Abstract
Type 2 diabetes mellitus (T2DM) and persistent cytomegalovirus (CMV) infection are postulated contributors to inflammatory processes that impact on the age-related decline in T-cell responses to influenza vaccination. Older subjects with T2DM (n = 30) and healthy aged controls (n = 40) were enrolled and received influenza vaccination in this study. Serum inflammatory markers and CMV serostatus were measured. Pre- to post-vaccination changes in serum antibody titers to the A/H3N2 strain, and levels of granzyme B (GrB, cytotoxic T lymphocytes) in lysates and cytokines in supernatants from influenza A/H3N2-challenged peripheral blood mononuclear cells were measured. We found no difference between the T2DM and healthy groups in the immune responses measured. However, CMV serostatus was a key determinant of the GrB response to influenza challenge; CMV+ subjects had low levels of inducible GrB (iGrB) activity in response to influenza challenge. In contrast, the serum antibody response to the A/H3N2 vaccine strain did not differ with CMV serostatus, and serum levels of the inflammatory marker, β2-microglobulin, were positively correlated with age, T2DM, and serum IL-10 levels. In conclusion, CMV seropositivity associated with a decline in GrB responses to influenza may predict increased susceptibility to influenza in older adults.
Keywords: Cytomegalovirus (CMV), Older adults, T cell, Granzyme B, Influenza vaccination
The decline in influenza vaccine efficacy has been attributed to changes in the immune response with aging, and the chronic inflammation associated with aging (so-called “inflammaging”), which contributes to or is the result of age-associated diseases such as type 2 diabetes mellitus (T2DM) (1). The dysregulation of inflammatory responses is also associated with reactivation of cytomegalovirus (CMV), and immune senescence, particularly in the CD4+ T-cell subset (2). CMV has also been shown to be a major driver of the terminal differentiation of CD8+ T cells (3) with an accumulation of cells that express and release the cytolytic mediator, granzyme B (GrB), in the absence of perforin, in both the resting and activated state (4,5). We have defined GrB activity that is present in the resting (baseline) state of T cells as “bGrB,” the activity of which is increased three- to fourfold in CMV-seropositive older adults relative to CMV-seronegatives, and correlates with an increase in CD28− CD8+ T cells (ie, those which no longer express CD28 costimulatory molecules) and expression of late or terminal differentiation markers on CD8+ T cells (6) and reported herein. Other studies suggest that when GrB is released in the absence of perforin, it remains in the extracellular space, it is toxic (7) and contributes to a proinflammatory state (8). We have thus postulated that the increase in bGrB activity in resting T cells that is associated with persistent CMV infection in older adults, negatively impacts their T-cell response to influenza virus. Because T2DM has been associated with a decline in influenza vaccine responsiveness, we compared healthy (nondiabetic) older adults to those with T2DM.
Despite the impact of CMV, there remains a population of effector memory CD8+ T cells in older adults that can respond to influenza vaccination with an increase in cells that produce both GrB and perforin when challenged ex vivo with live influenza virus, and are cytolytic in functional assays (9). However, this response to current inactivated influenza vaccines is modest and short-lived, and protection may have more to do with CD8+ T-cell memory from prior influenza infections (5). We have shown that the ex vivo response to live influenza A/H3N2 virus challenge in older adult peripheral blood mononuclear cell (PBMC) correlates with protection against influenza A/H3N2 strains (10–12). Recent reports highlight the association between CMV seropositivity and a heightened antibody response to pandemic influenza H1N1 (pdmH1N1) but the results vary in young and older adults (13–16). It should be noted that influenza attack rates in vaccinated older adults are too low to detect differences between groups when pdmH1N1 or B strains are circulating, while differences when A/H3N2 strains are circulating have been repeatedly demonstrated in our studies (10–12) and in large clinical trials (17,18). Thus, the present study focused on the response to influenza A/H3N2 in vaccinated older adults, which is by far the leading cause of influenza-related morbidity and mortality in this population (19,20).
It has been suggested that elevated serum levels of the inflammatory mediators, C-reactive protein (CRP) and interleukin-6 (IL-6), suppress immune function in older adults, while β2-microglobulin has been shown to perform better than serum CRP and IL-6 levels for risk stratification of mortality in older adults (21). Elevated levels of these inflammatory mediators have been associated with accelerated immune senescence in chronic diseases such as T2DM and chronic infections such as CMV (reviewed in Franceschi and Campisi (1)). Thus, we were interested in these inflammatory biomarkers as a potential measure of the effect of T2DM and/or CMV seropositivity on our previously established immunologic correlates of protection in older adults. Our study was designed to determine the association between serum levels of these inflammatory markers and the influence of T2DM on the T-cell response to influenza vaccination in older adults. Herein, we report how T2DM may interact with CMV serostatus, serum inflammatory markers, and antibody and T-cell responses to influenza A/H3N2 in vaccinated older adults, using previously established assays in influenza-challenged PBMC. We conclude that CMV appears to be a major determinant of a poor cytolytic T-cell response to influenza challenge in older adult PBMC.
Experimental Procedures
Study Population
A total of 70 participants (≥65 years of age; 45 males, 25 females) were enrolled from the community of Greater Vancouver, Canada, during the 2010–2011 influenza season: 30 participants with well-controlled T2DM (≥65 years of age; 9 females, 22 males with one drop-out in the study), and 40 healthy (16 females, 24 males) participants. All participants were community-dwelling, high functioning, nonsmoking, and free of other underlying chronic diseases. Written informed consent for participation in the study was obtained from the participants and ethics approval was obtained from the Clinical Research Ethics Board for the University of British Columbia.
