Abstract
Background
Previous studies have shown that individuals with diabetes exhibit accelerated cognitive decline. However, methodological limitations have limited the quality of this evidence. Heterogeneity in study design, cognitive test administration, and methods of analysis of cognitive data have made it difficult to synthesize and translate findings to practice. We analyzed longitudinal data from the Ginkgo Evaluation of Memory Study to test our hypothesis that older adults with diabetes have greater test-specific and domain-specific cognitive declines compared to older adults without diabetes.
Methods
Tests of memory, visuo-spatial construction, language, psychomotor speed, and executive function were administered. Test scores were standardized to z-scores and averaged to yield domain scores. Linear random effects models were used to compare baseline differences and changes over time in test and domain scores among individuals with and without diabetes.
Results
Among the 3,069 adults, aged 72–96 years, 9.3% reported diabetes. Over a median follow-up of 6.1 years, participants with diabetes exhibited greater baseline differences in a test of executive function (trail making test, Part B) and greater declines in a test of language (phonemic verbal fluency). For the composite cognitive domain scores, participants with diabetes exhibited lower baseline executive function and global cognition domain scores, but no significant differences in the rate of decline.
Conclusions
Identifying cognitive domains most affected by diabetes can lead to targeted risk modification, possibly in the form of lifestyle interventions such as diet and physical activity, which we know to be beneficial for improving vascular risk factors, such as diabetes, and therefore may reduce the risk of executive dysfunction and possible dementia.
Keywords: Diabetes, Domain-specific cognition, Epidemiology, Executive function, Psychomotor speed
Cognitive function declines with age, and diabetes may accelerate this rate of cognitive decline (1). Cognitive impairment is a concern because it results in disability (2) and increased healthcare costs (3). In the setting of diabetes, cognitive impairment can also adversely affect disease self-management, resulting in further complications (4).
Accumulating evidence demonstrates that diabetes is associated with lower cognitive function (5); however, the cognitive domains identified to be associated with diabetes have been inconsistent (6–13). Specifically, a recent meta-analysis highlighted decrements in processing speed, executive function and motor function to show the largest effect size estimates with diabetes compared to controls in cross-sectional analyses (5). In one longitudinal analysis of change in cognition from the Whitehall II cohort, diabetes was associated with the greatest declines in memory, followed by reasoning and global cognition (14). Contrary to these longitudinal findings, a sample of 1,290 individuals followed for 12 years from the Maastracht Aging Study showed that diabetes was associated with the largest declines in processing speed, executive function, and delayed word recall (15). Furthermore, of these studies with longitudinal measures of cognition, several do not have repeated measures across multiple tests within a domain of cognition, but rather rely on one cognitive test per cognitive domain (8–10). Another limitation of these existing longitudinal studies is their inclusion of only a measure of global cognitive function. Executive function is believed to be more affected by vascular disease (eg, hypertension and stroke) than by neurodegenerative disease (16–19), and is expected to decline disproportionally among individuals with diabetes due to their high vascular risk factor burden; however, decrements in this domain are not adequately captured with tests of global cognition, such as the Mini-Mental State Examination (MMSE) test.
A systematic review from a National Institutes of Health (NIH) conference stated that the quality of evidence for an association between diabetes and cognitive decline is low, but affirmed that a higher risk for cognitive decline among individuals with diabetes is probable (20). Furthermore, the NIH Diabetes Mellitus Interagency Coordinating Committee’s 2010 strategic planning report recommends the need for incorporating validated neuropsychological instruments in epidemiological studies to increase the evidence base for the detection of cognitive dysfunction in diabetes and elucidate mechanisms (21). Hypothesizing those individuals with diabetes experience greater cognitive decline, we compared the baseline and rates of change in global and domain-specific cognition among older adults with and without diabetes using prospective data from the Ginkgo Evaluation of Memory Study (GEMS), a 7-year study with high-quality neuropsychological assessments. Given the literature suggesting executive function declines to be more prominent in the setting of vascular brain damage (16–19), we specifically hypothesize individuals with diabetes to exhibit the greatest declines in the executive function domain due to their higher vascular risk factor burden.
Methods
Study Population and Design
GEMS was a randomized, double-blinded, placebo-controlled trial of 3,069 participants aged 72–96 years, designed to examine the preventive effect of ginkgo biloba on dementia in community-dwelling, cognitively unimpaired or mildly impaired (ie, mild cognitive impairment [MCI]) older adults (22). A participant was classified with MCI if they met the following criteria: (a) impaired at or below the 10th percentile of Cardiovascular Health Study normative data, stratified by age and education, on at least 2 of 10 selected neuropsychological test scores from each cognitive domain, including memory, language, visuospatial abilities, attention, and executive function; and (b) CDR global score of 0.5. Participants with prevalent dementia as determined by a Diagnostic and Statistical Manual of Mental Disorders [Fourth Edition] [DMS-IV] criteria or a score >0.5 on the Clinical Dementia Rating Scale (CDR) were excluded. Participants with neurological disorders/neurodegenerative diseases that would have an important contribution to cognitive function or risk of dementia were also excluded. Additional exclusions included: currently taking anticoagulant warfarin; taking cholinesterase for cognitive problems or dementia; unwillingness to discontinue over-the-counter ginkgo biloba treatment; treatment with tricyclic antidepressants, antipsychotics, or other medications with psychotropic or central cholinergic effects; daily use of more than 400-IU vitamin E; history of bleeding disorders; hospitalization for depression; history of Parkinson’s disease or taking anti-Parkinson medications; abnormal thyroid or liver function tests; low baseline vitamin B12 levels (≤210 pg/mL); hematocrit level <30%; platelet count <100 μL × 103 μL; disease-related life expectancy <5 years; or known allergy to ginkgo biloba. Participants were recruited from four U.S. communities: Hagerstown, Maryland; Pittsburgh, Pennsylvania; Sacramento, California; and Winston-Salem/Greensboro, North Carolina. Participants were randomized to receive either a twice-daily dose of 120-mg extract of ginkgo biloba or an identical-appearing placebo. The mean age at study entry was 79 years. Participants were predominantly white (95.5%) with some college or higher education (64%). The prevalence of hypertension was 54.3%, while 9.3% had diabetes. Additional study details are described elsewhere (22).
