Abstract
The loss of muscle strength with age has been studied from the perspective of a decline in muscle mass and neuromuscular junction (NMJ) stability. A third potential factor is force transmission. The purpose of this study was to determine the changes in the force transfer apparatus within aging muscle and the impact on membrane integrity and NMJ stability. We measured an age-related loss of dystrophin protein that was greatest in the flexor muscles. The loss of dystrophin protein occurred despite a twofold increase in dystrophin mRNA. Importantly, this disparity could be explained by the four- to fivefold upregulation of the dystromir miR-31. To compensate for the loss of dystrophin protein, aged muscle contained increased α-sarcoglycan, syntrophin, sarcospan, laminin, β1-integrin, desmuslin, and the Z-line proteins α-actinin and desmin. In spite of the adaptive increase in other force transfer proteins, over the 48 hours following lengthening contractions, the old muscles showed more signs of impaired membrane integrity (fourfold increase in immunoglobulin G-positive fibers and 70% greater dysferlin mRNA) and NMJ instability (14- to 96-fold increases in Runx1, AchRδ, and myogenin mRNA). Overall, these data suggest that age-dependent alterations in dystrophin leave the muscle membrane and NMJ more susceptible to contraction-induced damage even before changes in muscle mass are obvious.
Keywords: Force transmission, Skeletal muscle, Dystrophin-associated glycoprotein complex, Aging, Regeneration
On average, individuals lose 45% of their muscle mass between their mid 20s and 80s (1). This loss of muscle mass in the absence of disease is known as sarcopenia (2). The decline in muscle mass is accompanied by a rapid loss in muscle strength (2). Furthermore and of greater significance, the loss of strength has been identified as a predictor of all-cause mortality in healthy men (3). Neural activation also declines with aging. However, Thompson and Brown (4) demonstrated that single muscle fibers produced less specific force at 24 months of age, a time when neither cross-sectional area (CSA) nor innervation was affected. This indicates that components other than muscle mass and neural activity are impaired with aging.
One component of aging muscle that has received relatively little attention is the cytoskeletal network that is necessary for force transfer. Using frog muscle, Street (5) elegantly demonstrated that the cytoskeleton of muscle transmits force both along the length of each muscle fiber (longitudinally) and from the center to the outside of the fiber (laterally). The longitudinal force transfer apparatus includes a myriad of proteins surrounding the Z-line that connect thin filaments of adjacent sarcomeres (6). These proteins form part of the series elastic component of the fiber and are associated with the rate of force development (6). By contrast, the lateral force transfer apparatus passes load perpendicular to the line of force, from the center of the muscle fiber to the overlying connective tissue and extracellular matrix (ECM). Since these proteins link adjacent fibers through the ECM, their loss is associated with a propensity for membrane injury and neuromuscular junction (NMJ) instability (5,7).
NMJ instability describes the process where established contacts between the presynaptic and postsynaptic membranes become unstable, resulting in either transient or permanent denervation. Transient periods of denervation increase the expression of Runx1 (also called AML1 (8)), a transcription factor whose expression is inhibited by electrical activity and functions to promote reinnervation and prevent muscle wasting (9)). A decrease in electrical activity also removes the transcriptional inhibition on the myogenic factor myogenin (10,11). One of the transcriptional targets of myogenin is the delta subunit of the acetylcholine receptor (AchRδ) (12,13). Therefore, the expression of Runx1, myogenin, and the AchRδ subunit genes can be used to assess NMJ instability.
Dystrophin and the dystrophin-associated glycoprotein complex (DGC) proteins are central to lateral force transmission, promoting stable and strong interactions between the cytoskeleton, sarcolemma, and ECM (14). As such, the loss of dystrophin is associated with an increase in damage to the sarcolemma (15) as well as increased contraction-induced NMJ instability (16). In fact, Pratt and colleagues (16) showed that neuromuscular failure after contraction-induced muscle injury increased threefold in mdx mice that lack dystrophin, whereas it was not changed in wild type animals, suggesting that NMJ stability is directly influenced by dystrophin levels. Ramaswamy and colleagues (7) beautifully demonstrated that in senescent muscle dystrophin and other lateral force transmission proteins are lost. The low dystrophin in these very old rats resulted in decreased lateral force transmission, increased sarcomere instability, and contraction-induced muscle injury (7). Essentially, these very old rats showed signs of muscular dystrophy. Over time, the repeated contraction-induced injury and repair in dystrophic muscle results in a progressive loss of muscle mass and strength that ultimately leads to fibrosis and impacts locomotion and respiration (17). Whether a loss of dystrophin could contribute to sarcopenia remains to be determined.
When one protein within the DGC is lost, other force transmission proteins increase in an attempt to compensate. For example, in the mdx mouse model of Duchene’s muscular dystrophy, utrophin (18) and β1-integrins (19,20) are upregulated and can partially compensate for the loss of dystrophin. Crossing the mdx mice with either utrophin or β1-integrin, knockout mice produces a more severe muscular dystrophy, indicating that compensation can decrease the impact of the loss of dystrophin (18,20). β1-Integrins are a central component of the focal adhesion complex, a family of cytoskeleton proteins that work alongside the DGC to transmit force laterally. In skeletal muscle, the integrins within the focal adhesion complex function as cell surface adhesion receptors that can protect the sarcolemma from injury (19). The predominant integrin in adult skeletal muscle is α7β1, with the α7 subunit responsible for binding to laminin within the basal lamina and the β1 subunit involved with linking to actin through various subsarcolemmal proteins such as α-actinin, desmin, and paxillin (for a detailed review see ref. (21)). The β1-integrins are expressed in mammalian muscle within costameres at the sarcolemmal membrane and at the NMJ. The integrin complexes are altered during muscle contraction and hypertrophy and appear to play a role in attenuating contraction-induced muscle injury (19,22).
Even though Ramaswamy and colleagues demonstrated that extremely old animal show a loss of dystrophin and other lateral force transfer proteins, how and when this drop of dystrophin occurs remains unknown. Recently, Cacchiarelli and colleagues (23) discovered a microRNA (miRNA) in dystrophic muscles that could specifically target dystrophin mRNA and prevent its translation through miRNA silencing. These authors elegantly showed that miR-31 could bind to the 3′-untranslated region of dystrophin and prevent its translation (23). Since the initial discovery, at least two other miRNAs, the so-called dystromirs (miR-146b and miR-374), have been discovered (24). However, whether these miRNAs are affected by aging and what effect they would have on the muscle remains to be determined.