Procedures
Study participants were characterized according to demographics, presence or absence of T2DM, medications, and functional measures. Healthy participants without T2DM were screened by glucose tolerance tests to confirm the absence of a diagnosis of diabetes. All subjects received the 2010–2011 preparation of the standard tri-valent, split virus dose of seasonal influenza vaccine containing: A/California/7/2009 (H1N1)-like strain, A/Perth/16/2009 (H3N2)-like strain, and B/Brisbane/60/2008-like strain (NACI, 2010). Blood samples for serum and PBMC were collected prevaccination (0 week), and 4, 10, and 20 weeks postvaccination.
CMV Serology
The CMV serostatus and CMV-specific IgG titers were determined in serum by ELISA, using a CMV IgG ELISA kit (Omega Diagnostics, Alva, Scotland).
PBMC Isolation and Stimulation
To measure cellular immune responses, PBMC were stimulated with live influenza virus to measure cytokine levels and GrB activity, as previously published in detail (22). Briefly, human PBMC were isolated from heparinized venous blood samples using Ficoll-Hypaque gradient purification. Cell suspensions containing 1.8 × 106 PBMCs were prepared and infected with live influenza virus (A/H3N2) at a multiplicity of infection (MOI) = 2 in AIM V medium (Life Technologies, Burlington, Canada). After 20 hours incubation at 37°C, PBMC supernatants and lysates were prepared and frozen at −80°C for end of study assays. Lysates of CD3+ T cells isolated by negative selection from previously frozen unstimulated PBMC samples using magnetic beads (Stem Cell Technologies, Vancouver, Canada) were used to determine baseline GrB (bGrB) activity as previously described (22).
Granzyme B Assays
GrB activity was measured in PBMC lysates based on the enzyme’s unique cleavage of the peptide, IEPDpNA (EMD Millipore, St. Charles, MO), which causes a colorimetric change with the release of paranitroanilade (pna), and measured as Biomol units (U). GrB activity is adjusted for the total amount of protein in the PBMC lysate using the BCA (bicinchoninic acid) assay kit (Pierce, ThermoFisher Scientific), and reported as U/mg of protein, which correlates with cytolytic activity as previously described (23). Inducible GrB (iGrB) ratio levels were calculated as log(iGrB) = log(GrB in virus stimulated PBMC) − log(bGrB in unstimulated T cells).
Cytokine Measurement
Milliplex human multiplex assay kits (Millipore, Toronto, Canada) were used to measure cytokine levels in PBMC culture supernatants and serum samples according to the manufacturer’s instructions using a Luminex Instrumentation system (Bio-Rad Laboratories, Mississauga, Canada) according to standard curves using Bioplex Manager software. Minimum level of detection (MLD) for each of the biomarkers/cytokines assayed are as follows: β2-microglobulin [0.023 ng/mL], CRP [0.0012 ng/mL], IL-5 [0.1 pg/mL], IL-6 [0.4 pg/mL], IL-10 [0.3 pg/mL], and interferon-γ (IFN-γ) [0.4 pg/mL]. Cytokine levels below the minimum level of detection were assigned a value of one-half of the minimum level of detection in the analysis.
Hemagglutination Inhibition Assay
Serum antibody titers measured by hemagglutination inhibition assays were performed as previously described (24) using twofold dilutions of serum from 1/10 to 1/1024 and of the A/H3N2 vaccine strain. Geometric mean titers were calculated using log10 conversion for each dilution.
Statistical Analysis
Power calculations based on previous validation and demonstrated robustness of the cytokine and GrB assays from influenza-challenged PBMC showed that a 25% difference between groups can be detected with just 13 subjects per group for the GrB assay and 33 subjects per group for the IFNγ:IL-10 ratio (25). In contrast, more than 60 subjects/group are needed to detect this same difference in the individual cytokine levels in influenza-challenged PBMC. Thus, when divided into four groups by CMV and T2DM status, our study was adequately powered to detect differences in bGrzB and iGrzB levels, and differences in the IFNγ:IL-10 ratio in the comparison of healthy and diabetic groups. Only trends in differences in individual cytokine levels in the comparison of the four groups were expected.
Statistical analyses were performed by t-tests, analysis of variance test (ANOVA), and analysis of covariance (ANCOVA) to determine statistical significance for categorical variables between groups, age, gender, and sex, with a p-value ≤.05 for statistical significance. In serum, cytokine levels below the detection level were set at one-half of the minimum level of detection. For comparison of cytokine levels between different groups measured by multiplex assays, we used the nonparametric Kruskal–Wallis test because of the small number of subjects in each group. The associations between age, gender, CMV seropositivity, T2DM, GrB levels, cytokine levels, and other clinical factors in response to influenza vaccination were investigated using multiple linear regression models. A value of p < .05 was required for statistical significance in all analyses.
