Abstract
Background:
There are relatively few prospective studies evaluating the combined effect of abdominal obesity and low muscle strength on mortality, hospitalization, and incident disability. The aim of this study was to prospectively evaluate the prognostic value of dynapenic abdominal obesity on incident disability, hospitalization, and mortality in the population of the InCHIANTI study.
Methods:
In 370 men and 476 women aged between 65 and 95 years, handgrip strength, waist circumference (WC), body mass index, interleukin-6, C-reactive protein, education, medications, smoking status, and comorbidities were evaluated at the baseline. Difficulties in performing basic activities of daily living were assessed at baseline and at 3-, 6-, and 9-year follow-ups, using a standardized questionnaire. Hospitalization and mortality rates were evaluated during an 11-year follow-up. The study population was categorized as nondynapenic nonabdominal obese (ND/NAO, reference group), dynapenic nonabdominal obese (D/NAO), nondynapenic abdominal obese (ND/AO), and dynapenic abdominal obese (D/AO), according to handgrip strength/WC tertiles.
Results:
D/AO participants presented more than a twofold increase in risk of worsening disability (odds ratio = 2.10; 95% confidence interval [CI]: 1.14–3.88) and significantly higher risk of hospitalization (1.36; 95% CI: 1.04–1.78) compared with ND/NAO participants. After adjustment for potential confounders, the relative risk of death was 1.47 (95% CI: 1.09–1.97) for D/NAO compared with the ND/NAO group.
Conclusions:
Dynapenic abdominal obese participants are at higher risk of worsening disability and hospitalization than ND/NAO participants. Mortality risk was higher in participants with dynapenia without central fat distribution compared with the reference group.
Keywords: Sarcopenic obesity, Muscle strength, Dynapenia, Disability, Mortality
Aging is characterized by an increase in fat mass and a decrease of muscle mass with important consequences on strength and physical performance (1). Moreover, this body composition change, and in particular the so called sarcopenic obesity, characterized by the concurrence of low muscle mass and high amount of fat mass, is associated with disability (2–4), but the relationship with other important outcomes has been less investigated (5,6).
In recent years, the concept of dynapenic abdominal obesity (D/AO), defined as the combination of low muscle strength and central fat distribution, has been introduced (7,8). Separately dynapenia and abdominal obesity have shown associations with important outcomes in older adults, such as worsening disability and mortality (9–16), but only a few studies have investigated health risk associated with the simultaneous presence of both conditions (17–19). In a cross-sectional study of 2,039 men and women aged 55 years and older, where leg extension strength was measured with a dynamometer, Bouchard and Janssen observed that dynapenic obese participants had a lower walking speed compared with nondynapenic and nonobese participants (17). In a population of 3,594 adults ranging in ages between 50 and 91 years followed up for 33 years, Stenholm and colleagues observed instead that both low handgrip strength and obesity independently predict the risk of death (18). Moreover, in a recent study D/AO showed increased falls risk compared with reference group (19).
D/AO participants show an unfavorable metabolic profile, high cardiovascular risk, and are at higher risk of falls, also when compared with sarcopenic obese participants, suggesting that in obese individuals low strength is a predictor of mortality stronger than low muscle mass (19,20). D/AO participants show also an increased risk of worsening disability and mortality than participants with dynapenia or central fat distribution only (8), but this relationship has not been investigated in large, representative populations. Moreover, there is no information supporting the hypothesis that dynapenic abdominal obesity may influence the risk of hospitalizations in older adults.
Using the data from the InCHIANTI study, we tested the hypothesis that participants with simultaneous presence of low muscle strength, evaluated with handgrip, and abdominal obesity, defined as high waist circumference (WC), are at higher risk of worsening disability, hospitalization, and mortality.
Materials and Methods
Study Population
The InCHIANTI study is an epidemiological, population-based study of randomly selected older people living in the Chianti area, Tuscany, Italy. The study was designed to identify risk factors for late-life disability, as previously described (21). Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. In 1998, 1,453 persons who were randomly selected agreed to participate in the project. The Italian National Research Council on Aging Ethical Committee ratified the study protocol, and participants provided written consent to participate. For this analysis, we used data from the baseline (1998–2000) to the 9-year follow-up (2007–2009). Data for deaths and hospitalizations were available up to April 2010 (mean follow-up of 11 years) and therefore were included in the analysis. Of the 1,453 participants enrolled, we excluded 297 participants because they were younger than 65 years and 310 participants for whom information on handgrip strength and WC were not available. The analysis was therefore performed in 846 persons (age 65–95 years), 370 men and 476 women. 14 participants were not evaluated at follow-up because they emigrated from Tuscany to another health care system and 38 participants refused home interviews.