Data collection for GEMS began in September 2000. Follow-up time was defined as the time from enrollment to incident dementia, death, or end of study. Because GEMS was designed to study the effect of ginkgo biloba on the outcome of incident dementia, participants were censored from further follow-up after an incident dementia diagnosis. Dropout and loss to follow-up in GEMS was low (6.3%). Information collected at baseline and every 6-month clinic visit included vital signs, current medication use/adherence, medical history, adverse events, and a functional assessment. Cognitive screening measures included the Modified Mini-Mental State Examination (3MSE), CDR Scale for participants and informant, cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog), and an Informant Questionnaire for Cognitive Decline in the Elderly (IQCoDe) for the informant (22,23). Participants were administered the neuropsychological exam and the Center for Epidemiologic Studies Depression Scale (CES-D) at baseline and again at annual visits starting 4 years postrandomization (22,23). Written informed consent was obtained from all study participants and all institutional review boards approved the study.
Assessment of Diabetes Mellitus
Ascertainment of diabetes was based on self-reported physician diagnosis or use of diabetes medication.
Neuropsychological Testing
The neuropsychological battery was chosen for the GEM Study in order to assess multiple cognitive domains. These tests were chosen because they are sensitive to cognitive deficits observed in early/mild dementia. This same test battery was used in the Cardiovascular Health Study cognition substudy (24) and in the Alzheimer’s disease Research Center at the University of Pittsburgh to diagnose Alzheimer’s disease (AD) and other dementias (25). The tests are categorized into their conventionally appropriate domains based on the existing literature on neuropsychological assessment by Lezak and colleagues (26). Applying similar domain groupings across studies allows for the comparison of findings and yields greater generalizability. The cognitive domains and tests used in GEMS are outlined in Table 1.
Table 1.
GEM Study Neuropsychological Battery of Tests and Cognitive Domains (22)
| Domain | Test |
|---|---|
| Memory | California verbal learning test, long delayed free recall |
| Rey–Osterrieth complex figure test, delayed recall | |
| Visuo-spatial Construction | Rey–Osterrieth complex figure test, copy condition |
| Wechsler Adult Intelligence Scale– Revised (WAIS-R) Block Design | |
| Language | 30-item Boston naming test |
| Verbal fluency (letters F, A, and S) | |
| Animal fluency | |
| Psychomotor Speed/Attention | WAIS-R digit span forward |
| Trail making test (TMT), part A | |
| Executive Function | WAIS-R digit span backward |
| TMT, part B | |
| Stroop color/word test, interference condition |
Scoring for the Trail making test (TMT), Parts A and B, is based on time (in seconds) to task completion, with a possible range in scores from 0 to 240 seconds and 0 to 360 seconds, respectively, with lower scores indicating better performance. Participants who did not complete the task were assigned the maximum allotted time (ie, 240 seconds) as their score. To account for non-normality of the TMT, Parts A and B, test scores were converted to number of connections per minute in keeping with previous work conducted in cohort studies of older adults (2). For the transformed score, a higher score indicates better performance. To make relative comparisons across cognitive domains and to accommodate differences in test units and scales, the raw test scores were standardized to z-scores based on the means and standard deviations (SD) at baseline (27). Given that there were no differences in cognition and dementia risk (23), the control and Ginkgo groups were combined for analyses.
Covariates
Covariates were selected based on a priori theory, prior literature and univariate analyses suggesting their association with both diabetes and cognition. All covariates were measured at the baseline GEMS visit. Age (years), education (years) were modeled as continuous variables. Race was dichotomized as white or nonwhite race. Smoking status was categorized as never, former, or current smoker. A history of stroke, hypertension, and myocardial infarction (yes/no) were based on self-report only. Depressed mood was measured using the CES-D and analyzed continuously. Analyses involving the TMT, Part B, and executive function domain (which included the TMT, Part B) were further adjusted for continuous values of the TMT, Part A, to partition out the speeded component of the TMT, Part B, an otherwise executive functioning task (2,28–30).
Statistical Analysis
An initial descriptive analysis utilized chi-square and ANOVA tests to test for significant differences in baseline cognitive test scores and participant characteristics between individuals with and without diabetes. Nonparametric trajectories were characterized using empirical growth plots to determine whether the test and domain scores were linear throughout follow-up. Since linearity throughout follow-up was observed through visual inspection and model fit was not improved with inclusion of quadratic or spline terms, none were included in the main analysis.