Results
Association Between T2DM, CMV Serostatus, and Inflammatory Markers
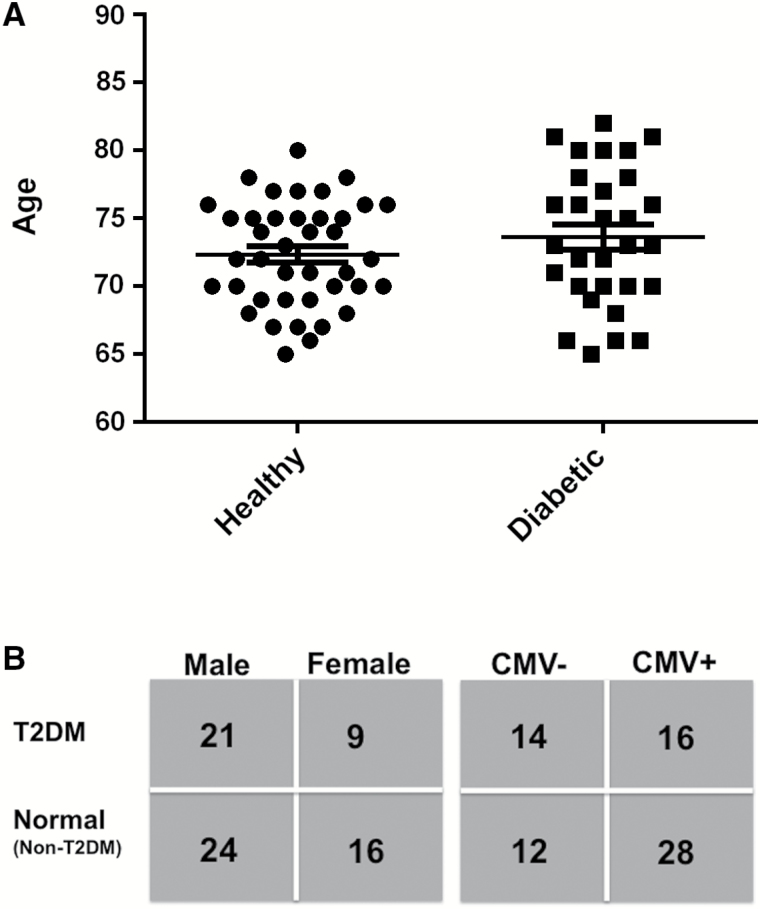
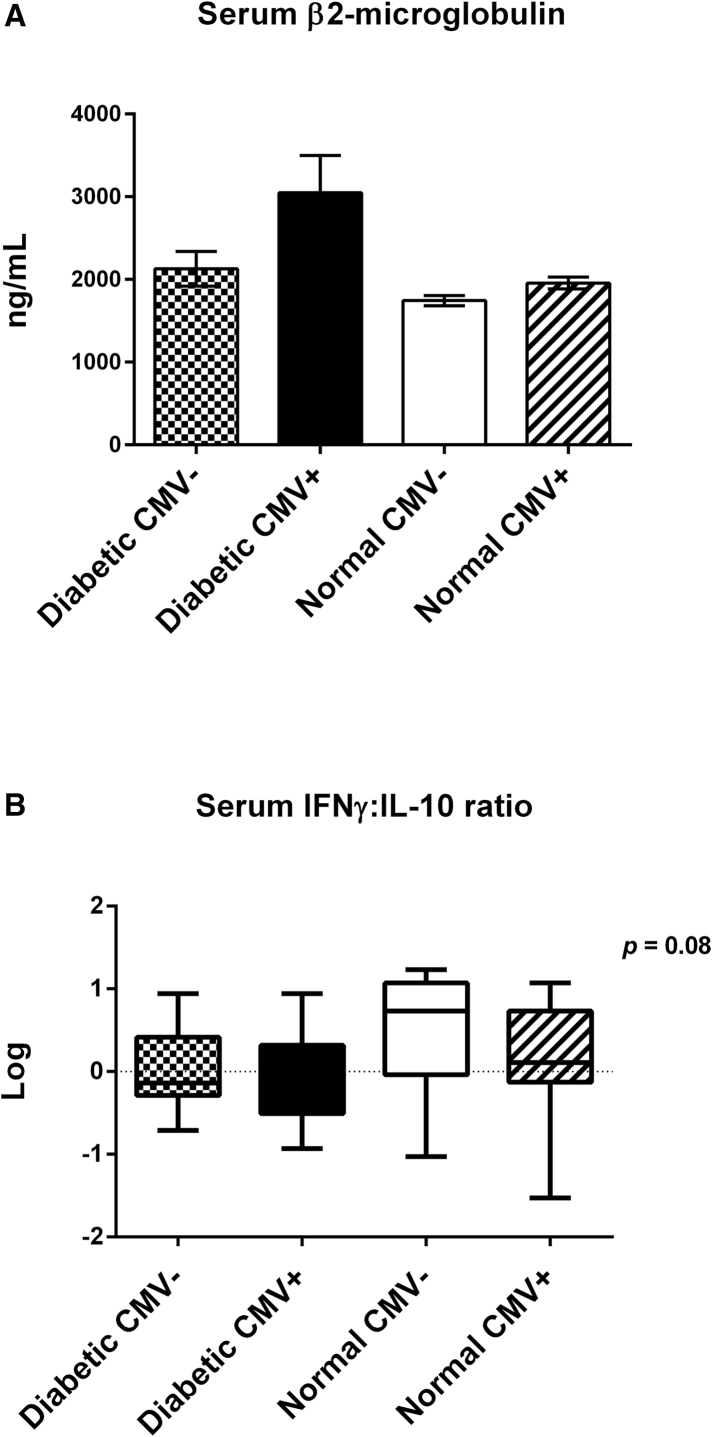
Healthy older adults and those with T2DM had a similar age distribution (Figure 1A). Distribution according to sex and CMV status are shown in Figure 1B. Clinical characteristics of this study cohort shown in Table 1 highlight the expected differences between T2DM versus healthy adults. In contrast, there were no significant differences in the clinical characteristics of CMV+ versus CMV− older adults (Supplementary Table 1). In our overall study cohort, two-thirds of the subjects were CMV+, consistent with CMV prevalence in our previous studies of people aged more than 65 years of age. However, only 50% of subjects in the T2DM group were CMV+ compared to 75% in the control group, which raises a question of selection bias in the recruitment of diabetic subjects because older adults with insulin-requiring diabetes excluded from the study may be more likely to be CMV+ in association with more advanced diabetes. The T2DM group overall had well-controlled diabetes (mean % glycosylated hemoglobin (HbA1c) = 6.33 ± 0.14). Of the three inflammatory markers, CRP, IL-6, and β2-microglobulin, only β2-microglobulin (predictor of mortality) showed a significant increase in diabetic compared to the healthy groups and was associated with a significantly lower serum IFN-γ:IL-10 ratio in the diabetic group (Table 2). There were no differences in the levels of these inflammatory markers based on CMV serostatus (Supplementary Table 2). A multivariate regression analysis showed that β2-microglobulin increased with age (R = .343, p = .004), diabetes (R = .281, p = .017), and serum IL-10 levels (R = .319, p = .01); sex was insignificant in this model. A breakdown of these results showed that β2-microglobulin levels were the lowest (Figure 2A) and serum IFN-γ:IL-10 ratios highest (Figure 2B) in the healthy, CMV-seronegative subset compared to the other three subsets (although not statistically significant). Serum levels of single cytokines were not statistically different in the T2DM versus healthy groups (Table 2), nor within the CMV+ versus CMV− subsets (Supplementary Table 1) but the study was underpowered for these comparisons. Taken together, these results are consistent with the notion that both T2DM and CMV contribute to an inflammatory state associated with increased serum β2-microglobulin levels while the IFN-γ:IL-10 ratio reflects the healthier state of no T2DM and CMV seronegative status.
