Abstract
Background
The apolipoprotein E polymorphism ε4 allele (ApoE4) and gait impairment are both known risk factors for developing cognitive decline and dementia. However, it is unclear the interrelationship between these factors, particularly among older adults with mild cognitive impairment (MCI) who are considered as prodromal for Alzheimer’s disease. This study aimed to determine whether ApoE4 carrier individuals with MCI may experience greater impairment in gait performance.
Methods
Fifty-six older adults with MCI from the “Gait and Brain Study” who were identified as either ApoE4 carriers (n = 20) or non-ApoE4 carriers (n = 36) with 1 year of follow-up were included. Gait variability, the main outcome variable, was assessed as stride time variability with an electronic walkway. Additional gait variables and cognitive performance (mini-mental state examination [MMSE] and Montreal Cognitive Assessment [MoCA]) were also recorded. Covariates included age, sex, education level, body mass index, and number of comorbidities.
Results
Baseline characteristics were similar for both groups. Repeated measures analysis of covariance showed that gait stride time and stride length variabilities significantly increased in ApoE4 carriers but was maintained in the non-ApoE4 carriers. Similarly, ApoE4 carriers showed greater decrease in MMSE score at follow-up.
Conclusions
In this sample of older adults with MCI, the presence of at least one copy of ApoE4 was associated with the development of both increased gait variability and cognitive decline during 1 year of follow-up. ApoE4 genotype might be considered as a potential mediator of decline in mobility function in MCI; future studies with larger samples are needed to confirm our preliminary findings.
Keywords: Apolipoprotein E, Gait variability, Cognition, Mild cognitive impairment, Longitudinal study
Mild cognitive impairment (MCI) is conceptualized as a transitional phase between normal aging to dementia, particularly Alzheimer’s disease (AD). Although numerous studies have indicated an accelerated rate of progression to AD in MCI older adults, almost one third of older adults with MCI will remain clinically stable or even revert to normal (1). A better understanding of this heterogeneity can help to accurately detect which individuals with MCI will progress to dementia.
A growing body of literature indicates that impaired motor performance is associated with an increased risk for cognitive decline. For example, it has been indicated that slow gait speed is associated with an increased risk for MCI and dementia syndromes (2–4). Previous studies have also shown that temporal and spatial gait variability tend to increase in older adults with MCI (5), and these quantitative gait abnormalities predict risk of cognitive decline and dementia in initially nondemented older adults (6).
Another factor associated with age-related cognitive impairment and AD is the apolipoprotein E polymorphism ε4 allele (ApoE4). This genetic variation is also a known genetic risk factor for cerebral amyloid angiopathy (7). A previous meta-analysis has shown that the odds of AD among ApoE4 carriers compared with noncarriers (e.g., E3/E3) increases to 14.9 times (7). It is also well documented that conversion of MCI to AD is accelerated by ApoE4 (8). Although the underlying mechanism(s) of this causal relationship is not fully understood, evidence has been presented suggesting ApoE4’s involvement with amyloid beta aggregation related to brain damage and atrophy (9).
Given the well-established association between cognitive performance and gait (10–12), it has been hypothesized that ApoE4 may also be associated with impairment of gait performance. A number of epidemiological findings, indeed, suggest a relationship between gait performance and ApoE4 carrier status. Verghese et al. showed that the presence of ApoE4 was not significantly associated with overall gait speed decline, but it was associated with faster gait speed decline in older men (13). Buchman et al. showed that ApoE4 is associated with rapid motor decline, such as muscle weakness, among older adults (14). More recently, MacAulay et al. showed that the presence of ApoE4 was significantly associated with shorter stride length and greater dual-task decrement in stride length in older adults (15).
