Abstract
Background
Decreased muscle strength is strongly associated with future mobility limitations in older adults. Homocysteine is a risk factor for vascular disease and may exacerbate muscle strength decline. The present study aimed to examine the association between homocysteine levels and muscle strength in adults aged 50 years or older.
Methods
Data were from 1,101 participants of The Baltimore Longitudinal Study of Aging between December 2004 and March 2015. Muscle strength was measured using grip strength. Mixed effects linear regression was used to estimate the association between homocysteine and muscle strength in men and women, separately.
Results
Total mean follow-up time was 4.7 ± 3.1 years, range from 0 to 10.1 years. Baseline mean grip strength was 39.9 kg for men and 25.5 kg for women. Grip strength declined over the follow-up time for both men and women. Among women, there was a significant inverse relationship between homocysteine and grip strength, where grip strength declined as a function of increasing homocysteine over time (β = −0.05, p = .031). Among men, an increase of 1 μmol/L in homocysteine was associated with −0.10 kg decrease in grip strength, though not significantly.
Conclusions
In this study of healthy older adults aged 50 years or older, higher homocysteine was related to lower muscle strength in women. This is the first study to characterize the relationship over a long follow-up period. Future research should focus on assessing homocysteine as a marker of physical function decline and translating the relationship into clinical and public health practice.
Keywords: Muscle, Physical function, Longitudinal
Aging is associated with a progressive decline in skeletal muscle strength, which is critical to maintaining physical functioning and mobility in older age. Decreased muscle strength is strongly associated with future mobility limitations in adults aged 65 years and older (1). Previous research has shown muscle strength in women declines more slowly than in men (2,3), which may be reflective of women having a smaller percentage of muscle mass in their arms and thus less to lose (4). Grip strength correlates well with total body strength (5) and in a pooled analysis, muscle weakness, defined using grip strength <26 kg for men and <16 kg for women, was associated with increased odds of mobility impairment regardless of height, disease status, and body mass index (BMI) in 11 cohorts of adults aged 65 years and older (5).
Homocysteine is a sulfur-containing amino acid metabolized in both the remethylation and transulfuration metabolic pathways. Elevated homocysteine concentrations are a known risk factor for vascular disease (6,7) and have been associated with fractures, disability, frailty, slow gait speed, poor balance, and poor physical function (7–9). There is no standard cut point for elevated homocysteine concentrations but high homocysteine is frequently defined as >13 μmol/L (10) and the prevalence of high homocysteine among adults aged 60 years and older is nearly 20% (11). Concentrations of homocysteine are influenced by renal function (12), behavioral factors (smoking, alcohol use, and caffeine consumption), genetic mutations (MTHFR C677T), and nutritional intake (folate, vitamin B12, and vitamin B6) (13).
Recent research regarding the role of homocysteine in the decline of muscle strength in older adults is conflicting, with most finding inverse relationships between elevated homocysteine concentrations and decreased muscle strength (14–19) and noting gender differences (15,16,18). The use of different measures of muscle strength and study population specific categorizations of homocysteine limits comparability across studies (14–19). Additionally, repeated measures of either muscle strength or homocysteine have not been available in previous longitudinal studies of the relationship (14,16,18). With increasing age, homocysteine tends to increase and muscle strength decrease (20–24), but not necessarily in tandem, thus multiple measures over time are needed to accurately describe the relationship between the rate of change in grip strength and the rate of change in homocysteine.
Identifying risk factors contributing to accelerated decline of muscle strength is of public health importance and could aid in improving overall quality of life, and independence, as well as reduce the incidence of sarcopenia, falls and fractures, disability, mortality, and associated healthcare cost burden in older adults. The present study aimed to examine the association between total plasma homocysteine levels and muscle strength in persons aged 50 years or older participating in The Baltimore Longitudinal Study of Aging between December 2004 and March 2015.