Figure 1.
Overview of the study population. (A) Age distribution of healthy and T2DM groups (mean ± SE). (B) Number of individuals within in each group by gender and CMV status. The numbers based on age and sex according to CMV status are as follows: male, CMV+ (n = 29); male, CMV− (n = 16); female, CMV+ (n = 15); and female, CMV− (n = 10). Originally 71 participants were enrolled; however, one male with T2DM dropped out.
Table 1.
Clinical Parameters of Older Adults: Healthy Versus Type 2 Diabetes (T2DM)
| Clinical Parameters | Healthy (n = 40) | T2DM (n = 30) | Statistics (p Value) |
|---|---|---|---|
| Age (years) | 72.35 ± 3.8 | 73.37 ± 4.94 | NS |
| BMI (kg/m2) | 24.80 ± 0.79 | 29.58 ± 0.83 | <.0001 |
| Waist circumference (cm) | 92.09 ± 1.15 | 103.93 ± 1.85 | <.05 |
| Height (m) | 1.79 ± 0.08 | 1.72 ± 0.17 | NS |
| Weight (kg) | 74.85 ± 2.02 | 87.46 ± 2.50 | <.0001 |
| Total cholesterol (mmol/L) | 5.29 ± 0.22 | 4.12 ± 0.176 | <.0001 |
| Triglyceride (mmol/L) | 0.99 ± 0.07 | 1.62 ± 0.15 | <.0001 |
| HDL (mmol/L) | 1.70 ± 0.10 | 1.18 ± 0.08 | <.0001 |
| LDL (mmol/L) | 3.09 ± 0.14 | 2.16 ± 0.15 | <.0001 |
| TC/HDL | 3.26 ± 0.14 | 3.63 ± 0.19 | <.0001 |
| HbA1c (%)* | 5.76 ± 0.18 | 6.33 ± 0.14 | NS |
| FBS (mmol/L) | 5.49 ± 0.07 | 8.11 ± 0.76 | <.005 |
Notes: BMI = body mass index; HDL = high density lipoprotein; LDL = low density lipoprotein; TC/HDL = total cholesterol/high density lipoprotein; HbA1c = % glycosylated hemoglobin; FBS = fasting blood sugar. Data are reported as mean ± SD.
*Based on available data from healthy (n = 3) and T2DM (n = 21).
Table 2.
Serum Markers in Older Adults: Healthy Versus Type 2 Diabetes (T2DM)
| Serum Markers | Healthy (n = 40) | T2DM (n = 30) | p Value |
|---|---|---|---|
| β2-microglobulin (ng/mL) | 1895.56 ± 56.71 | 2635.17 ± 254.67 | <.05 |
| CRP (ng/mL) | 2666.75 ± 314.85 | 2700.33 ± 372.46 | NS |
| IL-5 (pg/mL) | 0.47 ± 0.29 | 0.17 ± 0.09 | NS |
| IL-6 (pg/mL) | 3.2 ± 1.19 | 2.38 ± 0.71 | NS |
| IFN-γ (pg/mL) | 1.66 ± 0.5 | 1.27 ± 0.17 | NS |
| IL-10 (pg/mL) | 2.36 ± 0.66 | 1.64 ± 0.27 | NS |
| IFN-γ:IL-10 | 4.48 ± 0.73 | 2.18 ± 0.53 | <.05 |
Notes: CRP = C-reactive protein; IL = interleukin; IFN = interferon. Data are reported as mean ± SE.
Figure 2.
The inflammatory profile of diabetic CMV+ older adults contrasts with that of healthy CMV− older adults. (A) Comparison of serum β2-microglobulin (a predictor of mortality) levels among the four groups shows a trend (Kruskal–Wallis, p > .05) toward increased levels in diabetic CMV+ older adults compared to the other three subsets. Error bars represent standard error of the mean. (B) Log transformed values for the IFN-γ:IL-10 ratio in serum show a trend toward higher ratios in healthy CMV− older adults compared to the other three subsets (Kruskal–Wallis, p = .08). Horizontal lines represent median values, boxes represent the 25th and 75th percentiles and vertical lines represent the 10th to 90th percentiles. The number of older adults in each subset is: diabetic CMV− (n = 14), diabetic CMV+ (n = 16), normal CMV− (n=12), and 28 normal CMV+ (n = 28).