In the current work, we hypothesized that: (1) the expression of cytoskeleton proteins involved in force transmission would be reduced in presarcopenic muscle (ie, before muscle wasting), (2) the loss of dystrophin with aging would be the result of an increase in dystromir levels, and (3) a reduction in dystrophin would cause impaired lateral force transmission making aged muscles more prone to membrane damage and NMJ instability.
Materials and Methods
Animals/Ethical Approval
Adult (9 months) and old (29 months) male Fischer 344 Brown Norway rats were obtained from the National Institute of Aging. The Fischer Brown Norway rat is a well-established rodent model for the study of aging due to their increased life span and lower incidence of disease pathologies (25). These ages were selected since Fischer Brown Norway animals become muscularly mature at 9 months and begin to show signs of sarcopenia at 30 months (26). Therefore, the animals in this study were at their muscular peak (9 months) and presarcopenic. Once in the facility, rats were allowed to acclimatize in their cages for at least 1 week prior to testing. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Lengthening Contraction Protocol
To determine the propensity for muscle injury, a subset of rats underwent a bout of acute unilateral resistance exercise using a protocol described previously (27). Briefly, rats were chemically restrained (2.5% isoflurane) and the right sciatic nerve was stimulated (100 Hz, 3–6 volts, 1ms pulse, 9ms delay) for 10 sets of 6 repetitions (repetition length = 2s). In this model, muscle fibers in the extensor digitorum longus (EDL) and tibialis anterior (TA) perform high-force lengthening contractions. Following exercise, animals were given an analgesic (buprenorphine, 0.1mg/kg) and returned to their cages for 6, 18, or 48 hours.
Muscle Collection
Rats were anesthetized with 2.5% inhaled isoflurane, and the following muscles were excised from both hind limbs: EDL, medial gastrocnemius (MG), plantaris (PLN), TA, vastus lateralis (VL), and semimembranosus (SM). For the lengthening contraction protocol, EDL and TA muscles were collected. All muscles were frozen in liquid nitrogen for biochemical analyses, except the right PLN and TA and both EDL muscles from the 48-hour post exercise group, which were pinned on cork at a length approximating Lo and frozen in liquid nitrogen-cooled isopentane for histological analyses. Soleus muscles were harvested as well for the determination of wet weight.
Immunohistochemistry
Serial cross sections (10 μm) were cut from the TA, EDL, and PLN muscles using a Leica CM 3050S cryostat (Leica Microsystems). EDL muscle sections were fixed in cold acetone for 5 minutes at −20°C, followed by 3- and 5-minute phosphate-buffered saline washes. Sections were then incubated with Alexa Fluor® 488 conjugated goat anti-rat immunoglobulin G (IgG; [H+L]; 1:100, Life Technologies) for 1 hour at room temperature (RT). After 3- and 5-minute phosphate-buffered saline washes, slides were coverslipped using ProLong Gold Antifade reagent with DAPI (Life Technologies). Slides were imaged on a Zeiss Axio Imager.M1 fluorescent microscope using the EC Plan-Neofluar 10× objective. For comparative analysis, exposure length remained fixed for all samples. Images were analyzed using FIJI software. Five to six animals were analyzed per age, for both control (nonstimulated contralateral leg) and stimulated EDL muscles. Blind analysis was performed for the number of IgG-positive fibers (those showing IgG staining in the cytoplasm) as an indicator of muscle fiber damage (28).
Fiber Type-Specific CSA
To determine fiber type-specific CSA, TA and PLN muscle sections were fixed in cold acetone for 5 minutes at −20°C. Between each step, sections underwent 3- and 5-minute phosphate-buffered saline washes with 0.1% Tween-20. Sections were blocked in 5% normal goat serum in phosphate-buffered saline washes with 0.1% Tween-20 (blocking buffer) for 30 minutes at RT, then incubated in primary antibody overnight at 4°C. BA-F8 (slow type Mm, IgG2B), SC-71 (myosin heavy chain 2A, Mm, IgG1), and BF-F3 (myosin heavy chain 2B, Mm, immunoglobulin M) were diluted 1:250 in blocking buffer. A polyclonal laminin antibody (1:500, Rb) was included for the determination of CSA. After incubation in primary antibody, sections were incubated in secondary antibody 30 minutes at RT and then coverslipped using ProLong Gold Antifade reagent (Life Technologies). For simultaneous detection of multiple mouse primary antibodies, fluorescently conjugated goat-anti-mouse immunoglobulin-specific secondary antibodies were used (Alexa Fluor® 350, 488, and 555, Life Technologies). Goat-anti-rabbit AlexaFluor® 647 secondary was used to detect laminin. Anti-myosin heavy chain antibodies were purchased from the Developmental Studies Hybridoma Bank (Iowa City, Iowa) and anti-laminin antibody was purchased from Sigma Aldrich (St Louis, MO). Slides were imaged using a Zeiss Axio Imager.M1 fluorescent microscope using the EC Plan-Neofluar 10× objective (Jena, Germany) and analyzed using Axiovision software. Fibers from five to six images of a single section were analyzed per muscle, per animal.