In MCI older adults, Doi et al. have recently shown a cross-sectional association between ApoE4 carrier status and slow gait speed (16). However, the longitudinal relationship of ApoE4 carrier status with gait changes including quantitative gait parameters remains still unclear. Revealing the influence of ApoE4 on longitudinal changes in gait parameters, such as gait velocity and gait variability, could lead to a better understanding of the underlying mechanism of gait and cognition interaction in aging and neurodegeneration. The present longitudinal analysis therefore assessed whether ApoE4 is associated with greater change in gait parameters, specifically, stride time variability as the main endpoint; and gait velocity, stride time and length, and cognition as secondary endpoints in a well characterized cohort of older adults with MCI.
Materials and Methods
Participants
The Gait and Brain Study is a prospective cohort study designed to determine whether quantitative gait impairments can predict incident cognitive and mobility decline and progression to dementia among community-dwelling older adults free of dementia at baseline (2). Design and logistics have been described in detail elsewhere (2,17,18) and additional information can be found at clinicaltrial.gov, study identifier NTC03020381.
To be included in this analysis, participants needed to have MCI, as defined below; ApoE4 genotyping tested, and partake in two subsequent assessments to complete at least 12 months of follow-up. Of a total of 70 participants with MCI included in the Gait and Brain study since 2010 when ApoE4 genotyping testing was started, 61 agreed to have their blood drawn and 56 were included in this study since they had baseline, 6 month, and 12 month assessments completed at the moment of our analyses.
MCI was ascertained by scoring 0.5 on the global rating of the Clinical Dementia Rating (CDR) scale and by satisfying the following four criteria (19): (i) subjective cognitive complaints; (ii) objective cognitive impairment in at least one of the following cognitive domains: memory, executive function, attention, and language (19,20); (iii) preserved activities of daily living confirmed by clinician’s interviews; and (iv) absence of dementia using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition revised. Exclusion criteria included having parkinsonism or any neurological disorder with residual motor deficit (e.g., stroke), musculoskeletal disorder detected by clinical examination which affects gait performance, active osteoarthritis affecting the lower limbs, use of psychotropic medication which can affect motor performance, and major depression.
Ethics approval was obtained from the University of Western Ontario Health Sciences Research Ethics Board and participants’ signed informed consents were obtained at enrollment prior to study assessments. Data collection occurred between July 2010 and March 2015.
Measurements
Data collected at baseline and during the 1 year of follow-up testing involved medical, cognitive, and gait assessments. ApoE4 genotype status was assessed at baseline visit. Cognition was assessed at each study visit using the Mini-Mental State Examination (MMSE, score ranging from 0 to 30) (21), and the Montreal Cognitive Assessment (MoCA, score ranging from 0 to 30) (22) with higher scores indicating higher overall cognitive function. The MoCA test has been validated in our cohort for MCI identification (23).
Gait assessments
Gait performance, including gait velocity (cm/s), stride time (sec), stride time variability (%), stride length (cm), and stride length variability (%) was assessed using an electronic walkway (GaitRite Systems, 600 cm long and 64 cm wide) at each visit. Start and end points were marked on the floor 1 meter from both the walkway start and end point. These 1-meter markings were used in order to avoid recording participants’ acceleration and deceleration phases on the walkway. Each participant performed one practice trial walking on the mat at their usual pace. For the recorded walk, participants were again instructed to walk on the walkway at their usual pace. The gait trials occurred in a quiet, well-lit room, and participants wore comfortable footwear without any additional attached monitors. This gait assessment protocol has been validated in our cohort (17). Stride time variability has been selected as the main endpoint since previous research has shown that it is sensitive to impairment in cortical brain controls. Additionally, we have previously used it as the main endpoint in cross-sectional studies in MCI (2,24–26).
ApoE4 genotyping
Genomic DNA was extracted from whole blood samples using the Puregene DNA isolation kit (Gentra Systems Inc, Mississauga, ON, Canada). Genotypes were determined with TaqMan allelic discrimination assays (Applied Biosystems) for two single nucleotide polymorphisms, specifically rs429358 (ApoE 112) and rs7412 (ApoE 158) using standard methods. ApoE genotypes were classified into E2/E2, E3/E2, E3/E3, E4/E2, E4/E3, and E4/E4 based on the allele combinations for the two assays.
