Abstract
Death from chronic lung disease is increasing and chronic obstructive pulmonary disease has become the third leading cause of death in the United States in the past decade. Both chronic and acute lung diseases disproportionately affect elderly individuals, making it likely that these diseases will become more frequent and severe as the worldwide population ages. Chronic lung diseases are associated with substantial morbidity, frequently resulting in exercise limiting dyspnea, immobilization, and isolation. Therefore, effective strategies to prevent or treat lung disease are likely to increase healthspan as well as life span. This review summarizes the findings of a joint workshop sponsored by the NIA and NHLBI that brought together investigators focused on aging and lung biology. These investigators encouraged the use of genetic systems and aged animals in the study of lung disease and the development of integrative systems-based platforms that can dynamically incorporate data sets that describe the genomics, transcriptomics, epigenomics, metabolomics, and proteomics of the aging lung in health and disease. Further research was recommended to integrate benchmark biological hallmarks of aging in the lung with the pathobiology of acute and chronic lung diseases with divergent pathologies for which advanced age is the most important risk factor.
Keywords: Lungs/pulmonary, Biology of aging, Age-related pathology
Introduction
Age is a risk factor for virtually every chronic medical condition. Indeed, age is such an important and seemingly inevitable risk factor for disease that it is often overlooked in clinical practice. Yet expected rapid growth in the population of elderly, including a projected tripling in the size of the global population over 80 years old by 2050, mandates a better biologic understanding of the intersection between the biology of aging and the susceptibility to disease (1). Perhaps nowhere is the link between age and the susceptibility to disease more dramatic than in the steadily increasing morbidity and mortality attributable to chronic lung disease in older individuals, with mortality attributable to chronic obstructive lung disease (COPD) rising whereas most other causes of mortality are falling (2). Although a portion of this increase can be attributed to environmental factors, the changes that develop in the lung during “normal aging” show remarkable similarities to the pathologies evident in patients with COPD and pulmonary fibrosis (3). It therefore seems likely that interventions that would slow the normal age-related decline in lung function and increase lung resilience would have a dramatic effect on the morbidity attributable to chronic lung disease.
Aging is a biologic process that unfolds over a lifetime. A major question in aging biology is whether this process is subject to selective evolutionary pressures, analogous to development, or represents drift from a normal program of maintenance. The regularity of embryonic development and maturation to achieve sexual reproduction, which involves the combination of two progenitor cells to form a new organism mostly free of the age-related changes in the parents, suggests that natural selection has driven the development of a mechanism to cope with the challenge of time. In addition, a growing body of evidence suggests that age-related phenotypes can be prevented or delayed even outside the context of reproduction. For example, several investigators have repeated experiments originally conducted 150 years ago employing the technique of heterochronic parabiosis, in which aging phenotypes are partially and/or bidirectionally reversed and the life span of the aged member of the pair is extended when the circulation of a young mouse is surgically paired with that of an older animal (4). Heterochronic parabiosis results in improvements in several age-related phenotypes in mice including the resolution of cardiac hypertrophy, improvements in muscle function and regeneration after injury and increased proliferation of neural progenitors in the central nervous system (5,6), findings partially recapitulated using serum transfer. Identification of these factors through systematic comparisons of proteins, lipids, and metabolites differentially present in young and aged blood is an active area of investigation.
A fundamental question that emerges from this research is whether we can harness this or similar biology to prevent or even reverse the aging phenotypes that increase the risk of human disease. In model organisms, the answer to this question is conditionally “yes.” Although no one has created an immortal organism, a growing list of genetic and pharmacologic interventions extend the life span of the nematode C. elegans, the fruit-fly Drosophila melanogaster or the laboratory mouse Mus musculus (7). Some of these interventions have been shown to extend life span in primates, and a recently convened expert panel of biology-of-aging researchers cautiously recommended a set of therapies that could be tested in healthy humans to delay the onset or progression of age-related phenotypes thereby extending healthspan (as a primary goal). These include protein restriction and fasting-mimetic diets, pharmacologic inhibitors of the Growth Hormone/Insulin-like Growth Factor-1 axis, of the TOR-S6K pathway, and of inflammation, modulators of certain sirtuin proteins, the use of spermidine and metformin (7).
Within this background, the National Institute of Aging and the National Heart Lung and Blood Institute jointly hosted a workshop entitled “The Intersection of Aging Biology and Pathobiology of Lung Diseases.” Several common themes emerged from the conference. First, the investigators recognized that developing interventions to prevent aging phenotypes in the lung requires a better understanding of the age-related changes that develop in the lungs of model organisms and humans using an interdisciplinary approach that combines systems biology and hypothesis-driven mechanistic experiments. Second, the group advocated for research to identify how the molecular changes that occur with aging alter the response to stress in the lung, including the heat shock response, the mitochondrial unfolded protein response, autophagy, stem cell dysfunction, senescence, extracellular matrix (ECM) dysfunction, genomic instability, epigenomic dysregulation, and the immune response to infection. Fourth, the participants supported research to determine how lung aging contributes to the development of the multiorgan frailty that develops in some older patients. Fifth, the group highlighted the importance of developing new animal models of common lung diseases for which age is a strong risk factor including emphysema, idiopathic pulmonary fibrosis (IPF), and an increased susceptibility to lung infection. Finally, the investigators acknowledged some of the limitations to murine models of aging and emphasized the need to include tissues from patients with age-related lung disease and develop nonmurine models of lung disease for preclinical research. The purpose of this document is to provide examples that reveal important intersections between aging biology and lung pathobiology from the perspective of both disciplines. These examples are not meant to be comprehensive, instead they are used as illustrations of how our understanding of lung pathobiology is enhanced by studies that acknowledge the importance of aging.
Pathologic and Physiologic Changes in the Aging Lung
Growth and alveolarization
The human lung grows progressively until at least early adulthood when it contains approximately 300–480 × 106 alveoli (8). There is little evidence to suggest that new alveoli are generated after adulthood, instead, aging is characterized by a progressive loss of alveolar surface area and enlargement of alveolar size (9). This age-related increase in alveolar size develops in the absence of overt inflammation, signs of destruction, or fibrosis (8). The “normal” timeline of structural and functional deterioration is variable and may depend of a number of genetic, environmental, and epigenetic factors. For example, genome-wide association studies have identified numerous loci that underlie the variation in FEV1, FVC, and FEV1/FVC in the general population (10). Likewise, decreased and/or increased DNA methylation of a variety of genes involved in inflammation and oxidative stress was associated with decreased lung function in a cohort of elderly men (11).