CMV Seropositivity Associated With Suppressed GrB Response to Influenza
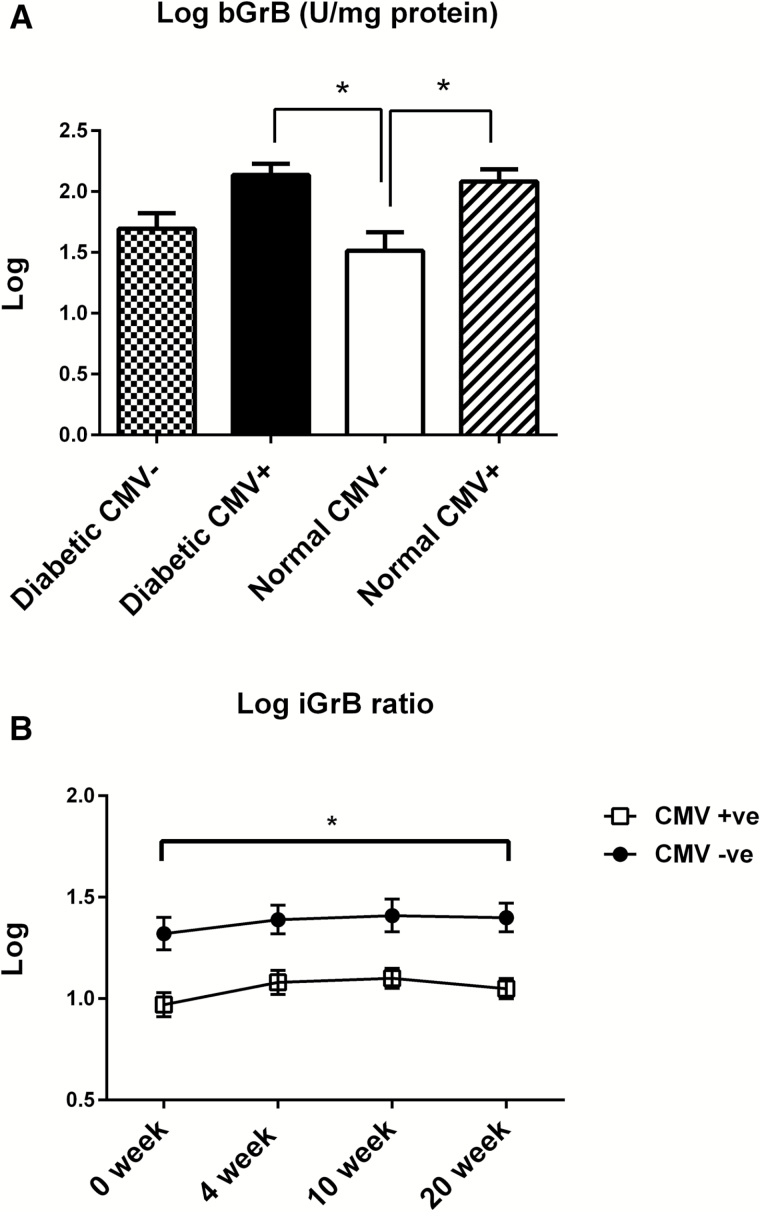
We have previously shown a three- to fourfold increase in bGrB activity in unstimulated T cells (bGrB) in CMV+ versus CMV− older adults (6) and that the level of bGrB activity is stable within subjects over the 20-week period following vaccination (unpublished observations). In this study, bGrzB activity was positively correlated with the proportion of total CD8+ T cells and the subsets expressing late or terminal differentiation markers, and negatively correlated with the proportion of different memory CD8+ T-cell subsets (Table 3). In contrast, total GrB activity in influenza-stimulated PBMC was not correlated with the frequency of any of these CD8+ T-cell subsets, consistent with the small percentage (1–2%) of responding influenza-specific memory CD8+ T cells relative to the large proportion CD8+ T cells that express late or terminal differentiation surface markers (up to 40%). We postulated that the inflammatory effects of T2DM would further increase bGrB levels, beyond those due to persistent CMV infection. Although there was a trend toward higher levels of bGrB in T2DM versus healthy adults, this effect was minimal in comparison to the effect of CMV with significantly higher bGrB levels in CMV+ older adults (both healthy and T2DM) compared to CMV− healthy older adults (Figure 3A). Thus, we calculated the response to live influenza virus as the mean fold increase in GrB activity as “iGrB” calculated as a ratio of GrB activity in influenza-stimulated PBMC to bGrB in resting T cells. There was a modest iGrB response to influenza vaccination with no difference in iGrB levels when comparing T2DM with healthy older adults (data not shown). In contrast, we observed significantly lower iGrB ratios in CMV+ versus CMV− subjects at all time points (Figure 3B). These lower iGrB ratios predict a poor T-cell response to influenza infection and increased risk of influenza illness. Taken together, these results suggest that persistent CMV infection contributes to an increased risk of influenza illness in older adults.
Table 3.
bGrB Activity Correlation With Frequency of Different CD8+ T-Cell Subsets
| CD8+ T-Cell Subsets | CD8+ T-Cell Phenotype | Pearson Correlation (r) | p Value |
|---|---|---|---|
| Total CD8+ T cells | CD3+CD8+ | 0.601 | .001 |
| Late or terminally differentiated CD8+ T cells | CD8+CD57+ | 0.586 | .001 |
| CD8+KLRG1+ | 0.555 | .002 | |
| CD8+/CD45RA+CCR7−CD27−CD28− | 0.553 | .002 | |
| Memory T cells | CD8+/CD28+ | −0.579 | .001 |
| CD8+/CD45RA−CCR7+CD27+CD28+ | −0.476 | .010 | |
| CD8+/CD45RA−CCR7−CD27+CD28+ | −0.627 | .0001 |
Figure 3.
Increased baseline GrB (bGrB) levels in CMV+ older adults are associated with lower levels of induced GrB (iGrB) in response to influenza challenge. (A) Levels of bGrB activity in unstimulated PBMC lysates among the four groups including diabetic CMV− (n = 14), diabetic CMV+ (n = 16), normal CMV− (n = 12), and 28 normal CMV+ (n = 28) are shown. *p ≤ .05, ANOVA between groups comparison. (B) Induced GrB (iGrB) activity in response to live influenza (A/H3N2) challenge pre-vaccination and at 4, 10, and 20 weeks postvaccination is lower among CMV+ (n = 44) compared to CMV− (n = 26) older adults. Error bars represent standard error of mean. *p ≤ .05, ANOVA comparing the CMV+ and CMV− subsets across the four time points.