Covariates
Relevant sociodemographic and clinical variables, including age, sex, education level, body mass index, and number of comorbidities were recorded (Table 1) and assessed as covariates of the relationship between ApoE4 genotype and longitudinal changes in gait variables. The selected covariates have been previously associated with gait performance and used in a previous cross-sectional study examining the association between ApoE4 and physical function among MCI older adults with MCI (16).
Table 1.
Baseline Characteristics Stratified by ApoE4 Carrier Status
| Variables | All participants | Non-ApoE4 carriers | ApoE4 carriers | |
|---|---|---|---|---|
| (n = 56) | (n = 36) | (n = 20) | p value | |
| Female, n (%) | 24 (42.9) | 16 (44.4) | 8 (40.0) | .747† |
| Age, mean (SD) | 73.8 (6.7) | 74.9 (7.2) | 71.9 (5.1) | .096‡ |
| Number of years of education, mean (SD) | 13.8 (2.7) | 14.3 (2.6) | 12.9 (2.7) | .064‡ |
| Body mass index, mean (SD) | 26.6 (4.4) | 26.7 (4.3) | 26.4 (4.6) | .788‡ |
| Number of medications, mean (SD) | 7.3 (4.1) | 6.8 (3.6) | 8.1 (5.0) | .277‡ |
| Number of comorbidities, mean (SD) | 6.2 (2.6) | 6.5 (2.4) | 5.8 (3.0) | .307‡ |
| Hypertension, n (%) | 32 (57.1) | 21 (58.3) | 11 (55.0) | .809† |
| Diabetes mellitus, n (%) | 8 (14.3) | 6 (16.7) | 2 (10.0) | .495† |
| Osteoporosis, n (%) | 6 (10.7) | 4 (11.1) | 2 (10.0) | .898† |
| Fall within the past year, n (%) | 18 (32.1) | 13 (36.1) | 5 (25.0) | .394† |
| Fear of falling, n (%) | 6 (10.7) | 2 (5.6) | 4 (20.0) | .094† |
| MMSE, mean (SD) | 28.2 (2.1) | 28.4 (1.8) | 27.9 (2.5) | .372‡ |
| MoCA, mean (SD) | 25.1 (3.3) | 25.2 (3.3) | 25.0 (3.5) | .796‡ |
| Gait velocity, cm/s, mean (SD) | 110.3 (22.3) | 108.5 (23.2) | 103.5 (20.8) | .421‡ |
| Gait stride time, ms, mean (SD) | 1148.3 (103.2) | 1151.3 (107.1) | 1143.0 (98.3) | .776‡ |
| Gait stride time variability, (CoV)% | 2.6 (1.6) | 2.7 (1.8) | 2.6 (1.31) | .871‡ |
| Gait stride length, cm, mean (SD) | 126.2 (20.5) | 124.8 (22.0) | 128.7 (17.9) | .498‡ |
| Gait stride length variability, (CoV)% | 3.1 (2.1) | 3.2 (2.2) | 3.0 (2.1) | .735‡ |
Notes: CoV = coefficient of variation; MMSE = mini-mental state examination; MoCA = Montreal cognitive assessment; SD = standard deviation.
†Chi-square test.
‡ t-test.
Statistical Analyses
Participants were assigned to either ApoE4 carriers (E4/E2, E4/E3, and E4/E4) or non-ApoE4 carriers (E2/E2, E3/E2, and E3/E3). Descriptive statistics of the differences between ApoE4 carriers and non-ApoE4 carriers were analyzed using Chi-square tests and t-tests. Repeated measures analyses of covariance [ANCOVAs; time (i.e., baseline and follow-up) and ApoE4 genotype (i.e., ApoE4 carriers and noncarriers) factors] adjusted for age, sex, education level, body mass index, number of comorbidities, and MoCA score (only for gait variables) were performed for the main endpoint, gait variability, assessed as stride time variability. Additional analyses were conducted for assessing associations between ApoE4 and gait velocity, stride time, stride length, stride length variability, and MMSE and MoCA. Statistical analyses were performed using IBM SPSS Statistics version 21.0 (SPSS Inc., Chicago, IL), with level of significance set at p <.05.
