic characterization of the largest collection of NEC and control strains enabled their discrimination, suggesting the existence of a specific signature. The hypothesis that other bacteria or clostridial species may share the same signature needs further investigation. Approaches have been proposed for the prevention of NEC, but the absence of consensus is partly due to the poorly understood pathogenesis of NEC. We propose that activation of the innate immune response may be a unifying causative mechanism for the development of NEC independent of a specific pathogenic organism. This work gives new insights into NEC pathophysiology that are needed for better diagnostics and strategies for implementing prevention of the disease.
MATERIALS AND METHODS
Patients.
PN were recruited in 20 French neonatal intensive care units (NICUs) that participated in the EPIFLORE (Etude épidémiologique de la FLORE) (5) and ClosNEC (Clostridium Necrotizing Enterocolitis) (ClinicalTrials.gov registration no. NCT02444624) cohorts. Approval was obtained from the National Data Protection Authority (Commission Nationale de l'Informatique et des Libertés, approval no. 8911009 and 915094, respectively) and the Consultative Committee on the Treatment of Information on Personal Health Data for Research Purposes (reference no. 10.626 and 15.055, respectively). Parents provided informed consent. NEC and control PN were matched for gestational age, birth weight, feeding, and mode of delivery and were present in the same NICU. NEC was defined as the presence of clinical evidence fulfilling modified Bell's stage 2 or 3 criteria for NEC (29) and was confirmed by the clinical team caring for the PN. PN who met criteria for spontaneous intestinal perforation were excluded. No NEC outbreak was declared during the inclusion period, and NEC cases were thus considered sporadic.
Fecal samples, bacterial strains, and growth conditions.
Stools were collected from diapers and placed into sterile tubes containing 0.5 ml brain heart infusion broth with 15% glycerol as a cryoprotective agent and immediately frozen at −80°C until analysis. Stool sampling of NEC cases was performed at the disease onset (first stool issued after the diagnosis). Clostridial strain isolation was performed on Columbia cysteine agar base (160 mg/liter) supplemented with sheep blood (5%), whole milk (5%), colistin (10 mg/liter), and neutral red (40 mg/liter). Medium incubation was performed for 48 h at 37°C under anaerobic conditions (CO2:H2:N2, 10:10:80) (MACS anaerobic chamber; bioMérieux, France). Species identification was performed by bacterial 16S rRNA gene sequencing as previously reported (10). Unless otherwise stated, strains were grown on Columbia cysteine agar medium (Oxoid, France) supplemented with 5% (vol/vol) sheep blood or in tryptone glucose yeast extract (TGY) broth (Bacto tryptone, 30 g/liter; glucose, 5 g/liter; yeast extract, 20 g/liter; and l-cysteine, 0.5 g/liter) at 37°C under anaerobic conditions for 24 h.
Phenotypic characterization.
Strain motility (swimming, swarming, or twitching), surface hydrophobicity, aerotolerance, tolerance to H2O2 (1%, vol/vol), porcine bile salts (0.3%, wt/vol), acid (pH 4.5), and NaCl (5% and 9%, wt/vol), and temperature viability (at 30°C and 50°C) were assessed as previously described by Schönherr-Hellec et al. (28).
Cell lines.
All medium components were purchased from Gibco (Fisher Scientific, France), unless otherwise stated. Human colon adenocarcinoma Caco-2 cells, 3T3 mouse embryo fibroblasts, and fibroblast-like green monkey kidney Vero cells (kindly provided by M. Popoff, Pasteur Institute, Paris, France) were grown at 37°C in a 5% CO2 humidified atmosphere. Vero cells were maintained in Dulbecco's modified Eagle medium (DMEM)/GlutaMax supplement, high glucose, supplemented with 10% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The same medium supplemented with 1% (vol/vol) nonessential amino acids was used for maintenance of 3T3 and Caco-2 cells. Medium was changed every day and trypsinization was performed using 0.05% Trypsin-EDTA solution at cell confluence. Cells were seeded in 96-well culture plates at 5 × 104 cells/well 24 h prior to cellular assays. Cellular assays were performed in media supplemented with 0.1% FBS.
Production of bacteria, bacterial components, and fecal filtrates.
Bacteria and bacterial components were prepared using an 18-h TGY bacterial culture. After centrifugation, the culture supernatants were filtered (0.2-μm-pore-size filter; Millipore), and the bacterial pellets were washed, resuspended in 1× phosphate-buffered saline (PBS), and divided into 2 aliquots, one being heat-treated at 100°C for 15 min. Additionally, bacterial cultures were lysed by sonication (Vibra-Cell 72434; Bioblock Scientific, France), and the bacterial debris and cytoplasmic contents were separated by centrifugation. For fecal filtrate preparation, 0.1 g/ml of each frozen stool sample was homogenized in 1× PBS, centrifuged, and filtered (0.2-μm-pore-size filter; Millipore).
Analysis of IL-8 secretion.