CMV Seropositivity Corresponds With Lower IL-2 and IL-10 Responses to Influenza Challenge
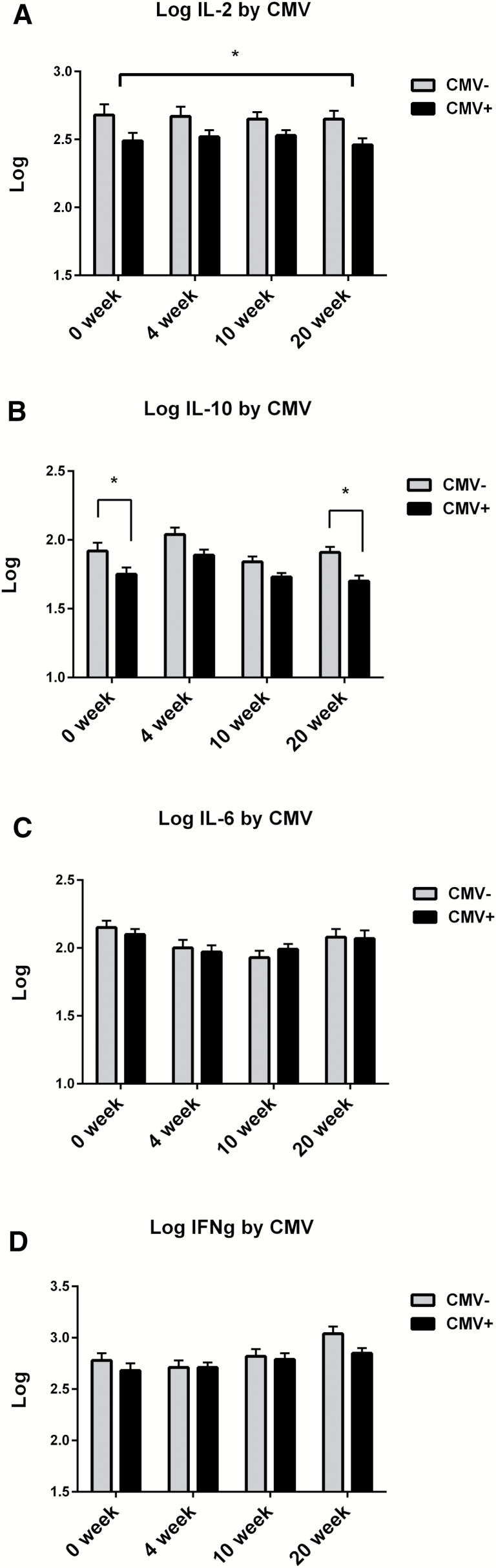
To examine the effect of T2DM and CMV serostatus on ex vivo T-cell responses, we measured cytokine levels in supernatants from influenza-stimulated PBMCs (Figure 4). It has previously been shown that CMV seropositivity in older adults is associated with a decline in the percentage of subjects manifesting a proinflammatory CD4 T-cell response to influenza peptides derived from the internal proteins, matrix, and nucleoprotein (26). Our results demonstrated that older adults who were CMV+ had lower levels of IL-2 at all time points (Figure 4A) independent of T2DM status (Supplementary Figure 1A) and would predict a poor proliferative response to influenza virus. We also found that IL-10 levels were also lower in the CMV+ subset with significant differences found pre-vaccination and at 20 weeks postvaccination (Figure 4B) and again were independent of T2DM status (Supplementary Figure 1B). The increase in IL-10 levels observed at 4-weeks postvaccination and declines by 10-weeks postvaccination, is a response to vaccination that we have previously observed and is thought to be mediated by regulatory T cells. In contrast to the IL-2 and IL-10 results, IL-6 and IFN-γ levels in influenza-challenged PBMC were not significantly affected by CMV seropositivity (Figure 4C and D) nor by T2DM status (Supplementary Figure 1C and D). However, the IFN-γ:IL-10 ratio declined with increasing age (R = −.296, p = .019) consistent with our previously published results (27).
Figure 4.
IL-2 and IL-10 responses to influenza challenge are lower in CMV+ subjects. Results are shown as the differences in the cytokine response (A, IL-2; B, IL-10; C, IL-6; and D, IFNγ) to ex vivo influenza challenge in older adult PBMC comparing CMV+ (n = 44) to CMV− (n = 26) older adults. Cytokine levels were calculated as pg/mL, and are presented as a log10 transformation. Error bars represent standard error of mean. *p ≤ .05, ANOVA comparing the CMV+ and CMV− subsets across the four time points for IL-2, and at two time points, prevaccination and 20-weeks postvaccination for IL-10.
Antibody Response to Influenza A/H3N2 Is Not Associated T2DM or CMV+ Status
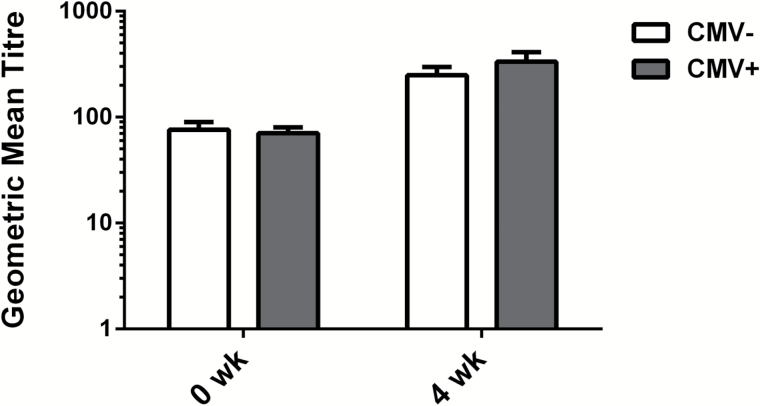
As in our previous report on hemagglutination inhibition assays of the antibody response to the A/H1N1 vaccine strain, the present study showed a similar lack of association between the presence of T2DM in older adults and the response to the A/H3N2 vaccine strain (Supplementary Figure 2). In contrast to the results showing an association between CMV seropositivity and an enhanced antibody response to the A/H1N1 strain (13–16), our results showed no difference in the antibody response to the A/H3N2 strain between CMV+ and CMV− subjects (Figure 5). Although the number of subjects was much smaller in our study, the antibody titers and responses to vaccination were virtually identical thus diminishing the chance of a Type II error in the interpretation of this result relative to the larger study.