Methods
The Baltimore Longitudinal Study of Aging (BLSA) began in 1958 and is currently sponsored and administered by the National Institute on Aging Intramural Research Program (24). The BLSA is an open-enrollment volunteer cohort of community-dwelling adults, at least 20 years old. Participants are free of disease, cognitive and functional impairments, non-morbidly obese (body mass index; BMI < 40 kg/m2), and have no reported difficulties in self-care or instrumental activities of daily living at the time of enrollment. BLSA follow-up schedules vary by participant age; those 20–59 years old are seen every 4 years, 60–79 years old are seen every 2 years, and 80 years or older are seen annually. More than 3,000 men and women have been enrolled since the start of the study.
This study includes adults aged 50 years or older participating in the BLSA with at least one complete observation with measurements for both homocysteine and grip strength between December 2004 and March 2015 (N = 1,101). There were 215 participants with a single observation, 218 with two observations, and 668 with three, or more observations. The study sample was reached after exclusion of 254 participants without homocysteine data, 16 participants without grip strength data, and 119 participants missing both measures. One participant with severe hyperhomocysteinemia (>100 µmol/L) was excluded.
This study was approved by the University of Texas Houston Health Science Center Committee for Protection of Human Subjects and National Institute of Environmental Health Sciences Institutional Review Boards (IRB). All participants signed BLSA IRB approved informed consent forms at enrollment and all follow-up visits.
Measurements
Grip strength was measured using the Jamar Hydraulic Hand Dynamometer, which measures maximum kilograms (kg) of force. Participants performed six trials, three trials with each hand, unless the participant had surgery on the affected hand in the past 3 months. The trials were done with the participant seated and tested arm extended at a 180° angle or shoulder height. The participants were instructed to squeeze as hard as they could for each measurement. The best of 6 trials was used in analyses.
At each BLSA visit fasting serum and plasma samples were collected according to standard protocols and stored at −80°C. Total plasma homocysteine was determined using a fully automated fluorescence polarization immunoassay (Abbott Diagnostics, Abbott Park, IL) (19).
Data on demographics, health behaviors, and medical conditions were collected through an interviewer-administered questionnaire at each visit. Variables included age, gender, marital status, household income, race, smoking status (current vs former/never), meeting physical activity guidelines (≥1000 kcal/wk) in the past 12 months (25,26), and self-reported physician diagnosed chronic diseases (cardiovascular disease, stroke, hypertension, osteoarthritis, Parkinson’s disease, any cancer, diabetes, and peripheral neuropathy). Measured height and weight were used to calculate BMI (kg/m2) and standard cut points for overweight and obesity used to categorize individuals. Supplement intake was collected via a food frequency questionnaire. Renal function was measured by serum creatinine and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (27).
Statistical analysis
Baseline characteristics of the study sample are presented as means and percentages by gender. Linear mixed effects models were used to estimate the association between homocysteine and grip strength while accounting for the lack of independence between repeated measures. Additionally, linear mixed effects models accommodate the BLSA unbalanced and unequally spaced observation intervals. Random effects for the intercept and slope (follow-up time in years) were utilized to account for the excess variation implicit in the study design. Analyses were stratified by gender given that muscle strength and the slope of its decline differ between men and women and homocysteine concentrations vary by gender (18,20).
An interaction between homocysteine and follow-up time was included to model whether changes in homocysteine predict longitudinal changes in grip strength. The beta coefficient for the interaction term should be interpreted as the rate of change of grip strength as a function of homocysteine change over time; whereas, the beta coefficient for homocysteine is the overall relationship between homocysteine and grip strength. Additional interaction terms with follow-up time were explored in gender-specific models to assess potential age effects and between each covariate for inclusion into the model.
Homocysteine and all other covariates, excluding baseline age category (50–59, 60–69, 70–79, ≥80), education, and race, were time-dependent. Splines were used to model follow-up time as a fixed effect. Restricted cubic and linear splines were assessed with knots placed at 2-year intervals (2, 4, and 6 years) and percentiles. Akaike information criterion indicated linear splines with knots at 2-year intervals was the best fit. Likelihood ratio tests and maximum likelihood estimator were used to test the removal of fixed effects, reducing the model to clinically and statistically significant covariates. Linear regression assumptions were checked and adjustments to the functional form of covariates done as necessary. All statistical analyses were performed using Stata v.12 (StataCorp, College Station, TX).