Results
Fifty-six participants (mean age 73.8 ± 6.7 years, 42.9% women) were included in this analysis. No significant differences in baseline characteristics in ApoE4 carriers when compared with noncarriers were found (Table 1). Distribution of ApoE genotypes were E2/E2, 1.8%; E3/E2, 8.9%; E3/E3, 53.6%; E4/E2, 1.8%; E4/E3, 28.6%; E4/E4, 5.4%.
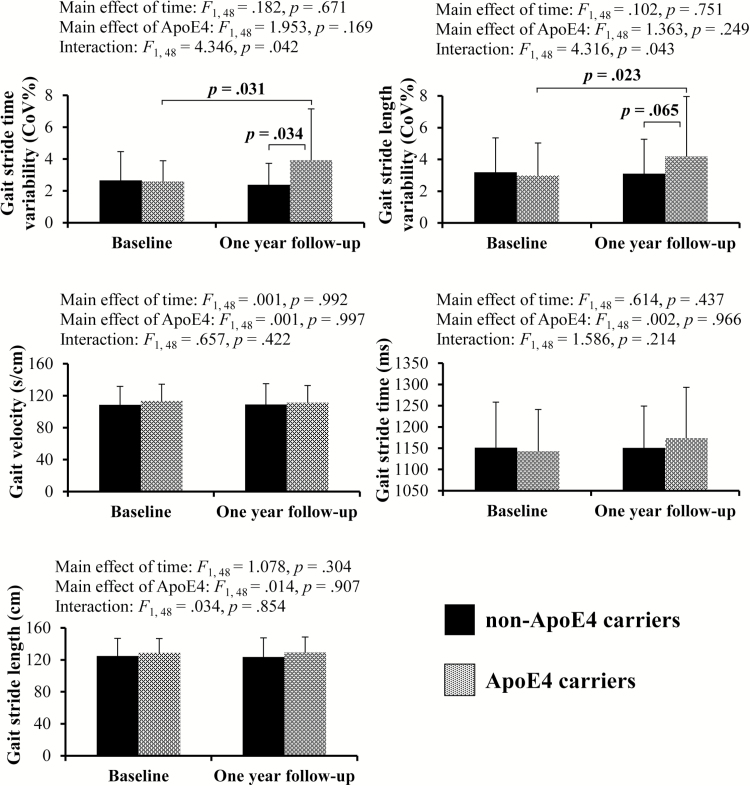
Figure 1 shows baseline and follow-up gait variables changes among ApoE4 carriers and noncarriers. Repeated measures ANCOVAs adjusted for age, sex, education level, body mass index, number of comorbidities, and MoCA score showed a significant interaction between ApoE4 genotype and time factors for gait stride time variability (F1, 48 = 4.346, p = .042) and gait stride length variability (F1, 48 = 4.316, p =. 043) without any significant main effects. Subsequent post-hoc tests demonstrated that ApoE4 carriers showed greater increases in both gait stride time variability (p = .031) and gait stride length variability (p = .023) at 1-year follow-up compared with baseline; and significant difference in gait stride time variability between the two groups at follow-up (p = .034). There were no significant main effects or interactions between the two factors for gait velocity, gait stride time, and gait stride length.
Figure 1.
Comparisons of gait variables at baseline and 1-year follow-up for ApoE4 carriers and non-ApoE4 carriers.