Linear random effects models for clustered longitudinal data (31) were used to examine test-specific and domain-specific cognitive declines from baseline to death, incident dementia diagnosis, or end of follow-up. We fit random intercept and random slope for time models with primary exposure variables time, diabetes status and the interaction of time, and diabetes status. This model yields a main effect of diabetes representing the differences in baseline cognitive outcomes comparing individuals with and without diabetes; and the interaction term is the difference in the slopes comparing individuals with and without diabetes. In addition, we adjusted for GEMS treatment group assignment and the above covariates/confounders suspected to be associated with the diabetes and cognition. To account for multiple comparisons, a Bonferroni adjusted p-value was estimated as α/n, where n=number of tests and α = 0.05. In order to reject the null hypothesis, a p-value <.0042 and p-value <.0083 for the cognitive tests and cognitive domains analyses, respectively, must be present. All analyses were conducted using STATA 13.0 (STATA Corp, College Station, TX).
Results
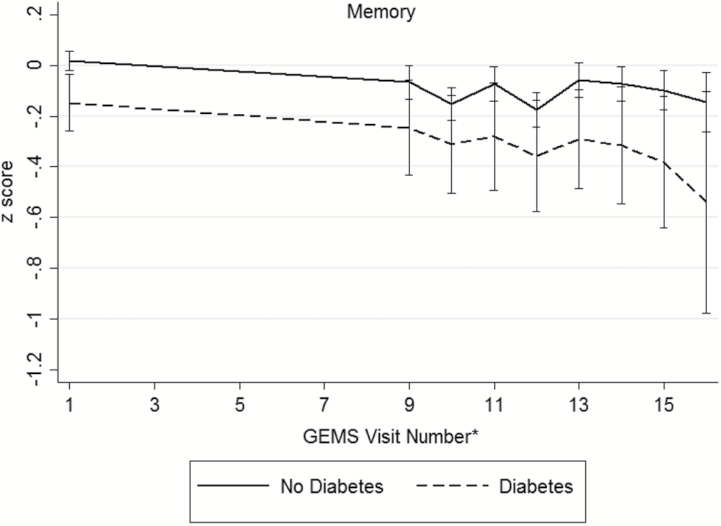
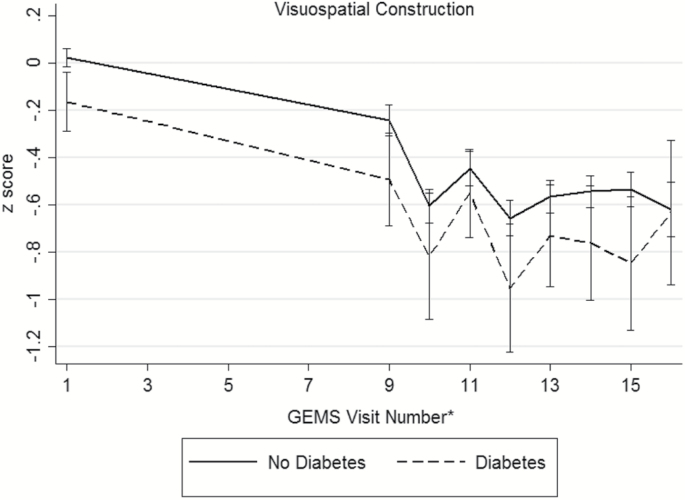
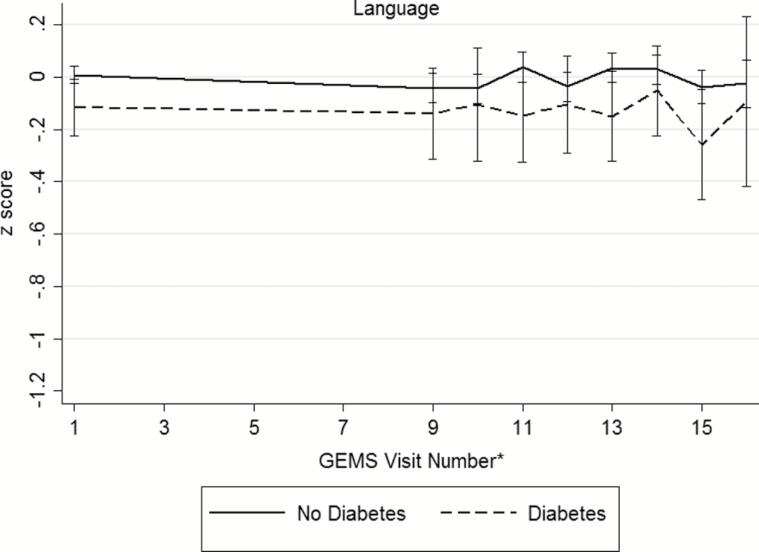
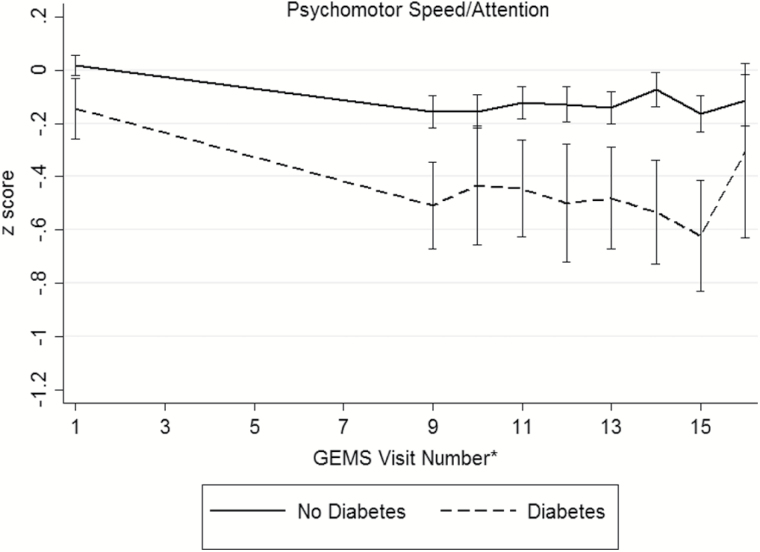
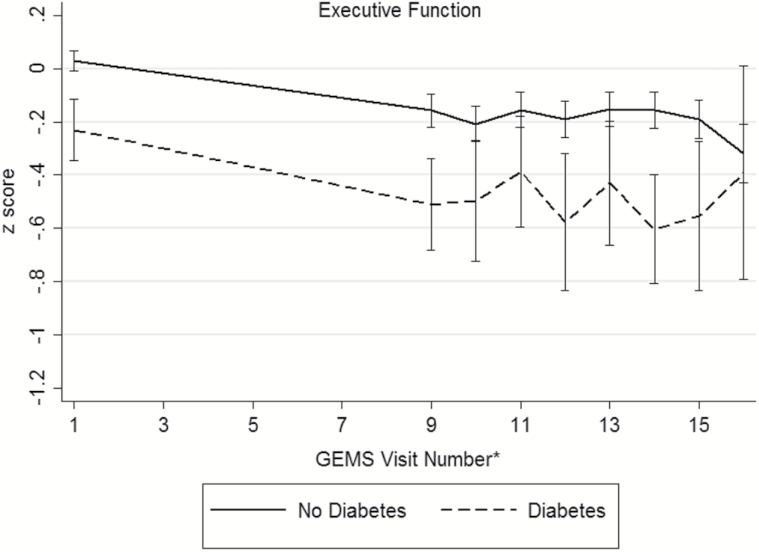
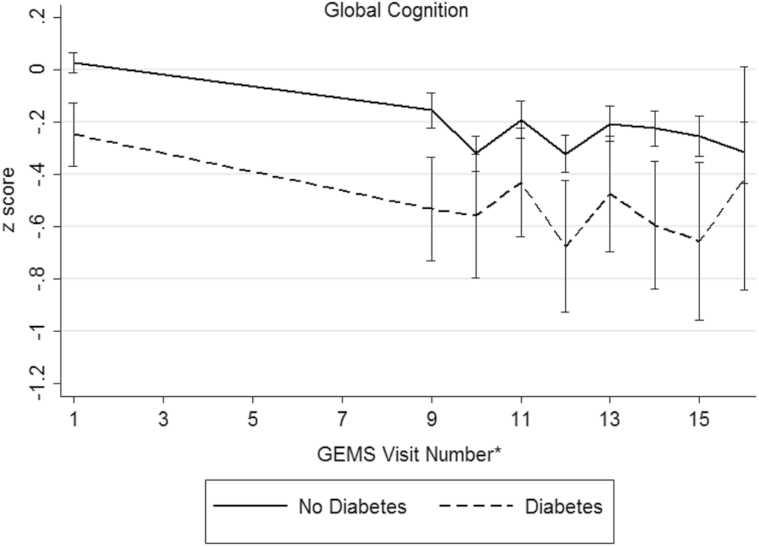
Baseline demographic and disease characteristics of the participants are presented in Table 2 (distributions of raw cognitive test scores, by diabetes status, presented in Supplementary Table 1). During the GEM study period, 523 participants (16.1%) were diagnosed with dementia and 379 died from a death of any cause. There were 195 participants (6.3%) who were either lost to follow-up or withdrew consent. An examination of participants that remained in the study versus those who were lost to follow-up only did not differ in regards to age, sex, race/ethnicity, baseline diabetes and disease (myocardial infarction, stroke, heart failure, cancer) status, or smoking status. Among the 3,027 participants, 286 (9%) were classified with diabetes. Individuals with diabetes were more often