Structural changes in the aging lung
Similar to emphysema, lung aging is characterized by a decrease in the density and an increase in the diameter of the membranous bronchioles, however, unlike emphysema, there are no differences in alveolar attachments (12). Aging lungs show uniform increase in alveolar size, with a mean linear intercept (Lm) 60% higher and an emphysema score 8-fold higher than young controls (13). Therefore, the aging lung is characterized by progressive enlargement of the alveolar ducts and spaces reminiscent of changes seen in patients with emphysema. These similarities pose a challenge for distinguishing normal aging and emphysema, which might be addressed with novel imaging techniques, including fractal dimension analysis, lung density measurements, and diffusion-weighted magnetic resonance imaging using hyperpolarized 3He (14).
Physiologic changes in the aging lung
There is a progressive, age-associated decrease in lung function, FEV1 declines by ~30 mL per year in men and women, whereas FVC begins to decline later and at a slower rate (20 mL per year) resulting in a decrease in the FEV1/FVC ratio. Consistent with the morphologic findings described previously, the residual volume and functional residual capacity increase with age; however, significant variability among healthy older individuals makes it difficult to establish a range of values that define “normal function” (15). Age-related changes in the pulmonary circulation result in an increase in pulmonary artery systolic pressure, increased ventilation-perfusion mismatching, and a progressive decrease in the DLCO in elderly (16,17).
Gaps in knowledge
Most measures of lung morphology and function in elderly have been performed on a relatively small numbers of individuals. Studies that incorporate advanced imaging and functional measures of lung health (rather than lung disease) combined with molecular profiling including genomic, epigenomic, transcriptomic, proteomic, and metabolomics data might better define aging phenotypes and provide insights into the underlying biology of aging in the lung. Although some of these data can be generated using surrogate tissues, for example, the peripheral blood or the nasal/proximal airways, it will be important to include studies of “normal” lung tissue across the life span. A combined anatomic, physiologic, and molecular definition of lung aging will provide an endpoint that can be used in proposed trials of interventions that seek to slow global processes of aging (7). This is important as some proposed interventions, for example, the administration of the mTOR inhibitor rapamycin, do not appear to slow the development of age-related changes in the lung in mice, and mTOR inhibitors have been reported to cause pneumonitis that can be fatal in humans (18,19). Studies of fundamental mechanisms of lung aging specifically are therefore necessary.
Molecular Hallmarks of Aging
Through the study of aging, including studies that employ laboratory animals with shorter versus longer life spans, investigators have made significant progress in understanding some of the fundamental biologic changes that develop with aging (20,21). These “hallmarks of aging” have been reviewed elsewhere (22). Integrating these hallmarks of aging into a conceptual framework that describes how age-related dysfunction in fundamental cellular processes (genomic instability, epigenetic alterations, mitochondrial dysfunction, altered nutrient sensing, or disruptions in proteostasis) contribute to the cellular phenotypes of aging (altered immune responses, senescence, stem cell exhaustion, altered intracellular communication, changes to the ECM), and how these cellular phenotypes of aging contribute to the physiologic decline and loss of resilience in the aging lung represents a major challenge in lung biology. Included below are four examples of how investigators have used some of these biologic hallmarks of aging (proteostasis, mitochondrial dysfunction, ECM changes, and inflammation) as a prism to understand age-related lung disease, followed by a discussion of how these and other hallmarks of aging might be used to inform our understanding of COPD and pulmonary fibrosis.
Age-Related Changes in Proteostasis
An emergent principle of cellular biology is that the protein fold is globally managed by the protein homeostasis or proteostasis program (23–26). Proteostasis refers to the dynamic process by which cells control the concentration, conformation, binding interactions, and stability of individual proteins making up the proteome through a system of regulated networks of interacting and competing biological pathways that influence protein synthesis, folding, trafficking, disaggregation, and degradation (23,26). Alterations in proteostasis can result in loss of protein function, for example, the generation of insufficient amounts of active protein, or the inappropriate degradation of functional proteins. Alternatively, altered proteostasis can result in a gain of toxic function, for example, the aggregation and accumulation of misfolded proteins. Dysfunction in the proteostasis network has been shown to contribute to the phenotypes of aging by impairing cellular function, reducing the capacity of the cell to respond to environmental or metabolic stress (23). Severe proteostatic stress leads to a premature aging proteome phenotype by enhancing the cellular sensitivity to signals that trigger senescence or apoptosis (27). We now appreciate from robust C. elegans genetic models that the cellular processes required to maintain proteostasis begin to decline early in life, precisely at the release of the first progeny, via a coordinated mechanism initiated in reproductive cells and disseminated through the entire organism via an epigenetic mark in a neuronal cell (28). The expression of a curated set of genes that comprise the known proteostasis network in aging human brains declines with age, suggesting a similar decline in the proteostasis network in other tissues (29). Whether a similar change occurs in the lung is not yet known; however, it is now possible to quantify age-related changes in the function of the proteostasis network using novel imaging approaches combined with top–down proteomic approaches (30). Newer techniques now allow a direct measure of proteostasis at the level of the cellular interactome. For example, investigators recently compared the interactome of the wild-type and mutant cystic fibrosis transmembrane receptor and identified a core set of hundreds of proteins that interact with the wild-type and mutant protein and a distinct set of proteins that interacted exclusively the mutant, some of which affected the function of the mutant (31). Other proteins that might be targeted using similar approaches include those implicated in emphysema pathogenesis (e.g., alpha-1 antitrypsin) or pulmonary fibrosis (e.g., MUC5b or Surfactant Protein C).