Results
A baseline comparison of included and excluded participants showed included participants were older than excluded participants (men: p = .001, women: p < .001). Among men, excluded participants had a higher mean BMI (p = 0.036) compared to included participants, but not among women (p = .826). Included women were more likely to be married (p = .003) and have a graduate degree (p = .042) and reported drinking less alcohol (p = .001) than excluded women; these differences were not seen among men. There were no significant differences in smoking, physical activity, income, and number of chronic diseases between excluded and included men and women.
Baseline characteristics for men and women included in the study sample are displayed in Table 1. Total mean follow-up time was 4.7 ± 3.1 years, range from 0 to 10.1 years. Baseline mean grip strength was 39.9 kg for men and 25.5 kg for women. The prevalence of clinically significant muscle weakness was low for both men and women, 5.0% and 4.4%, respectively. Homocysteine values ranged from 5.1 to 39.4 µmol/L at baseline. In men, 17.6% had high homocysteine (>13 µmol/L) and in women, only 7.0%. Overall, the majority of participants were white, well-educated, financially secure, physically active, non-smokers, and had a BMI < 30.0 kg/m2. Over 20% of both men and women had impaired renal function and most reported having at least one chronic disease.
Table 1.
Baseline Characteristics of BLSA Participants by Gender, N = 1,101, 2004–2015
| Men n = 556 |
Women n = 545 |
|
|---|---|---|
| Mean ± SD | ||
| Follow-up time, y | 4.5 ± 3.1 | 4.9 ± 3.0 |
| Age, y | 70.8 ± 10.4 | 67.5 ± 10.5 |
| Grip strength, kg | 39.9 ± 9.5 | 25.5 ± 6.3 |
| Homocysteine, µmol/L | 11.1 ± 3.2 | 9.3 ± 2.5 |
| BMI, kg/m2 | 27.6 ± 4.2 | 26.9 ± 5.2 |
| eGFRa | 71.8 ± 16.0 | 74.1 ± 17.5 |
| n (%) | ||
| Age category, y | ||
| 50–59 | 92 (16.5) | 138 (25.3) |
| 60–69 | 151 (27.2) | 187 (34.3) |
| 70–79 | 190 (34.2) | 131 (24.0) |
| 80+ | 123 (22.1) | 89 (16.3) |
| Weaknessb | 28 (5.0) | 24 (4.4) |
| High homocysteinec | 98 (17.6) | 38 (7.0) |
| eGFR <60 mL/min/1.73m2 | 116 (20.9) | 116 (21.3) |
| White | 413 (74.3) | 339 (62.2) |
| Household income ≥ $50,000 | 445 (81.2) | 379 (71.5) |
| Graduate degree | 357 (64.2) | 319 (58.5) |
| Married | 427 (77.2) | 316 (58.6) |
| BMI categories, kg/m2 | ||
| ≤24.9 | 164 (29.5) | 236 (43.3) |
| 25.0–29.9 | 262 (47.1) | 172 (31.6) |
| ≥30.0 | 130 (23.4) | 137 (25.1) |
| Adequate physical activityd | 335 (64.5) | 261 (49.0) |
| Alcoholic drinks per week | ||
| None | 80 (14.5) | 95 (17.8) |
| Three or less | 237 (43.1) | 286 (53.5) |
| 4–7 | 109 (19.8) | 106 (19.6) |
| Eight or more | 124 (22.5) | 49 (9.2) |
| Current smoker | 14 (2.6) | 14 (2.6) |
| Any reported chronic disease | 464 (83.6) | 439 (81.0) |
| Supplement intakee | 177 (80.1) | 200 (89.7) |
Missing: Household income (n = 23), alcohol intake (n = 16), married (n = 9), current smoking (n = 15), any reported chronic disease (n = 4), and physical activity (n = 19).
aeGFR: estimated glomerular filtration rate based on chronic kidney disease epidemiology collaboration equation.
bWeakness: grip strength <26 kg for men and <16 kg for women.
cHigh homocysteine: homocysteine >13 µmol/L.
dAdequate physical activity defined as meeting recommended physical activity guidelines, ≥1000 kcal/wk.
eSupplement Intake: men (n = 221) and women (n = 223).