Western Blotting
Frozen TA, MG, SM, and VL muscles were homogenized in sucrose lysis buffer (50mM Tris pH 7.5, 250mM sucrose, 1mM ethylenediaminetetraacetic acid, 1mM ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1% Triton X 100, and protease inhibitors). The supernatant was collected following centrifugation at 10,000g for 5 minutes and protein concentrations were determined in triplicate using the DC protein assay (Bio-Rad). Twenty micrograms of protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 4–20% Criterion Tris, glycine eXtended gels (Bio-Rad) and transferred to nitrocellulose membrane for 2 hours. Membranes were blocked in 1% fish skin gelatin in Tris-buffered saline with 0.1% Tween-20 for 1 hour and then probed with primary antibody overnight at 4°C. The next day, membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies at 1:10,000 for one hour at RT. Immobilon Western Chemiluminescent horseradish peroxidase substrate (Millipore) was then applied to the membranes for protein band visualization by chemiluminescence. Image acquisition and band quantification was performed using the ChemiDoc™ MP System and Image Lab 5.0 software (Bio-Rad). Total protein staining of the membrane (via Ponceau) was used as the normalization control (whole lane) for all blots. The following antibodies were used in this study at a concentration of 1:1000: dystrophin (Santa Cruz, Cat no. 365954), β-dystroglycan (Hybridoma Bank, Cat no. MANDAG2), α-sarcoglycan (Hybridoma BankSanta Cruz, Cat no. IVD3 A9), laminin-2α (Santa Cruz, Cat no. 20142), β1-integrin (Santa Cruz, Cat no. 6622), desmin (Hybridoma Bank, Cat no. D76), desmuslin (Santa Cruz, Cat no. 49651), α-actinin (Cell Signaling, Cat no. 6487), muscle ankryin repeat protein (Santa Cruz, Cat no. 138111), cardiac ankryin repeat protein (Santa Cruz, Cat no. 30181), and muscle LIN-11, ISL-1 and MEC-3 domain (LIM) protein (Santa Cruz, Cat no.166930). Both syntrophin and sarcospan cross-reacted with the antibody for dystrophin (Cat no. 365954) and were determined by molecular weight.
Titin Isoform and Nebulin Determination
The detection of titin isoforms and nebulin in adult and old MG and TA muscles was performed based on previously described procedures (29). Briefly, frozen tissue samples were homogenized in sample buffer (8M urea, 2M thiourea, 3% sodium docyl sulfate, 75mM dithiothreitol, 0.03% bromophenol blue, and 0.05M Tris-hydrogen chloride [pH 6.8]). Samples were heated at 60°C for 10 minutes, then vortexed, and subsequently centrifuged for 5 minutes at 13,200g. The supernatant was removed from the tubes and protein concentration was determined by 660nm protein assay (Thermo Scientific). Approximately 80 μg of protein was loaded into the gel. Rat cardiac muscle was prepared in the same way as skeletal muscle and loaded as a molecular weight ladder for titin bands (29). A 15-cm vertical gel system was used, with the gel being comprised of a 12% sodium dodecyl sulfate–polyacrylamide plug, 25mL of a 1% agarose and 5mL of a 2% agarose stacking gel. The lower chamber buffer contained 4L of 50mM Tris-base, 0.384M glycine, and 0.1% sodium dodecyl sulfate and the upper buffer contained 600mL of the same buffer plus 10mM 2-mercaptoethanol. The gel was run at 4°C using a 15-mA constant current for 5 hours, with optimum titin detection occurring when the dye front moved to the bottom of the acrylamide plug.
Prior to silver staining, the glass plates were removed, and the acrylamide plug and wells were cut off and discarded. Silver staining was performed per manufacturer’s instructions (Silver stain plus, Bio-Rad). The gels were fixed with 50% methanol, 10% acetic acid, 10% fixative enhancer, and 30% distilled water for 20 minutes. Following fixation, gels were rinsed in distilled water for 20 minutes and silver staining was performed until desired staining intensity (~15 minutes) was reached. A 5% acetic acid solution was added for 15 minutes to stop the reaction and subsequently the gels were rinsed with distilled water prior to image capture and analysis through the ChemiDoc™ MP System and Image Lab 5.0 software (Bio-Rad).
RNA Analysis
Prior to RNA isolation, aliquots of frozen muscle powder were weighed in order to calculate RNA per milligram of wet muscle tissue. Total RNA was extracted with Tri Reagent® Solution (Ambion Inc. Cat no. AM9738) according to manufacturer’s instructions. Following isolation, RNA concentrations were determined spectrophotometrically (Epoch Microplate Spectrophotometer, BioTek Instruments Inc.). For micro RNA analysis, one microgram of RNA from the MG and TA muscles was DNase treated (Invitrogen® Cat no. 18068-015) and then reverse transcribed using the Taqman® MiroRNA Reverse Transcription Kit (Applied Biosystems® Cat no. 4366596). Following reverse transcription, cDNA was preamplified using TaqMan® PreAmp Mastermix (Applied Biosystems® Cat no. 4488593). Reverse transcription, preamplification, and real-time quatitative polymerase chain reaction (RT-qPCR) were performed according to the manufacturer’s instructions (Applied Biosystems® publication part no. 4465407). qPCR was performed using TaqMan®–Universal MasterMix II, no UNG (Applied Biosystems® Cat no. 4440040) with 10 μL reaction volumes on a Bio-Rad CFX384 Touch™ RT PCR detection system. Gene expression was calculated using the delta, delta threshold cycle method using a TaqMan® miRNA Control Assay, small nucleolar RNA U87 (Assay ID #001712) as a housekeeping gene. Target and tested housekeeping genes for rat miRNA’s included TaqMan® miRNA’s: miR-31 (Assay ID #000185), miR-146b (Assay ID #002755), miR-374 (Assay ID #001319), U87 (Assay ID #001712). All miRNA data are expressed relative to adult control baseline time point, group average ± standard error mean.
For mRNA quantification, 1 µg of RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Cat no. 4368814) with thermal cycling according to the manufacturer’s instructions. cDNA was stored at −80°C and diluted 1:10 in nuclease-free water prior to qPCR analysis (SYBR Green Supermix from Bio-Rad Laboratories, Cat no. 172–5121; 7900HT Fast Real-Time PCR System, Thermo Scientific). Primer sequences are available on request. Gene expression was calculated using the delta threshold cycle method and then normalized to the amount of RNA per milligram of tissue used (30). Normalization was done this way since there was not a reference gene that remained constant throughout the exercise recovery time course, as determined using the reference gene panel R384 (BioRad, Benecia, CA).
Statistical Analysis
Results are presented as mean ± standard error of the mean. The percentage of injured fibers was analyzed by Student’s t test. The remaining data were analyzed using two-way analysis of variance using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Tukey’s post hoc analysis was used to determine the differences when interactions existed. Statistical significance was set at p < .05.
Results
29-Month-Old FBN Rats Are Presarcopenic
Muscles of the old animals tended to weigh less than those of the adult controls; however, there were no significant decreases in muscle mass (TA adult = 0.779±0.043, Old = 0.741±0.059; MG adult = 0.741±0.059, Old = 0.872±0.064; Supplementary Table S1). Similarly, fiber-type specific CSA was significantly lower in only the type IIb muscle fibers of the PLN muscle (Supplementary Table S2). Together, these data support the idea that the 29-month-old FBN rats were presarcopenic; that is, changes identified in muscles at this point could predispose the muscle to subsequent sarcopenia.
