Abstract
Background
The Foundation for the NIH Sarcopenia Project validated cutpoints for appendicular lean mass. We ascertained the relationship between low lean mass (LLM), obesity, and mortality and identified predictors in this subgroup.
Methods
A total of 4,984 subjects aged 60 years and older were identified from the National Health and Nutrition Examination Survey 1999–2004 linked to the National Death Index. LLM was defined using reduced appendicular lean mass (men < 19.75 kg; females < 15.02 kg). Obesity was defined using dual-energy x-ray absorptiometry body fat (males ≥ 25%; females ≥ 35%). LLM with obesity was defined using criteria for both LLM and obesity. Proportional hazard models determined mortality risk for LLM and LLM with obesity, separately (referent = no LLM and no LLM with obesity, respectively).
Results
Mean age was 71.1 ± 0.19 years (56.5% female). Median follow-up was 102 months (interquartile range: 78, 124) with 1,901 deaths (35.0%). Prevalence of LLM with obesity was 33.5% in females and 12.6% in males. In those with LLM, overall mortality risk was 1.49 (1.27, 1.73) in males and 1.19 (1.02, 1.40) in females. Mortality risk in LLM with obesity was 1.31 (1.11, 1.55) and 0.99 (0.85, 1.16) in males and females, respectively. Age, diabetes, history of stroke, congestive heart failure, cancer, and kidney disease were predictive of death.
Conclusions
Risk of death is higher in subjects with LLM than with LLM and obesity. Having advanced age, diabetes, stroke, heart failure, cancer, and renal disease predict a worse prognosis in both classifications.
Keywords: Low lean mass, Survival, Epidemiology
Sarcopenia, defined as the loss of muscle and function with aging, is a major risk factor for numerous adverse health consequences in older adults (1). While sarcopenia can be accelerated following an acute illness, a natural loss occurs during the aging process (2). However, its definition in the past has been fraught with considerable methodological challenges (3), prompting a consortium of experts to work with the Foundation for the National Institutes of Health (FNIH) to identify individuals in clinical practice at risk for clinical weakness using body composition and functional measures (1,4,5). Better characterizing sarcopenia is an essential first step in describing its natural history and long-term outcomes of those with this geriatric syndrome in older adults.
The emergence of the obesity epidemic in older adults potentially compounds the adverse outcomes of individuals with sarcopenia (6). Adults with obesity surviving to older adulthood are more likely to have declines in physical function (7), morbidity (8), and institutionalization (9). The interplay between sarcopenia and obesity in patients with both syndromes is referred to as sarcopenic obesity and is an area of intense research requiring further attention. Both disorders lead to adverse, unintended consequences and have limited therapeutic modalities to reverse such changes. The purpose of this study was to apply the new FNIH definitions of sarcopenia, using low lean mass (LLM) as its primary definition, to a representative cohort of U.S. adults with and without obesity based on different fat indices. We then aimed to ascertain the impact of these classifications on long-term mortality. An exploratory analysis was performed to identify baseline predictors of overall mortality in these LLM with obesity groups with the anticipation that this may allow clinicians to better target individuals at highest risk.
Methods
Study Design and Sample
The National Health and Nutrition Examination Surveys (NHANES) are cross-sectional survey data conducted by the Centers for Disease Control and Prevention of individuals representative of the U.S. population. NHANES oversamples minorities and older adults and is a multistage, complex stratified, probability sampling design. All procedural manuals and survey contents are publically accessible and available online at http://www.cdc.gov/nchs/nhanes. Survey data from 1999 to 2004 were used for this analysis after being downloaded in September 2015. Mortality data were downloaded in February 2016. NHANES had its own formal institutional review board process. Our institution exempted this study due to the nature of the de-identified data. We included 4,984 subjects after restricting our sample to individuals aged 60 years and older with body composition measures (see below) from an initial study cohort of 38,077 screened subjects. Of the 31,125 interviewed, 29,402 were evaluated in a mobile examination center by a physician. Our rationale for limiting to age older than 60 was that the prevalence of sarcopenia and sarcopenic obesity is much less in younger populations (3).
Demographic Covariates
All sociodemographic variables (age, sex, race, poverty income ratio, smoking status) and comorbid medical conditions were assessed using a self-reported questionnaire. These questionnaires were completed by the subject or their caregiver if participants were unable to do so. Individuals were classified by age category (60–69.9, 70–79.9, and 80+ years) and race (non-Hispanic White, non-Hispanic Black, Hispanic, and Other). Poverty income ratio is an index for the ratio of family income to poverty based on the Department of Health and Human service poverty guidelines. It was calculated by dividing family income by poverty guidelines specific to family size, as well as the appropriate year and state. Smoking status was defined as never smokers, former smokers of cigarettes, or current smokers. Presence of kidney disease was defined in individuals based on a Modification of Diet in Renal Disease Study formula for glomerular filtration rate of less than 60 mL/min/m2 (10).