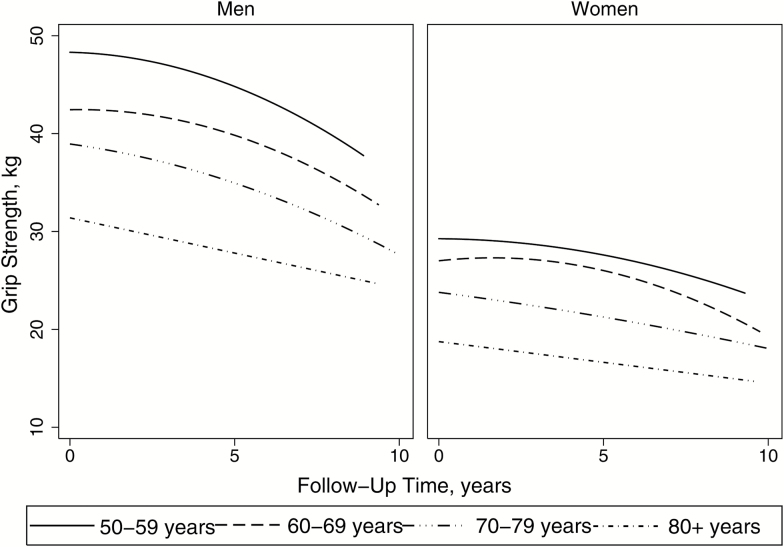
Grip strength declined over the follow-up time for both men and women (Figure 1). While women were weaker at baseline (p < .001), men had steeper grip strength decline over time for each age category (p < .001). For either men or women, the rate of decline did not vary significantly between baseline age categories but overall increasing age was associated with lower grip strength (p < .001, Figure 1).
Figure 1.
Grip strength over the follow-up time by gender and baseline age category, 2004–2015
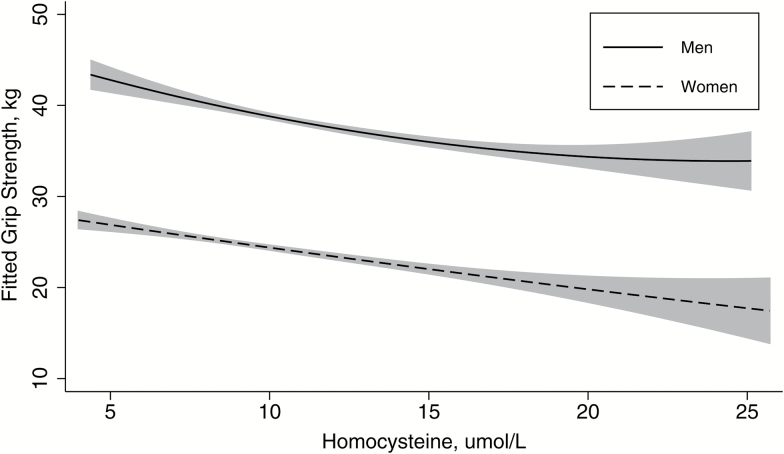
In both men and women, grip strength was inversely related to homocysteine, after adjustment of clinically and statistically significant variables (Figure 2). Among women, there was a significant relationship between the rate of change in homocysteine and rate of change of grip strength, where grip strength declined as a function of increasing homocysteine over time (β = −0.05, p = .031). Overall, grip strength declined −0.04 kg with every 1 μmol/L increase in homocysteine (p = .575). Among men, there was a slight decline in grip strength as homocysteine increased over time but not significantly (β = −0.02, p = .421). There was an overall trend (−0.10 kg, p = .174), though not significant, in grip strength decline with increasing homocysteine concentrations (Table 2).
