Abstract
External and internal stimuli cause modifications to gene and biochemical pathways. In turn, demonstrating that biological systems continuously make short-term adaptations both to set-points, and to the range of “normal” capacity, due to mild conditional changes, or to subtoxic, nondamaging levels of chemical agents. This is termed as “Adaptive Homeostasis,” defined with the following: “The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, nondamaging, signaling molecules or events, or the removal or cessation of such molecules or events.” Research from several laboratories, including our own, found that adaptive homeostasis declines with age in organisms as diverse as worms, flies, and mammals, and decreases with senescence in mammalian cell cultures. We suggest that diminishing adaptive homeostasis may play a causal role as a factor responsible for the aging phenotype. Furthermore, although studies of humans, animals, and model organisms are often limited to a single sex, and cell culture studies may even be conducted with lines whose donor’s sex was unknown, studies reveal distinct sexual dimorphism in adaptive homeostasis. Interestingly, although young males and females may exhibit dramatic differences in adaptive capacities and/or preferences, these distinctions are lost with age as adaptive homeostasis patterns converge.
Keywords: Adaptive homeostasis, sexual dimorphism, Nrf2-Keap1, oxidative stress, heat shock
Sexual Dimorphism—General Aspects in Mammals and Flies
Across species, females typically outlive males. Many current longevity interventions are beneficial in a sex-specific (female-favored) manner (1). These differences may be a consequence of the maternal transmission of the mitochondrial genome. There are several nonexclusive hypotheses for why it may be selectively advantageous for the mitochondrial genome to be preferentially transmitted through the female gamete (1). These hypotheses include: limiting the spread of parasitic genomes (2), limiting damage to the mitochondrial genome that might be greater in the relatively more metabolically active sperm, avoiding potential conflicts between different mitochondrial genome alleles in the zygote, and the potential to act as a driver of evolution, including evolution of the sexes (3). As a result, an asymmetric pattern of mitochondrial lineage arises, causing the mitochondrial genome to be best adapted for females, to the detriment of males. This is evident in mitochondrial polymorphisms, which have little impact on nuclear gene expression in females, but are detrimental to males, modifying nearly 10% of nuclear transcripts (4). Additionally, D. melanogaster strains with the same nuclear background, but different naturally occurring mitochondrial haplotypes, exhibit accelerated aging in a male-specific manner (5).
Moreover, sexual dimorphism in stress and disease sensitivity is common in mammals, including humans. For example, women are more resistant to ischemic heart disease (6) and ischemic stroke (7) than are men, and both these diseases involve oxidative stress. Sexual dimorphism in mammalian stress resistance appears to be partly due to sex-specific hormone levels (cell nonautonomous effects). For example, estrogen is generally reported to be beneficial for stress resistance, whereas testosterone effects are more mixed, and benefits may be specific to male cells (8). In addition, female cells and tissues are generally reported to be more resistant to stress, including oxidative stress, than are male cells, independent of circulating hormones (cell-autonomous effects) (1). In certain cases this cell-autonomous female advantage correlates with increased expression of X-linked genes important for stress responses, such as G6PD and XIAP (9,10). These results suggest that the female advantage in stress resistance relative to males may result in part from the increased copy number and expression of X-linked genes in females relative to males. The copy number and expression level of X-linked genes is also important in Drosophila physiology. For example, the larger body size of females is regulated in part by tra-on and in part by the copy number of the X-linked gene Myc (11).
In Drosophila, females are also reported to be generally more stress resistant than are males. For example, Drosophila females are relatively more resistant to heat stress (12), oxidative stress (13), and starvation stress (14). Part of this female advantage in stress resistance, relative to males, may be related to the larger size of females, which may provide greater nutrient reserves and/or cellular redundancy. In contrast, sex-specific stress adaptation appears to be regulated independent of body size, as discussed further in the following paragraphs.
Sex Determination in Drosophila Melanogaster
In Drosophila, sex determination is based on the chromosomal sex of the animal, where X/X is female and X/Y is male. The greater ratio of X chromosomes to autosomes in females activates the expression of a master X-linked regulatory gene (called Sex lethal, Sxl). Once activated, the Sxl protein maintains its own expression through positive-feedback regulation of its own alternative splicing, such that Sxl is maintained in the on-state and is actively expressed in females. In contrast, Sxl is in the off-state in males, and it is not expressed (Figure 1). The on/off state of the Sxl gene regulates dosage compensation (15). The Sxl protein inhibits the translation of the gene male specific lethal 2 (msl-2), thereby preventing the formation of the male-specific-lethal (MSL) transcriptional regulatory complex in females. In males, the msl-2 protein is expressed, the resultant MSL complex is targeted to the single male X chromosome, and this yields dosage compensation through mechanisms that are not yet entirely clear. The on/off state of Sxl also regulates the great majority of the rest of sexual differentiation. The Sxl protein regulates the alternative splicing of the gene transformer (tra), such that tra is in the on-state and actively expressed in females, and is in the off-state and not expressed in males.
Figure 1.