Anthropometric Measures
All measurements were performed on the right side of the body to the nearest tenth of a centimeter except if amputations, casts, or other factors prevented an assessment. A stadiometer measured height after deep inhalation and an electronic digital scale, calibrated in kilograms, assessed weight. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference (WC) was measured standing at the iliac crest (at the mid-axillary line) using a tape measure. Obesity based on BMI and WC were defined as greater than or equal to 30 kg/m2 and greater than or equal to 88/102 cm in men and women (11).
Measurement of LLM and Obesity
Dual-energy x-ray absorptiometry using a QDR-4500 Hologic scanner (Bedford, MA) provided all body composition variables of fat and appendicular lean mass (ALM) and their respective percentages. ALM was defined as the sum of the muscle mass of both arms and legs. For this assessment, NHANES excluded subjects whose height was above 192.5 cm or weight was above 136.4 kg. All metal objects were removed with the exception of false teeth and hearing aids. Each NHANES cycle consisted of similar operations procedures. The FNIH definition for sarcopenia was used to define LLM as an ALM below 19.75 kg and below 15.02 kg in men and women, respectively (1). A second FNIH definition of a ratio of ALM:BMI below 0.789 and below 0.512, respectively, was also considered (see the Statistical Analysis section). Obesity using body fat was defined as in our previous studies of 25% or above in men and 35% or above in women (12). LLM with obesity was defined as the combination of each definition of LLM with each respective definition of obesity.
Mortality Data
All mortality information was cross-linked to the NHANES datasets using National Death Index data. All data are publically available and contains de-identified death certificate data, updated through December 31, 2011. Death data were based upon a probabilistic match between NHANES and National Death Index data. Cause of death was classified as cardiovascular (including stroke) or other, following the International Statistical Classification of Disease, Injuries and Causes of Death guidelines with the 9th revision used for those dying in 1999, and the 10th revision for all others. Procedures are in place to harmonize the differences in definitions and causes of death. Time of follow-up was calculated in months from interview date to date of death or most recent vital record. Vital status was accounted for in more than 99% of our sample.
Statistical Analysis
A secondary analysis of the data was performed after merging all relevant files into a master file in February 2016. We followed standard NHANES analytic procedures for weighting, accounting for primary sampling unit, cluster, and strata. Continuous data were presented as means ± SE or counts (weight prevalence). Data are presented for the overall cohort and stratified by sex. A t test and chi-square compared continuous and categorical data, respectively, between survivors and decedents. A weighted prevalence of LLM using the ALM definition, obesity (using body fat), and LLM with obesity, using a combination of the LLM and obesity definition, was calculated. Rates were stratified by age group (60–69.9, 70–79.9, and ≥80 years). The primary analysis was the use of Cox’s proportional hazard models to assess the association of all-cause and cardiovascular mortality risk with LLM and LLM with obesity in separate models. This analysis was then stratified by sex. Three separate proportional hazard models adjusting for a priori covariates were constructed: Model 1 was unadjusted, Model 2 adjusted for age, sex (where appropriate), race, education, and smoking status; Model 3 additionally adjusted for diabetes, congestive heart failure, non-skin cancer, coronary artery disease, arthritis, and physical activity. Proportional hazard assumptions were confirmed. An exploratory analysis determined predictors of overall mortality in those with LLM and LLM with obesity using stepwise selection methods. A p value of .10 was the upper level for removal from the model, and a value of .05 was the lower level for the addition to the model. Lastly, as FNIH considered two definitions of sarcopenia (ALM and ALM:BMI), we replicated the above analytic method using ALM:BMI in lieu of ALM, as a sensitivity analysis. All analyses were conducted using STATA v. 12 (College Station, TX). A p value of less than 0.05 was considered statistically significant.