male (63.3%) and nonwhite race (9.4%). Hypertension, myocardial infarction, stroke, and depressed mood were more prevalent among individuals with diabetes. Baseline smoking status, GEMS treatment assignment, and prevalence of MCI did not differ between the groups. At baseline, participants with diabetes exhibited poorer performance on tests of verbal memory, visual-spatial construction, and executive function (Supplementary Table 1). No significant differences were seen on tests of language between the two groups. Unadjusted mean domains scores across follow-up are shown in Figure 1, by diabetes status. Across the cognitive domains, participants without diabetes appear to have higher baseline scores compared to those with diabetes.
Table 2.
Characteristics of the Study Population (n = 3,027), by Baseline Diabetes Status, Ginkgo Evaluation of Memory Study, 2000–2009
| Characteristic | No Diabetes, n = 2,741 | Diabetes,an = 286 |
Statistic t
or χ2 |
p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (range) | 78.6 (72–96) | 78.2 (74–93) | 1.9 | .06 |
| Female sex, n (%) | 1,290 (47.1) | 105 (36.7) | 11.2 | .001 |
| Education, years, mean (range) | 14.4 (1–20) | 14.3 (6–20) | 0.31 | .76 |
| Nonwhite race, n (%) | 108 (3.9) | 27 (9.4) | 18.4 | <.001 |
| Covariates | ||||
| Ginkgo treatment assignment, n (%) | 1,379 (50.3) | 142 (49.7) | 0.05 | .83 |
| Current smoker, n (%) | 125 (4.7) | 9 (3.2) | 1.3 | .52 |
| CES-D, median (range) | 3 (0–26) | 4 (0–26) | −4.3 | <.001 |
| Hypertension, n (%) | 1,114 (41.5) | 172 (61.4) | 41.0 | <.001 |
| Stroke, n (%) | 71 (2.6) | 15 (5.3) | 6.6 | .01 |
| Myocardial infarction, n (%) | 247 (9.1) | 49 (17.4) | 19.8 | <.001 |
| Mild cognitive impairment, n (%) | 420 (15.3) | 54 (18.9) | 2.5 | .12 |
| Insulin medication use, n (%) | — | 38 (13.3) | — | — |
| Oral hypoglycemic medication use, n (%) | — | 205 (71.7) | — | — |
| Anti-hypertensive medication use, n (%) | 1,495 (54.5) | 227 (79.4) | 65.1 | <.001 |
Note: To test differences between groups: Chi-square for categorical variables, Kruskal–Wallis for continuous variables. aDiabetes = self-reported physician diagnosis of diabetes or self-reported diabetes medication use at baseline.