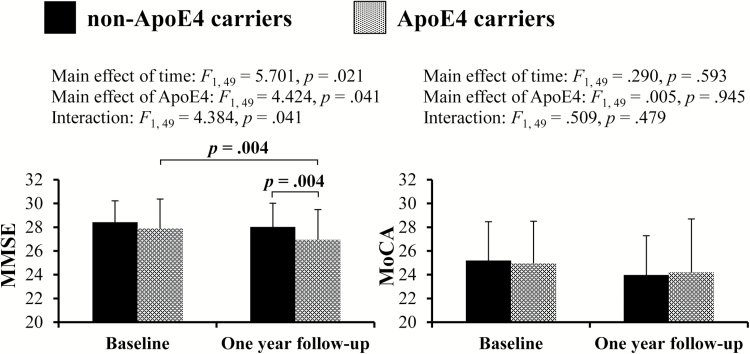
For cognitive functioning (Figure 2), repeated measures ANCOVAs adjusted for covariates showed significant main effects of time (F1, 49 = 5.701, p = .021) and ApoE4 genotype (F1, 49 = 4.424, p = .041) for MMSE score. A significant interaction also appeared between the two factors (F1, 49 = 4.384, p = .041). Subsequent post-hoc tests demonstrated that ApoE4 carriers showed greater decrease in MMSE score at 1 year follow-up compared with baseline (p = .004) There was no significant interaction between the two factors for MoCA score.
Figure 2.
Comparisons of cognitive measurements at baseline and 1-year follow-up for ApoE4 carriers and non-ApoE4 carriers.
Discussion
The present findings demonstrate that the presence of at least one copy of ApoE4 was a risk factor for impairment of gait control among MCI older adults. Gait stride time and stride length variability, valid markers of gait instability and previously associated with MCI (2,17,24), significantly increased in ApoE4 carriers but was maintained in the non-ApoE4 carriers. Moreover, ApoE4 carriers showed greater decrease in MMSE score at follow-up compared with the baseline. These two parallel lines of findings are in agreement with the concept that ApoE4 may mediate the decline in both gait and cognitive functions seen in MCI (16). To our knowledge, no previous studies have shown the longitudinal association of ApoE4 carrier status with the development of higher gait variability in older adults with MCI.
Gait variability in stride time is thought to reflect the ability to generate consistent rhythmical step cycles by central control mechanisms, which are key to regulate gait and maintain a steady and stable walking pattern (24). Increased gait variability is associated with low executive function, poor performance in dual-task gait and with increased risk of falling (10,24). It has also been proposed that gait variability can be a marker of neurodegenerative processes (10,24–28) and specifically in MCI populations, increased gait variability has been associated with structural and neurochemistry brain changes (29,30).
Previous imaging studies in high-functioning community-dwelling older adults demonstrated that greater gait variability is associated with white matter hyperintensities (WMH) severity (31). ApoE4 is also shown to increase the risk of brain microvascular damage, resulting in WMH (9,32). Indeed, ApoE4 carriers with higher WMH volume are at higher risk of developing dementia (33). Thus, white matter lesions in ApoE4 carriers may be an underlying mechanism in the greater increase in gait stride time and stride length variability seen in our study.
Brain accumulation of beta amyloid is another possible mechanism to explain the associations between ApoE4 carrier status and increase in gait variability in our study (34). Beta amyloid accumulates not only in cognitive brain regions, but also in motor-related regions, including basal ganglia (35–37). Therefore, deposition of beta amyloid in the brain regions involved in gait control, such as basal ganglia, may explain why ApoE4 carriers may have increased gait variability.
In the present study, no relationship between ApoE4 and a longitudinal change in gait speed was found. This is partly inconsistent with previous findings in which carriers of ApoE4 among MCI older adults had lower gait speed than noncarriers (16). Although the reason for the difference between the present findings and previous findings can be related to lack of power of our sample size, studies examining the association of ApoE4 genotype and gait speed among nondementia older adults have also been inconsistent. A previous larger study including 622 older adults failed to show a longitudinal association between ApoE4 genotype and gait speed (38). Similarly, no association between ApoE4 genotype and gait speed was found in a cross-sectional study including 1,010 participants (39). Conversely, two longitudinal studies showed that ApoE4 was associated with gait speed decline (13,40). Finally, one study reported only cross-sectional association between the presence of ApoE4 and slower gait speed but not longitudinal association (41). Discrepancies in the previous findings could be explained by the number of ApoE4 alleles (e.g., E4/E3 or E4/E4), comorbidities, and level of executive function. Further studies are needed to examine the determinants of these different findings.