Results
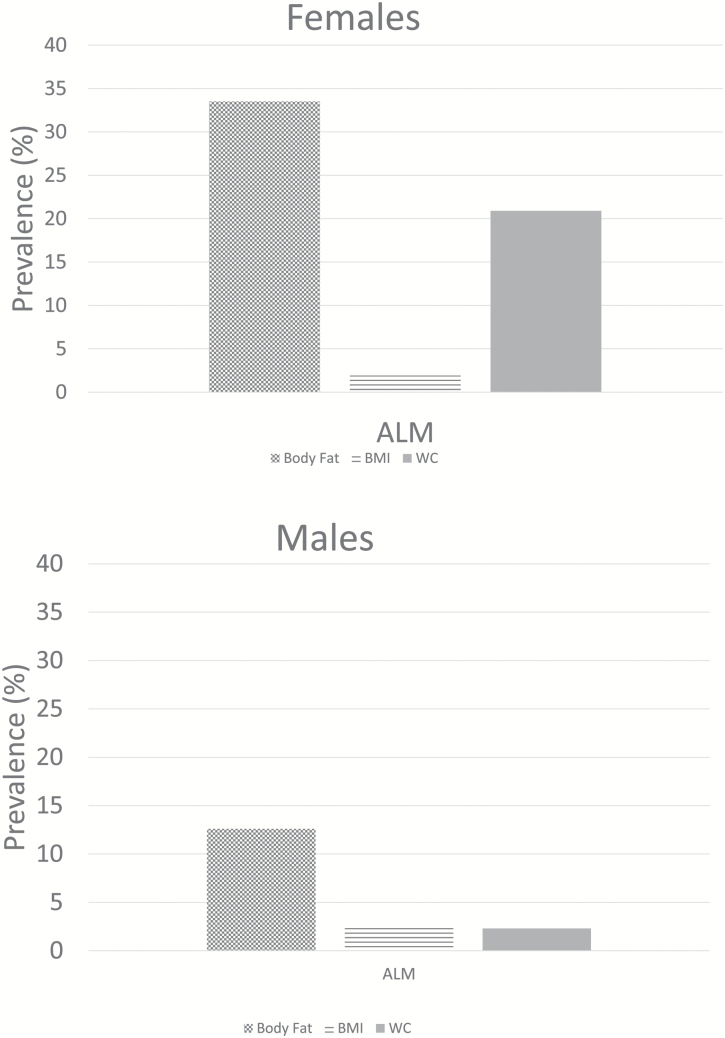
Of the 4,984 subjects, the mean age was 71.1 ± 0.19. Median follow-up time was 102 months (interquartile range: 78, 124). Table 1 presents data on the overall cohort, by sex, and the differences in sociodemographic characteristics by vital status. Table 2 highlights comorbidities and anthropometric characteristics of the cohort, by vital status. Generally, decedents had a higher comorbidity burden than those alive across both sexes. Table 3 presents data on the overall prevalence rates of LLM and LLM with obesity. Rates were lower in males than in females. Rates increased with age across both sexes and the definition of sarcopenic obesity using body fat resulted in the highest prevalence for both sexes (Figure 1a and b). The ALM:BMI rates are represented in Supplementary Appendix 1. Using BMI or WC as other obesity indices, rates of LLM with obesity were considerably lower (Supplementary Appendix 2).
Table 1.
Sociodemographic Characteristics of 4,984 Subjects Aged 60 and Older in NHANES 1999–2004 Cohort
| Overall | Overall | Males | Overall | Females | |||||
|---|---|---|---|---|---|---|---|---|---|
| >60 y | Males | Survivors | Decedents | p Value | Females | Survivors | Decedents | p Value | |
| N = 4,984 | N = 2,453 | N = 1,404 | N = 1,048 | N = 2,531 | N = 1,676 | N = 853 | |||
| Age, y ± SE | 71.1 ± 0.19 | 70.5 ± 0.18 | 68.1 ± 0.16 | 74.3 ± 0.32 | <.001 | 71.6 ± 0.25 | 69.4 ± 0.22 | 76.3 ± 0.34 | <.001 |
| Weight, kg | 77.7 ± 0.30 | 85.1 ± 0.43 | 87.0 ± 0.56 | 82.1 ± 0.69 | <.001 | 72.0 ± 0.35 | 73.5 ± 0.44 | 68.8 ± 0.70 | <.001 |
| Hispanics | 1,202 (7.3) | 579 (6.8) | 388 (6.9) | 191 (6.6) | 623 (7.6) | 465 (8.3) | 158 (6.3) | ||
| Whites | 2,846 (81.2) | 1,427 (82.5) | 764 (82.5) | 662 (82.5) | .06 | 1,419 (80.2) | 879 (79.3) | 539 (82.0) | .20 |
| Blacks | 811 (8.3) | 386 (7.7) | 210 (7.0) | 176 (8.8) | 425 (8.9) | 283 (8.6) | 141 (9.3) | ||
| Current smoker | 611 (11.9) | 374 (13.9) | 183 (11.4) | 191 (18.0) | 1,543 (10.3) | 183 (9.3) | 91 (12.5) | ||
| Never smoker | 2,327 (46.7) | 784 (30.6) | 476 (33.2) | 308 (26.5) | .002 | 746 (59.1) | 476 (61.3) | 488 (54.8) | .008 |
| Former smoker | 2,035 (41.4) | 1,289 (55.4) | 741 (55.4) | 547 (55.4) | 237 (30.6) | 146 (29.5) | 273 (32.6) | ||
| Activity: sits | 1,569 (29.3) | 763 (28.6) | 313 (20.1) | 450 (42.3) | 806 (29.8) | 399 (22.6) | 407 (45.2) | ||
| Activity: walks | 2,756 (55.6) | 1,316 (53.4) | 824 (58.2) | 491 (45.8) | <.001 | 1,440 (57.4) | 1,059 (62.3) | 379 (46.7) | <.001 |
| Activity: light | 541 (13.1) | 288 (14.6) | 205 (18.1) | 83(9.2) | 253 (11.9) | 195 (13.9) | 58 (7.7) | ||
| Activity: heavy | 106 (2.0) | 80 (3.3) | 59 (3.7) | 21 (2.8) | 26 (0.1) | 21 (1.2) | 5 (0.3) | ||
| PIR | 2.93 ± 0.05 | 2.99 ± 0.07 | 3.28 ± 0.07 | 2.54 ± 0.08 | <.001 | 2.53 ± 0.07 | 2.69 ± 0.07 | 2.20 ± 0.08 | <.001 |
| >College education | 1,676 (40.6) | 884 (46.1) | 554 (38.2) | 330 (51.2) | <.001 | 792 (36.4) | 556 (31.3) | 235 (38.8) | .01 |
Note: Data are mean ± SEs or counts (%). Data are weighted according to the National Health and Nutrition Examination Survey protocol. PIR = poverty income ratio.