Figure 1.
Unadjusted mean (95% confidence interval) domain scores for (a) memory, (b) visuospatial construction, (c) language, (d) psychomotor speed/attention, (e) executive function, and (f) global cognition across follow-up, by diabetes status. *The full neuropsychological assessment was performed at the GEMS baseline visit, 4 years after baseline, and annually thereafter.
Longitudinal Analyses
Participants were followed for a median of 6.1 years (maximum of 7 years). For each cognitive test (Supplementary Table 2) and domain score (Table 3), we estimated the difference in the average baseline scores comparing individuals with and without diabetes and the difference in the average 7-year rate of change in scores comparing individuals with and without diabetes. Negative values of the estimated differences indicate worse cognitive performance among individuals with diabetes. After accounting for multiple comparisons, specific tests within the domain and the domain scores for memory and visuo-spatial construction were neither associated with baseline cognition nor rates of change in cognition between those with and without diabetes.
Table 3.
Adjusted Baseline Cognitive Domain z-Scores and Rates of Change in Cognitive Domain z-Scores by Diabetes Status, Ginkgo Evaluation of Memory Study, 2000–2009
| Domain, Mean of Test z-Scores | Baseline Function, No Diabetes, β (95% CI) | Baseline Function, Diabetes, β (95% CI) | Difference in Baseline Function, β (95% CI) | p a,c | 7-Year Rate of Change, No Diabetes, β (95% CI) | 7-Year Rate of Change, Diabetes, β (95% CI) | Difference in 7-Year Rate of Change, β (95% CI) | p b,c |
|---|---|---|---|---|---|---|---|---|
| Memory | 0.27 (0.20, 0.35) | 0.18 (0.04, 0.32) | −0.09 (−0.2, 0.03) | .13 | −0.41 (−0.46, −0.37) | −0.44 (−0.59, −0.29) | −0.03 (−0.2, 0.1) | .71 |
| Visuo-spatial Construction | 0.17 (0.09, 0.24) | 0.02 (−0.12, 0.16) | −0.15 (−0.3, −0.02) | .02 | −0.85 (−0.90, −0.79) | −0.81 (−0.97, −0.64) | 0.04 (−0.1, 0.2) | .68 |
| Language | −0.02 (−0.08, 0.05) | −0.13 (−0.25, −0.01) | −0.1 (−0.2, −0.01) | .03 | −0.19 (−0.23, −0.16) | −0.22 (−0.33, −0.11) | −0.03 (−0.1, 0.09) | .64 |
| Psychomotor Speed/ Attention | 0.09 (0.02, 0.16) | −0.06 (−0.19, 0.07) | −0.15 (−0.3, −0.04) | .01 | −0.30 (−0.34, −0.26) | −0.45 (−0.58, −0.32) | −0.15 (−0.3, −0.02) | .03 |
| Executive Function | 0.60 (0.53, 0.68) | 0.41 (0.27, 0.54) | −0.20 (−0.3, −0.09) | .001 | −0.41 (−0.44, −0.37) | −0.47 (−0.59, −0.36) | −0.07 (−0.2, 0.05) | .27 |
| Global Cognition | 0.14 (0.06, 0.21) | −0.08 (−0.21, 0.06) | −0.21 (−0.3, −0.1) | <.001 | −0.64 (−0.68, −0.61) | −0.68 (−0.81, −0.55) | −0.04 (−0.2, 0.1) | .59 |
Note: Models are adjusted for age, sex, years of education, race, baseline smoking status, depressed mood, history of stroke, history of hypertension, history of myocardial infarction, TMT, part A (executive function domain only), and GEMS treatment group. Bold values indicate Bonferroni adjusted p-value for test of significance = .0083. aMain effect of diabetes: difference in baseline function between diabetic and nondiabetic groups. bInteraction of time and diabetes status: difference in 7-year rate of change between diabetic and nondiabetic groups. cTo account for multiple comparisons, a Bonferroni adjusted p-value for test of significance = .0083.
Among tests of language (30-item Boston naming test, phonemic verbal fluency, semantic verbal fluency), no significant differences at baseline were observed. Longitudinal analyses showed that phonemic verbal fluency decreased at a significantly faster rate among individuals with compared to those without diabetes (estimated difference in 7-year rate of change = −1.5, 95% confidence interval [CI]: −2.6, −0.5). Individuals with diabetes decreased at a rate of roughly 0.3 words per year (or 2.1 words over 7 years), while those without diabetes only decreased at a rate of 0.07 words per year (or 0.5 words over 7 years). Although participants with diabetes performed, on average, 0.1 SD (95% CI: −0.2, −0.01) lower in the language domain at baseline, the rate of change in the language score was not significantly different compared to those without diabetes.