Among the proteostatic systems, degradation and recycling of cellular components in the lysosomes through autophagy play a central role in the cellular response to proteostatic challenges. Autophagy participates in the reorganization of the cell’s proteome, in organelle turnover and in the response to metabolic and oxidative stress. Recent studies have shown decreased autophagy in the aging lung and in aging-associated diseases of the respiratory system, such as IPF (32,33). Reduction of autophagic activity in lung fibroblasts and epithelial cells might not only account for an inefficient response to proteostatic stress, with the subsequent accumulation of damaged and aggregated proteins in this organ, but might also result in accelerated cell senescence.
Mitochondrial Stress During Aging
There is an increased realization that alterations in mitochondrial function might contribute to age-related lung pathology. For instance, a recent study demonstrated that abnormal mitochondria appear to accumulate in alveolar type II cells of patients with IPF (34). This accumulation appears to be due to a defect in the clearance of damaged mitochondria through the process of mitophagy. One key regulator of mitophagy is the PTEN-inducible putative kinase-1 (PINK1). Indeed, PINK1-deficient mice were shown to have an increased susceptibility for the development of lung fibrosis (34). Interestingly, PINK1-deficient mice were protected against cigarette smoke-induced empysema highlighting the complexity of the function of PINK1 in chronic lung disease (35). An accumulation of damaged mitochondria can also be seen in models of cigarette smoke where again a decline in mitophagic flux appears to be involved in driving airway epithelial cells toward a senescent phenotype (36); similarly, in sepsis-induced lung injury and multiple organ dysfunction, impaired mitophagy results in the accumulation of damaged mitochondria that activate the inflammasome (37). New methods to detect mitophagy in vivo should, therefore, be very helpful in dissecting how this process contributes to a wide range of age-related lung pathologies (38).
The mechanism through which damaged or dysfunctional mitochondria contribute to diseases of aging remains an important question. The answer to this question will likely come from developing a more nuanced view of how mitochondria function within the cell, tissue, and organ. Although classically viewed as the powerhouse of the cell, it is becoming increasingly clear that the mitochondria function as more than simple generators of intracellular ATP. A large body of recent evidence suggests that beyond their role in bioenergetics, mitochondria also act as crucial signaling organelles (39). Although bioenergetic defects are often observed in age-related diseases, dysregulation of mitochondrial signaling might be as important, or perhaps more important, in disease initiation and progression. As part of this signaling function, it is clear that alterations in the mitochondrial genome or accumulation of misfolded proteins within the mitochondrial matrix can trigger a nuclear transcriptional response. This response, called the mitochondrial unfolded protein response (UPRmt), has garnered increasing interest, as activation of the UPRmt in lower organisms appears to be linked to increased longevity (40). In addition, this pathway has been recently linked to the age-dependent decline in mammalian stem cell function (41). Whether or not the UPRmt plays a role in age-related lung pathology remains untested.
Inflammation and Aging
In elderly individuals, the levels of proinflammatory mediators, including C-reactive protein, IL-1β, IL-6, IL-8, and TNF-α are increased, yet the immune system is simultaneously hyporesponsive to specific antigens, a combination of findings collectively referred to as “inflammaging” (42). A variety of hypotheses have been put forward to explain both these phenotypes. Enhanced inflammation has been suggested to result from the release of damage-associated molecular pattern molecules (DAMPs) from dying cells, or ineffective clearance of DAMPs due to impaired autophagy/mitophagy. These DAMPs can activate Toll-like receptors or intracellular pattern recognition receptors to induce the expression of inflammation-related genes, or can prime or activate the inflammasome (43). The consequences of the resulting low level chronic inflammation are not entirely clear, but chronic low level inflammation has been suggested to contribute to the development of cardiovascular disease in the elderly and to the development of COPD (44,45).
The observed hyporesponsiveness of the immune system to antigen stimulation is best documented in the weak response to influenza infection in elderly compared with younger individuals (46). This loss of adaptive immunity might occur through cell autonomous mechanisms. For example, investigators have reported reduced proliferative capacity of hematopoietic progenitor cell populations and a shift toward increased production of myeloid cells in older animals as well as reduced proliferative potential of differentiated lymphocyte populations (47,48). These changes are accompanied by alterations in the numbers and relative composition of the circulating leukocyte pool as well as the cellular populations in the bone marrow, lymph nodes, spleen, and thymus (49). In addition, investigators have observed clonal expansion of subpopulations of T and B cells in lymphoid tissues from older compared with younger individuals (50). These clonally expanded populations might impair the function of neighboring cells through homeostatic feedback or simple dilution. Hyporesponsiveness of the adaptive immune system might also result from an age-related loss in signals from key stromal populations in the bone marrow, thymus, lymph node, or spleen, which are necessary to maintain the stem cell niche or to preserve the function of differentiated cells (51,52). Therapies that target the age-related decline in adaptive immune responses in the lung would be predicted to have an important clinical impact. For example, individuals between 65 and 74 have a 30-fold higher risk of death after influenza A infection compared with individuals aged 25–49, and a similar increase in risk has been reported with other respiratory viruses (53).
Aging and the ECM
The ECM is a complex network of cross-linked proteins that provides essential physical scaffolding and is a reservoir for growth factors, cytokines, and ECM-remodeling enzymes that transduce signals for the differentiation, proliferation, survival, polarity, and migration of cells. As such, age-related changes in the composition or structure of the matrix might initiate a process of disordered repair as seen in emphysema or fibrosis. Since ECM proteins are large, complex, and assembled into cross-linked insoluble matrices, defining their biochemical composition has been challenging and our knowledge of the lung matrisome remains incomplete, although advances in mass spectroscopy-based proteomics offer promise (54). Using unbiased microarray analysis of normal lungs, investigators observed age-related increases in collagen among the 40 genes that were increased in patients in their sixth or seventh compared with their third and fourth decade of life (55). Investigators have also observed increases in the levels of matrix collagen in the lungs of aged rodents (56). At the level of the matrix proteome, the problem is more complex as age-related changes in matrix composition might alter the susceptibility of the tissue to solubilization. Within these limits, increased expression of fibrillar collagens and advanced glycation end-products accompanied by a loss of elastic fibers are often associated with tissue aging (57). Studies using unbiased proteomic approaches are underway that will overcome these limitations (54).
