Abstract
The mouse clinical frailty index and the mouse frailty phenotype assessment are two recently developed tools used to assess frailty in mice. The objectives of this study were to investigate whether the same mice are identified as frail with both tools and to examine the association of each of the assessment tools with age and frailty-related outcomes. Frailty was measured using both tools in old (~24 months; n = 36) C57BL/6 male mice. After 2 weeks, blood pressure and heart rate were measured and serum samples were collected for analysis of alanine aminotransferase, creatinine, and albumin levels. The mouse frailty phenotype assessment identified no mice as frail but modification of the assessment tool identified six mice as frail. The mouse clinical frailty index identified 16 mice as frail and the agreement between the two scales was 50.0%. Increasing clinical frailty index scores were correlated with low serum alanine aminotransferase, as well as decreased heart rate, and reduced heart rate variance. We conclude that, consistent with equivalent frailty assessment scales in humans, both tools have value but do not necessarily identify the same mice as frail.
Keywords: Frailty index, Frailty phenotype, Deficit index, Deficit accumulation, Animal models
Frailty is generally considered a state of high vulnerability for adverse global health outcomes and a reduced capacity to react to stressors (1). Frailty is associated with old age and increases the risk of falls, dependency, disability, institutionalization, hospitalization, and mortality in humans (2–4).
Two of the most commonly used clinical frailty assessments in humans are the phenotype model (5) and the frailty index (FI) (6). The phenotype model was developed by Fried and colleagues (5) and they define frailty as the presence of three or more criteria including weight loss, exhaustion, weakness, slow walking speed, and low physical activity. It conceptualizes frailty as a biological syndrome and defines patients as nonfrail (no criteria present), prefrail (one or two criteria present), or frail (three or more criteria present). The FI was originally developed by Rockwood and colleagues (6) and conceptualizes frailty as a “multidimensional risk state” (7). It measures the proportion of accumulated health deficits in a person, with a focus on the number of deficits rather than the precise nature of the deficits (8,9). A higher FI indicates a greater degree of frailty. When these two frailty assessment tools have been investigated in the same populations, they identify some but not all of the same patients as frail (10,11). The scales do, however, both predict adverse outcomes (7,12,13).
There has been a recent focus on developing mouse frailty assessment tools (14), to enable preclinical research into frailty. Parks and colleagues (15) developed a tool based on the clinical FI, which assesses a mouse as frail based on measures of deficits including activity, body composition, hemodynamic factors, and metabolic status. Whitehead and colleagues (16) continued this work by developing a noninvasive mouse FI based on 31 clinically evident health deficits. Liu and colleagues (17) developed a tool based on the clinical phenotype model, which assesses a mouse as frail based on its grip strength, walking speed, physical activity, and endurance. As these tools are newly developed, studies have not yet investigated the relationships between them. The relationship between these tools and potential serum markers of poor prognosis and aging including serum alanine aminotransferase (ALT) (18,19), albumin or creatinine (20,21), or with changes in cardiovascular factors that have been clinically associated with aging or frailty, such as heart rate or blood pressure (22), has not been explored.
This aim of this study was to determine, in a cohort of ~2-year-old C57BL/6 mice, whether the mouse frailty phenotype assessment and the mouse clinical FI identify the same mice as frail. Furthermore, we aimed to correlate the scales with serum markers and cardiovascular outcomes previously associated with aging or frailty. We also suggest potential ways the assessment tools could be optimized for use in aging and frailty research in animal models.
Methods
Animals
C57BL/6 male mice were obtained from and aged at the Kolling Institute of Medical Research, Sydney, Australia, until 101.8 ± 2.9 weeks of age (n = 36). This corresponds to an age of about 70–75 years in humans (23) and mice in this age range have been used in previous frailty studies (24,25). The primary outcomes (polypharmacy intervention effects) for n = 21 of the mice (cohort 1) have been reported previously (26) and this is a secondary analysis of the control data for these mice. Data from the other n = 15 mice has not been previously published. Animals were maintained on a 12-hour light–dark cycle, in boxes of 1–4 with ad libitum access to food (Standard Meat Free Mouse and Rat Feed, Specialty Feeds, Western Australia, Australia) and water. The study was approved by the Northern Sydney Local Health District Animal Care Ethics Committee, Sydney, Australia.