A comparison of the participants excluded from the present analysis (n = 310) with those who were included shows that those excluded were older (p < .001), had higher WC (p = .01), and had more prevalent disability (p < .001), with lower values on the Mini-Mental State Examination (MMSE; p < .001) and higher C-reactive protein (CRP) values (p < .01), along with higher mortality (p < .001), whereas there were no significant differences in age, sex distribution, handgrip, comorbidity, worsening disability, or time to first hospitalization.
Assessment of Dynapenic Abdominal Obesity
WC was measured to the nearest 0.5 cm by using a nonelastic plastic tape, with the participant standing upright, at the midpoint between the lower rib margin and the iliac crest (normally umbilical level). Muscle strength was assessed by grip strength, measured using a hand-held dynamometer (hydraulic hand BASELINE; Smith & Nephew, Agrate Brianza, Milan, Italy). Participants were asked to perform the task twice with each hand, and the maximum strength attained during the four trials was used for the present analyses.
Classification of Groups
Similar to Stenholm and colleagues (7) and Bouchard and Janssen (17), sex-specific tertiles of handgrip strength were created: Individuals in the lowest tertile of handgrip strength (<33 kg in men and <19 kg in women, respectively) were considered as dynapenic, whereas those in the second and third tertiles were considered as nondynapenic. As previously reported by Stenholm and colleagues (7) and Rossi and colleagues (8), sex-specific cutoffs based on WC tertiles were used to classify individuals as abdominal obese or nonabdominal obese (99 and 95 cm in men and women, respectively). Individuals were finally classified into four groups based on sex-specific strength and WC tertiles: nondynapenic nonabdominal obese (ND/NAO), dynapenic nonabdominal obese (D/NAO), nondynapenic abdominal obese (ND/AO), and dynapenic abdominal obese (D/AO) (8).
Outcomes
Worsening disability
Difficulties in six basic activities of daily living (ADL; eating, bathing, transferring, dressing, toileting, and continence) were evaluated through a standardized interview-administered questionnaire. At baseline, prevalent disability was defined as the presence of any difficulty in one or more ADL (22). At the 3, 6, and 9 years of follow-up, ADL status was reassessed using the same questionnaire: Worsening disability in ADL was defined as the development of new ADL disability among participants free of ADL disabilities at baseline, or increase in the number of ADL difficulties among those who already had prevalent ADL disability at baseline. Disability status was available at follow-up for 771 participants (91% of the baseline study population).
Hospitalization
Information on hospitalization was collected using hospital discharge records extracted from the administrative archives of the Tuscany Health Care System. For this analysis, we considered the first hospital admission after the baseline visit.
Mortality
At the end of the field data collection, mortality data of the original InCHIANTI cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region and death certificates deposited immediately after death at the Registry office in the municipality of residence.
Other covariates
Weight and height were measured by using standard techniques. Body mass index was calculated as weight (kg) divided by the square of height (m).
Sociodemographic variables (age, gender, and education) and number of medications were assessed through survey questions. Smoking habit was assessed by self-report, and, on the basis of the answers, participants were categorized into never smokers, and former or current smokers.
The baseline prevalence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study (23). Cognitive status was explored using the MMSE.
Inflammatory markers
Blood samples were drawn in the morning after a 12-hour overnight fast and resting period. Aliquots of serum were stored at −80°C. Serum interleukin-6 (IL-6) was measured in duplicate by high-sensitivity enzyme-linked immunoabsorbent assays (ELISAs; kits from BIOSOURCE, Camarillo, CA) with a sensitivity of 0.1 pg/mL and a intra-assay coefficient of variations less than 6%. CRP was measured using a high-sensitivity ELISA, a competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies (sensitivity 0.03 µg/mL and inter-assay coefficient of variations < 5%).
Statistical Analysis
For descriptive purposes, baseline characteristics of the four groups were compared using chi-square test for categorical variables and analysis of variance model or Kruskal–Wallis nonparametric test for continuous variables with normal or skewed distribution. Differences in hospitalization and mortality rates across the four groups of participants were preliminarily evaluated fitting Kaplan–Meier survival curves adjusted for age. Cox proportional hazard models and logistic regression models were used to assess the risk of both death and hospitalization, and worsening disability, respectively.