Effect of Aging on Dystrophin
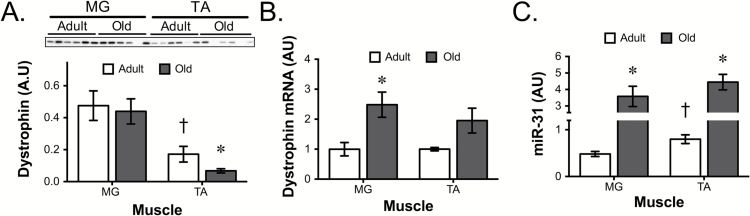
Since impaired lateral force transmission, accompanied by decreases in dystrophin, has previously been observed in very old rats (7), we determined the influence of aging on dystrophin protein. Even in adult muscle, the amount of dystrophin protein varied between muscles. There was significantly more dystrophin in the MG than the TA muscle (Figure 1A). Aged muscle demonstrated a further significant reduction in dystrophin protein in the TA muscle. The differences in dystrophin protein across muscles or ages could not be explained by differences in mRNA. In the adult MG and TA, there were equivalent amounts of dystrophin mRNA and in both muscles this increased with age (Figure 1B), even though protein was decreasing in the TA muscle with age. A possible explanation for the disconnect between dystrophin protein and mRNA was the expression of the dystromir miR-31. miR-31 was slightly higher in the adult TA muscle and increased four- to fivefold in both the aged muscles (Figure 1C). The other known dystromirs, miR-146b and miR-374, were not different across muscles or ages (Supplemental Figure 1).
Figure 1.
Dystrophin is posttranscriptionally controlled with age. Quantification of (A) dystrophin protein, (B) dystrophin mRNA, and (C) miR-31 levels in adult and old MG and TA muscles. Total protein for western blot analysis was determined by ponceau stain and was used to normalize protein expression. *p < .05 old vs. adult muscle. †p < .05 between adult muscles. Data presented as mean ± SEM. N=5/6 per group. MG = medial gastrocnemius; TA = tibialis anterior; SEM = standard error of mean.
Other Lateral Force Transfer Proteins
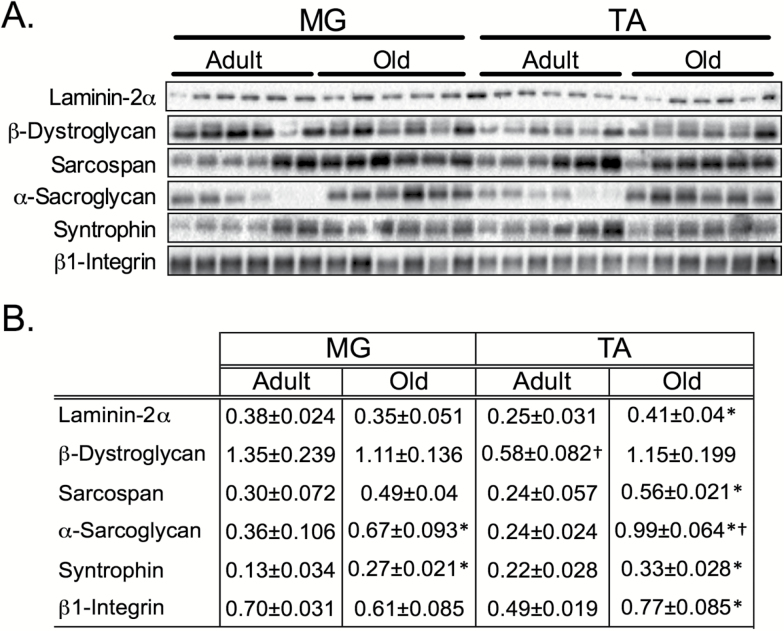
The loss of dystrophin protein in aged TA muscle was accompanied by a significant increase in the dystrophin-associated glycoprotein complex-associated proteins laminin-2α, β-dystroglycan, sarcospan, α-sarcoglycan, and syntrophin (Figure 2). These changes paralleled increases in the integrin complex subunit, β1-integrin (Figure 2). Most of these proteins showed a pattern that was the inverse of dystrophin, being higher in the old TA muscle and not changing in the MG. However, sarcospan, α-sarcoglycan, and syntrophin were elevated in the old MG muscle as well.
Figure 2.
DGC complex and focal adhesion protein levels in adult and old gastrocnemius (MG) and tibialis anterior (TA) muscles. (A) Representative western blots for laminin-2α, the DGC complex, and β1-integrin proteins from adult and old extensor (MG) and flexor (TA) muscles. (B) Quantification of total protein for western blot analysis was normalized to ponceau stain. *p < .05 old vs. adult muscle. †p < .05 between adult muscles. Data presented as mean ± SEM. N = 6 per group. DGC = dystrophin-associated glycoprotein; MG = medial gastrocnemius; TA = tibialis anterior.
Since differences were observed between the flexor (TA) and the extensor (MG) muscles of the distal hind limb, we extended the study to include the flexor (SM) and extensor (VL) muscles of the thigh. The flexor/extensor difference was also evident in the thigh, where dystrophin levels were significantly lower in the aged SM muscle (Supplemental Figure 2A), with no significant differences observed between adult and aged VL muscle or adult VL versus adult SM muscle (Supplemental Figure 2A). β1-Intergrin and laminin-2α levels showed the same inverse pattern in the VL and SM muscles with age, tending to start lower in the flexor and increasing in this muscle with age (Supplemental Figure 2B and C). β-Dystroglycan tended to decrease in both the aged VL and SM muscles (Supplemental Figure 2D), whereas α-sarcoglycan (Supplemental Figure 2E) and syntrophin (Supplemental Figure 2F) were significantly increased in the old, regardless of muscle.