Assessments
At 101.8 ± 2.9 weeks of age, frailty assessments were performed on all mice as described below. For a subset of mice from cohort 1 (n = 8), after 2 weeks, blood pressure and heart rate were measured and mice were euthanized for collection of blood. Blood pressure and heart rate were measured by the tail cuff method using the CODA Non-invasive Blood Pressure System (Kent Scientific Corporation, CT), with appropriate acclimatization and training (27). In brief, mice were exposed to the machine for 15 minutes for 3 days prior to testing. Testing was completed on 2 consecutive days and mice were acclimatized to the machine for 15 minutes, then 10 acclimatization cycles were completed before the testing cycles for each experiment. An estimate of heart rate variability was determined from the standard deviation of 8–20 heart rate measurements for each mouse. For one mouse, heart rate and heart rate variability was not available. Serum ALT, creatinine, and albumin were measured by the Pacific Laboratory Medicine Services (Sydney, Australia). Vitamin B6 was added to the ALT assay to account for age-related decline in this cofactor of the assay (28). Further details can be found in Huizer-Pajkos and colleagues (26).
Mouse Frailty Phenotype Assessment
The mouse frailty phenotype assessment was completed based on the article by Liu and colleagues, with minor adaptations to allow assessment with available equipment (Table 1). The functional phenotype assessments included walking speed, grip strength, physical activity, and endurance. The differences in assessment protocols between the study by Liu and colleagues and the current study are summarized in Table 1. Details of functional assessments are found in ref. (26). We evaluated the performance of each mouse in each of these criteria according to the methods outlined by Liu and colleagues (17) to determine if a mouse was nonfrail or frail. In brief, we calculated a cutoff point 1.5 SD below the baseline cohort mean for each criterion. A mouse was assessed as frail if measures for three or more criteria were below this cutoff point. A mouse was also assessed as prefrail if two criteria were below the cutoff point.
Table 1.
Comparison of Criteria Used for Frailty Phenotype Assessments Clinically and in Mice
| Human Frailty Phenotype Assessment | Mouse Frailty Phenotype Assessment | Modified Mouse Frailty Phenotype Assessment | |
|---|---|---|---|
| Reference | Fried et al. (5) | Liu et al. (17) | Current study |
| Cutoff value | Lowest 20% of cohort | 1.5 SD below cohort mean (approximately lowest 7%) | 0.8 SD below cohort mean (approximately lowest 20%) |
| Criteria | Grip strength | Four paw inverted hang (seconds to fall) | Front paw wire hang (seconds to fall) |
| Activity | Voluntary wheel running (daily running distance) | Open-field assessment (distance moved in 5 min) | |
| Walking speed | Rotarod - training protocol (maximum speed) | Rotarod - no training protocol (maximum speed) | |
| Endurance | Derived from four paw hang and rotarod measures (seconds) | Derived from wire hang and rotarod measures (seconds) | |
| Weight loss | Not included | Low body weight |
Note: SD = standard deviation.
As described in the Results section, no mice in either cohort were identified as frail and only one mouse was identified as prefrail, with the previously reported frailty phenotype assessment criteria (17). The standard deviations and coefficients of variance of the four functional assessments were compared between the Liu and colleagues (19) data set and the current data set to understand this difference. Analysis was also completed in the current data set with a modified frailty phenotype assessment in which a cutoff point 0.8 SD below the cohort mean was used and a weight loss criterion was added. A cutoff point of 0.8 SD below the mean identifies approximately the lowest 20% of the cohort for each criteria, as was done in the original frailty phenotype assessment in humans (5). The addition of weight loss criteria also more closely mimics the original clinical assessment in humans (5) (Table 1). A continuous score counting the number of criteria (0–5) below the 0.8 SD cutoff point using the modified frailty phenotype assessment was used for correlation analysis.