Odds ratios (ORs) and 95% confidence intervals (95% CI) were estimated to investigate the association of the four study groups with the risk of worsening disability. Hazard ratios (HRs) and 95% CIs were estimated to investigate the association of the four study groups with the risk of hospitalization and mortality. Three models were fitted for each outcome: unadjusted, age and gender adjusted, and adjusted for all potential confounders. Candidate variables to be included in the third model were selected on the basis of the biological and clinical plausibility as risk factors for hospitalization and mortality (age, sex, smoking status, education, congestive heart failure, diabetes, stroke, number of medications, chronic obstructive pulmonary disease [COPD], and coronary heart disease). All analyses were performed using Stata 11.0 for Windows (StataCorp, College Station, TX).
Results
Baseline characteristics of the 846 participants according to dynapenia/abdominal obesity groups at baseline are presented in Table 1. Mean age of the study participants was 74.5 ± 6.9 years, and 56.3% were women.
Table 1.
Baseline Characteristics of Study Population According to the Handgrip Strength and Abdominal Obesity Status
| Nondynapenic/ Nonabdominal Obese | Nondynapenic/ Abdominal Obese | Dynapenic/ Nonabdominal Obese | Dynapenic/ Abdominal Obese | p Value | |
|---|---|---|---|---|---|
| (n = 394) | (n = 159) | (n = 203) | (n = 90) | ||
| Age (years) | 73.0 ± 6.2 | 72.5 ± 4.9 | 78.4 ± 7.6* | 76.3 ± 6.7* | <.001 |
| Sex (female) (%) | 57.1 | 56.6 | 50.7 | 64.4 | .166 |
| BMI (kg/m2) | 25.8 ± 3.0 | 31.4 ± 3.3* | 25.8 ± 3.0 | 30.9 ± 4.3* | <.001 |
| Waist circumference (cm) | 87.3 ± 7.7 | 103.8 ± 5.5* | 88.0 ± 7.3 | 103.7 ± 5.9* | <.001 |
| Handgrip strength (kg) | 33.6 ± 10.7 | 33.5 ± 10.6 | 19.8 ± 7.4* | 18.0 ± 7.5* | <.001 |
| Education (years), median (IQR) | 5 (4–6) | 4 (4–6) | 5 (3–5)* | 5 (4–5)** | <.001 |
| Medications (n), median (IQR) | 1(0–3) | 2 (1–3)*** | 2 (1–4)* | 3 (1–4)* | <.001 |
| ADL disability (%) | 1.5 | 1.9 | 11.8* | 10.0* | <.001 |
| Smoking habit (%) | 40.9 | 39.0 | 42.4 | 41.1 | .936 |
| MMSE, median (IQR) | 26 (24–28) | 26 (24–28) | 25 (21–27)* | 24 (23–27)* | <.001 |
| Stroke (%) | 4.1 | 8.2*** | 10.3** | 10.0*** | .016 |
| CHF (%) | 2.5 | 5.7 | 6.9*** | 4.4 | .075 |
| CHD (%) | 4.6 | 11.9** | 9.8*** | 6.7 | .011 |
| COPD (%) | 6.6 | 7.5 | 11.3*** | 5.6 | .175 |
| Diabetes (%) | 6.8 | 17.0* | 10.3 | 17.8** | .001 |
| Leg arthritis (%) | 10.9 | 13.8 | 11.8 | 12.2 | .815 |
| IL-6 (pg/mL), median (IQR) | 1.27 (0.8–1.1) | 1.49 (0.91–1.21)*** | 1.61 (0.9–2.09)* | 1.94 (1.09–2.67)* | <.001 |
| CRP (µg/mL), median (IQR) | 2.08 (1.07–3.24) | 3.29 (1.94–5.06)* | 2.55 (1.38–4.36)** | 4.51 (2.1–6.38)* | <.001 |
Notes: ADL = activities of daily living; BMI = body mass index; CHD = coronary heart disease; CHF = chronic heart failure; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; IL-6 = interleukin-6; IQR = interquartile range; MMSE = Mini-Mental State Examination.
In comparison with the reference category (Nondynapenic/Nonabdominal obese), *p < .001, **p < .01, and ***p < .05.