Table 2.
Baseline Comorbid and Anthropometric Characteristics of 4,984 Subjects Aged 60 and Older in NHANES 1999–2004 Cohort
| Overall | Overall | Males | Overall | Females | |||||
|---|---|---|---|---|---|---|---|---|---|
| >60 y | Males | Survivors | Decedents | p Value | Females | Survivors | Decedents | p Value | |
| Hypertension | 2,326 (87.7) | 1,050 (87.9) | 570 (86.0) | 479 (90.5) | .04 | 1,276 (87.6) | 814 (86.1) | 461 (9.5) | .07 |
| Diabetes mellitus | 1,060 (18.3) | 542 (19.8) | 286 (18.6) | 255 (21.4) | .15 | 518 (17.2) | 301 (18.6) | 216 (22.5) | <.001 |
| CHF | 373 (7.1) | 203 (8.0) | 68 (4.4) | 135 (13.7) | <.001 | 170 (6.5) | 54 (2.9) | 116 (14.1) | <.001 |
| Non-skin cancer | 916 (21.7) | 506 (24.6) | 227 (21.1) | 279 (17.0) | <.001 | 410 (19.4) | 237 (30.1) | 173 (24.6) | <.001 |
| Stroke | 405 (7.6) | 222 (7.6) | 85 (4.6) | 137 (12.3) | <.001 | 183 (7.6) | 83 (4.9) | 100 (13.2) | <.001 |
| COPD | 496 (11.8) | 212 (9.5) | 84 (5.8) | 128 (15.3) | <.001 | 284 (13.6) | 160 (11.8) | 123 (17.2) | .01 |
| Osteoporosis | 149 (2.9) | 75 (3.0) | 34 (2.4) | 41 (4.0) | .15 | 74 (2.7) | 23 (1.1) | 51 (6.2) | <.001 |
| Kidney disease | 81 (4.2) | 47 (5.5) | 12 (2.4) | 35 (8.9) | .006 | 34 (3.3) | 9 (2.0) | 25 (5.3) | .04 |
| CAD | 870 (18.3) | 521 (23.0) | 233 (19.1) | 288 (29.4) | <.001 | 349 (14.7) | 185 (11.7) | 164 (21.3) | <.001 |
| Arthritis | 2,379 (50.2) | 965 (41.0) | 527 (38.2) | 438 (45.5) | .002 | 1,414 (57.3) | 894 (54.3) | 519 (63.6) | <.001 |
| % Body fat | 37.2 ± 0.11 | 30.9 ± 0.12 | 30.8 ± 0.17 | 31.0 ± 0.21 | .49 | 42.0 ± 0.13 | 42.5 ± 0.15 | 40.9 ± 0.26 | <.001 |
| ALM | 19.7 ± 0.09 | 24.1 ± 0.12 | 24.9 ± 0.15 | 22.9 ± 0.18 | <.001 | 16.3 ± 0.09 | 16.6 ± 0.10 | 15.7 ± 0.17 | <.001 |
| ALM:BMI | 0.71 ± 0.03 | 0.87 ± 0.003 | 0.88 ± 0.004 | 0.84 ± 0.005 | <.001 | 0.58 ± 0.003 | 0.58 ± 0.003 | 0.58 ± 0.004 | <.001 |
| BMI, kg/m2 | 28.2 ± 0.10 | 28.2 ± 0.11 | 28.5 ± 0.15 | 27.6 ± 0.22 | .002 | 28.3 ± 0.13 | 28.6 ± 0.16 | 27.5 ± 0.28 | .002 |
| WC, cm | 100.1 ± 0.22 | 104.4 ± 0.32 | 104.9 ± 0.36 | 103.6 ± 0.59 | .07 | 96.7 ± 0.30 | 97.0 ± 0.39 | 96.0 ± 0.61 | .17 |
Note: Data are mean ± SEs or counts (%). Data are weighted according to the National Health and Nutrition Examination Survey protocol. ALM = appendicular lean mass; BMI = body mass index; CAD = coronary artery disease; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; WC = waist circumference. Blacks = Non-Hispanic Blacks; Hispanics = Hispanic American; Whites = Non-Hispanic Whites.
Table 3.