Mouse Clinical FI Assessment
A FI score was calculated for each mouse using the 31-item FI (16). Cutoff points were applied to the FI scores to estimate the number of frail, and nonfrail, mice determined with this assessment tool. The cutoff points used were: <0.18 = nonfrail and ≥0.18 = frail, which corresponds to the upper 50% confidence interval of the mean FI score for this cohort of mice (29). The value is also similar to cutoff points used for FI scores in previous clinical studies (30–32).
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Agreement of the two scales was assessed using Cohen’s κ. Correlations were assessed using the Pearson correlation coefficient (r) and comparisons across frail and nonfrail groups were assessed using Student’s t tests. Data analysis was completed using the statistics program SPSS (Version 21.0, SPSS, Chicago, IL) and SigmaPlot (Version 11.0, Systat Software, Germany).
Results
Mice Identified as Frail With the Two Frailty Assessments
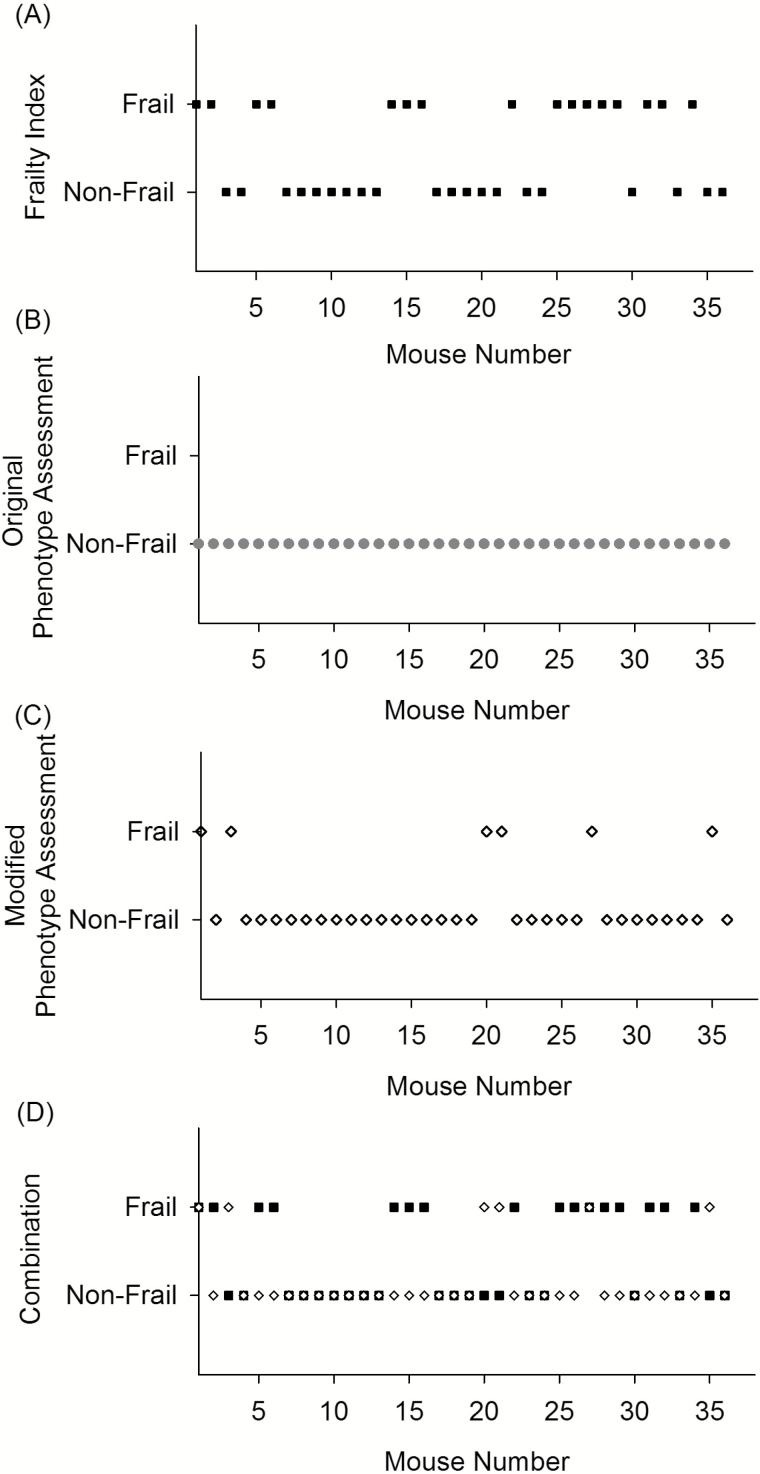
To determine which mice were identified as frail with the two assessment tools, FI values and phenotype scores were determined for each mouse. The application of a cutoff point for the mouse clinical FI (16), identified 16 mice as frail (Figure 1A). The mean FI for all of the mice was 0.19 ± 0.06 (n = 36). The number of mice scored for each of the individual FI items is shown in Supplementary Table 1.