Age-Related Obstructive Lung Disease, COPD, and Asthma
Although the mortality attributable to heart disease, cancer, stroke, and other common diseases have declined over the past decade, both the prevalence and mortality attributable to COPD are increasing worldwide (58). Environmental factors, most importantly cigarette smoke exposure, are important drivers of this increase; however, the incidence of COPD increases exponentially with age making it likely that the expanding population of elderly worldwide is contributing to the increasing health burden of COPD. Furthermore, because COPD is often associated with dyspnea on exertion that lasts for years prior to death, it is an important limitation on healthspan. There are many similarities between the pathologic and physiologic features of normal aging in the lung and COPD (see the discussion of normal aging described previously). Although still an area of controversy, these similarities have led many to suggest that exposure to cigarette smoke and other environmental stressors over the life span accelerate biologic processes associated with normal aging (3). Furthermore, recent epidemiological observations have suggested that about half of patients with COPD fail to achieve full lung function in adolescence and early adulthood. In these individuals, COPD might develop as a consequence of the “normal” decline in lung function with age, highlighting the need for longitudinal data that capture lung function and anatomy in early adulthood (59).
In support of the hypothesis that COPD may represent an accelerated (or normal) form of lung aging, most of the biologic hallmarks of aging have been associated with COPD in animal models or human samples (3). Investigators have identified several lines evidence suggesting telomere shortening is causally linked to the development of COPD; telomeres are shortened in the lungs of patients with COPD, mutations in telomerase genes have been linked to the development of emphysema in familial and population-based genetic studies, mice with short telomeres are prone to cigarette smoke-induced emphysema, and the prevalence of telomerase mutations appears to rival that of α-1 antitrypsin deficiency in severe emphysema (60). In patients with α-1 antitrypsin deficiency, chronic proteostatic stress induced by expression of the misfolded mutant is implicated in emphysema pathology (61). In contrast, investigators have observed markers of autophagy in the lungs of patients with COPD, and genetic inhibition of Egr1-dependent autophagy protected against cigarette smoke-induced emphysema in mice (62). Mitochondrial reactive oxygen species generation can be directly induced by cigarette smoke exposure or through ineffective clearance of dysfunctional mitochondria through mitophagy (35). Epigenetic changes including DNA methylation, histone modifications, and altered expression of noncoding RNA molecules have been reported in patients with COPD where they are associated with the risk of developing COPD, and with responses to therapy (63). Telomere shortening, increased mitochondrial ROS generation and epigenetic changes might all contribute to the genomic instability observed in the lungs of patients with COPD, which likely contributes to the coincident risk of lung cancer in these patients (3). Dysfunction in these pathways may also contribute to the increased numbers of senescent cells observed in the lungs of patients with COPD, which have been suggested to contribute to pathogenesis through impaired lung regeneration (stem cell exhaustion), an enhanced susceptibility to apoptosis, and the release of senescence-associated secreted proteins (altered intracellular communication) (3). There is ample evidence that the numbers of inflammatory cells and the production of proinflammatory cytokines and chemokines are increased in the lungs of patients with COPD, perhaps exacerbated by the enhanced basal inflammation that is a component of “inflammaging” (3).
Asthma is relatively common in adults more than 60 years of age with a reported prevalence between 4% and 13% (64), and carries one of the highest rates of asthma-attributable morbidity and mortality, with 50% to 66% of all asthma deaths occurring in adults more than 65 years of age (65). In older individuals, the airway inflammation and airway reactivity characteristic of asthma are often combined with the fixed obstruction characteristic of COPD (66). Limited data regarding airway inflammation in aged adults with asthma suggest that there are increased levels of airway neutrophils, decreased eosinophil effector function, and reduced numbers of Tregs (65). Establishing the characteristics of airway inflammation in elderly patients with asthma and the asthma COPD overlap syndrome is an important step toward formulating more effective age-directed approaches to treatment.
Lung Aging and Fibrosis
IPF is the most common form of the idiopathic interstitial pneumonias, and the most severe chronic interstitial lung disease usually displaying a progressive, irreversible, and lethal course (67,68). The disease occurs in middle-aged and elderly adults and the frequency rises markedly with age (69,70). For example, advanced age emerged from a multidimensional prognostic staging system as one of the four selected variables most strongly associated with mortality and similar findings were reported for fibrotic nonspecific interstitial pneumonia (71). In rodent models of fibrosis, advanced age increases the severity of lung fibrosis and/or impairs capacity for resolution (72). However, the mechanisms linking aging to the pathogenesis of IPF remain elusive.
Similar to COPD, many of the biologic hallmarks of aging have been described in the context of lung fibrosis. Genetic defects in telomerase and telomere genes markedly increase the risk of IPF and is in fact the most commonly identifiable cause of familial pulmonary fibrosis accounting for at least one-third of cases, and is a constitutional finding in at least half of IPF patients (73). Epigenetic alterations including the expression of long noncoding RNAs and micro-RNAs have emerged from unbiased transcriptomic data obtained from the lungs of patients with IPF (74). Disordered proteostasis including mutations in Surfactant Protein C that lead to misfolding of the protein has been identified in families with pulmonary fibrosis (75). Increased expression of senescence markers have been observed in the lungs of patients from IPF and myofibroblasts obtained from the lungs of patients with IPF demonstrate a senescence-associated resistance to apoptosis (72). Mitochondrial ROS and a loss of antioxidant defenses have been implicated in TGF-β signaling of NOX4, a critical mediator of lung fibrosis (76,77). While highlighting the link between aging biology and chronic lung diseases, the fact that similar biologic hallmarks of aging are linked with the apparently discordant phenotypes of COPD and IPF raises important questions and is a fertile area for future research.