Baseline Prevalence of ALM-Defined Low Lean Mass, Obesity, and Low Lean Mass With Obesity
| Classification | Overall (>60 y) | ALM | |||
|---|---|---|---|---|---|
| 60–70 y | 70–80 y | 80+ y | |||
| Females | Low lean mass | 1,028 (59.4) | 343 (30.0) | 309 (42.5) | 376 (61.1) |
| Obesity (high BF%) | 2,169 (88.7) | 1,009 (91.0) | 678 (90.1) | 482 (81.1) | |
| Low lean mass with obesity | 859 (33.5) | 301 (25.1) | 263 (36.0) | 295 (48.0) | |
| Males | Low lean mass | 459 (16.0) | 98 (8.9) | 186 (19.1) | 175 (32.9) |
| Obesity (high BF%) | 2,026 (87.5) | 864 (86.7) | 722 (88.4) | 440 (87.9) | |
| Low lean mass with obesity | 364 (12.6) | 75 (6.7) | 142 (14.9) | 147 (27.5) | |
Note: Data are counts (prevalence rates), after weighting and accounting for strata and primary sampling units. ALM = appendicular lean mass; BF = body fat.
Figure 1.
(a,b) Sex-specific prevalence of low lean mass with obesity, by obesity definition, 1999–2004. Rates of low lean mass obesity using the ALM definitions of sarcopenia from the Foundation for the National Institutes of Health and three definitions of obesity (body fat percent, waist circumference, and body mass index). Obesity defined using body fat cutpoints were ≥25% in men and ≥35% in females; waist circumference ≥88/102 cm in men/women and body mass index ≥30 kg/m2. Sarcopenia is defined as ALM <19.75 kg and <15.02 kg in men and women. ALM = appendicular lean mass.
The survival analyses are presented in Table 4 for LLM. There were 1,901 deaths (35.0%) of which 521 (9.0%) were classified as cardiovascular. In the overall population, participants with LLM were at higher adjusted risk of death (hazard ratio [HR] 1.35 [1.21, 1.51]). Stratifying by sex demonstrated that males may be at higher risk (males: HR 1.47 [1.27, 1.73]; females: 1.19 [1.02, 1.40]). Individuals with LLM and body fat-defined obesity had a marginally higher (yet still significant) risk of mortality in the overall cohort (1.14 [1.02, 1.28]). Males were at marginally higher risk than females (males: HR 1.31 [1.11, 1.565]; females: 0.99 [0.85, 1.16]). Subjects with body fat-defined obesity had lower risks of death in the overall sample (HR 0.72 [0.63, 0.82]) as well as by sex (males: HR 0.75 [0.63, 0.90]); females: HR 0.71 [0.58, 0.87]). Cardiovascular mortality using LLM was higher in males (HR 1.75 [1.31, 2.33]) than that in females (HR 1.13 [0.83, 1.55]). Similar, yet lower magnitude risks were observed in those with LLM with obesity (males: HR 1.59 [1.17, 2.09]; females: 1.00 [0.74, 1.35]). Supplementary Appendix 3 presents the ALM:BMI definition data estimates that trend those using the ALM definition. Defining LLM with obesity using BMI or WC did not demonstrate any higher risk of mortality (Supplementary Appendix 4). Tables 5 and 6 and Supplementary Appendices 5 and 6 demonstrate the univariate and multivariate predictors of mortality in subjects meeting criteria for LLM or LLM with obesity. Consistently, multivariate predictors suggested that age, diabetes mellitus, non-Hispanic Black, poverty income ratio, history of non-skin cancer, and kidney disease were all predictive of death.
Table 4.
Mortality Models for ALM-Defined Low Lean Mass, Obesity, and Low Lean Mass With Obesity
| Low Lean Mass (ALM) | Low Lean Mass With Obesity (BF%) | Obesity | |||
|---|---|---|---|---|---|
| Overall mortality | Overall | Model 1 | 1.59 (1.44, 1.75) | 1.34 (1.21, 1.48) | 0.66 (0.58, 0.75) |
| N = 1,901 (35.0%) | Model 2 | 1.25 (1.11, 1.40) | 1.07 (0.95, 1.21) | 0.82 (0.71, 0.94) | |