Figure 1.
Visualization of which old (101.8 ± 2.9 weeks, n = 36) male C57BL/6 mice were identified as nonfrail or frail by either the mouse clinical frailty index (A, black square), the original mouse frailty phenotype assessment (B, gray circle), the modified mouse frailty phenotype assessment (C, white diamond), or by the mouse clinical frailty index and/or the modified mouse frailty phenotype assessment (D).
The mouse frailty phenotype assessment (17) identified no mice as frail (Figure 1B). One mouse was identified as prefrail (with two criteria below the cutoff point). The standard deviations and coefficients of variance were compared between the original (19) and current data set for the assessments included in the phenotype tool (Supplementary Table 2). There was less variance in the current data set, compared to the original study and to account for this and more closely mimic the clinical assessment, we re-evaluated using a modified mouse frailty phenotype assessment with a cutoff 0.8 SD below the cohort mean and the addition of a weight loss criterion. This identified six mice as frail (Figure 1C). Seven additional mice were assessed as prefrail. Using the modified frailty phenotype assessment, the number of mice identified as either frail or nonfrail by both assessments was 18/36 (50%), as shown in Figure 1D. There was no agreement between the two scales compared with Cohen’s kappa, κ = −0.08 (p = .55).
Correlation of Frailty With Serum Markers
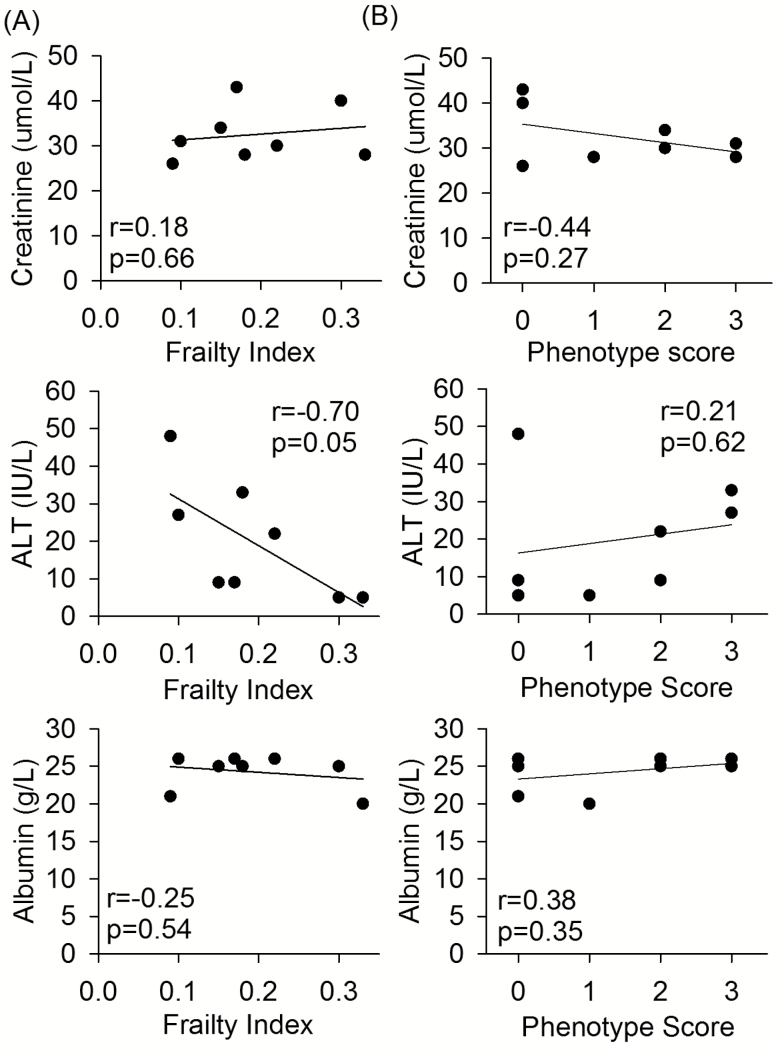
Mouse clinical FI values and the modified frailty phenotype scores (0–5) were correlated with potential serum markers of frailty (Figure 2A and B). There was a negative correlation between mouse clinical FI scores and serum ALT (r = −0.70, p = .05) but no correlation between FI and serum creatinine or albumin (Figure 2A). There was no correlation between the frailty phenotype score and any serum marker (Figure 2B).