The ApoE4 has been involved in cognitive impairment seen with aging (42,43). This may be especially prominent in MCI older adults (8). Previous studies showed that ApoE4 is associated with AD pathology, such as decreased hippocampal volume and entorhinal cortex thickness (44,45); likewise, MCI older adults who are ApoE4 carriers showed a higher risk of progression to AD (8,46). In line with these previous studies, we found that presence of ApoE4 alleles was significantly associated with greater decreases in MMSE score. This implies the possibility that ApoE4 carriers among older adults with MCI may be at higher risk of both cognitive decline and increased gait variability and mobility decline. Overall, our findings also raise the hypothesis that ApoE4 may modulate falls risk in MCI. In order to better address the role of ApoE4 in the increased risk of falls seen in MCI, further studies are needed. Interestingly, ApoE4 carriers did not show greater impairment of MoCA score. One possible explanation for this divergence can be related to the low sensitivity of MMSE for MCI which increases the likelihood of individuals with MCI to score within the normal range. Indeed, mean baseline MMSE score of our participants was 28.2. Therefore, among MCI older adults, MMSE scores might be sensitive to a significant decrement during disease progression because baseline values were high.
Baseline gait and cognitive performances did not differ by ApoE4 carrier status in our study, whereas a difference was observed at follow-up. This is in line with previous cross-sectional studies using large samples which demonstrated that there were no significant associations between ApoE carrier status and physical (39) and cognitive (16) functions. A possible explanation can be related to the fact that, at cohort inception, participants were recently diagnosed as MCI and thus, at this early stage, ApoE4 carriers might not yet manifest impeding cognitive and mobility decline. Additionally, there may be a possibility that the influence of ApoE4 on physical and cognitive function is modulated by the interaction between aging and other life style-related factor or comorbidities, such as a disorder of lipid metabolism (32).
Our study has limitations. First, our relatively small number of ApoE4 carriers could have affected the power to find significant additional associations with gait variables, such as gait velocity. Confirmation of our findings in a larger sample of subjects with MCI is needed. Second, 1 year of follow-up may not be long enough to detect larger influences of ApoE4 on gait performance. Finally, while our results were independent of important comorbidities, the role of metabolic abnormalities, including increased fasting plasma glucose and serum cholesterol was not explored (9,32). Despite these limitations, to the authors’ knowledge, this is the first report of longitudinal association between ApoE4 carrier status in MCI and gait performance decline.
Conclusion
Having at least one copy of the ApoE4 allele was associated with both future decline in gait performance, assessed as gait stride time variability and gait stride length variability, and global cognition among older adults with MCI. The findings of this study imply the possibility that ApoE4 may be a possible candidate risk factor for impairment of gait control in MCI. Our findings may also help to explain the increased risk of falling seen in MCI populations (10). Further studies are needed to confirm our results in other MCI cohorts.
Funding
The “Gait and Brain Study” is funded by an operating grant from the Canadian Institutes of Health Research (CIHR), MOP 211220.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Our gratitude goes to Larry Stitt for his valuable assistance in our statistical analyses and to Yanina Sarquis-Adamson for her help in editing the final version of the manuscript. Dr. Ryota Sakurai holds a scholarship award from the Japan Society for the Promotion of Science (JSPS) while conducting a postdoctoral research fellowship at the Gait and Brain Lab, University of Western Ontario. Dr. Montero-Odasso’s program in “Gait and Brain Health” is supported by grants from the Canadian Institute of Health and Research (CIHR), the Ontario Ministry of Research and Innovation, The Ontario Neurodegenerative Diseases Research Initiative (ONDRI), the Canadian Consortium on Neurodegeneration in Aging (CCNA), and by Department of Medicine Program of Experimental Medicine (POEM) Research Award, University of Western Ontario. He is the first recipient of the Schulich Clinician-Scientist Award and holds the CIHR New Investigator Award.