| Model 3 | 1.31 (1.16, 1.47) | 1.10 (0.98, 1.24) | 0.74 (0.64, 0.85) | ||
| Males | Model 1 | 2.14 (1.86, 2.47) | 1.89 (1.62, 2.21) | 0.79 (0.66, 0.93) | |
| N = 1,048 (38.8%) | Model 2a | 1.39 (1.19, 1.64) | 1.23 (1.03, 1.46) | 0.86 (0.71, 1.03) | |
| Model 3a | 1.41 (1.20, 1.66) | 1.25 (1.05, 1.48) | 0.79 (0.65, 0.96) | ||
| Females | Model 1 | 1.59 (1.38, 1.83) | 1.27 (1.10, 1.47) | 0.55 (0.46, 0.67) | |
| N = 853 (32.2%) | Model 2a | 1.07 (0.91, 1.26) | 0.93 (0.79, 1.09) | 0.80 (0.65, 0.97) | |
| Model 3a | 1.16 (0.98, 1.37) | 0.96 (0.81, 1.14) | 0.72 (0.58, 0.88) | ||
| Cardiovascular mortality | Overall | Model 1 | 1.78 (1.48, 2.14) | 1.57 (1.29, 1.90) | 0.78 (0.60, 1.01) |
| N = 521 (9.0%) | Model 2 | 1.35 (1.09, 1.68) | 1.22 (0.98, 1.51) | 0.93 (0.71, 1.22) | |
| Model 3 | 1.40 (1.12, 1.75) | 1.22 (0.97, 1.52) | 0.81 (0.61, 1.08) | ||
| Males | Model 1 | 2.52 (1.95, 3.27) | 2.34 (1.78, 3.09) | 0.92 (0.65, 1.30) | |
| N = 292 (10.4%) | Model 2a | 1.59 (1.19, 2.13) | 1.48 (1.10, 2.01) | 1.01 (0.70, 1.46) | |
| Model 3a | 1.61 (1.19, 2.17) | 1.49 (1.09, 2.04) | 0.93 (0.64, 1.37) | ||
| Females | Model 1 | 1.75 (1.33, 2.30) | 1.45 (1.10, 1.91) | 0.66 (0.44, 0.97) | |
| N = 229 (7.9%) | Model 2a | 1.03 (0.74, 1.43) | 0.95 (0.69, 1.30) | 0.95 (0.62, 1.44) | |
| Model 3a | 1.12 (0.80, 1.56) | 0.95 (0.69, 1.31) | 0.80 (0.52, 1.21) | ||
Note: Values represented are hazard ratios (95% confidence intervals) in adults aged 60 y and older. Model 1: no adjustment; Model 2: Model 1 adjusted for age, sex, race, poverty income ratio, and smoking; Model 3: Model 2 adjusted for diabetes mellitus, congestive heart failure, non-skin cancer, coronary artery disease, arthritis, physical activity, and smoking status. ALM = appendicular lean mass; BF = body fat.
Models not adjusted for gender.
Table 5.
Univariate Predictors of Overall Mortality in ALM-Defined Low Lean Mass
| Predictor | HR (95% CI) | p Value | Predictor | HR (95% CI) | p Value |
|---|---|---|---|---|---|
| Age | 1.08 (1.08, 1.10) | <.001 | CAD | 1.50 (1.27, 1.77) | <.001 |
| Sex | 0.52 (0.45, 0.60) | Kidney disease | 2.57 (1.67, 3.94) | <.001 | |
| Weight | 0.99 (0.99, 1.00) | .18 | Arthritis | 1.08 (0.94, 1.23) | .30 |
| Race | Protein intake | 1.00 (1.00–1.00) | .38 | ||
| NHW | 1.67 (1.42, 1.97) | <.001 | Smoking | ||
| NHB | 2.14 (1.65, 2.78) | <.001 | Current | 1.50 (1.29, 1.74) | <.001 |
| Other | 0.67 (0.40, 1.11) | .12 | Former | 1.35 (1.10, 1.66) | .004 |
| PIR | 0.92 (0.87, 0.98) | .006 | Physical activity | ||
| Hypertension | 1.23 (0.90, 1.67) | .19 | Walks | 0.47 (0.41, 0.54) | <.001 |
| Diabetes | 1.35 (1.13, 1.60) | .001 | Light loads | 0.32 (0.24, 0.44) | <.001 |
| Stroke | 2.18 (1.80, 2.64) | <.001 | Heavy work | 0.27 (0.11, 0.65) | .004 |
| CHF | 2.35 (1.92, 2.88) | <.001 | % Body fat | 0.96 (0.95, 0.97) | <.001 |
| COPD | 1.42 (1.16, 1.73) | .001 | Body mass index | 0.95 (0.93, 0.97) | <.001 |
| Cancer | 1.50 (1.27, 1.77) | <.001 | Waist circumference | 1.01 (1.00, 1.01) | .07 |
| Osteoporosis | 2.54 (1.93, 3.35) | <.001 |
Note: All values represent univariate HRs with 95% CIs. Kidney disease was determined using the Modification of Diet in Renal Disease formula and classified if glomerular filtration rate was <60 mL/min/m2. ALM = appendicular lean mass; CAD = coronary artery disease; CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio; NHB = non-Hispanic black; NHW = non-Hispanic white; PIR = poverty income ratio.
Table 6.