Figure 2.
The correlation of (A) mouse clinical frailty index scores and (B) modified frailty phenotype scores, with potential poor prognostic serum markers creatinine, alanine aminotransferase (ALT), and albumin, for old (99.6 ± 1.2 weeks) male C57BL/6 mice. Pearson correlation coefficient values (r) and p values are shown on each of the graphs. n = 8 for all graphs.
Mean values for the serum markers were also compared between frail and nonfrail mouse groups identified with the two tools but there were no significant differences for any of the markers for either assessment tool (Supplementary Table 3).
Correlation of Frailty With Cardiovascular Outcomes
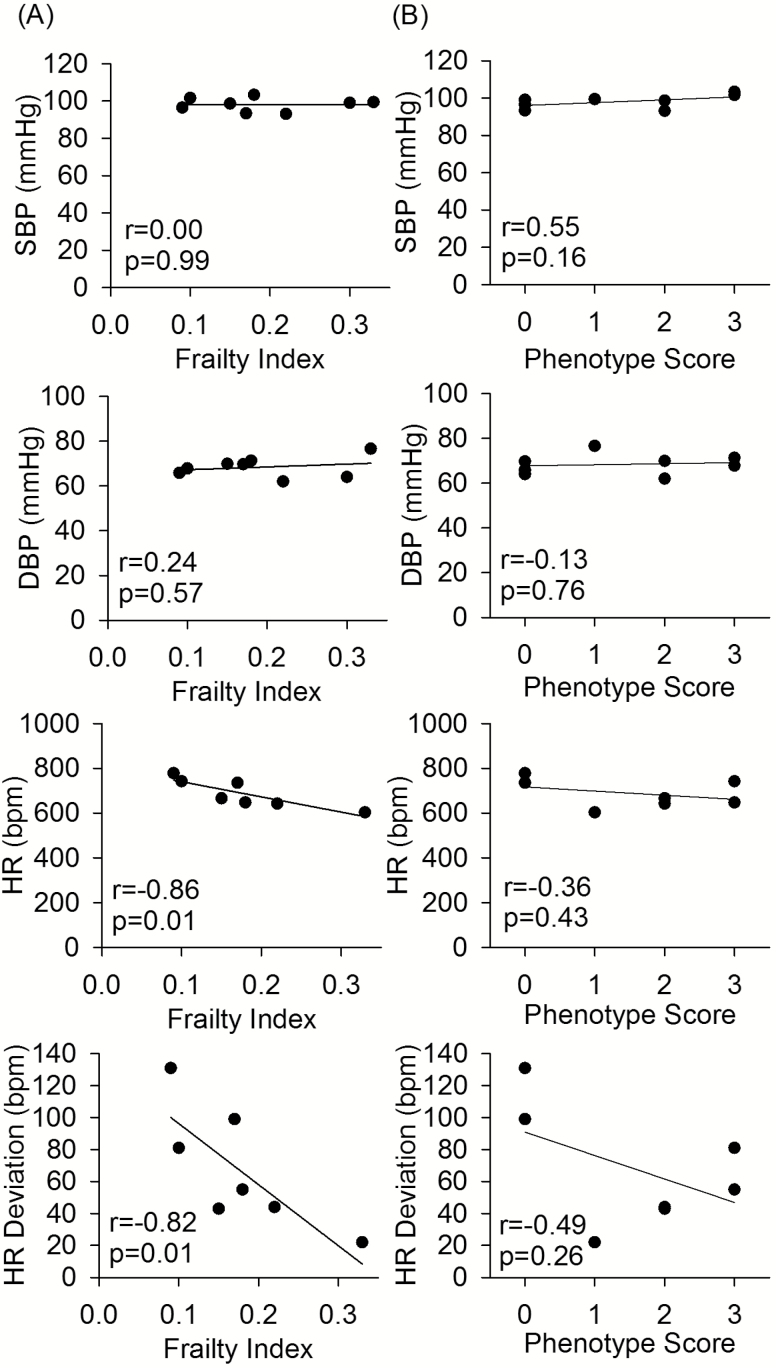
To investigate the association of each frailty assessment tool with age-related cardiovascular changes, the correlations between the frailty scores and systolic blood pressure, diastolic blood pressure, heart rate, and variation in heart rate were determined. There was no association between either mouse frailty phenotype score or mouse clinical FI and blood pressure. Mouse clinical FI scores were negatively correlated with heart rate and heart rate deviation (Figure 3A). Similar trends were seen for the frailty phenotype scores (Figure 3B), although this was not statistically significant.
Figure 3.
The correlation of (A) mouse clinical frailty index scores and (B) modified frailty phenotype scores, with systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and HR deviation, for old (99.6 ± 1.2 weeks) male C57BL/6 mice. Pearson correlation coefficient values (r) and p values are shown on each of the graphs. n = 8 for all BP graphs and n = 7 for HR graphs.
Mean values for the cardiovascular changes were also compared between frail and nonfrail mouse groups identified with the two frailty assessment tools. Heart rate and heart rate variance were significantly lower in frail mice compared to nonfrail mice as identified with the mouse clinical FI but not the phenotype assessment (Supplementary Table 3).
Discussion