References
- 1. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi:10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 2. Montero-Odasso M, Oteng-Amoako A, Speechley M, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–1421. doi:10.1093/gerona/glu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi:10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montero-Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive-frailty: results from the Gait and Brain Study. J Gerontol A Biol Sci Med Sci. 2016;71:1476–1482. doi:10.1093/gerona/glw044 [DOI] [PubMed] [Google Scholar]
- 5. Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. doi:10.1016/j.gaitpost.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 6. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi:10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. doi:10.1001/jama.1997.03550160069041 [PubMed] [Google Scholar]
- 8. Scarabino D, Broggio E, Gambina G, Maida C, Gaudio MR, Corbo RM. Apolipoprotein E genotypes and plasma levels in mild cognitive impairment conversion to Alzheimer’s disease: a follow-up study. Am J Med Genet B Neuropsychiatr Genet. 2016;171:1131–1138. doi:10.1002/ajmg.b.32495 [DOI] [PubMed] [Google Scholar]
- 9. Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi:10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakurai R, Fujiwara Y, Yasunaga M, et al. Regional cerebral glucose metabolism and gait speed in healthy community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2014;69:1519–1527. doi:10.1093/gerona/glu093 [DOI] [PubMed] [Google Scholar]
- 12. Hooghiemstra AM, Ramakers IH, Sistermans N, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4C study. J Gerontol A Biol Sci Med Sci. 2017. doi:10.1093/gerona/glx003 [DOI] [PubMed] [Google Scholar]
- 13. Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB. Role of APOE genotype in gait decline and disability in aging. J Gerontol A Biol Sci Med Sci. 2013;68:1395–1401. doi:10.1093/gerona/glt115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23:63–69. doi:10.1097/WAD.0b013e31818877b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacAulay RK, Allaire T, Brouillette R, Foil H, Bruce-Keller AJ, Keller JN. Apolipoprotein E genotype linked to spatial gait characteristics: predictors of cognitive dual task gait change. PLoS One. 2016;11:e0156732. doi:10.1371/journal.pone.0156732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doi T, Shimada H, Makizako H, Tsutsumimoto K, Uemura K, Suzuki T. ApolipoproteinE genotype and physical function among older people with mild cognitive impairment. Geriatr Gerontol Int. 2015;15:422–427. doi:10.1111/ggi.12291 [DOI] [PubMed] [Google Scholar]
- 17. Montero-Odasso M, Casas A, Hansen KT, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. doi:10.1186/ 1743-0003-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson DA, Savva GM, Coen RF, Kenny RA. Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc. 2014;62:2118–2124. doi:10.1111/jgs.13111 [DOI] [PubMed] [Google Scholar]
- 19. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi:10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 20. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi:10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi:10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 23. Montero-Odasso M, Muir SW. Simplifying detection of mild cognitive impairment subtypes. J Am Geriatr Soc. 2010;58:992–994. doi:10.1111/j.1532-5415.2010.02823.x [DOI] [PubMed] [Google Scholar]
- 24. Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil. 2005;2:19. doi:10.1186/1743-0003-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Hum Mov Sci. 2007;26:555–589. doi:10.1016/j.humov.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13:428–437. doi:10.1002/mds.870130310 [DOI] [PubMed] [Google Scholar]
- 27. Lord S, Howe T, Greenland J, Simpson L, Rochester L. Gait variability in older adults: a structured review of testing protocol and clinimetric properties. Gait Posture. 2011;34:443–450. doi:10.1016/j.gaitpost.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 28. Montero-Odasso M. Gait as a biomarker of cognitive impairment and dementia syndromes. Quo vadis? Eur J Neurol. 2016;23:437–438. doi:10.1111/ene.12908 [DOI] [PubMed] [Google Scholar]