At baseline, dynapenic participants compared with the ND/NAO group were older, used higher number of medications, had lower MMSE score, and had higher prevalence of both disability in ADL and previous stroke. Participants with abdominal obesity, regardless of the dynapenic status, had higher body mass index and diabetes prevalence. No difference in the use of corticosteroids, ACE inhibitors, oral hypoglycemics, and insulins or analogs was observed across study groups. Reported mean weight loss during the 12 months before baseline evaluation was not significantly different between study groups.
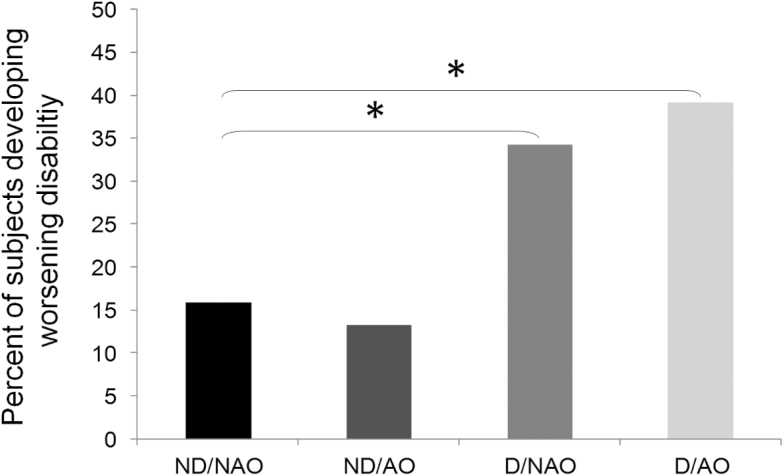
Of the original 846 participants, 4.4% showed an improvement in disability and 22.5% developed worsening disability between baseline and 9-year follow-up; D/AO participants had significantly higher risk of worsening disability compared with ND/NAO participants (39% vs 16%, p < .001; Figure 1).
Figure 1.
Percentage of participants developing worsening disability between baseline and 9-year follow-up according to study groups, nondynapenic nonabdominal obese (ND/NAO), abdominal obese (ND/AO), dynapenic (D/NAO), and dynapenic abdominal obese (D/AO). *p < .001.
Logistic regression analysis adjusted for gender and age showed that compared with the ND/NAO category, the D/AO group was the only subgroup with a significantly higher risk of worsening disability (OR = 2.44; 95% CI: 1.36–4.39), compared with the reference group: This association persisted in the fully adjusted model (OR = 2.10; 95% CI: 1.14–3.88; Table 2). Even after adjustment for CRP and IL-6, this association was still significant (OR = 2.20; 95% CI: 1.08–3.67).
Table 2.
Worsening Disability Risk According to Dynapenic Abdominal Obesity Groups, Using Nondynapenic Nonabdominal Obese Group as Reference
| No. of Participants | Events (%) | Model 1 (unadjusted) | Model 2 (age and sex adjusted) | Model 3 (fully adjusted)a | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| Nondynapenic/Nonabdominal obese | 394 | 59 (9,15) | 1 | — | 1 | — | 1 | — |
| Nondynapenic/Abdominal obese | 203 | 20 (2,13) | 0.81 | 0.47–1.40 | 0.90 | 0.50–1.60 | 0.73 | 0.40–1.34 |
| Dynapenic/Nonabdominal obese | 159 | 58 (3,34) | 2.77 | 1.82–4.23 | 1.53 | 0.95–2.49 | 1.25 | 0.76–2.07 |
| Dynapenic/Abdominal obese | 90 | 31 (2,39) | 3.43 | 2.01–5.82 | 2.44 | 1.36–4.39 | 2.10 | 1.14–3.88 |
Notes: CI = confidence interval; OR = odds ratio.
aAdjusted for age, sex, smoking habit, education, medications, diabetes, congestive heart failure, stroke, chronic obstructive pulmonary disease, and coronary heart disease.