Z-line Proteins
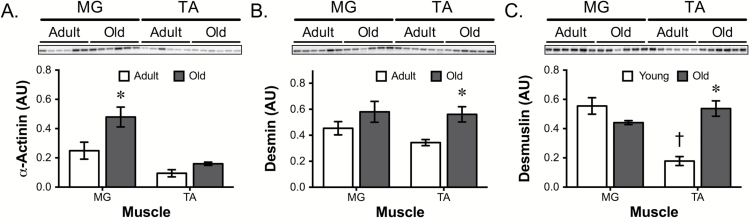
Other proteins that are involved in longitudinal force transfer are those cytoskeleton proteins that provide structural links between the thin filaments of adjacent sarcomeres (31–33). Of these proteins, we investigated the effect of aging on desmin, desmuslin (also known as synemin), and α-actinin. α-Actinin levels in aged MG muscle were double the levels of adult (Figure 3A). The protein pattern for desmin and desmuslin was similar to laminin-2α, β-dystroglycan, and β1-integrin (Figure 3B and C), somewhat lower in the adult TA and elevated, especially in the TA, with age. Analysis of the VL and SM muscles revealed that α-actinin increased (although not significantly) in the aged muscle compared with adult animals (Supplemental Figure 3A). In both VL and SM muscles, the same flexor/extensor pattern was observed for desmin (Supplemental Figure 3B). Desmuslin displayed a decrease in the aged VL muscle, which was similar to the MG muscle (Supplemental Figure 3C), whereas the SM levels were the same between adult and old muscles, in contrast to the significant increase observed in the aged TA muscle.
Figure 3.
Alterations in α-actinin, desmin, and desmuslin in aged flexor and extensor muscles. Representative western blots for (A) α-actinin, (B) desmin, and (C) desmuslin in adult and old MG and TA muscles. Total protein for western blot analysis was determined by ponceau stain and was used to normalize protein expression. *p < .05 old vs. adult muscle. †p < .05 between adult muscles. Data presented as mean ± SEM. N = 5/6 per group. MG = medial gastrocnemius; TA = tibialis anterior.
Alterations in Longitudinal Force Transfer with Aging
Since there was a significant change in lateral force transfer proteins, we also measured the levels of proteins involved in longitudinal force transmission. Titin content was increased in the TA of old rats compared with their adult counterparts (Supplemental Figure 4A). Furthermore, aged muscle contained more of the longer isoform (N2AL) compared with adult counterparts where the shorter isoform predominated (N2AS; Supplemental Figure 4B). In terms of nebulin content, no significant differences were observed between ages or muscles (Supplemental Figure 4C). Muscle LIM protein and muscle ankryin repeat protein levels were not changed with aging in either the MG or TA muscle (Supplemental Figure 4D and E). However, cardiac ankryin repeat protein levels were decreased in aged MG muscle compared with adult counterparts (Supplemental Figure 4F).
Susceptibility for Membrane Injury and NMJ Instability
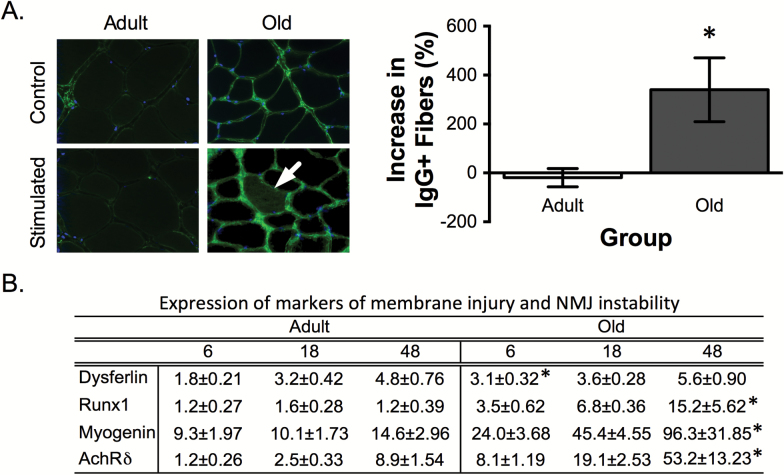
The decrease of dystrophin levels in the aged TA muscle suggested that old muscles might be more susceptible to contraction-induced membrane (15) and NMJ injury (16). To test this hypothesis, the ankle flexor muscles underwent 60 lengthening contractions as part of a 20-minute exercise bout. In the exercised TA muscles, we determined the expression of the membrane repair enzyme dysferlin (34) and markers of denervation including Runx1 (8), the AchRδ, and the myogenic factor myogenin (11). In addition, we also established the percent of fibers that showed membrane injury (staining positive for IgG within the muscle fiber) in the stimulated and contralateral control EDL muscles 48 hours after stimulation (Figure 4). Consistent with the hypothesis that lower dystrophin levels make the older muscle fibers more prone to membrane injury, aged TA muscles showed 70% greater increase in dysferlin 6 hours after eccentric loading and aged EDL muscles demonstrated a fourfold increase in IgG-positive fibers, whereas there was no change in IgG-positive fibers in adult muscles (Figure 4). The markers of NMJ instability were similarly affected following eccentric loading. Runx1, AchRδ, and myogenin all progressively increased in the old TA muscle following lengthening contractions showing a 15.2-, 53.2-, and 96.3-fold increase, respectively at 48 hours (Figure 4).
Figure 4.
Muscle susceptibility to contraction-induced membrane injury and NMJ instability in adult and old muscle. Visible membrane damage (IgG-positive fibers) in EDL muscles post 48h after contraction-induced muscle injury. White arrow on the ×40 image indicates IgG-positive muscle fibers. The adjacent panel shows the quantification of the percent increase in injured fibers (ie, number of injured fibers in RT leg − number of injured fibers in the unstimulated LT leg/number of injured fibers in the unstimulated LT leg). Expression of a marker of membrane injury, dysferlin, and markers of denervation including, Runx1, myogenin, and AchRδ 6, 18, and 48 hours post exercise. *p < .05 vs. adult muscle. Data are represented as mean ± SEM. N = 5/6 per group. IgG = immunoglobulin G; EDL = extensor digitorum longus; AchRδ = acetylcholine receptor δ-subunit; RT = right; LT = left.