TMT, Part A (psychomotor speed, attention), but not the WAIS-R Digit Span Forward (attention) test yielded significant differences in baseline cognitive test scores. No differences in rates of change on individual tests of psychomotor speed and attention were observed. Participants with diabetes performed, on average, 0.15 SD (95% CI: −0.3, −0.04) lower in psychomotor speed/attention at baseline. Over the 7-years of follow-up, individuals with diabetes declined roughly 0.15 SD (95% CI: −0.3, −0.02) more in psychomotor speed compared to individuals without diabetes.
Three tests comprise the executive function domain, WAIS-R digit span backward, TMT, Part B, and stroop color/word interference tests. For the digit span backward test, there was no significant baseline or longitudinal differences among those with and without diabetes. However, baseline differences were observed in the TMT, Part B (estimated baseline difference = −1.04, 95% CI: −1.6, −0.5). Individuals with diabetes took on average 1.04 seconds longer to complete the task compared to those without diabetes (p = .001). The differences in annual rate of change over 7 years were not statistically significant between the two groups on the TMT, Part B; however, individuals with diabetes exhibited a greater 7-year rate of decline on the stroop color/word interference test compared to those without diabetes (estimated difference in 7-year rate of change = −3.8, 95% CI: −7.0, −0.6). Significant baseline differences, but not rates of change, in the composite executive function domain score were observed between individuals with and without diabetes.
Significant baseline differences, but not rates of change, in global cognition domain z-scores were observed between individuals with and without diabetes. Although the differences were not statistically significant (Table 3), the 7-year rate of change in global cognitive function that we observed was −0.64 among individuals without diabetes and −0.68 among individuals with diabetes (difference: −0.04, 95% CI: −0.2, 0.1), that is, a 6.25% greater decline among persons with diabetes (−0.04/−0.64 = 6.25%).
Discussion
Diabetes is often accompanied by other metabolic dysregulations and vascular risk factors (eg, history of hypertension and stoke); therefore, we hypothesized diabetes to have a greater effect on domains most impacted by vascular pathways, which includes executive function. Consistent with this a priori hypothesis, we found that individuals with diabetes performed worse on a test of executive function (TMT, Part B) at baseline and observed steeper declines in a test of language (phonemic verbal fluency) and executive function (stroop color/word interference test) compared to individuals without diabetes; and in no instances did those with diabetes outperform those without diabetes in comparisons of raw cognitive test scores or domain z-scores. The results support previous findings of significant differences in executive function, language, and psychomotor speed/attention (5,15); but contradict prior findings suggesting declines in global cognitive function and memory in comparison to participants with versus those without diabetes (32,33). Specifically, our null results for changes in global cognition by diabetes status differ from those observed in the community-based Atherosclerosis Risk in Communities (ARIC) study, where diabetes in midlife was associated with greater 20-year declines in global cognition compared to those without diabetes (7). These differences may be attributable to the differences in the baseline age of participants (57 years in ARIC and 78 years in GEMS). Larger effect size estimates for cognitive outcomes when comparing vascular risk factors measured in midlife as compared to those measured in late-life have been previously documented (34), and are supported by the comparison of our results in GEMS (6.25% greater decline among persons with diabetes) to those observed in ARIC (19% greater decline among persons with diabetes (7)). However, novel to our study is the comprehensive neuropsychological battery of 12 tests administered longitudinally to examine the rate of change across multiple tests and domains of cognition and to estimate the differential associations by diabetes status.
In a sample of older women, also screened to be healthy and free of dementia at baseline, authors showed that declines in executive function preceded that of memory (35). Similar findings have been documented in other observational studies (36) and in vivo studies of the aging brain (37). This observed pattern may be exacerbated in the presence of diabetes. Executive functioning is particularly important for self-care and maintaining independence as it has been linked to impairments in instrumental activities of daily living (eg, preparing meals) and disability (2). Individuals with executive functioning impairments may have difficulty carrying out complex self-management behaviors, such as carbohydrate counting, insulin administration, and planning meals and exercise routines. Over time, poor self-management may lead to poor metabolic control and increase the risk of diabetes-related complications.
Similar to the findings from a recent meta-analysis (5), we observed differences in effect size estimates across tests within domains (ie, larger effect size estimates for 7-year rate of cognitive decline for the stroop interference test compared to the TMT, Part B). These differences in effect size estimates highlight the potentially informative differences in the sensitivity of the neuropsychological tests to detect differences in cognitive function between individuals with and without diabetes.
Several mechanisms may explain the link between diabetes and impaired cognition. One well-supported mechanism involves increased insulin resistance, which results in chronically increased levels of blood glucose. Insulin resistance can affect both cerebrovascular and noncerebrovascular mechanisms for cognitive impairment in diabetes. In a noncerebrovascular pathway, insulin resistance can induce chronic hyperinsulinemia in the brain, increasing levels of beta amyloid peptide-42, the primary component of amyloid plaques (38). Amyloid plaques are a hallmark characteristic for AD and amnestic cognitive impairment (39). In a cerebrovascular pathway, insulin resistance is associated with vascular risk factors, such as hypertension. These vascular factors increase the risk for small vessel cerebrovascular damage, which has been linked to vascular cognitive impairment and vascular dementia (40). Although preliminary investigations of these mechanisms are under way, there is still a need for studies, especially large cohort studies, to use brain imaging to further elucidate mechanisms and help confirm diagnoses of a specific cognitive disorder.