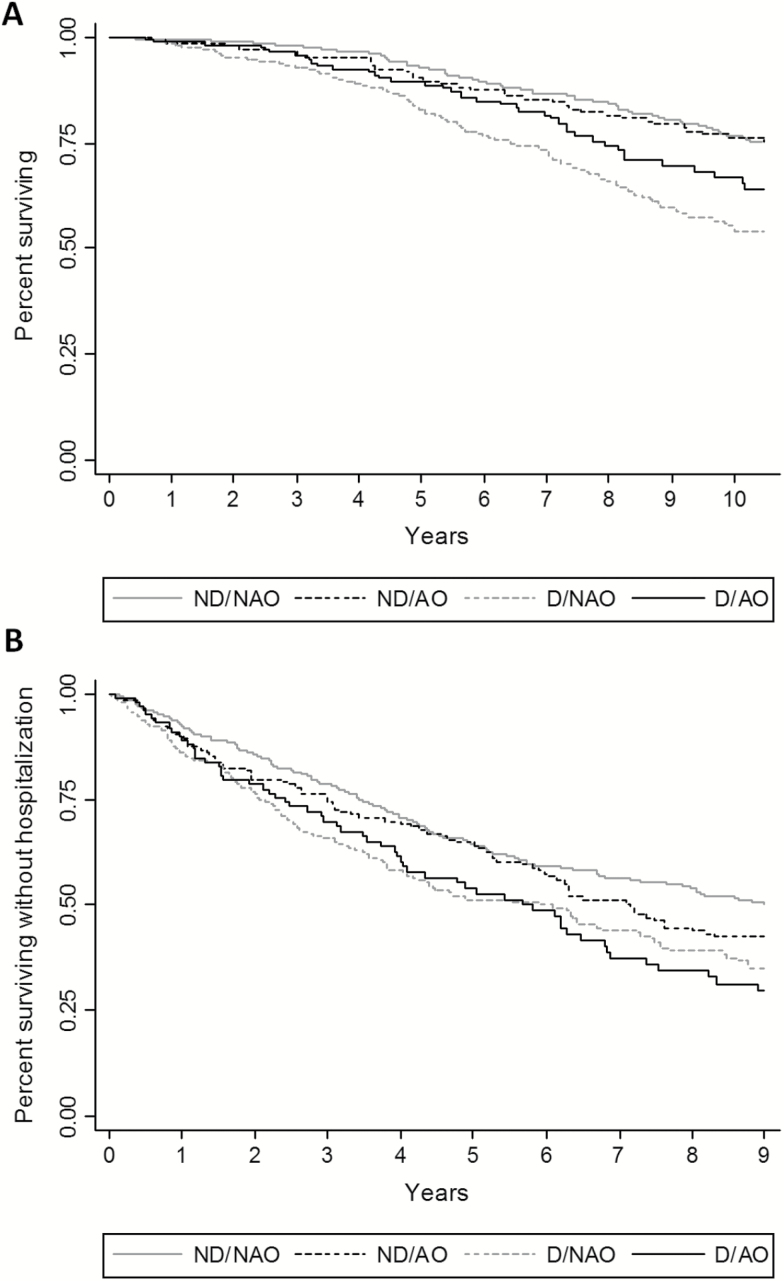
In Figure 2A, adjusted Kaplan–Meier curves show that D/NAO and D/AO participants had the shorter survival free of urgent hospitalization. These findings were confirmed in multivariable Cox proportional hazard models (Table 3) where, after adjusting for potential confounders, dynapenic abdominal obesity was significantly associated with hospital admission (HR = 1.36; 95% CI: 1.04–1.78), with no significant changes when CRP and IL-6 were added to the model.
Figure 2.
Kaplan–Meier survival curves for (A) hospitalization and (B) all-cause mortality according to study groups, nondynapenic nonabdominal obese (ND/NAO), abdominal obese (ND/AO), dynapenic (D/NAO), and dynapenic abdominal obese (D/AO).
Table 3.
Hospitalization Risk According to Dynapenic Abdominal Obesity Groups, Using Nonabdominal Obese Group as Reference
| No. of participants | Events (%) | Model 1 (unadjusted) | Model 2 (age and sex adjusted) | Model 3 (fully adjusted)a | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Nondynapenic/Nonabdominal obese | 394 | 197 (50.0) | 1 | — | 1 | — | 1 | — |
| Nondynapenic/Abdominal obese | 203 | 85 (53.5) | 1.20 | 0.97–1.48 | 1.23 | 1.00–1.51 | 1.18 | 0.96–1.46 |
| Dynapenic/Nonabdominal obese | 159 | 134 (66.0) | 1.65 | 1.36–2.00 | 1.32 | 1.08–1.62 | 1.21 | 0.98–1.49 |
| Dynapenic/Abdominal obese | 90 | 60 (66.7) | 1.60 | 1.23–2.07 | 1.48 | 1.14–1.93 | 1.36 | 1.04–1.78 |
Notes: CI = confidence interval; HR = hazard ratio.
aAdjusted for age, sex, smoking habit, education, medications, diabetes, congestive heart failure, stroke, chronic obstructive pulmonary disease, and coronary heart disease.