In this study, we report for the first time on the relationship between the mouse clinical FI (16) and the mouse frailty phenotype assessment (17). We found that, as with the equivalent frailty assessment scales used in humans, these tools do not necessarily identify the same mice as frail. Increasing mouse clinical FI scores were correlated with low serum ALT, as well as decreased heart rate, and reduced heart rate variance.
The use of the original mouse frailty phenotype assessment in this study classified none of the mice as frail and only one mouse (2.8%) as prefrail. This may be because the mice were 3–4 months younger in the current study compared to the original study (17) (23–24 vs 27–28 months). This age difference represents an approximately 75% survival rate compared to a 50% survival rate in terms of the mouse life span (33) and would correspond to approximately 70–75 years old compared to 80–85 years old for humans (17,23). However, the original human frailty phenotype assessment identified 3%–10% of people as frail and up to 46% as prefrail even at ages of 65–75 years (5). The age difference or the larger sample size of the current study may also explain the reduced variance seen in the current data set compared to the original study (17). Modification of the frailty phenotype assessment to more closely resemble the clinical tool (5) identified 16.6% of the mice as frail. By comparison, 44.4% of mice were identified as frail with the mouse clinical FI. Interestingly, these numbers are similar to human studies that identified 6%–16% of older adults (70–85 years old) as frail with the phenotype and 22%–32% with the FI (65 years and older) (1,34). The agreement between the two assessment tools in our study was also reasonably similar to human studies, with 50% agreement seen in this mouse study (using the modified phenotype assessment) compared to 51% (7) and 38.2% (11) in human studies. The mouse studies in the current article seem to support the conclusion, as with the human studies, that the different frailty assessments identify different subpopulations as frail (13,34).