Discussion
During the aging process, skeletal muscle undergoes a rapid decline in strength that precedes the reduction in mass and neural activation (4). Here we provide evidence of a posttranscriptional decrease in the cytoskeletal protein dystrophin that begins before the onset of sarcopenia in a muscle-specific manner. This decrease in dystrophin protein is accompanied by a four- to fivefold increase in the dystromir miR-31 that is known to prevent the translation of dystrophin mRNA into protein (23). Unlike in senescent muscle, where other force transfer proteins are also lost (7,35), presarcopenic muscle appears to show a compensatory increase in many lateral force transmission, transitional, and z-line proteins. In spite of this increase in other force transmission proteins, old muscle, like the muscle of dystrophic animals, is more susceptible to contraction-induced injury of the sarcolemma and NMJ. These data suggest that old muscle is similar in many ways to dystrophic muscle.
Ramaswamy and colleagues (7) highlighted that the loss of dystrophin with age resulted in impaired lateral force transmission. Interestingly, in their 34- to 35-month old rats they also observed a decrease in other force transfer proteins such as β-dystroglycan. Similarly, in cachexic human muscle, Taub and colleagues (35) found a drop in all of the lateral force transmission proteins. By contrast, in presarcopenic rats (29 vs. 34- to 35-month old), we demonstrate a muscle-specific reduction in dystrophin occurred concomitant with increases in other components of the dystrophin-associated glycoprotein (such as β-dystroglycan, α-sarcoglycan, and sarcospan) and the focal adhesion (β1-integrin and laminin-2α) complexes. We hypothesize that these proteins initially increase in an attempt to compensate for the loss of dystrophin. However, as muscle loss continues, other lateral transmission proteins are lost as well. The increase in β1-integrin is seen in both dystrophic muscle (36), and in very old rats (7), suggesting that this adaptation is maintained as wasting progresses. Further, the compensatory increase in β1-integrin when dystrophin is low is functionally important, as it is known to slow the progression of muscular dystrophy (20). Together, these data suggest that a muscle-specific loss of dystrophin is partially compensated for through increases in β1-integrin, laminin-2α, β-dystroglycan, sarcospan, α-sarcoglycan, syntrophin, desmuslin, desmin, and α-actinin. Interestingly, this line of proteins leads all the way from the ECM to the thick and thin filaments and suggests that the upregulation of these proteins occurs to strengthen the mechanical link between the ECM, sarcolemma, and contractile proteins (7,37,38). Therefore, our data suggest that the age-associated loss of dystrophin results in a compensatory increase in other members of the dystrophin-associated and focal adhesion complexes in an effort to maintain lateral force transmission and prevent injury (19,39). However, a more extensive time course that tracks these proteins over time is needed to completely understand this process.
Even though there is an attempt to compensate for the decrease in dystrophin with age, as with muscular dystrophy, we demonstrate impaired membrane integrity and NMJ instability. Insufficient dystrophin protein is well-known to leave the sarcolemma prone to injury (15,40). Following sarcolemmal wounding, dysferlin interacts with the lipid- and calcium-binding annexins and the E3 ligase MG53 to seal the membrane and prevent further secondary injury (41). Both dysferlin and MG53 are upregulated in muscular dystrophy (42). Here, we show that dysferlin mRNA goes up following eccentric loading in both adult and old animals, with old animals showing a 70% greater increase in the stimulated leg at the 6-hour time point. Consistent with an increase in membrane wounding in the older animals, we noted a significant increase in fibers that stained IgG positive in the old animals 48 hours after eccentric loading. Increased mononuclear cell infiltration is an indicator of either increased membrane injury or impaired membrane repair (43). Even though we hypothesize that dysferlin and IgG-positive fibers are indicators of increased sarcolemmal injury, we provide no data to definitively support that hypothesis.
Another hallmark of both aged and dystrophic muscle is denervation (44), and here our data are more definitive. Contraction-induced injury to the quadriceps of mdx mouse caused significant changes in NMJ morphology, and neuromuscular transmission, whereas the same lengthening contraction protocol had no effect on the NMJs in wild type animals (16). Interestingly, in healthy young muscle, eccentric loading results in an increase in size of AchR clusters (45). Since large clusters of AchRs require the presence of dystrophin (46), it is not surprising that this would not occur in old muscles after eccentric loading. Instead, old muscle shows NMJ instability in the form of greater expression of indicators of denervation (15.2- and 96.3-fold 48 hours after lengthening contractions) in the TA muscle of old animals but not adult animals. These data suggest that the age-associated loss of dystrophin in the TA muscle makes the muscle prone to NMJ instability. Interestingly, Pannérec and her colleagues recently demonstrated that sarcopenia did not progress uniformly (47). In their case, they found that some muscles were prone to sarcopenia, whereas others were resistant. The difference that they identified on the protected muscles was an intact neuromuscular system. Interestingly, one of the genes they identified as being up in muscle that would rapidly develop sarcopenia was dystrophin (47). We also observe an increase in dystrophin mRNA in aged muscles where dystrophin protein is being lost. This raises the possibility that the lack of dystrophin drives increased contraction-induced neuromuscular instability leading to impaired innervation and sarcopenia.
The possibility that increased susceptibility to contraction-induced muscle injury could underlie muscle loss with age was first proposed by Holloszy and colleagues (48). In muscle wasting conditions, such as cancer cachexia (49), and myopathies associated with diabetes and heart failure (50), a similar loss of dystrophin is evident. This suggests that the loss of dystrophin and the subsequent increased susceptibility to membrane injury and neuromuscular instability might be central to all muscle wasting conditions. According to this paradigm, chronic muscle injury would lead to repeated cycles of injury and repair that eventually leads to the substitution of myofibrillar protein with nonfunctional fibrotic tissues, as observed in severe muscular dystrophies (51). In fact, a similar increase in nonfunctional fibrotic tissues has been observed in aged muscle (52).
One of the more novel findings in this paper is that the level of dystrophin protein in muscle is regulated in a posttranscriptional manner. Cacchiarelli and colleagues (23) were the first to show that there were specific miRNAs that could target and decrease dystrophin translation through miRNA silencing. Since then, this finding has been confirmed and extended by Fiorillo and colleagues (24) who showed that there were other miRNAs that could similarly affect dystrophin translation. These miRNAs have now been termed dystromirs for their ability to modify dystrophin protein levels. Here, for the first time, we show that miR-31, but not the other dystromirs miR-146b and 374, is differentially regulated in adult muscle and transcriptionally upregulated with age. This finding is significant because it could provide a novel target for the prevention of sarcopenia.