Figure 5.
Antibody titers and response to influenza vaccination are independent of CMV serostatus. Geometric mean antibody titres against the A/H3N2 vaccine strain (A/Perth/16/2009) at prevaccination (0 week) and 4 weeks postvaccination are shown for the CMV+ (n = 44) and CMV− (n = 26) older adult subsets. Error bars represent standard error of the mean.
Discussion
The purpose of the study was to evaluate the influence of T2DM on immune responses to influenza vaccination. T2DM has been associated with a decline in vaccine responsiveness (28) and increased susceptibility to influenza and pneumonia in older adults (29) but may not be the case for older adults with well-controlled T2DM as represented in our study. We also wanted to evaluate the effect of CMV on the T-cell response to influenza given earlier findings that persistent CMV infection is associated with the accumulation of late-differentiated memory T cells, particularly in the CD8+ T cell subset (reviewed in Pawelec (30)). Further, CMV is associated with the shift in the immune system toward a low-grade inflammation, described as “inflamm-aging” (1).
Antibody responses (HI assays) have been used as a gold standard to estimate vaccine efficacy but may be limited as a sole measure of protection in older adults (31). In the present study, we found that T2DM had no effect on the antibody response to the A/H3N2 vaccine strain. There was also no association between CMV serostatus and the A/H3N2 antibody response as has been found in other studies (32). However, CMV seropositivity was associated with an enhanced A/H1N1 response in our previously published results (16). Other studies have also shown an enhanced A/H1N1 antibody response in CMV seropositive young adults (13,14) but suppressed B-cell responses to A/H1N1 in CMV seropositive older adults (15). These differing results may need to be explored in the context of original antigenic sin whereby vaccination re-stimulates immunologic memory from childhood exposure to a similar strain, and may explain the relative protection of older adults against the current pandemic H1N1 strains (33). This phenomenon has not been explored in the context of persistent CMV infection, but taken together, these results highlight the importance of identifying which subtype of influenza is being studied and standardizing the definition for a vaccine antibody “responder,” going beyond the current accepted World Health Organization definition.
We have shown that T-cell responses, including the IFN-γ:IL-10 ratio and GrB activity in influenza-challenged PBMCs, are more sensitive correlates of protection in this population (10–12). Here, we found no effect of T2DM on these T-cell responses to influenza vaccination in older adults with well-controlled diabetes, whereas CMV-seropositivity was associated with a lower iGrB response to influenza A/H3N2 with no effect on the antibody response to the A/H3N2 vaccine strain. GrB plays a major role in apoptosis of virus-infected cells; however, cytolytic function is dependent upon the co-expression of perforin for GrB to gain entry into the cytoplasm of the virus-infected target cells. When GrB is expressed in the absence of perforin, it has been shown to disrupt extracellular matrix remodeling in tissue repair mechanisms and stimulates an inflammatory response (34). Previous studies have reported that persistent CMV infection is associated with an accumulation of late differentiated CD8+CD28− T cells with aging (35), the frequency of which correlates with bGrB activity (6), and with a decline in effector memory CD8+ T cell responses to influenza (36).
We postulate that bGrB levels are a measure of immunologic burden of these putatively terminally differentiated T cells and that they increase with aging, CMV infection, and chronic diseases. In accordance with these studies, we observed significantly higher bGrB levels in participants who were CMV+, which appears to suppress the iGrB response to influenza challenge. We have shown that the GrB response to influenza challenge occurs in the effector memory (CD45RA+CCR7−) CD8+ T-cell subset but we have yet to correlate this response with iGrB levels in CMV+ versus CMV− older adults. Given that influenza virus stimulation not only increases the frequency of influenza-specific T cells but also the amount of GrB produced on a per cell basis, differences between CMV− and CMV+ older adults in the correlation between iGrB and the frequency of influenza-specific effector memory CD8 T cells in response to influenza challenge would be an important next step in these studies. These results suggest that persistent CMV infection is an important determinant of the CTL response to influenza vaccination as a correlate of protection, but requires studies with a much larger sample size and influenza surveillance to detect laboratory-confirmed influenza illness, to support this hypothesis.
In this study, we found no significant differences in cytokine responses to ex vivo influenza challenge in PBMC from healthy older adults and those with T2DM. However, we observed a marked decrease in the IL-2 response to influenza challenge in CMV+ relative to CMV− individuals, which predicts a poor proliferative response. The IL-10 response was also found to be lower in the CMV+ subset, suggesting a defect in counter-regulatory mechanisms in the T-cell response to influenza. Other studies in older adults have shown that proinflammatory cytokine production by monocytes in response to influenza vaccination is associated with increased levels of IL-10 in monocytes, perhaps surprisingly found to be highest 4-weeks postvaccination (37). Although CMV serostatus was not evaluated in that study, monocyte production of proinflammatory cytokines has been positively correlated with CMV antibody titers (38). Taken together, these results suggest that there is an interaction between the innate immune response in monocytes and the adaptive immune response to influenza vaccination.
Our findings shed light on the interaction between T2DM and persistent CMV infection in the immune response to A/H3N2 influenza and influenza vaccination among older adults. We show that older adults with well-controlled T2DM have similar CTL responses to influenza vaccination when compared to healthy older adults. However, CMV is associated with higher levels of GrB in resting T cells that do not express perforin, and thus may contribute to a suppressed cytolytic T-cell response in older adults, and to greater inflammatory side effects caused by extracellular GrB toxicity. Additional studies are needed to explore a causal relationship between persistent CMV infection and T-cell responses at a cellular level.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This study was funded under MOP-89729 (J.E.M., Principal Investigator) from the Canadian Institutes of Health Research.