The mouse clinical FI was associated with the potential serum marker of poor prognosis, ALT. Low serum ALT has been linked to frailty, as assessed with the phenotype tool, in previous clinical studies (18). It may be that ALT is a surrogate marker for reduced liver size, changing morphology and generally reduced function that is associated with ageing (19). The mouse clinical FI was also associated with decreased heart rate and a decline in heart rate variability. Although some rodent and clinical studies have seen no difference in resting heart rate with age (22), other mouse studies have seen reduced heart rate with age (35). Reduced heart rate variance has been seen in human studies with aging (22) and frailty as assessed with the phenotype tool (36,37). A similar trend was seen for the mouse frailty phenotype assessment for these outcomes and perhaps with a larger sample size an association may be seen. The association of the mouse clinical FI with these changes previously seen in aging and frailty in humans, provides further support of the index as a validated measure of frailty in normal mouse aging, and a valuable research tool.
As with their equivalent human frailty scales, each of the mouse frailty assessment tools has advantages and limitations to its use in research. Both scales have been shown to detect changes in frailty with interventions (24,38), which is an important factor in their use in frailty research. Additionally, both assessments are noninvasive and can be performed at multiple time points in long-term studies. The clinical FI requires no specialized equipment and is very fast to perform (16). By contrast, the frailty phenotype assessment (17) requires some specialized equipment and time-consuming assessments but provides a cutoff to categorize a mouse as nonfrail or frail. The current study used different assessment protocols to identify certain criteria used in the frailty phenotype assessment, compared to the original study (17) (Table 1). However, the effect of this on the interpretation of results should be minimal as the overall outcomes of activity, grip strength, and walking speed were assessed in both studies and the results stratified within the different mouse cohorts to identify the worst performers. The adaptability of the criteria assessments to the available tools in each research laboratory would be an advantage of the phenotype assessment, enabling it to be a relevant and widely used tool. This flexibility is consistent with the use of available tools for analysis of the frailty phenotype in clinical studies. No mice were identified as frail by the original phenotype assessment in this old mouse cohort. The modification of the frailty phenotype assessment used in the current study, with the alignment of the cutoff values with the clinically used tool and the inclusion of a weight loss criterion as in humans, identified more mice as frail and prefrail. These modifications may increase the applicability and use of this tool in aging mouse studies. The mouse FI, as with the human FI, provides a continuous value for frailty, rather than a cut-off, which Cesari and colleagues (39) identify as a major strength. The current study did use cutoff points for the FI to create ordinal values that could be compared with the phenotype results. Cutoff points for the index have also been used in clinical FI studies (30–32), however more research is needed to confirm the appropriate cutoff points that may be used for the mouse clinical FI.
Another, more conceptual, difference between the two scales is that the phenotype assessment identifies mouse as frail based on their performance relative to the current cohort of old mice (17). The FI, however, compares to a young healthy mouse in determining if a factor is counted as a deficit (16). This is consistent with the equivalent clinical assessments but may explain some of the difference in frailty assessment with the two different scales.
A limitation of this study is a lack of direct adverse outcomes such as mortality or stress responses. Further studies are needed to explore the association of these factors with the two mouse frailty assessments. Another limitation of this study is the small group numbers for some assessments, as this was a secondary analysis of these data considering only control groups (main results of this study published in ref. (26)). The relationship between the two scales should be confirmed in a larger cohort of old mice.
In conclusion, this study found that, as with clinical frailty assessment scales, both the mouse frailty phenotype assessment and the mouse clinical FI have value in frailty research but predict and identify different populations of mice as frail. Care is needed in choosing and interpreting mouse frailty assessments and outcomes in research.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This study was funded by the Penney Ageing Research Unit, Royal North Shore Hospital, Australia. S.E.H. is supported by Canadian Institute for Health Research grant (MOP 126018). S.J.M. and R.C. are supported by the National Institute on Aging, National Institutes of Health. A.E.K. is supported by the Reynolds Postdoctoral Fellowship from the Department of Pharmacology, Dalhousie University.
Supplementary Material
Acknowledgments
The authors thank Caitlin Logan and Quinn Cretney-Ross for their assistance with open-field analysis for this paper.