Multivariate Predictors of Overall Mortality in ALM-Defined Low Lean Mass
| Multivariatea | p Value | |
|---|---|---|
| HR (95% CI) | ||
| Age | 1.07 (1.05, 1.11) | <.001 |
| Sex | 0.79 (0.30, 1.67) | .54 |
| Diabetes | 2.68 (1.59, 4.52) | <.001 |
| Protein | 0.99 (0.99, 1.00) | .10 |
| % Body fat | 0.65 (0.42, 1.00) | .05 |
| Racecat_2 | 1.99 (1.20, 3.30) | .007 |
| IPAQ180_2 | 0.56 (0.36, 0.87) | .01 |
| PIR | 0.76 (0.64, 0.92) | .004 |
| Kidney disease | 3.73 (1.86, 7.49) | <.001 |
| CHF | 1.66 (0.94, 2.93) | .08 |
| Cancer | 2.76 (1.59, 4.80) | <.001 |
| Osteoporosis | 2.58 (0.92, 7.25) | .07 |
| Stroke_1 | 1.74 (0.96, 3.15) | .07 |
Note: All values represent HRs with 95% CIs. Kidney disease was determined using the Modification of Diet in Renal Disease formula and classified if glomerular filtration rate was <60 mL/min/m2. ALM = appendicular lean mass; CHF = congestive heart failure; CI = confidence interval; HR = hazard ratio; PIR = poverty income ratio.
Stepwise regression models integrating all of the above key variables. We present only predictors that were significant in the multivariate models A p value of .10 was the upper level for removal from the model, and a value of .05 was the lower level for the addition to the model.
Discussion
We observed a higher risk of death in individuals with LLM using the ALM definition of sarcopenia particularly in men. The use of body fat as a measure for adiposity attenuated the mortality risk in those identified as having LLM with obesity. Cardiovascular mortality was higher in males than in females.
To our knowledge, only two studies have examined the impact of sarcopenia (or LLM) on long-term mortality (4,13) using the FNIH criteria. The initial FNIH study demonstrated inconsistent results of a pooled HR using ALM over a 10-year period of HR 1.37 (1.03, 1.82) in men and 1.07 (0.81, 1.41) in women. Hirani et al. (13) demonstrated a HR of 1.65 (1.30, 2.09) in those with low ALM alone on long-term mortality with a median follow-up of 7 years. Clinical weakness over low ALM is likely the predominant predictor of clinical outcomes and is that which factors in the trajectory of decline and is implicated in the mortality process.
Previous studies examining the relationship of sarcopenia with mortality used different defining characteristics of sarcopenia (14). A recent meta-analysis demonstrated that in 10 studies, there was an 81% higher risk of mortality (95% CI: 1.61, 2.18) in those fulfilling criteria of sarcopenia as compared to those in the non-sarcopenia group. This analysis had a limited follow-up time of 4.17 years and sampled fewer individuals (n = 3,797) than ours (15). Sarcopenia has emerged as a predictor of death in cancer and renal disease populations (16–18) and we accounted for these confounding conditions by adjusting for them in our multivariable analysis. Our previous report using NHANES III assessed the relationship of LLM defined using bioelectrical impedance data (19). However, rates of LLM and LLM with obesity were much lower than those observed in this current study which used dual-energy x-ray absorptiometry for assessment of body composition and women had higher mortality risks than that of men. Sex-specific biologic differences on time-dependent mortality are unclear as well as whether the duration of inflammation may potentially explain our results.
A paucity of studies examine sarcopenia defined using LLM with obesity as a predictor for death. A meta-analysis demonstrated significant study heterogeneity with a marginally higher risk of all-cause mortality, based on earlier definitions of sarcopenia and obesity (HR 1.24 [1.12–1.37]), which was higher in males than females (20). Our results do conflict with those observed by Atkins and Chuang that showed no relationship between cardiovascular mortality and sarcopenic obesity (21,22). However, these authors used WC and mathematical thresholds unique to their population to assess adiposity, which we have previously debated. By using body fat cutoffs for adiposity assessment rather than conventional anthropometric measures, we avoid the poor sensitivity these markers have in ascertaining adiposity (23). This allows valid classification of individuals with obesity, which in conjunction with LLM, can assist in correctly categorizing individuals with LLM with obesity.
Our results suggest that body fat-defined obesity may indeed be protective in some individuals. Such older individuals may not be susceptible to obesity-related complications and may have survived, while others less resistant have died. Older cohorts are also at higher risk of competing mortality risks than younger cohorts. We also adjusted for a number of comorbidities known to negatively impact mortality which could partially explain these results. Another hypothesis is that late-life energy reserves may be protective; the interplay between adipose and skeletal muscle tissue on practical clinical outcomes requires future investigation. Our results suggest that subjects with LLM with obesity may not necessarily be at higher risk of adverse events.
Our exploratory analysis provides some interesting insight into potential mortality predictors, irrespective of sarcopenia definition. Generally, age, diabetes, non-Hispanic Black race, cancer, and kidney diseases were strong predictors of death. We acknowledge that we limited this current analysis to those with LLM or LLM with obesity measurements at baseline. Our sample was also limited by the number of deaths in each group to allow us to perform such predictive analytics. These predictors are not surprising in that each of these disease entities, and age itself, strongly predict future death. While preliminary, these results suggest the importance of maintaining function and optimizing disease-specific risk in these aforementioned populations.