Muscle Dysfunction in Patients With Lung Disease and Aging
With age, skeletal muscle strength and endurance decrease and the prevalence of sarcopenia increases (78). In patients with chronic lung diseases the presence of sarcopenia is associated with increased morbidity and impaired the quality of life (79,80). Skeletal muscle dysfunction is common in critically ill patients and patients with the acute respiratory distress syndrome (ARDS) (80). Multisystem organ dysfunction (MODS) can manifest as skeletal muscle weakness in critically ill patients and negatively impacts the quality of life in critical illness survivors. In older patients with respiratory diseases limb and respiratory muscle dysfunction can contribute to hypoventilatory respiratory insufficiency which results in longer hospitalizations and protracted circle of progressive loss of muscle mass and dysfunction (81). Muscle dysfunction following critical illness is more severe in older individuals. For example, Herridge and colleagues found that in patients with an ICU length of stay longer than 2 weeks older patients had higher mortality and survivors had worse disability compared with younger patients with comparable severity of illness (82).
Skeletal muscle is continuously subject to cycles of protein degradation and muscle cell loss followed by regeneration. Myogenesis during muscle regeneration in the adult is carried out by satellite cells that are closely associated with muscle fibers. In response to muscle injury, satellite cells are stimulated to undergo proliferation and differentiation to form new muscle fibers (83). This process is known to be impaired during aging, which might be attributable to cell autonomous changes in the satellite cell pool or result from changes in the levels of circulating myokines or changes in resident and circulating immune cells (83). These latter mechanisms might be altered in patients with critical illness and/or chronic lung disease. In addition, a number of studies have identified a key role of the ubiquitin-proteosome system in muscle degradation in patients with critical illness and chronic lung disease. In particular, increased expression and activity of the muscle-specific ubiquitin E3 ligases MuRF-1 and Atrogin1 are consistently observed in muscle biopsy specimens from patients with critical illness and chronic lung disease skeletal muscle from patients with lung diseases (84,85). Accordingly, changes in the function of the proteasome have been suggested to contribute to impaired muscle regeneration during aging (86). Interventions that enhance satellite cell function and reduce ubiquitin-proteasome mediated muscle cell degradation offer the promise of improving muscle function and reducing morbidity in elderly patients with critical illness and chronic lung disease.
A Systems Biology Approach to Lung Aging
Systems biology is a useful tool for generating hypotheses about cause-and-effect relationships but to be effective it requires large data sets. In 2004, the National Human Genome Research Institute began to fund projects with a goal of reducing the cost of sequencing a human genome to less than $1000, and 11 years later, this goal has been reached using commercially available sequencing technologies that are within the reach of most research institutions and many individual laboratories (87). As an offshoot of these efforts, reliable and reproducible methods have been developed to sequence the entire transcriptome of a given tissue or cell population, including the ability to detect rare splice variants and noncoding RNAs (RNA-seq) (88). When combined with flow cytometry technologies, next generation sequencing technology allows for measurement of the transcriptomes of up to several 100 individual cells (single cell RNA-seq) and newer microfluidic platforms allow examination of the individual transcriptomes of 1000s of cells simultaneously (e.g., Drop-Seq) (89). The same next generation sequencing technologies have been adapted to measure histone modifications across the genome in an effort to understand the epigenetic regulation of cell-specific gene expression (ChIP-Seq) (90). These data complement advancing technologies to measure DNA methylation at the level of the genome, improvements in metabolomics, and advances in mass spectroscopy that put top–down proteomic approaches in reach.
As a result of these technologies, examination of lung aging over the life span at the level of the entire tissue will generate large parallel data sets that will require novel “transomic” systems biology-based approaches for their interpretation (91). A complex system like the aging lung presents an even larger challenge, as changes in whole lung “omic” data may be driven by changes in the number or function of only a subset of the more than 50 cell types that comprise the lung. Small scale heterogeneity that might develop within specific cellular populations during aging (e.g., the emergence of a small population of senescent progenitor cells) can be detected through the analysis of cellular populations identified using flow cytometry or via the analysis of single cells (92,93). Both of these approaches dramatically increase the amount of data generated. Advances in data analysis, storage, manipulation, and access will be required to fully realize the potential of these rich resources.
Examination of age-related changes in DNA methylation, an epigenetic modification implicated in aging biology, highlights some of these complexities. DNA methylation data can be complex to interpret and aging is well-known to be accompanied by changes in subsets of individual cell types (e.g., changes in immune cell profiles are altered over time) (94). These cell-type-specific changes be accounted for in discovery-based studies of the association of alterations of DNA methylation with aging. In data dense interrogations of DNA methylation at CpG sites (e.g., Illumina arrays or sequence-based methods) systematic changes in the cellular composition of the tissue could lead to changes in the methylation pattern. These changes in cellular composition might be mistakenly interpreted as aging or environmentally induced methylation alterations. This occurs because epigenetic modifications, specifically including DNA methylation, dictate programmed differentiation within the somatic lineages, including, for example, the immune system (95). Studies of blood are particularly prone to this problem. Remodeling the epigenome during development leads to progressively restricted immune subtypes and DNA methylation provides a chemically stable mark for these cell fate decisions (96).
As an example, DNA methylation arrays can be applied to study isolated leukocyte subtypes and these data have led to the discovery that all lineage-specific immune cells in the peripheral blood can be distinguished by a signature or “fingerprint” of differentially methylated regions. Statistical algorithms for estimating leukocyte number and type solely by reference to DNA methylation data have been developed and undergone extensive validation (97). These approaches continue to evolve as ever more sophisticated bioinformatic methods for immunomethylomics will allow for greater accuracy in defining different cell types (97,98). Using pure cell type reference DNA methylation data (cell type libraries), one can deconvolute separate target DNA methylation data sets into constituent cell-type proportions, but this approach cannot localize changes in the whole blood methylation signature with aging to an individual cellular constituent in the blood. Although there are numerous cell types and changes involved, given the rapidly advancing technology systems biology is highly likely to generate important insights into the molecular changes taking place with aging in the lung.