References
- 1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Žugich J. The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi:10.1016/j.exger.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 4. Romero-Ortuno R. The frailty instrument for primary care of the survey of health, ageing and retirement in Europe predicts mortality similarly to a frailty index based on comprehensive geriatric assessment. Geriatr Gerontol Int. 2013;13:497–504. doi:10.1111/j.1447- 0594.2012.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3 [DOI] [PubMed] [Google Scholar]
- 6. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi:10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi:10.1111/jgs.12420. [DOI] [PubMed] [Google Scholar]
- 8. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460. doi:10.1016/S0047-6374(02)00082-9 [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi:10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 10. Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi:10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hogan DB, Freiheit EA, Strain LA, et al. Comparing frailty measures in their ability to predict adverse outcome among older residents of assisted living. BMC Geriatr. 2012;12:56. doi:10.1186/1471-2318-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi:10.1111/j.1532-5415.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi:10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 14. Howlett SE, Rockwood K. Ageing: develop models of frailty. Nature. 2014;512:253. doi:10.1038/512253d. [DOI] [PubMed] [Google Scholar]
- 15. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227. doi:10.1093/gerona/glr193. [DOI] [PubMed] [Google Scholar]
- 16. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi:10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69:1485–1491. doi:10.1093/gerona/glt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Couteur DG, Blyth FM, Creasey HM, et al. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci. 2010;65:712–717. doi:10.1093/gerona/glq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elinav E, Ackerman Z, Maaravi Y, Ben-Dov IZ, Ein-Mor E, Stessman J. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc. 2006;54:1719–1724. doi:10.1111/j.1532-5415.2006.00921.x. [DOI] [PubMed] [Google Scholar]
- 20. Schalk BW, Visser M, Deeg DJ, Bouter LM. Lower levels of serum albumin and total cholesterol and future decline in functional performance in older persons: the Longitudinal Aging Study Amsterdam. Age Ageing. 2004;33:266–272. doi:10.1093/ageing/afh073. [DOI] [PubMed] [Google Scholar]
- 21. Odden MC, Shlipak MG, Tager IB. Serum creatinine and functional limitation in elderly persons. J Gerontol A Biol Sci Med Sci. 2009;64:370–376. doi:10.1093/gerona/gln037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keller KM, Howlett SE. Sex differences in the biology and pathology of the aging heart. Can J Cardiol. 2016;32:1065–1073. doi:10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 23. Weon BM, Je JH. Trends in scale and shape of survival curves. Sci Rep. 2012;2:504. doi:10.1038/srep00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kane AE, Hilmer SN, Boyer D, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2016;71:333–339. doi:10.1093/gerona/glu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feridooni HA, Sun MH, Rockwood K, Howlett SE. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2015;70:686–693. doi:10.1093/gerona/glu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huizer-Pajkos A, Kane AE, Howlett SE, et al. Adverse geriatric outcomes secondary to polypharmacy in a mouse model: the influence of aging. J Gerontol A Biol Sci Med Sci. 2016;71:571–577. doi:10.1093/gerona/glv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009;27:2–4. doi:10.3791/1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bordoni A, Hrelia S, Lorenzini A, et al. Dual influence of aging and vitamin B6 deficiency on delta-6-desaturation of essential fatty acids in rat liver microsomes. Prostaglandins Leukot Essent Fatty Acids. 1998;58:417–420. [DOI] [PubMed] [Google Scholar]
- 29. Romero-Ortuno R. An alternative method for frailty index cut-off points to define frailty categories. Eur Geriatr Med. 2013;4:1–11. doi:10.1016/j.eurger.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockwood K, Song X, Mitnitski AB. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;138:487–494. doi:10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi:10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 32. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi:10.1186/s12916-014-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi:10.1093/gerona/54.11.B492 [DOI] [PubMed] [Google Scholar]
- 34. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi:10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 35. Xing S, Tsaih SW, Yuan R, et al. Genetic influence on electrocardiogram time intervals and heart rate in aging mice. Am J Physiol Heart Circ Physiol. 2009;296:H1907–H1913. doi:10.1152/ajpheart.00681.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaves PH, Varadhan R, Lipsitz LA, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc. 2008;56:1698–1703. doi:10.1111/ j.1532-5415.2008.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varadhan R, Chaves PH, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64:682–687. doi:10.1093/gerona/glp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70:1045–1058. doi:10.1093/gerona/glu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi:10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.