In both of the aged flexor muscles that were studied (tibialis anterior and semimembranosus), we observed less dystrophin compared with their adult counterparts. The fact that age-related differences tended to be smaller or nonexistent in the more frequently loaded extensor muscles suggests that the load placed upon the muscle may play a role in the maintenance of dystrophin levels. Rice and colleagues (53) also examined dystrophin levels with age in the extensor (soleus) and flexor (EDL) muscles. These authors reported no change in dystrophin in the soleus muscle at 30 months. However, by 36 months of age, dystrophin levels fell in the soleus, possibly reflecting the drop in locomotor activity that occurs with increasing age (54). Interestingly, they did not observe a decrease in dystrophin in the EDL muscle, which is functionally similar to the TA, at 30 months of age. Further, the authors (53) found an increase in dystrophin in the EDL at 36 months old. The differences between studies could reflect the different antibodies used or the fact that direct comparisons between muscles were not made in the Rice study. Either way, the differences need to be clarified through further work in the area. Even though loading history seems to play a role in dystrophin levels, neither resistance exercise training for 8 (55) or 16 (56) weeks nor endurance training for 12 weeks (57) was sufficient to increase dystrophin levels in young men. However, all of these data were collected in a primary extensor muscle (VL), which may be less sensitive to the increase in loading. Further, Kosek and Bamman (56) showed a trend for an increase in dystrophin following 16 weeks of training in older (60–75 years) men, consistent with the hypothesis that increased loading could prevent the drop in dystrophin that occurs with sedentary aging. Future studies need to address the impact of mechanical loading and unloading on dystrophin, specifically in aged muscle.
Summary
Here we show that the level of the lateral force transmission protein dystrophin decreases with age in a muscle-specific manner. The decrease in dystrophin protein is a posttranscriptional event that may be regulated by the dystromir miR-31. The decrease in dystrophin protein in presarcopenic flexor muscles is associated with a compensatory increase in other lateral force transmission proteins compared with adult counterparts. In spite of the attempted compensation, the age-associated loss of dystrophin is associated with an increase in membrane damage and NMJ instability. Overall, these data suggest that alterations in the force transfer apparatus with aging may contribute to sarcopenia.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by a project grant to K. Baar from the National Institute on Aging of the National Institutes of Health under award number R01AG045375 and a Merit Award from the United States Department of Veterans Affairs to S. C. Bodine under award number VARR&D 1I01RX000673-01A1. D. W. D. West was supported by postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada.
Conflict of Interest
The authors have no conflict of interest to declare.
Supplementary Material
Acknowledgments
Conception and design of the experiments: D. C. Hughes, D. W. D. West, L. M. Baehr, S. C. Bodine, and K. Baar. Collection, analysis and interpretation of data: D. C. Hughes, G. R. Marcotte, A. G. Marshall, D. W. D. West, L. M. Baehr, M. A. Wallace, P. M. Saleh, S. C. Bodine, and K. Baar. Drafting the article or revising it critically for important intellectual content: D. C. Hughes, G. R. Marcotte, A. G. Marshall, D. W. D. West, L. M. Baehr, M. A. Wallace, P. M. Saleh, S. C. Bodine, and K. Baar. All authors read and approved the final version of the manuscript and all authors listed qualify for authorship.
References
- 1. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:451–456. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength: A quantitative review. Front Physiol. 2012;3:260. doi:10.3389/fphys.2012.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: Prospective cohort study. BMJ. 2008;337:a439. doi:10.1136/bmj.a439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol (1985). 1999;86:881–886. [DOI] [PubMed] [Google Scholar]
- 5. Street SF. Lateral transmission of tension in frog myofibers: A myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi:10.1002/jcp.1041140314 [DOI] [PubMed] [Google Scholar]
- 6. Hughes DC, Wallace MA, Baar K. Effects of aging, exercise, and disease on force transfer in skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309:E1–E10. doi:10.1152/ajpendo.00095.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramaswamy KS, Palmer ML, van der Meulen JH, et al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol. 2011;589:1195–1208. doi:10.1113/jphysiol.2010.201921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu X, Yeadon JE, Burden SJ. AML1 is expressed in skeletal muscle and is regulated by innervation. Mol Cell Biol. 1994;14:8051–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Blagden C, Fan J, et al. Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev. 2005;19:1715–1722. doi:10.1101/gad.1318305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu Y, Hall ZW. Characterization of acetylcholine receptor subunits in developing and in denervated mammalian muscle. J Biol Chem. 1988;263:12878–12885. [PubMed] [Google Scholar]
- 11. Eftimie R, Brenner HR, Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci USA. 1991;88:1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prody CA, Merlie JP. The 5’-flanking region of the mouse muscle nicotinic acetylcholine receptor beta subunit gene promotes expression in cultured muscle cells and is activated by MRF4, myogenin and myoD. Nucleic Acids Res. 1992;20:2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chahine KG, Walke W, Goldman D. A 102 base pair sequence of the nicotinic acetylcholine receptor delta-subunit gene confers regulation by muscle electrical activity. Development. 1992;115:213–219. [DOI] [PubMed] [Google Scholar]
- 14. Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol. 2000;279:C1290–C1294. [DOI] [PubMed] [Google Scholar]
- 15. Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratt SJ, Shah SB, Ward CW, Inacio MP, Stains JP, Lovering RM. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J Physiol. 2013;591:559–570. doi:10.1113/jphysiol.2012.241679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moser H. Duchenne muscular dystrophy: Pathogenetic aspects and genetic prevention. Human Genet. 1984;66:17–40. [DOI] [PubMed] [Google Scholar]
- 18. Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: A model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. [DOI] [PubMed] [Google Scholar]
- 19. Liu J, Milner DJ, Boppart MD, Ross RS, Kaufman SJ. Beta1D chain increases alpha7beta1 integrin and laminin and protects against sarcolemmal damage in mdx mice. Human Mol Genet. 2012;21:1592–1603. doi:10.1093/hmg/ddr596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi:10.1242/jcs.02952 [DOI] [PubMed] [Google Scholar]