Summary and Recommendations
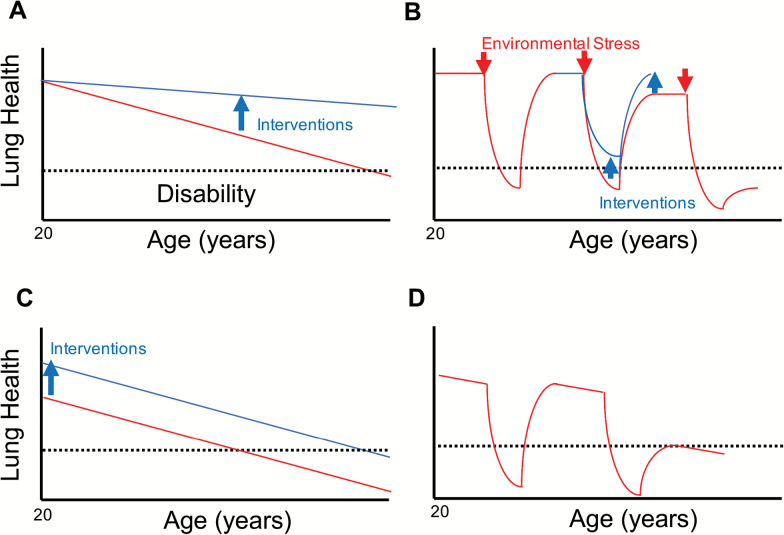
The growing importance of age-related lung diseases in limiting both life span and healthspan highlight the importance of understanding lung aging as part of an overall strategy to enhance health, lengthen life, and reduce illness and disability. Lung health during aging might be improved through strategies that slow the progressive age-related decline in lung function, improve childhood lung development, and enhance lung resilience to environmental challenges (Figure 1). The strong clinical association between advanced age and the incidence of acute and chronic lung disease suggests a fundamental link between the biology of aging and many lung diseases. Hence, the study of lung aging needs to extend beyond “aging researchers” to encompass the much larger community of researchers studying lung disease.
Figure 1.
Models for the age-related decline in lung health. Red lines represent age-related changes in lung health, blue lines the effect of potential therapies to preserve lung health during aging. A. Progressive decline. According to this model, the rate of accumulation of age-related damage to the lung over time determines whether, and at what age, an individual will develop respiratory disability (dashed line). Effective interventions should slow the rate of age-related decline in lung funciton to preserve lung health over the life span (blue arrow). B. Loss of resilience. Even in individuals who maintain their lung function over time, injury induced by environmental challenges (infections, inhaled toxins, etc) over the life span might be followed by incomplete resolution, resulting in intermittent step-declines in lung health. Interventions to improve lung resilience could reduce the severity of lung damage in response to a given environmental challenge or improve lung regeneration after injury (blue arrows). C. Impaired lung development. Data from recent longitudinal studies of lung function suggest that some individuals fail to achieve normal lung function during early adulthood. These individuals might be particularly prone to the age-related decline in lung health. Interentions that minimize antenatal and postnatal exposures to environmental factors that impair lung development is predicted to preserve lung health during aging in these individuals (blue arrow). D. Combined age-related progressive lung dysfunction and loss of lung resilience, particularly in patients with impaired lung development, can dramatically reduce the age at which respiratory disability develops.
Investigators have made key insights that provide a glimpse of the molecular mechanisms underlying aging at the cellular and organismal level, but additional research is required to link these processes and pathways with disease phenotypes in the aging lung and with the susceptibility to lung disease. Four key research challenges and opportunities emerged from the conference. First, it is essential to use aged animals for the study of lung disease; in some cases, findings in aged animals better recapitulate phenotypes of the human disease and aged models allow for interventions that selectively target aging- and disease-related processes. Second, we need to better understand normal aging in the lung in the absence of clinically identifiable disease: When does aging in the lung begin? Which cellular populations drive aging phenotypes? What are the lung-specific effects of interventions that extend healthspan and are being considered for therapies to delay aging in healthy humans? Third, how can we develop integrative systems-based platforms that can dynamically incorporate data sets that describe the genomics, transcriptomics, epigenomics, metabolomics, and proteomics of the aging lung and generate interfaces through which investigators without a bioinformatic background can interact with the data to generate testable hypotheses and develop novel biomarkers? Fourth, how do we reconcile the observations that benchmark biologic hallmarks of aging are associated with divergent lung phenotypes—COPD and pulmonary fibrosis in different individuals?
Funding
Funding provided by the National Institute on Aging and the National Heart, Lung and Blood Institute.
Conflict of Interest
The authors declared no conflict of interest.
References
- 1. Thannickal VJ, Murthy M, Balch WE et al. . Blue journal conference. Aging and susceptibility to lung disease. Am J Respir Crit Care Med. 2015;191:261–269. doi:10.1164/rccm.201410-1876PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous. Deaths: Preliminary data for 2005. Atlanta, GA: Centers for Disease Control, National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 3. Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70:482–489. doi:10.1136/thoraxjnl-2014-206084 [DOI] [PubMed] [Google Scholar]
- 4. Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell. 2013;12:525–530. doi:10.1111/acel.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loffredo FS, Steinhauser ML, Jay SM et al. . Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi:10.1016/j.cell.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villeda SA, Luo J, Mosher KI et al. . The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi:10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Longo VD, Antebi A, Bartke A et al. . Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi:10.1111/acel.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ochs M, Nyengaard JR, Jung A et al. . The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi:10.1164/rccm.200308-1107OC [DOI] [PubMed] [Google Scholar]
- 9. Weibel ER, Gomez DM. Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures. Science. 1962;137:577–585. doi:10.1126 [DOI] [PubMed] [Google Scholar]
- 10. Loth DW, Soler Artigas M, Gharib SA et al. . Genome-wide association analysis identifies six new loci associated with forced vital capacity. Nat Genet. 2014;46:669–677. doi:10.1038/ng.3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lepeule J, Baccarelli A, Motta V et al. . Gene promoter methylation is associated with lung function in the elderly: the Normative Aging Study. Epigenetics. 2012;7:261–269. doi:10.4161/epi.7.3.19216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992;101:793–799. doi:10.1378 [DOI] [PubMed] [Google Scholar]
- 13. Verbeken EK, Cauberghs M, Lauweryns JM, van de Woestijne KP. Anatomy of membranous bronchioles in normal, senile and emphysematous human lungs. J Appl Physiol (1985). 1994;77:1875–1884. [DOI] [PubMed] [Google Scholar]
- 14. Paulin GA, Ouriadov A, Lessard E, Sheikh K, McCormack DG, Parraga G. Noninvasive quantification of alveolar morphometry in elderly never- and ex-smokers. Physiol Rep. 2015;3:e12583. doi:10.14814/phy2.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quanjer PH, Stanojevic S, Cole TJ et al. ; ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Report of the Globals Lung Function Initiative, ERS task force to establish improved lung funciton reference values. Eur Respir J. 2012;40:1324–1343. doi:10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cardús J, Burgos F, Diaz O et al. . Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156(2 Pt 1):648–653. doi:10.1164/ajrccm.156.2.9606016 [DOI] [PubMed] [Google Scholar]