During an average follow-up of 11 years, 298 participants (35.2%) died. Figure 2B shows that participants with dynapenia had the shorter survival as compared with the nondynapenic counterpart.
Estimates derived from the Cox proportional hazard models, adjusted for age and gender, confirmed the results of the survival analysis, whereas, after adjustment for potential confounders, the association was significant only for the D/NAO subgroup (HR = 1.47; 95% CI: 1.09–1.97; Table 4). When CRP and IL-6 were added to the model, a slight increase in risk was observed for D/NAO (HR = 1.51; 95% CI: 1.12–2.03) with no significant changes for the other groups (data not shown in the table).
Table 4.
Mortality Risk According to Dynapenic Abdominal Obesity Groups, Using Nondynapenic Nonabdominal Obese Group as Reference
| No. of participants | Events (%) | Model 1 (unadjusted) | Model 2 (age and sex adjusted) | Model 3 (fully adjusted)a | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| Nondynapenic/Nonabdominal obese | 394 | 101 (25.6) | 1 | — | 1 | — | 1 | — |
| Nondynapenic/Abdominal obese | 203 | 35 (22.0) | 0.86 | 0.58–1.26 | 0.94 | 0.64–1.38 | 0.86 | 0.58–1.27 |
| Dynapenic/Nonabdominal obese | 159 | 121 (59.6) | 3.26 | 2.50–4.25 | 1.69 | 1.27–2.26 | 1.47 | 1.09–1.97 |
| Dynapenic/Abdominal obese | 90 | 41 (45.5) | 2.17 | 1.51–3.12 | 1.59 | 1.10–2.30 | 1.36 | 0.93–1.97 |
Notes: CI = confidence interval; HR = hazard ratio.
aAdjusted for age, sex, smoking habit, education, medications, diabetes, congestive heart failure, stroke, chronic obstructive pulmonary disease, and coronary heart disease.
IL-6 and CRP were both associated with mortality; however, in the multivariate analysis only CRP was still statistically associated. CRP was not statistically associated when interaction with dynapenic obesity was tested (p = .79). Only CRP was associated in the outcome with hospitalization risk, but when interaction with dynapenic obesity was tested, it was found not to be statistically associated (p = .56).
IL-6 and CRP were both included in the fully adjusted model because they were associated with worsening disability, but when included in the multivariate analysis they both lost significance.
Discussion
The results of this study suggest that in older community-dwelling people, dynapenia is associated with an increased risk of hospitalization and mortality regardless of the coexistence of abdominal obesity, whereas the likelihood of worsening disability associated with dynapenia is strongly enhanced by the presence of abdominal obesity.
We showed that, after adjustment for confounding factors, dynapenic abdominal obese participants experience a more than doubled risk of worsening disability, in line with the findings of Baumgartner and colleagues (2) who demonstrated that older participants with sarcopenic obesity at baseline had more than twofold higher risk of developing instrumental ADL disability compared with those who were not sarcopenic obese at baseline.
Our work also confirms and expands previous results from Rossi and colleagues in a smaller Italian population (8), who showed that participants with dynapenic abdominal obesity at baseline had a more than threefold increased risk of worsening disability and from Bouchard and Stenholm that, in different populations, observed that dynapenic obese participants are at greater risk for decline in physical performance (7).
In the current study, we also observed an independent negative effect of D/AO on the risk of hospitalization. Both D/AO and D/NAO participants showed an increased risk of hospitalization and a shorter time to first hospitalization compared with other groups, but after adjustment for potential confounders, only D/AO showed a significantly increased risk. Our results are partially in agreement with those of Cawthon and colleagues (24), who observed in the population of the Health ABC Study that the highest risk of hospitalization was related with lower extremity extension strength. Few studies have looked directly at the relationship between adiposity indexes and hospital admission rates (25,26), and there is a lack of studies considering the coexistence of dynapenia and central adiposity as a risk factor for this outcome.