- 21. Mayer U. Integrins: Redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi:10.1074/jbc.R200022200 [DOI] [PubMed] [Google Scholar]
- 22. Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1623–1630. doi:10.1152/ajpregu.00089.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cacchiarelli D, Incitti T, Martone J, et al. miR-31 modulates dystrophin expression: New implications for Duchenne muscular dystrophy therapy. EMBO Rep. 2011;12:136–141. doi:10.1038/embor.2010.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fiorillo AA, Heier CR, Novak JS, et al. TNF-alpha-induced microRNAs control dystrophin expression in becker muscular dystrophy. Cell Rep. 2015;12:1678–1690. doi:10.1016/j.celrep.2015.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway× Fischer 344, and Fischer 344× brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64:618–628. doi:10.1093/gerona/glp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamilton DL, Philp A, MacKenzie MG, Baar K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One. 2010;5:e11624. doi:10.1371/journal.pone.0011624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen PM, Murray JD, Schill KE, et al. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One. 2014;9:e88360. doi:10.1371/journal.pone.0088360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi:10.1002/elps.200305392 [DOI] [PubMed] [Google Scholar]
- 30. Heinemeier KM, Olesen JL, Schjerling P, et al. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: Differential effects of specific contraction types. J Appl Physiol (1985). 2007;102:573–581. doi:10.1152/japplphysiol.00866.2006 [DOI] [PubMed] [Google Scholar]
- 31. Palmisano MG, Bremner SN, Hornberger TA, et al. Skeletal muscle intermediate filaments form a stress-transmitting and stress-signaling network. J Cell Sci. 2015;128:219–224. doi:10.1242/jcs.142463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia-Pelagio KP, Muriel J, Desmond PF, et al. Myopathic changes in murine skeletal muscle lacking synemin. Am J Physiol Cell Physiol. 2015;308:C448–C462. doi:10.1152/ajpcell.00331:ajpcell. 00331.02014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seto JT, Lek M, Quinlan KG, et al. Deficiency of alpha-actinin-3 is associated with increased susceptibility to contraction-induced damage and skeletal muscle remodeling. Hum Mol Genet. 2011;20:2914–2927. [DOI] [PubMed] [Google Scholar]
- 34. Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi:10.1074/jbc.M307247200 [DOI] [PubMed] [Google Scholar]
- 35. Taub PR, Ramirez-Sanchez I, Ciaraldi TP, et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and type 2 diabetes: Restorative effects of (-)-epicatechin-rich cocoa. Clin Sci. 2013;125:383–389. doi:10.1042/CS20130023 [DOI] [PubMed] [Google Scholar]
- 36. Hodges BL, Hayashi YK, Nonaka I, Wang W, Arahata K, Kaufman SJ. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci. 1997;110 (Pt 22):2873–2881. [DOI] [PubMed] [Google Scholar]
- 37. Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi:10.1152/ajpcell.00199.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol. 2000;150:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol. 2006;290:C1660–C1665. doi:10.1152/ajpcell.00317.2005 [DOI] [PubMed] [Google Scholar]
- 40. Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lek A, Evesson FJ, Lemckert FA, et al. Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair. J Neurosci. 2013;33:5085–5094. doi:10.1523/JNEUROSCI.3560-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waddell LB, Lemckert FA, Zheng XF, et al. Dysferlin, annexin A1, and mitsugumin 53 are upregulated in muscular dystrophy and localize to longitudinal tubules of the T-system with stretch. J Neuropathol Exp Neurol. 2011;70:302–313. doi:10.1097/NEN.0b013e31821350b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roche JA, Lovering RM, Roche R, Ru LW, Reed PW, Bloch RJ. Extensive mononuclear infiltration and myogenesis characterize recovery of dysferlin-null skeletal muscle from contraction-induced injuries. Am J Physiol Cell Physiol. 2010;298:C298–C312. doi:10.1152/ajpcell.00122.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harris J, Wilson P. Denervation in murine dystrophy. Nature. 1971;229:61–62. [DOI] [PubMed] [Google Scholar]
- 45. Warren GL, Ingalls CP, Shah SJ, Armstrong RB. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol. 1999;515 (Pt 2):609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong J, Anderson JE. Dystrophin is required for organizing large acetylcholine receptor aggregates. Brain Res. 1999;839:298–304. [DOI] [PubMed] [Google Scholar]
- 47. Pannérec A, Springer M, Migliavacca E, et al. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging (Albany NY). 2016; 8:712–729. doi:10.18632/aging.100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holloszy JO, Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: Contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50:124–129. [DOI] [PubMed] [Google Scholar]
- 49. Acharyya S, Butchbach ME, Sahenk Z, et al. Dystrophin glycoprotein complex dysfunction: A regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi:10.1016/j.ccr.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 50. Gutierrez-Salmean G, Ciaraldi TP, Nogueira L, et al. Effects of (-)-epicatechin on molecular modulators of skeletal muscle growth and differentiation. J Nutr Biochem. 2014;25:91–94. doi:10.1016/j.jnutbio.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316:3050–3058. doi:10.1016/j.yexcr.2010.05.035 [DOI] [PubMed] [Google Scholar]
- 52. Hidestrand M, Richards-Malcolm S, et al. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J Gerontol A Biol Sci Med Sci. 2008;63:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rice KM, Preston DL, Neff D, Norton M, Blough ER. Age-related dystrophin–glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. J Gerontol A Biol Sci Med Sci. 2006;61:1119–1129. [DOI] [PubMed] [Google Scholar]
- 54. Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–328. [DOI] [PubMed] [Google Scholar]
- 55. Woolstenhulme MT, Conlee RK, Drummond MJ, Stites AW, Parcell AC. Temporal response of desmin and dystrophin proteins to progressive resistance exercise in human skeletal muscle. J Appl Physiol (1985). 2006;100:1876–1882. doi:10.1152/japplphysiol.01592.2005 [DOI] [PubMed] [Google Scholar]
- 56. Kosek DJ, Bamman MM. Modulation of the dystrophin-associated protein complex in response to resistance training in young and older men. J Appl Physiol (1985). 2008;104:1476–1484. doi:10.1152/japplphysiol.00708.2007 [DOI] [PubMed] [Google Scholar]
- 57. Parcell AC, Woolstenhulme MT, Sawyer RD. Structural protein alterations to resistance and endurance cycling exercise training. J Strength Cond Res. 2009;23:359–365. doi:10.1519/JSC.0b013e318198fd62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.