- 17. McQuillan BM, Picard MH, Leavitt M, Weyman AE. Clinical correlates and reference intervals for pulmonary artery systolic pressure among echocardiographically normal subjects. Circulation. 2001;104:2797–2802. doi:10.1161 [DOI] [PubMed] [Google Scholar]
- 18. White DA, Camus P, Endo M et al. . Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403. doi:10.1164/rccm.200911-1720OC [DOI] [PubMed] [Google Scholar]
- 19. Neff F, Flores-Dominguez D, Ryan DP et al. . Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim EB, Fang X, Fushan AA et al. . Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223–227. doi:10.1038/nature10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi:10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi:10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 24. Powers ET, Balch WE. Diversity in the origins of proteostasis networks–a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013;14:237–248. doi:10.1038/nrm3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Oosten-Hawle P, Porter RS, Morimoto RI. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell. 2013;153:1366–1378. doi:10.1016/j.cell.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balch WE, Sznajder JI, Budinger S et al. . Malfolded protein structure and proteostasis in lung diseases. Am J Respir Crit Care Med. 2014;189:96–103. doi:10.1164/rccm.201306-1164WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth DM, Balch WE. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol. 2011;23:126–134. doi:10.1016/j.ceb.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Labbadia J, Morimoto RI. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol Cell. 2015;59:639–650. doi:10.1016/j.molcel.2015.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brehme M, Voisine C, Rolland T et al. . A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 2014;9:1135–1150. doi:10.1016/j.celrep.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta R, Kasturi P, Bracher A et al. . Firefly luciferase mutants as sensors of proteome stress. Nat Methods. 2011;8:879–884. doi:10.1038/nmeth.1697 [DOI] [PubMed] [Google Scholar]
- 31. Pankow S, Bamberger C, Calzolari D et al. . ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature. 2015;528:510–516. doi:10.1038/nature15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sosulski ML, Gongora R, Danchuk S, Dong C, Luo F, Sanchez CG. Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell. 2015;14:774–783. doi:10.1111/acel.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romero Y, Bueno M, Ramirez R, Alvarez D, Sembrat JC, Goncharova EA et al. . mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell. 2016;15:1103–1112. doi:10.1111/acel.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bueno M, Lai YC, Romero Y et al. . PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi:10.1172/JCI74942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mizumura K, Cloonan SM, Nakahira K et al. . Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi:10.1172/JCI74985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahmad T, Sundar IK, Lerner CA et al. . Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi:10.1096/fj.14-268276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Q, Kuang H, Chen C et al. . The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nat Immunol. 2015;16:458–466. doi:10.1038/ni.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun N, Yun J, Liu J et al. . Measuring In Vivo Mitophagy. Mol Cell. 2015;60:685–696. doi:10.1016/j.molcel.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015;22:204–206. doi:10.1016/j.cmet.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 40. Houtkooper RH, Mouchiroud L, Ryu D et al. . Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi:10.1038/nature12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohrin M, Shin J, Liu Y et al. . Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347:1374–1377. doi:10.1126/science.aaa2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferrucci L, Corsi A, Lauretani F et al. . The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi:10.1182/blood-2004-07-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldberg EL, Dixit VD. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev. 2015;265:63–74. doi:10.1111/imr.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi:10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi:10.1172/JCI28139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi:10.1016/j.vaccine.2005.08.105 [DOI] [PubMed] [Google Scholar]
- 47. Pang WW, Price EA, Sahoo D et al. . Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA. 2011;108:20012–20017. doi:10.1073/pnas.1116110108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–2703. doi:10.1084/jem.20111490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sansoni P, Cossarizza A, Brianti V et al. . Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- 50. Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi:10.1084/jem.20040437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi:10.1172/JCI64096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Labrie JE 3rd, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi:10.1084/jem.20040845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ortiz JR, Neuzil KM, Rue TC et al. . Population-based incidence estimates of influenza-associated respiratory failure hospitalizations, 2003 to 2009. Am J Respir Crit Care Med. 2013;188:710–715. doi:10.1164/rccm.201212-2341OC [DOI] [PubMed] [Google Scholar]
- 54. Schiller HB, Fernandez IE, Burgstaller G et al. . Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol. 2015;11:819. doi:10.15252/msb.20156123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gruber MP, Coldren CD, Woolum MD et al. . Human lung project: evaluating variance of gene expression in the human lung. Am J Respir Cell Mol Biol. 2006;35:65–71. doi:10.1165/rcmb.2004-0261OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Misra V, Lee H, Singh A et al. . Global expression profiles from C57BL/6J and DBA/2J mouse lungs to determine aging-related genes. Physiol Genomics. 2007;31:429–440. doi:10.1152/physiolgenomics.00060.2007 [DOI] [PubMed] [Google Scholar]
- 57. Rolewska P, Al-Robaiy S, Navarrete Santos A, Simm A, Silber RE, Bartling B. Age-related expression, enzymatic solubility and modification with advanced glycation end-products of fibrillar collagens in mouse lung. Exp Gerontol. 2013;48:29–37. doi:10.1016/j.exger.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 58. Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance–United States, 1999-2011. Chest. 2013;144:284–305. doi:10.1378/chest.13-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lange P, Celli B, Agustí A et al. . Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi:10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 60. Alder JK, Guo N, Kembou F et al. . Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi:10.1164/rccm.201103-0520OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Silverman GA, Pak SC, Perlmutter DH. Disorders of protein misfolding: alpha-1-antitrypsin deficiency as prototype. J Pediatr. 2013;163:320–326. doi:10.1016/j.jpeds.2013.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen ZH, Kim HP, Sciurba FC et al. . Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi:10.1371/journal.pone.0003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schamberger AC, Mise N, Meiners S, Eickelberg O. Epigenetic mechanisms in COPD: implications for pathogenesis and drug discovery. Expert Opin Drug Discov. 2014;9:609–628. doi:10.1517/17460441.2014.913020 [DOI] [PubMed] [Google Scholar]