In a recent study, Scott and colleagues (19) showed that the risk of falling is increased in participants with dynapenic abdominal obesity, with serious health consequences, such as head injury or vertebral and femoral fractures, events that frequently lead to hospitalization (27). Both the higher rate of worsening disability and the falls risk associated with dynapenic abdominal obesity might at least in part explain the increase in hospitalization risk observed in our study. An alternative explanation for the observed increased risk of hospitalization might be the unfavorable metabolic profile associated with abdominal obesity and dynapenia, leading to an increased incidence of conditions that are a common cause of hospitalization in older patients, including but not limited to congestive heart failure and cardiovascular and cerebrovascular diseases (20).
Mortality risk during the 11 years of follow-up was similar for D/NAO and D/AO participants, but after adjustment for potential confounders, the association between mortality and the simultaneous presence of dynapenia and central obesity was no longer statistically significant although the point estimates were quite similar. Muscle strength is considered a very important marker of mortality risk in old age, but previous studies failed to capture the biological mechanism underlying this association (28,29). In our study population, in the multivariate analysis CRP, but not IL-6, was statistically associated with mortality and hospitalization risk, but the former biomarker was not statistically associated when interaction with dynapenic obesity was tested. Therefore, CRP seems to be a confounder in the relationship between dynapenic obesity and both, mortality and hospitalization risk, but we cannot exclude it is a mediator of the observed association.
It is likely that in older people low muscle strength might represent a final common pathway capturing the detrimental effect of several age-related biological and pathological mechanisms eventually increasing the likelihood of death.
From this point of view, these results seem to be partially in contrast with those of Rossi and colleagues (8), which reported an increased mortality risk in D/AO and not in D/NAO participants, but actually some differences between the two studies must be considered. Firstly of all, the InCHIANTI population was larger, nearly 4 years older, showed higher prevalence of men, cardiovascular disease, and COPD, and the sex distribution across study groups was different. Secondly, it must be noted that only after adjustment for comorbidity and other confounding variables, the association between mortality and dynapenic abdominal obesity was no longer significant. Because the D/AO group had the smaller sample size and low number of cases, it is possible that a reduced statistical power might also explain the lack of statistical association in the more complex multivariate model.
Thirdly, similar to previous reports (7,8), we chose to categorize the study population on the basis of muscle strength and WC tertiles. Obtained cutoff for abdominal obesity in women was higher and more conservative in our study population compared with that of Rossi and colleagues (95 vs 87 cm) and ND/AO and D/AO percentages were higher. This could partially account for the differences in association with mortality observed in the two studies.
Some potential limitations of the present study should be acknowledged. Firstly, as previously highlighted, the D/AO subgroup was relatively small, and therefore it is possible that a reduced statistical power might have at least partially influenced our findings. Therefore, the obtained results must be considered with caution and confirmed in larger populations.
Second, we did not consider the causes of hospitalization death, in part because of the limited power. It is conceivable that these analyses would have provided useful additional information on the underlying biological mechanisms.
As a result, the occurrence of a selection bias due to the high number of participants with missing data in our study sample cannot be ruled out. In fact, participants not included in the present analysis showed more prevalent disability, were older, presented higher mortality, and had higher values of coefficient of variations and CRP, and thus could have influenced the number of events observed in our study population and the relationships with considered outcomes.
In conclusion, our results suggest that dynapenia is related to the risk of death regardless of the presence of central obesity, whereas abdominal obesity strongly increases the risk of disability and hospitalization associated with low muscle strength. Identification of elderly participants with central fat distribution and simultaneous low muscle strength could help, with easily available and inexpensive tools, to select a subgroup of participants with the highest risk of functional decline and loss of independence.
Funding
The InCHIANTI study baseline (1998 and 2000) was supported as a “targeted project”(ICS110.1/RF97.71) by the Italian Ministry of Health, and in part by the National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336). The Follow-up 1 (2001 and 2003) was funded by the U.S. National Institute on Aging (Contracts: N.1AG-1-1 and N.1-AG-1-2111); the Follow-up 2 and 3 studies (2004 and 2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002). The study was also supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland.
Conflict of Interest
None.