Conflict of Interest
Janet McElhaney has participated on advisory boards for GlaxoSmithKline, Sanofi Pasteur, and Pfizer and on data monitoring boards for Sanofi Pasteur; she has participated in clinical trials sponsored by GlaxoSmithKline and has received honoraria and travel and accommodation reimbursements for presentations sponsored by Merck, GlaxoSmithKline, Sanofi Pasteur, and Pfizer, and travel reimbursement for participation on a publication steering committee for GlaxoSmithKline. Graham Pawelec has participated on advisory boards for GlaxoSmithKline and Sanofi Pasteur and has received honoraria and travel and accommodation reimbursements for presentations sponsored by GlaxoSmithKline, Sanofi Pasteur, Astellas, Cellgene, Clasado, and Pfizer. Graydon Meneilly has received speaking fees from Eli Lilly and Boehringer-Ingelheim, has participated on Advisory Boards for Merck and Sanofi-Aventis, and has received research support from Sanofi. K.H., T.F., G.T., B.G., H.G., and A.K. have declared no conflicts of interest.
Supplementary Material
References
- 1. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 2. Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age (Dordr). 2013;35:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derhovanessian E, Larbi A, Pawelec G. Biomarkers of human immunosenescence: impact of Cytomegalovirus infection. Curr Opin Immunol. 2009;21:440–445. [DOI] [PubMed] [Google Scholar]
- 4. McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(suppl 1):S10–S25. [DOI] [PubMed] [Google Scholar]
- 5. Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Granville DJ. Granzymes in disease: bench to bedside. Cell Death Differ. 2010;17:565–566. [DOI] [PubMed] [Google Scholar]
- 8. Afonina IS, Tynan GA, Logue SE, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell. 2011;44:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou X, Hopkins JW, Wang C, et al. IL-2 and IL-6 cooperate to enhance the generation of influenza-specific CD8 T cells responding to live influenza virus in aged mice and humans. Oncotarget. 2016;7:39171–39183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. [DOI] [PubMed] [Google Scholar]
- 11. McElhaney JE, Ewen C, Zhou X, et al. Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010;28:6145–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wald A, Selke S, Magaret A, Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol. 2013;85:1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furman D, Jojic V, Sharma S, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015;7:281ra243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine. 2015;33:1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McElhaney JE, Garneau H, Camous X, et al. Predictors of the antibody response to influenza vaccination in older adults with type 2 diabetes. BMJ Open Diabetes Res Care. 2015;3:e000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McElhaney JE, Beran J, Devaster JM, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13:485–496. [DOI] [PubMed] [Google Scholar]
- 18. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. [DOI] [PubMed] [Google Scholar]
- 19. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. [DOI] [PubMed] [Google Scholar]
- 20. Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 21. Shinkai S, Chaves PH, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch Intern Med. 2008;168:200–206. [DOI] [PubMed] [Google Scholar]
- 22. McElhaney JE, Gentleman B. Cell-mediated immune response to influenza using ex vivo stimulation and assays of cytokine and granzyme B responses. Methods Mol Biol. 2015;1343:121–141. [DOI] [PubMed] [Google Scholar]
- 23. Ewen CL, Rong J, Kokaji AI, Bleackley RC, Kane KP. Evaluating antigen-specific cytotoxic T lymphocyte responses by a novel mouse granzyme B ELISPOT assay. J Immunol Methods. 2006;308:156–166. [DOI] [PubMed] [Google Scholar]
- 24. WHO Collaborating Center for Influenza BPD. The hemagglutination inhibition test for influenza viruses. Atlanta, GA: DHEW, PHS, CDC, Center for Infectious Disease, revised version 31; 1981:1–21. [Google Scholar]
- 25. Gijzen K, Liu WM, Visontai I, et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine. 2010;28:3416–3422. [DOI] [PubMed] [Google Scholar]
- 26. Derhovanessian E, Maier AB, Hahnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol. 2014;193:3624–3631. [DOI] [PubMed] [Google Scholar]
- 27. Skowronski DM, Hottes TS, McElhaney JE, et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis. 2011;203:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: systematic review and meta-analysis. BMC Med. 2015;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Millett ER, De Stavola BL, Quint JK, Smeeth L, Thomas SL. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open. 2015;5:e008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pawelec G. Immunosenenescence: role of cytomegalovirus. Exp Gerontol. 2014;54:1–5. [DOI] [PubMed] [Google Scholar]
- 31. Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. [DOI] [PubMed] [Google Scholar]
- 32. den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine. 2011;29:4869–4874. [DOI] [PubMed] [Google Scholar]
- 33. Boon AC, Debeauchamp J, Krauss S, et al. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 influenza mouse model. J Virol. 2010;84:7662–7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hiebert PR, Boivin WA, Abraham T, Pazooki S, Zhao H, Granville DJ. Granzyme B contributes to extracellular matrix remodeling and skin aging in apolipoprotein E knockout mice. Exp Gerontol. 2011;46:489–499. [DOI] [PubMed] [Google Scholar]
- 35. Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. [DOI] [PubMed] [Google Scholar]
- 36. Xie D, McElhaney JE. Lower GrB+ CD62Lhigh CD8 TCM effector lymphocyte response to influenza virus in older adults is associated with increased CD28null CD8 T lymphocytes. Mech Ageing Dev. 2007;128:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mohanty S, Joshi SR, Ueda I, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;211:1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Pablo-Bernal RS, Canizares J, Rosado I, et al. Monocyte phenotype and polyfunctionality are associated with elevated soluble inflammatory markers, cytomegalovirus infection, and functional and cognitive decline in elderly adults. J Gerontol A Biol Sci Med Sci. 2016;71: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.