Figure 2.
Fitted grip strength and homocysteine by gender, adjusted for follow-up time, baseline age category, race, physical activity, and BMI categories
Table 2.
Linear Mixed Effects Regression Results for Associations Between Homocysteine and Grip Strength by Gender
| Men | Women | |||
|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | |
| Homocysteine, µmol/L | −0.10 (−0.24, 0.04) | .174 | −0.04 (−0.19, 0.10) | .575 |
| Homocysteine*Time | −0.02 (−0.06, 0.02) | .421 | −0.05 (−0.09, −0.004) | .031 |
| Age category, y | ||||
| 50–59 | Reference | Reference | ||
| 60–69 | −5.46 (−7.43, −3.50) | <.001 | −2.11 (−3.18, −1.04) | <.001 |
| 70–79 | −9.05 (−10.96, −7.13) | <.001 | −5.48 (−6.65, −4.30) | <.001 |
| 80+ | −16.34 (−18.49, −14.20) | <.001 | −9.19 (−10.56, −7.82) | <.001 |
| White | −1.18 (−2.66, 0.29) | .117 | −2.31 (−3.19, −1.43) | <.001 |
| BMI categories, kg/m2 | ||||
| ≤24.9 | Reference | Reference | ||
| 25.0–29.9 | −0.41 (−1.25, 0.44) | .348 | 0.60 (−0.16, 1.36) | .121 |
| ≥30.0 | −0.54 (−1.74, 0.65) | .373 | 1.72 (0.81, 2.63) | <.001 |
| Physical activity | 0.83 (0.17, 1.49) | .014 | −0.03 (−0.56, 0.49) | .910 |
Discussion
In this study of healthy older adults aged 50 years or older, elevated homocysteine was related to lower muscle strength over an average follow-up period of 4.7 years in women. These results are consistent with some but not all previous research on the association of homocysteine and muscle strength (14–16,18,19). In agreement with the current analysis, cross-sectional studies have found significant inverse associations between elevated homocysteine and decreased muscle strength (14,15,18,19). Similarly, among studies stratified by gender, a statistically significant association was observed only in women (15,18). Only one previous study has assessed muscle strength, as measured by grip strength, and homocysteine longitudinally in older adults (mean age 75.6 ± 6.6 years, range 55–85 years) over a 3-year follow-up period and, contrary to the present study, found no relationship in men or women (16). As well, homocysteine has been previously associated with decline in overall physical function, which is often an aggregate measurement of balance, coordination, and muscle strength (14,18). The relationship between elevated homocysteine and decreased physical function may be mediated by the inverse relationship between homocysteine and muscle strength.
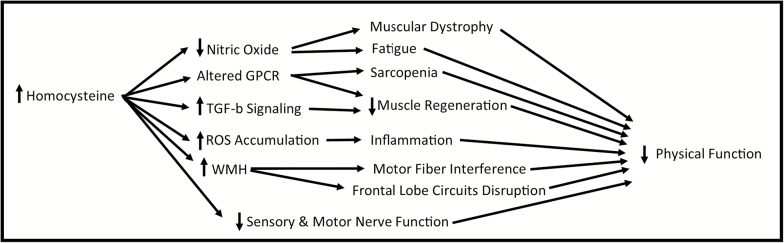
Homocysteine could be contributing to muscle strength decline through various pathologies (Figure 3) (6). Elevated homocysteine levels can lead to greater concentrations of reactive oxygen species, which can damage mitochondria and lead to inflammation, and altered G-protein coupled receptor and increased TGF-b signaling both leading to reduced muscle regeneration (6). Homocysteine also decreases the bioavailability of nitric oxide, which regulates blood flow to muscle cells; this can lead to a reduction in endurance, greater fatigue, muscular dystrophy, and ischemia (6).