There are a number of strengths that should be noted. Most importantly, while previous research has shown a risk of lower cognitive function among individuals with diabetes, most prior studies do not have a comprehensive neuropsychological test battery administered longitudinally to examine the rate of change across multiple tests of cognition.
Limitations regarding our analyses should be noted. First, data were not available on type of diabetes (type 1 or type 2), diabetes duration, or severity of diabetes (eg, hemoglobin A1c or fasting glucose). Some studies have suggested that duration and severity of diabetes is associated with cognitive impairment (41) and that the type of diabetes is associated with different domains of cognition (42). Second, ascertainment of diabetes was based on self-report. Although not ideal for a diagnosis of diabetes, validation studies have suggested that self-report of diabetes is sufficiently accurate for population-based studies (43). Third, obesity and/or body mass index data were not available at baseline in GEMS. Obesity is a strong correlate of diabetes and may be an important confounder in the associations between diabetes and cognition. However, whether obesity has an association with cognition that is independent of cardio-metabolic abnormalities (eg, diabetes, hypertension) remains unclear (44). Therefore, we do not anticipate a lack of adjustment for obesity to bias our results. There is a possibility that our effect size estimates are underestimated due to the censoring of participants after a dementia diagnosis in follow-up. However, in our study, the incidence of dementia did not differ from participants with and without diabetes. Our findings may have limited generalizability to the entire US population since participants in this trial were primarily Caucasian with high levels of education. Participants were further self-selected due to their willingness to participate in a 7-year randomized controlled trial; and participants were screened extensively to rule out moderate to severe cognitive impairment at baseline. Finally, given that the mean age of our study sample at baseline was 79 years, our analyses are likely restricted to those individuals who survived to older age without dementia, which may therefore bias our results toward the null.
In conclusion, this prospective study, which included 7 years of longitudinal follow-up and extensive neurocognitive data, identified specific cognitive tests and domains associated with diabetes. Future studies examining the longitudinal associations between diabetes and cognitive function should include an evaluation of diabetes severity, exploration of sex and race/ethnic differences, and whether there are differences in cognitive trajectories in incident versus prevalent diabetes and in diabetes with early life onset. More comprehensive evaluations, such as the one performed in this study, of the relative performances on cognitive domains and tests are needed so that we may determine which cognitive functions are most affected. Identifying which cognitive domains are most affected by diabetes can lead to targeted risk modification, possibly in the form of cognitive rehabilitation therapies or lifestyle interventions, including physical activity and exercise, which are known to target executive functioning (45), are good for improving vascular risk factors and obesity (46), and which may reduce the risk for dementia (47).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The study was sponsored by the Ginkgo Evaluation of Memory Study (GEMS) and was supported by grant number U01 AT000162 from the NCCAM and the Office of Dietary Supplements, and support from the National Institute on Aging, National Heart, Lung, and Blood Institute, the University of Pittsburgh Alzheimer’s Disease Research Center (P50AG05133), the Roena Kulynych Center for Memory and Cognition Research, and the National Institute of Neurological Disorders and Stroke. The first author (P.P.) was supported by a predoctoral training fellowship from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Training Grant in Clinical Research and Epidemiology in Diabetes and Endocrinology (T32 DK062707) and a postdoctoral training fellowship from the National Heart, Lung, and Blood Institute Training Grant in Cardiovascular Epidemiology, Biostatistics and Preventive Medicine (T32 HL007055).
Supplementary Material
References
- 1. Yaffe K, Falvey C, Hamilton N et al. Diabetes, glucose control and 9 year cognitive decline among non-demented older adults. Arch Neurol. 2012;69:1170–1175. doi:10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol Psychol Sci Soc Sci. 1999;54:S262–S270. doi:10.1093/geronb/54B.5.S262 [DOI] [PubMed] [Google Scholar]
- 3. Handels RL, Wolfs CA, Aalten P, Verhey FR, Severens JL. Determinants of care costs of patients with dementia or cognitive impairment. Alzheimer Dis Assoc Disord. 2013;27:30.–. doi:10.1097/WAD.0b013e318242da1d. [DOI] [PubMed] [Google Scholar]
- 4. Punthakee Z, Miller ME, Launer LJ et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012;35:787–793. doi:10.2337/dc11-1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278–291. doi:10.1017/S1355617713001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bangen KJ, Gu Y, Gross AL et al. Relationship between type 2 diabetes mellitus and cognitive change in a multiethnic elderly cohort. J Am Geriatr Soc. 2015;63:1075–1083. doi:10.1111/jgs.13441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rawlings AM, Sharrett AR, Schneider AL et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi:10.7326/M14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wessels AM, Lane KA, Gao S, Hall KS, Unverzagt FW, Hendrie HC. Diabetes and cognitive decline in elderly African Americans: a 15-year follow-up study. Alzheimers Dement. 2011;7:418–424. doi:10.1016/j.jalz.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ravona-Springer R, Luo X, Schmeidler J et al. Diabetes is associated with increased rate of cognitive decline in questionably demented elderly. Dement Geriatr Cogn Disord. 2010;29:68–74. doi:10.1159/000265552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maggi S, Limongi F, Noale M et al. Diabetes as a risk factor for cognitive decline in older patients. Dement Geriatr Cogn Disord. 2009;27:24–33. doi:10.1159/000183842 [DOI] [PubMed] [Google Scholar]