We recognize a number of limitations in our analysis. First, our data are dependent on self-reported measures. Second, we relied solely on body composition data and were unable to integrate muscle strength of functional data into our analysis. Third, results are only generalizable to the noninstitutionalized adults living in the United States not those residing in nursing homes who may have higher degrees of LLM or obesity. Fourth, our results use a fixed baseline data point with a long-term mortality endpoint. While helpful to characterize the longitudinal relationships, the analysis does not account for weight-cycling which is known to impact functional and mortality estimates.
Using a national representative cohort, our results suggest the importance of sarcopenia defined using the FNIH ALM definition, particularly in males, both in terms of overall and cardiovascular mortality. Our results suggest that the FNIH defined ALM:BMI can also be considered, but a harmonized definition is critically needed. Obesity defined using body fat may mitigate the risk of death from sarcopenia, suggesting the possibility of an obesity paradox. Our exploratory analysis demonstrates consistent mortality predictors in this population that are not otherwise surprising but should prompt clinicians to continue to focus on modalities to improve function and muscle mass in these individuals. Longitudinally designed studies with intermittent data points can account for variations in medical, physical, and social determinants of long-term health.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
J.A.B. received funding from Health Resources Services Administration (UB4HP19206-01-00) for medical geriatric teaching, the Junior Faculty Career Development Award, the Department of Medicine, Dartmouth-Hitchcock Medical Center, and the Dartmouth Centers for Health and Aging. He is currently supported in part by the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.J.B. receives funding from the National Institute of Mental Health (K12 HS0217695 [AHRQ], NIMH: T32 MH073553, R01 MH078052, R01 MH089811; R24 MH102794 CDC U48DP005018). R.T.E. supported by the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of Interest
There are no conflicts of interest pertaining to this manuscript
Supplementary Material
Acknowledgment
Work submitted for presentation to the 2017 American Geriatrics Society Annual Meeting, San Antonio, TX.
References
- 1. Studenski SA, Peters KW, Alley DE, et al. The FNIH Sarcopenia Project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi:10.1111/jgs.12260 [DOI] [PubMed] [Google Scholar]
- 4. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. doi:10.1093/gerona/glu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woo J, Leung J. Anthropometric cut points for definition of sarcopenia based on incident mobility and physical limitation in older Chinese people. J Gerontol A Biol Sci Med Sci. 2016;71:935–940. doi:10.1093/gerona/glv197 [DOI] [PubMed] [Google Scholar]
- 6. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi:10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 7. Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res. 2015;35:1031–1039. doi:10.1016/j.nutres.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang IA, Llewellyn DJ, Alexander K, Melzer D. Obesity, physical function, and mortality in older adults. J Am Geriatr Soc. 2008;56:1474–1478. doi:10.1111/j.1532-5415.2008.01813.x [DOI] [PubMed] [Google Scholar]
- 9. Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res. 2002;10:816–823. doi:10.1038/oby.2002.110 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82:509–524. doi:10.1038/sj.clpt.6100355 [DOI] [PubMed] [Google Scholar]
- 12. Batsis JA, Singh S, Lopez-Jimenez F. Anthropometric measurements and survival in older Americans: results from the third National Health and Nutrition Examination Survey. J Nutr Health Aging. 2014;18:123–130. doi:10.1007/s12603-013-0366-3 [DOI] [PubMed] [Google Scholar]
- 13. Hirani V, Blyth F, Naganathan V, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: the Concord Health and Ageing in Men Project. J Am Med Dir Assoc. 2015;16:607–613. doi:10.1016/j.jamda.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 14. Filippin LI, Teixeira VN, da Silva MP, Miraglia F, da Silva FS. Sarcopenia: a predictor of mortality and the need for early diagnosis and intervention. Aging Clin Exp Res. 2015;27:249–254. doi:10.1007/s40520-014-0281-4 [DOI] [PubMed] [Google Scholar]
- 15. Chang SF, Lin PL. Systematic literature review and meta-analysis of the association of sarcopenia with mortality. Worldviews Evid Based Nurs. 2016;13:153–162. doi:10.1111/wvn.12147. [DOI] [PubMed] [Google Scholar]
- 16. Go SI, Park MJ, Song HN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24:2075–2084. doi:10.1007/s00520-015-2997-x [DOI] [PubMed] [Google Scholar]
- 17. Onesti JK, Wright GP, Kenning SE, et al. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016;16:284–289. doi:10.1016/j.pan.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 18. Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30:1718–1725. doi:10.1093/ndt/gfv133 [DOI] [PubMed] [Google Scholar]
- 19. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi:10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 20. Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2016;16:155–166. doi:10.1111/ggi.12579 [DOI] [PubMed] [Google Scholar]
- 21. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi:10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chuang SY, Hsu YY, Chen RC, Liu WL, Pan WH. Abdominal obesity and low skeletal muscle mass jointly predict total mortality and cardiovascular mortality in an elderly Asian population. J Gerontol A Biol Sci Med Sci. 2016;71:1049–1055. doi:10.1093/gerona/glv192 [DOI] [PubMed] [Google Scholar]
- 23. Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond). 2016;40:761–767. doi:10.1038/ijo.2015.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.