Acknowledgments
The specific responsibilities of the authors were as follows—L.B., S.B., S.V., A.P.R., and M.Z.: analysis and interpretation of data; A.P.R., L.B., S.V., and M.Z.: concept and design of study and manuscript preparation; L.F. and S.B.: study design consultation, participant recruitment, and manuscript edits; S.V., L.B., and M.Z.: manuscript edits; L.F. and S.B.: participant acquisition, data collection, and manuscript review.
Professor Mark Newman corrected the final version of the manuscript.
References
- 1. Baumgartner RN. Body composition in healthy aging. Ann NY Acad Sci. 2000;904:437–448. doi:10.1111/j.1749-6632.2000.tb06498.x [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi:10.1038/oby.2004.250 [DOI] [PubMed] [Google Scholar]
- 3. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi:10.1016/j.numecd.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 4. Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi:10.1111/jgs.12260 [DOI] [PubMed] [Google Scholar]
- 5. Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi:10.1038/ejcn.2014.117 [DOI] [PubMed] [Google Scholar]
- 6. Atkins J, Whincup P, Morris R, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62:253–260. doi:10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI Study. Int J Obes. 2009;33:635–644. doi:10.1038/ijo.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossi AP, Fantin F, Caliari C, et al. Dynapenic abdominal obesity as predictor of mortality and disability worsening in older adults: a 10-year prospective study. Clin Nutr. 2015:S0261–5614. doi:10.1016/j.clnu.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 9. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi:10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 10. Fantin F, Di Francesco V, Fontana G, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci. 2007;62:1375–1381. doi:10.1093/gerona/62.12.1375 [DOI] [PubMed] [Google Scholar]
- 11. Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clin Exp Res. 2007;19:277–283. doi:10.1007/BF03324702 [DOI] [PubMed] [Google Scholar]
- 12. Stenholm S, Rantanen T, Heliövaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc. 2008;56:462–469. doi:10.1111/j.1532-5415.2007.01567.x [DOI] [PubMed] [Google Scholar]
- 13. Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement aged adults. Int J Obes. 2006;30:364–373. doi:10.1038/sj.ijo.0803130 [DOI] [PubMed] [Google Scholar]
- 14. Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135–142. doi:10.1016/j.clnu.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 15. Koster A, Leitzmann MF, Schatzkin A, et al. Waist circumference and mortality. Am J Epidemiol. 2008;167:1465–1475. doi:10.1093/aje/kwn079 [DOI] [PubMed] [Google Scholar]
- 16. Zoico E, Di Francesco V, Guralnik JM, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–241. doi:10.1038/sj.ijo.0802552 [DOI] [PubMed] [Google Scholar]
- 17. Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:71–77. doi:10.1093/gerona/glp159 [DOI] [PubMed] [Google Scholar]
- 18. Stenholm S, Mehta NK, Elo IT, Heliovaara M, Koskinen S, Aromaa A. Obesity and muscle strength as long-term determinants of all-cause mortality—a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obes. 2014;38:1126–1132. doi:10.1038/ijo.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott D, Sanders KM, Aitken D, Hayes A, Ebeling PR, Jones G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity. 2014;22:1568–1574. doi:10.1002/oby.20734 [DOI] [PubMed] [Google Scholar]
- 20. Sénéchal M, Dionne IJ, Brochu M. Dynapenic abdominal obesity and metabolic risk factors in adults 50 years of age and older. J Aging Health. 2012;24:812–826. doi:10.1177/0898264312440324 [DOI] [PubMed] [Google Scholar]
- 21. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 22. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi:10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. (Eds.). The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD, National Institute on Aging; 1995. [Google Scholar]
- 24. Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. doi:10.1111/j.1532-5415.2009.02366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borné Y, Hedbland B, Essén B, Engstrom G. Anthropometric measures in relation to risk of heart failure hospitalization: a Swedish population-based cohort study. Eur J Pubbl Health. 2014;24:215–220. doi:10.1093/eurpub/cks161 [DOI] [PubMed] [Google Scholar]
- 26. Volpato S, Romagnoni F, Soattin L, et al. Body mass index, body cell mass, and 4-year all-cause mortality risk in older nursing home residents. J Am Geriatr Soc. 2004;52:886–891. doi:10.1111/j.1532-5415.2004.52254.x [DOI] [PubMed] [Google Scholar]
- 27. Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. In J Prev. 2006;12:290–295. doi:10.1136/ip.2005.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Newman B, Varant K, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi:10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 29. Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi:10.1034/j.1600-0579.2003.00207.x [DOI] [PubMed] [Google Scholar]