- 64. McHugh MK, Symanski E, Pompeii LA, Delclos GL. Prevalence of asthma among adult females and males in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2001-2004. J Asthma. 2009;46:759–766. doi:10.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Skloot GS, Busse PJ, Braman SS et al. ; ATS ad hoc Committee on Asthma in the Elderly An Official American Thoracic Society Workshop Report: Evaluation and Management of Asthma in the Elderly. Ann Am Thorac Soc. 2016;13:2064–2077. doi:10.1513/AnnalsATS.201608-658ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Postma DS, Rabe KF. The Asthma-COPD Overlap Syndrome. N Engl J Med. 2015;373:1241–1249. doi:10.1056/NEJMra1411863 [DOI] [PubMed] [Google Scholar]
- 67. King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi:10.1016/S0140-6736(11)60052-4 [DOI] [PubMed] [Google Scholar]
- 68. Raghu G, Collard HR, Egan JJ et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi:10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi:10.1164/rccm.200602-163OC [DOI] [PubMed] [Google Scholar]
- 70. Selman M, Pardo A. Revealing the pathogenic and aging-related mechanisms of the enigmatic idiopathic pulmonary fibrosis. an integral model. Am J Respir Crit Care Med. 2014;189:1161–1172. doi:10.1164/rccm.201312-2221PP [DOI] [PubMed] [Google Scholar]
- 71. Ley B, Ryerson CJ, Vittinghoff E et al. . A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi:10.7326/0003-4819-156-10-201205150-00004 [DOI] [PubMed] [Google Scholar]
- 72. Hecker L, Logsdon NJ, Kurundkar D et al. . Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi:10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stanley SE, Chen JJ, Podlevsky JD et al. . Telomerase mutations in smokers with severe emphysema. J Clin Invest. 2015;125:563–570. doi:10.1172/JCI78554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tzouvelekis A, Kaminski N. Epigenetics in idiopathic pulmonary fibrosis. Biochem Cell Biol. 2015;93:159–170. doi:10.1139/bcb-2014-0126 [DOI] [PubMed] [Google Scholar]
- 75. Lawson WE, Grant SW, Ambrosini V et al. . Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59:977–980. doi:10.1136/thx.2004.026336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jain M, Rivera S, Monclus EA et al. . Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288:770–777. doi:10.1074/jbc.M112.431973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hecker L, Vittal R, Jones T et al. . NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi:10.1038/nm.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fantin F, Di Francesco V, Fontana G et al. . Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci. 2007;62:1375–1381. [DOI] [PubMed] [Google Scholar]
- 79. Barreiro E, Sznajder JI, Nader GA, Budinger GR. Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med. 2015;191:616–619. doi:10.1164/rccm.201412-2189OE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maltais F, Decramer M, Casaburi R et al. ; ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi:10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dres M, Dube BP, Mayaux J et al. . Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical ICU patients. Am J Respir Crit Care Med. 2017;195:57–66. doi:10.1164/rccm.201602-0367OC [DOI] [PubMed] [Google Scholar]
- 82. Dos Santos C, Hussain SN, Mathur S et al. ; MEND ICU Group; RECOVER Program Investigators; Canadian Critical Care Translational Biology Group Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016;194:821–830. doi:10.1164/rccm.201512-2344OC [DOI] [PubMed] [Google Scholar]
- 83. Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142:1572–1581. doi:10.1242/dev.114223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Puthucheary ZA, Rawal J, McPhail M et al. . Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi:10.1001/jama.2013.278481 [DOI] [PubMed] [Google Scholar]
- 85. Doucet M, Russell AP, Léger B et al. . Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi:10.1164/rccm.200605-704OC [DOI] [PubMed] [Google Scholar]
- 86. Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122:659–667. doi:10.1016/j.cell.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 87. Bentley DR, Balasubramanian S, Swerdlow HP et al. . Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi:10.1038/nature07517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nagalakshmi U, Wang Z, Waern K et al. . The transcriptional landscape of the yeast genome defined by RNA sequencing. Science. 2008;320:1344–1349. doi:10.1126/science.1158441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Macosko EZ, Basu A, Satija R et al. . Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi:10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kharchenko PV, Tolstorukov MY, Park PJ. Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nat Biotechnol. 2008;26:1351–1359. doi:10.1038/nbt.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yugi K, Kubota H, Hatano A, Kuroda S. Trans-Omics: how to reconstruct biochemical networks across multiple “Omic” layers. Trends Biotechnol. 2016;34:276–290. doi:10.1016/j.tibtech.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 92. Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–510. doi:10.1165/rcmb.2013-0086MA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Du Y, Guo M, Whitsett JA, Xu Y. “LungGENS”: a web-based tool for mapping single-cell gene expression in the developing lung. Thorax. 2015;70:1092–1094. doi:10.1136/thoraxjnl-2015-207035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Houseman EA, Kim S, Kelsey KT, Wiencke JK. DNA methylation in whole blood: uses and challenges. Curr Environ Health Rep. 2015;2:145–154. doi:10.1007/s40572-015-0050-3 [DOI] [PubMed] [Google Scholar]
- 95. Ji H, Ehrlich LI, Seita J et al. . Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 2010;467:338–342. doi:10.1038/nature09367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Knight AK, Craig JM, Theda C et al. . An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol. 2016;17:206. doi:10.1186/s13059-016-1068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Houseman EA, Kelsey KT, Wiencke JK, Marsit CJ. Cell-composition effects in the analysis of DNA methylation array data: a mathematical perspective. BMC Bioinformatics. 2015;16:95. doi:10.1186/s12859-015-0527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Accomando WP, Wiencke JK, Houseman EA, Nelson HH, Kelsey KT. Quantitative reconstruction of leukocyte subsets using DNA methylation. Genome Biol. 2014;15:R50. doi:10.1186/gb-2014-15-3-r50 [DOI] [PMC free article] [PubMed] [Google Scholar]