Figure 3.
Potential mechanisms of decreased physical function through elevated homocysteine. GPCR = G-protein coupled receptor; ROS = reactive oxygen species; WMH = white matter hyperintensities.
The reason for a lack of a significant association in men, seen here and in the literature, is not fully known but gender-specific hormones could play a role. Estrogen is inversely and testosterone is positively correlated with homocysteine concentrations (28). In postmenopausal women, reduced estrogen has been associated with muscle strength decline. And among older men, a meta-analysis concluded testosterone or dihydrotestosterone replacement therapy resulted in a moderate increase in muscle strength (29). The role of gender-specific hormones in the relationship between homocysteine and muscle strength have yet to be studied.
Kidney dysfunction results in elevated homocysteine concentrations (12). As expected, homocysteine concentrations (Table 1) were higher for participants who had kidney dysfunction for men (13.5 vs 10.5 μmol/L, p < .001) and women (11.4 vs 8.7 μmol/L, p < .001). Additionally, 20% of both men and women had kidney dysfunction (eGFR < 60ml/min/1.73m2). The relationship between kidney dysfunction and reduced physical function and performance in older adults is well established (30). In this study population, adjustment for kidney dysfunction during model building did not meaningfully change the results of the final models. Given the strong biological association between homocysteine and renal disease, it is possible that homocysteine lies on the causal pathway between kidney dysfunction and poor physical function.
Vitamin B12, vitamin B6, and folate are involved in the intracellular metabolism and removal of homocysteine resulting in an inverse relationship between concentrations of homocysteine and these nutritional factors (21,23,31). In 1998, the Food and Drug Administration issued a regulation requiring grain products to be fortified with folic acid in an effort to reduce neural tube defects. Since folic acid fortification, vitamin B12 deficiency is usually the main nutritional cause of elevated homocysteine. In this population of healthy and active older adults, the prevalence of low vitamin B12 (<200 pg/ml) was less than 1% and serum folate inadequacy (≤3 ng/mL) was 0%. Vitamin B6 and fat intake are also possible contributors to homocysteine variation. As noted in a previous analysis of this study population, vitamin B6 intake from food and supplements was negatively correlated with homocysteine concentrations (32). Additionally, in the presence of high vitamin B12, very-long-chain n-3 fatty acids are inversely associated with homocysteine concentrations (32,33). Thus, for populations, such as the BLSA, with high levels of folate and vitamin B12, fat or vitamin B6 could be influential nutritional determinants of homocysteine concentrations.
Conceivably lowering homocysteine concentrations could delay muscle strength decline. Supplementation therapy with folic acid and vitamin B12 to reduce homocysteine and delay muscle strength and physical function decline has been the focus of two recent clinical trials in older adults, mean ages 75.7 and 74.1 years old (34,35). Both studies found lower concentrations of homocysteine in the treatment groups compared to the placebo groups. However, the supplementation therapy did not result in significant differences in decline of muscle strength and physical function between study groups. Dosages of folic acid and vitamin B12 were similar between the two studies and were above dietary reference intakes (36). These studies did not find gender differences, yet the reduction in homocysteine through folic acid supplementation has been shown to be larger in women than men (37). The null results seen in clinical trials compared to epidemiologic studies may indicate that homocysteine is a marker of muscle strength decline and not causally related to decline. Observational epidemiologic studies are inherently limited by the inability to show causality, thus the results seen here and in other studies could be from reverse causality, residual confounding, or both.
There are some limitations to be considered when interpreting results of this study. The BLSA study population is a robust and healthy group of older adults with low prevalences of obesity and smoking and thus may not be comparable to the general population (38,39). Women aged 60 years or older, in the current study population had a much lower prevalence of muscle weakness compared to previous reports (4.4% vs. 18.0%) (5). As well, the prevalence of high homocysteine was lower in the present study population than the general population (11). As such, the current study was unable to analytically assess relationships between these clinically relevant cutpoints. The magnitude of the estimated effect sizes, while statistically significant, might not be clinically relevant. Yet, any factor that exacerbates the progressive decline in muscle strength with aging, regardless of size, could be considered relevant.