Caco-2 cells in 96-well plates (5 × 104 cells/well) were exposed for 24 h to bacteria (dilution, 1/20), bacterial culture supernatants (2-fold serial dilutions up to 1/16), heat-treated bacteria (dilution, 1/20), bacterial debris (dilution, 1/20), or cytoplasmic contents (dilution, 1/2). After centrifugation, the cellular culture medium was collected and used for IL-8 concentration determination using the human IL-8 enzyme-linked immunosorbent assay (ELISA) Ready-Set-Go kit (eBioscience, USA) according to the manufacturer's specifications. Bacteria and bacterial components were prepared as described in the supplemental material.
Cytotoxicity assay.
Cytotoxicity activity was tested on Caco-2, 3T3, and Vero cells in 96-well plates (5 × 104 cells/well). Morphological changes were best visualized in 3T3 cells compared to cells receiving 1× PBS (negative control) or toxigenic Clostridium difficile filtrate (positive control) after 24 h of contact. Cellular cytotoxicity was observed using an inverted microscope (200× magnification). Twofold serial dilutions up to 1/320 of bacteria, culture supernatants, bacterial debris, and cytoplasmic contents were tested. Twofold serial dilutions (up to 1/1,000) of fecal filtrates either treated or not treated by heat (100°C for 15 min) or by PMSF (1 mM) were also tested. Bacteria, bacterial components, and fecal filtrate preparation are described in the supplemental material.
Confocal microscopy.
3T3 cells (2 × 104 cells/well) in a Lab-Tek 8-chamber slide system (Fisher Scientific) were stained with Alexa Fluor 488 phalloidin (Fisher Scientific), and cell nuclei were counterstained with To-Pro-3 (Invitrogen, France). Cells were observed on a Leica TCS SP2 confocal microscope (Leica Microsystems). The two color-coded channels were merged using ImageJ software (30).
Statistical analysis.
XLSTAT 2014 add-on software was used for statistical analysis. The Wilcoxon-Mann and Whitney U test for ranked data (two-tailed, P < 0.05) was used to compare the quantitative values between strain populations. The Fisher exact test (P < 0.05) was used for categorical values. A quadratic discriminant analysis (multivariate test) was performed using NEC and control strain phenotypic and inflammatory data as explanatory variables.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the French National Research Agency (grant ANR-PRTS-0018) and by the French government under École Doctorale MTCI no. 563.
We thank all the pediatricians that participated in the EPIFLORE and ClosNEC study groups, and the Cellular and Molecular Imaging Facility, INSERM UMS 025—CNRS UMS 3612, Faculté de Pharmacie de Paris, Université Paris Descartes, Paris, France, for their help with confocal microscopy.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02428-17.
REFERENCES
- 1.Blencowe H, Cousens S, Chou D, Oestergaard MZ, Say L, Moller AB, Kinney M, Lawn J. 2012. 15 million preterm births: priorities for action based on national, regional and global estimates, p 17–31. In Howson CP, Kinney MV, Lawn JE (ed), Born too soon: the global action report on preterm birth. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Niño DF, Sodhi CP, Hackam DJ. 2016. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol 13:590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. 2017. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5:31. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho SX, Berger PJ, Nold-Petry CA, Nold MF. 2016. The immunological landscape in necrotising enterocolitis. Expert Rev Mol Med 18:e12. doi: 10.1017/erm.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozé J-C, Ancel P-Y, Lepage P, Martin-Marchand L, Nabhani ZA, Delannoy J, Picaud J-C, Lapillonne A, Aires J, Durox M, Darmaun D, Neu J, Butel M-J, Nutrition EPIPAGE 2 Study Group, EPIFLORE Study Group. 2017. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr 106:821–830. doi: 10.3945/ajcn.117.152967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander VN, Northrup V, Bizzarro MJ. 2011. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 159:392–397. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, Moelbak L, Buddington RK, Weström BR, Holst JJ, Burrin DG. 2006. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130:1776–1792. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Cassir N, Benamar S, Khalil JB, Croce O, Saint-Faust M, Jacquot A, Million M, Azza S, Armstrong N, Henry M, Jardot P, Robert C, Gire C, Lagier J-C, Chabrière E, Ghigo E, Marchandin H, Sartor C, Boutte P, Cambonie G, Simeoni U, Raoult D, Scola BL. 2015. Clostridium butyricum strains and dysbiosis linked to necrotizing enterocolitis in preterm neonates. Clin Infect Dis 61:1107–1115. doi: 10.1093/cid/civ468. [DOI] [PubMed] [Google Scholar]