- 11. Debette S, Seshadri S, Beiser A et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi:10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayeda ER, Haan MN, Yaffe K, Kanaya AM, Neuhaus J. Does type 2 diabetes increase rate of cognitive decline in older Mexican Americans? Alzheimer Dis Assoc Disord. 2015;29:206–212. doi:10.1097/WAD.0000000000000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayeda ER, Haan MN, Neuhaus J et al. Type 2 diabetes and cognitive decline over 14 years in middle-aged African Americans and whites: the ARIC Brain MRI Study. Neuroepidemiology. 2014;43:220–227. doi:10.1159/000366506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuligenga RH, Dugravot A, Tabák AG et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2:228–235. doi:10.1016/S2213-8587(13)70192-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spauwen PJ, Köhler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care. 2013;36:1554–1561. doi:10.2337/dc12-0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuang YF, Eldreth D, Erickson KI et al. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2014;35:1396–1403. doi:10.1016/j.neurobiolaging.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular disease risk factors and cognition in the elderly. Curr Cardiovasc Risk Rep. 2011;5:407–412. doi:10.1007/s12170-011-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC. Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry. 2002;72:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santiago C, Herrmann N, Swardfager W et al. White matter microstructural integrity is associated with executive function and processing speed in older adults with coronary artery disease. Am J Geriatr Psychiatry. 2015;23:754–763. doi:10.1016/j.jagp.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 20. Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi:10.7326/0003-4819-153-3-201008030-00258 [DOI] [PubMed] [Google Scholar]
- 21. National Institutes of Health. Advances and Emerging Opportunities in Diabetes Research: A strategic planning report of the Diabetes Mellitus Interagency Coordinating Committee. Bethesda, MD: National Institutes of Health; 2011. [Google Scholar]
- 22. DeKosky ST, Fitzpatrick A, Ives DG et al. The Ginkgo evaluation of memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238–253. doi:10.1016/j.cct.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 23. DeKosky ST, Williamson JD, Fitzpatrick AL et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi:10.1001/jama.2008.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 67110 [DOI] [PubMed] [Google Scholar]
- 25. Lopez OL, Becker JT, Klunk W et al. Research evaluation and diagnosis of possible Alzheimer’s disease over the last two decades: II. Neurology. 2000;55:1863–1869. [DOI] [PubMed] [Google Scholar]
- 26. Lezak M, Howieson D, Loring D.. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 27. Meador KJ, Loring DW, Sethi KD, Yaghmai F, Styren SD, DeKosky ST. Dementia associated with dorsal midbrain lesion. J Int Neuropsychol Soc. 1996;2:359–367. [DOI] [PubMed] [Google Scholar]
- 28. Krall JR, Carlson MC, Fried LP, Xue QL. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180:838–846. doi:10.1093/aje/kwu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palta P, Xue QL, Deal JA, Fried LP, Walston JD, Carlson MC. Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2015;70:873–878. doi:10.1093/gerona/glu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yasar S, Ko JY, Nothelle S, Mielke MM, Carlson MC. Evaluation of the effect of systolic blood pressure and pulse pressure on cognitive function: the Women’s Health and Aging Study II. PLoS One. 2011;6:e27976. doi:10.1371/journal.pone.0027976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 2002. [Google Scholar]
- 32. Reijmer YD, van den Berg E, Ruis C, Kappelle LJ, Biessels GJ. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507–519. doi:10.1002/dmrr.1112 [DOI] [PubMed] [Google Scholar]
- 33. Espeland MA, Miller ME, Goveas JS et al. Cognitive function and fine motor speed in older women with diabetes mellitus: results from the women’s health initiative study of cognitive aging. J Womens Health (Larchmt). 2011;20:1435–1443. doi:10.1089/jwh.2011.2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80:1194–1201. doi:10.1136/jnnp.2009.176818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2009;64:110–117. doi:10.1093/gerona/gln008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harrington MG, Chiang J, Pogoda JM et al. Executive function changes before memory in preclinical Alzheimer’s pathology: a prospective, cross-sectional, case control study. PLoS One. 2013;8:e79378. doi:10.1371/journal.pone.0079378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi:10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 38. Matsuzaki T, Sasaki K, Tanizaki Y et al. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi:10.1212/WNL.0b013e3181eee25f [DOI] [PubMed] [Google Scholar]
- 39. Klunk WE. Amyloid imaging as a biomarker for cerebral beta-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol Aging. 2011;32(suppl 1):S20—S36. doi:10.1016/j.neurobiolaging.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi:10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 41. Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009;57:1816–1824. doi:10.1111/j.1532-5415.2009.02431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocrine Rev. 2008;29:494–511. doi:10.1210/er.2007-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drzezga A, Grimmer T, Henriksen G et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–633. doi:10.1016/j.neuroimage.2007.09.020 [DOI] [PubMed] [Google Scholar]
- 44. Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9:93–113. doi:10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 45. Carlson MC, Varma VR. Activity and Neurocognitive Health in Older Adults. In: Waldstein SR, Elias MF, eds. Neuropsychology of Cardiovascular Disease. 2nd ed. New York/London: Taylor and Francis Psychology Press; 2015. [Google Scholar]
- 46. Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation. 2003;107:e2–e5. doi:10.1161/01.CIR.0000048890.59383.8D [DOI] [PubMed] [Google Scholar]
- 47. Grande G, Vanacore N, Maggiore L et al. Physical activity reduces the risk of dementia in mild cognitive impairment subjects: a cohort study. J Alzheimers Dis. 2014;39:833–839. doi:10.3233/JAD-131808 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.