Still, this study has many strengths. The BLSA has a long follow-up time for both measures of muscle strength and homocysteine. Grip strength was used to measure muscle strength and correlates well with total body strength (5). As well, homocysteine was assessed using valid laboratory procedures.
In conclusion, this study is the first to find a significant longitudinal relationship between elevated homocysteine concentrations and muscle strength decline in older adult women. Future research should be focused on assessing homocysteine’s role as a causal factor or a marker for decline and translating that relationship into clinical and public health practice.
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging (Grant 03-AG-0325).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi:10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 2. Wu Y, Zhang D, Pang Z et al. . Gender-specific patterns in age-related decline in general health among Danish and Chinese: a cross-national comparative study. Geriatr Gerontol Int. 2012;12:431–439. doi:10.1111/j.1447-0594.2011.00784.x [DOI] [PubMed] [Google Scholar]
- 3. Goodpaster BH, Park SW, Harris TB et al. . The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61: 1059–1064. doi:10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 4. Hughes VA, Frontera WR, Wood M et al. . Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. doi:10.1093/gerona/56.5.B209 [DOI] [PubMed] [Google Scholar]
- 5. Alley DE, Shardell MD, Peters KW et al. . Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi:10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013;14:15074–15091. doi:10.3390/ijms140715074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP, Lipsitz LA. The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol A Biol Sci Med Sci. 2005;60:1190–1201. doi:10.1093/gerona/60.9.1190 [DOI] [PubMed] [Google Scholar]
- 8. Ng TP, Aung KC, Feng L, Scherer SC, Yap KB. Homocysteine, folate, vitamin B-12, and physical function in older adults: cross-sectional findings from the Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2012;96:1362–1368. doi:10.3945/ajcn.112.035741 [DOI] [PubMed] [Google Scholar]
- 9. Rolita L, Holtzer R, Wang C, Lipton RB, Derby CA, Verghese J. Homocysteine and mobility in older adults. J Am Geriatr Soc. 2010;58:545–550. doi:10.1111/j.1532-5415.2010.02718.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–1454. doi:10.1056/NEJM199905133401901 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Second national report on biochemical indicators of diet and nutrition in the US population: 2012. Atlanta, GA: US Department of Health and Human Services; 2012. [Google Scholar]
- 12. Robinson K, Dennis VW. Homocysteine and renal disease. In: Homocysteine and Vascular Disease 2000. Springer: Netherlands; 253–270. [Google Scholar]
- 13. Refsum H, Nurk E, Smith AD et al. . The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136(6 Suppl):1731S–1740S. [DOI] [PubMed] [Google Scholar]
- 14. Kado DM, Bucur A, Selhub J, Rowe JW, Seeman T. Homocysteine levels and decline in physical function: MacArthur Studies of Successful Aging. Am J Med. 2002;113:537–542. doi:10.1016/S0002-9343(02)01269-X [DOI] [PubMed] [Google Scholar]
- 15. Swart KM, Enneman AW, van Wijngaarden JP et al. . Homocysteine and the methylenetetrahydrofolate reductase 677C–>T polymorphism in relation to muscle mass and strength, physical performance and postural sway. Eur J Clin Nutr. 2013;67:743–748. doi:10.1038/ejcn.2013.97 [DOI] [PubMed] [Google Scholar]
- 16. Swart KM, van Schoor NM, Heymans MW, Schaap LA, den Heijer M, Lips P. Elevated homocysteine levels are associated with low muscle strength and functional limitations in older persons. J Nutr Health Aging. 2013;17:578–584. doi:10.1007/s12603-013-0047-2 [DOI] [PubMed] [Google Scholar]