- 9.Coggins SA, Wynn JL, Weitkamp J-H. 2015. Infectious causes of necrotizing enterocolitis. Clin Perinatol 42:133–154. doi: 10.1016/j.clp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraris L, Butel MJ, Campeotto F, Vodovar M, Rozé JC, Aires J. 2012. Clostridia in premature neonates' gut: incidence, antibiotic susceptibility, and perinatal determinants influencing colonization. PLoS One 7:e30594. doi: 10.1371/journal.pone.0030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfa MJ, Robson D, Davi M, Bernard K, Van Caeseele P, Harding GKM. 2002. An outbreak of necrotizing enterocolitis associated with a novel Clostridium species in a neonatal intensive care unit. Clin Infect Dis 35:S101–S105. doi: 10.1086/341929. [DOI] [PubMed] [Google Scholar]
- 12.Han VKM, Sayed H, Chance GW, Brabyn DG, Shaheed WA. 1983. An outbreak of Clostridium difficile necrotizing enterocolitis: a case for oral vancomycin therapy? Pediatrics 71:935–941. [PubMed] [Google Scholar]
- 13.Heida FH, van Zoonen AGJF, Hulscher JBF, te Kiefte BJC, Wessels R, Kooi EMW, Bos AF, Harmsen HJM, de Goffau MC. 2016. A necrotizing enterocolitis-associated gut microbiota is already present in the meconium: results of a prospective study. Clin Infect Dis 62:863–870. doi: 10.1093/cid/ciw016. [DOI] [PubMed] [Google Scholar]
- 14.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li M-S, Haddad M, Langford PR, Cookson WOCM, Moffatt MF, Kroll JS. 2015. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clin Infect Dis 60:389–397. doi: 10.1093/cid/ciu822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Shan G, Sodergren E, Weinstock G, Walker WA, Gregory KE. 2015. Longitudinal analysis of the premature infant intestinal microbiome prior to necrotizing enterocolitis: a case-control study. PLoS One 10:e0118632. doi: 10.1371/journal.pone.0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittmar E, Beyer P, Fischer D, Schäfer V, Schoepe H, Bauer K, Schlösser R. 2008. Necrotizing enterocolitis of the neonate with Clostridium perfringens: diagnosis, clinical course, and role of alpha toxin. Eur J Pediatr 167:891–895. doi: 10.1007/s00431-007-0614-9. [DOI] [PubMed] [Google Scholar]
- 17.Smith B, Bodé S, Petersen BL, Jensen TK, Pipper C, Kloppenborg J, Boyé M, Krogfelt KA, Mølbak L. 2011. Community analysis of bacteria colonizing intestinal tissue of neonates with necrotizing enterocolitis. BMC Microbiol 11:73. doi: 10.1186/1471-2180-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Cochetière M-F, Piloquet H, des Robert C, Darmaun D, Galmiche J-P, Rozé J-C. 2004. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr Res 56:366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 19.Tomas CA, Welker NE, Papoutsakis ET. 2003. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcriptional program. Appl Environ Microbiol 69:4951–4965. doi: 10.1128/AEM.69.8.4951-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Nie X, Ravcheev DA, Rodionov DA, Sheng J, Gu Y, Yang S, Jiang W, Yang C. 2014. Redox-responsive repressor Rex modulates alcohol production and oxidative stress tolerance in Clostridium acetobutylicum. J Bacteriol 196:3949–3963. doi: 10.1128/JB.02037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasaki S, Nakagawa T, Nishiyama Y, Benno Y, Uchimura T, Komagata K, Kozaki M, Niimura Y. 1998. Effect of oxygen on the growth of Clostridium butyricum (type species of the genus Clostridium), and the distribution of enzymes for oxygen and for active oxygen species in clostridia. J Ferment Bioeng 86:368–372. doi: 10.1016/S0922-338X(99)89006-0. [DOI] [Google Scholar]
- 22.Imlay JA. 2002. How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv Microb Physiol 46:111–153. doi: 10.1016/S0065-2911(02)46003-1. [DOI] [PubMed] [Google Scholar]
- 23.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. doi: 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm R, Staneck JL, Stauffer LR, Neblett WW. 1980. Neonatal necrotizing enterocolitis associated with penicillin-resistant, toxigenic Clostridium butyricum. Pediatrics 66:928–931. [PubMed] [Google Scholar]
- 26.Popoff MR, Jolivet-Reynaud C, Carlier JP. 1987. Cytotoxic activity of Clostridium butyricum supernatants induced by butyrate. FEMS Microbiol Lett 43:95–100. doi: 10.1111/j.1574-6968.1987.tb02104.x. [DOI] [Google Scholar]
- 27.Waligora-Dupriet A-J, Dugay A, Auzeil N, Huerre M, Butel M-J. 2005. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res 58:629–635. doi: 10.1203/01.PDR.0000180538.13142.84. [DOI] [PubMed] [Google Scholar]
- 28.Schönherr-Hellec S, Klein G, Delannoy J, Ferraris L, Friedel I, Rozé JC, Butel MJ, Aires J. 2017. Comparative phenotypic analysis of “Clostridium neonatale” and Clostridium butyricum isolates from neonates. Anaerobe 48:76–82. doi: 10.1016/j.anaerobe.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Walsh MC, Kliegman RM. 1986. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.