- 29. Annweiler C, Montero-Odasso M, Bartha R, Drozd J, Hachinski V, Beauchet O. Association between gait variability and brain ventricle attributes: a brain mapping study. Exp Gerontol. 2014;57:256–263. doi:10.1016/j.exger.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 30. Annweiler C, Beauchet O, Bartha R, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain. 2013;136:859–871. doi:10.1093/brain/aws373 [DOI] [PubMed] [Google Scholar]
- 31. Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi:10.1159/000111582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. [DOI] [PubMed] [Google Scholar]
- 33. Brickman AM, Schupf N, Manly JJ, et al. APOE ε4 and risk for Alzheimer’s disease: do regionally distributed white matter hyperintensities play a role? Alzheimers Dement. 2014;10:619–629. doi:10.1016/j.jalz.2014.07.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi:10.1073/pnas.0600549103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horoupian DS, Wasserstein PH. Alzheimer’s disease pathology in motor cortex in dementia with Lewy bodies clinically mimicking corticobasal degeneration. Acta Neuropathol. 1999;98:317–322. doi:10.1007/s004010051087 [DOI] [PubMed] [Google Scholar]
- 36. Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi:10.1002/ana.20723 [DOI] [PubMed] [Google Scholar]
- 37. Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–231. [DOI] [PubMed] [Google Scholar]
- 38. Skoog I, Hörder H, Frändin K, et al. Association between APOE genotype and change in physical function in a population-based Swedish cohort of older individuals followed over four years. Front Aging Neurosci. 2016;8:225. doi:10.3389/fnagi.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasunilashorn S, Glei DA, Lin YH, Goldman N. Apolipoprotein E and measured physical and pulmonary function in older Taiwanese adults. Biodemography Soc Biol. 2013;59:57–67. doi:10.1080/19485565.2013.778703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alfred T, Ben-Shlomo Y, Cooper R, et al. ; HALCyon Study Team Associations between APOE and low-density lipoprotein cholesterol genotypes and cognitive and physical capability: the HALCyon programme. Age (Dordr). 2014;36:9673. doi:10.1007/s11357-014-9673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Melzer D, Dik MG, van Kamp GJ, Jonker C, Deeg DJ. The apolipoprotein E e4 polymorphism is strongly associated with poor mobility performance test results but not self-reported limitation in older people. J Gerontol A Biol Sci Med Sci. 2005;60:1319–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caselli RJ, Reiman EM, Locke DE, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64:1306–1311. doi:10.1001/archneur.64.9.1306 [DOI] [PubMed] [Google Scholar]
- 43. Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol Aging. 2011;32:63–74. doi:10.1016/j.neurobiolaging.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 44. Spampinato MV, Langdon BR, Patrick KE, Parker RO, Collins H, Pravata’ E; Alzheimer’s Disease Neuroimaging Initiative Gender, apolipoprotein E genotype, and mesial temporal atrophy: 2-year follow-up in patients with stable mild cognitive impairment and with progression from mild cognitive impairment to Alzheimer’s disease. Neuroradiology. 2016;58:1143–1151. doi:10.1007/s00234-016-1740-8 [DOI] [PubMed] [Google Scholar]
- 45. Shi J, Leporé N, Gutman BA, et al. ; Alzheimer’s Disease Neuroimaging Initiative Genetic influence of apolipoprotein E4 genotype on hippocampal morphometry: An N = 725 surface-based Alzheimer’s disease neuroimaging initiative study. Hum Brain Mapp. 2014;35:3903–3918. doi:10.1002/hbm.22447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ. Predictive value of APOE-ε4 allele for progression from MCI to AD-type dementia: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1149–1156. doi:10.1136/jnnp.2010.231555 [DOI] [PubMed] [Google Scholar]