- 17. McDermott MM, Ferrucci L, Guralnik JM et al. . Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol. 2007;50:897–905. doi:10.1016/j.jacc.2007.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Schoor NM, Swart KM, Pluijm SM et al. . Cross-sectional and longitudinal association between homocysteine, vitamin B12 and physical performance in older persons. Eur J Clin Nutr. 2012;66:174–181. doi:10.1038/ejcn.2011.151 [DOI] [PubMed] [Google Scholar]
- 19. Kuo HK, Liao KC, Leveille SG et al. . Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J Gerontol A Biol Sci Med Sci. 2007;62:434–439. doi:10.1093/gerona/62.4.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562. doi:10.1016/j.annepidem.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 21. Ganji V, Kafai MR. Population reference values for plasma total homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr. 2006;84:989–994. [DOI] [PubMed] [Google Scholar]
- 22. Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi:10.1093/gerona/52A.5.B267 [DOI] [PubMed] [Google Scholar]
- 23. Selhub J, Jacques PF, Rosenberg IH et al. . Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991-1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131:331–339. doi:10.7326/0003-4819-131-5-199909070-00003 [DOI] [PubMed] [Google Scholar]
- 24. Shock NW. Normal human aging: The Baltimore Longitudinal Study of Aging. JAMA. 1986; 255:960. [Google Scholar]
- 25. Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. Am J Prev Med. 2011; 40:454–461. doi:10.1016/j.amepre.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 26. Havlik RJ, Simonsick EM, Sutton-Tyrrell K et al. . Association of physical activity and vascular stiffness in 70-to 79-year-olds: The Health ABC Study. J Aging Phys Act 2003; 11:156–166. doi:10.1123/japa.11.2.156 [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dierkes J, Jeckel A, Ambrosch A, Westphal S, Luley C, Boeing H. Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam study. Metabolism. 2001;50:640–645. doi:10.1053/meta.2001.23286 [DOI] [PubMed] [Google Scholar]
- 29. Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: A meta-analysis. J Am Geriatr Soc. 2006;54:1666–1673. doi:10.1111/j.1532-5415.2006.00938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69:315–322. doi:10.1093/gerona/glt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clarke R, Grimley Evans J, Schneede J et al. . Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33:34–41. doi:10.1093/ageing/afg109 [DOI] [PubMed] [Google Scholar]
- 32. Vidoni ML, Gabriel KP, Luo ST, Simonsick EM, Day RS. Vitamin B12 and homocysteine associations with gait speed in older adults: The Baltimore Longitudinal Study of Aging. J Nutr Health Aging 2017:1–8. doi:10.1007/s12603-017-0893-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berstad P, Konstantinova SV, Refsum H et al. . Dietary fat and plasma total homocysteine concentrations in 2 adult age groups: the Hordaland Homocysteine Study. Am J Clin Nutr. 2007;85:1598–1605. [DOI] [PubMed] [Google Scholar]
- 34. Lewerin C, Matousek M, Steen G, Johansson B, Steen B, Nilsson-Ehle H. Significant correlations of plasma homocysteine and serum methylmalonic acid with movement and cognitive performance in elderly subjects but no improvement from short-term vitamin therapy: a placebo-controlled randomized study. Am J Clin Nutr. 2005;81:1155–1162. [DOI] [PubMed] [Google Scholar]
- 35. Swart KM, Ham AC, van Wijngaarden JP et al. . A randomized controlled trial to examine the effect of 2-year vitamin B12 and folic acid supplementation on physical performance, strength, and falling: additional findings from The B-PROOF Study. Calcif Tissue Int. 2016; 98(1):18–27. doi:10.1007/s00223-015-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 37. Homocysteine Lowering Trialists’ Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82:806–812. [DOI] [PubMed] [Google Scholar]
- 38. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi:10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 39. Shay CM, Ning H, Allen NB et al. . Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003-2008. Circulation. 2012;125:45–56. doi:10.1161/CIRCULATIONAHA.111.035733 [DOI] [PMC free article] [PubMed] [Google Scholar]