Drosophila sex determination. In males (X/Y), Sxl gene is not expressed and tra gene is not expressed. The dsx and fru genes undergo default splicing to produce the male isoforms (“M”), which then regulate male cell differentiation. In females (X/X), Sxl is expressed and negatively regulates MSL-2 expression to regulate dosage compensation. Sxl protein also regulates alternative splicing of tra such that tra is expressed. The tra protein in turn regulates female-specific splicing of dsx and fru to produce the female isoforms (“F”) and female cell differentiation. In addition, tra protein acts through as-yet unclear mechanisms (“?”) to regulate female gene expression and cell differentiation. Please see text for details.
The tra protein in turn regulates the alternative splicing of the transcription factor genes doublesex (dsx) and fruitless (fru), such that a female-specific isoform (“F”) of each protein is expressed in females. In males, the dsx and fru genes undergo a default splicing pattern, thereby yielding the male-specific isoforms (“M”) of each protein. These sex-specific transcription factors then regulate a large portion of the structural, functional, and behavioral differences between males and females. In addition, it has recently been reported that the tra protein also regulates female gene expression and sexual differentiation independent of its function as a splicing regulator, through mechanisms that are not yet clear but which might include direct transcriptional regulation (16). Consistent with the central regulatory role of tra protein, forced expression of tra protein in X/Y animals produces a female-like differentiation (“pseudo-females”), and in turn knock down of tra expression in X/X animals produces a male-like differentiation, (“pseudo-males”) (17).
Mammalian sexual differentiation is related to Drosophila sexual differentiation in several ways, including the fact that females are X/X and males are X/Y. In mammals, dosage compensation is also regulated by the on/off state of a master X-linked regulatory gene (called Xist). Xist is in the on-state and expressed on one of the X chromosomes in females, and cis-inactivates most (but not all) genes on that X chromosome to achieve dosage compensation (18); the genes that “escape” from X inactivation include several implicated in stress response pathways and disease. Another similarity is that a gene related to Drosophila dsx is expressed in a male-specific pattern and is required for male differentiation in mammals (called Doublesex and Mab3 Related Transcription factor 1, or DMRT1) (19) as well as in zebrafish (20) and C. elegans (called Mab3).
Adaptive Homeostasis and Stress Resistance
Homeostasis is the concept that internal conditions are maintained in a constant state within living organisms, despite varying external and even internal influences. The term homeostasis comes to us from the Harvard physiologist Walter Bradford Cannon, who built on the original concept of milieu intérieur, or a constant interior bodily environment, which was developed by the celebrated French physiologist Claude Bernard in 1865. The word “homeostasis” was coined by Cannon in 1926 to describe and extend Bernard’s milieu intérieur concept, and popularized (in 1932) in his very successful book, The Wisdom of the Body (21). Cannon combined two words from Ancient Greek ὅμος (hómos, “similar”) + ιστημι (histēmi, “standing still”)/stasis (from στάσις) into a Modern Latin form to invent his term homeostasis.
Homeostasis explains how, even when summer temperatures soar to 100°F, or winter temperatures plunge to −20°F, human beings can maintain internal core body temperature of 98.6°F. In some ways, Bernard’s concept of a constant milieu intérieur, and Cannon’s incorporation of stasis into his term homeostasis, has biased subsequent generations of physiologists to think of normal functions as being unvarying. Yet we know that “normal” core temperature is not one specific number but rather exhibits a range of 97.6–99.6°F. Under certain conditions, even that range can expand or contract somewhat. In point of fact, Cannon (21) actually wrote, “…..The word (homeostasis) does not imply something set and immobile, a stagnation. It means a condition – a condition which may vary, but which is relatively constant.”
In recent years, it has become clear that organisms from bacteria to humans can transiently and reversibly adapt to a wide variety of stresses, if they are first exposed to a sufficiently low level, i.e., subtoxic or only mildly toxic, stress, and allowed a sufficient period of time in which to “turn on” various protective systems. Thus, all organisms, so far studied, exhibit powerful adaptive responses to heat stress, cold stress, glucose stress, oxidative stress, reductive stress, food deprivation, hypoxia or anoxia, chemical toxins, heavy metals, exercise, mechanical stress, salt stress, alcohol, osmolarity, emotional and psychosocial stresses, and many more (22,23).
Many have considered that the transient adaptive responses referred to above result from a damage-repair process in which damage to nucleic acids, proteins, and/or lipids can turn-on responses that essentially overcompensate and provide excess protection for a period of time before being reset to basal levels. Indeed, certain DNA repair processes, for example in response to oxidative damage, do fall into this category (24). Along the same lines, the term hormesis was suggested by Southam and Erlich (25) to describe the process by which sublethal damage caused by small doses of a toxin or poison would produce an exaggerated repair response in which the organism actually becomes stronger than it was previously. Hormesis appears to be a biological corollary of the views of philosopher Friedrich Wilhelm Nietzsche, who stated: “That which does not kill us makes us stronger.”
Hormesis has been eloquently and extensively championed by physiologist and toxicologist, Edward J. Calabrese, who has made major contributions to our understanding of the importance of biphasic dose–response curves (26). The problem with hormesis, however, is its association with repair or restoration of damage, to produce a stronger organism. Although this may apply for select cases of DNA repair (24), we now have numerous examples of situations in which the homeostatic range for multiple functions is transiently expanded or contracted, without any damaging initiating stimulus and, therefore, with no repair process.
In experiments conducted during recent years, we have discovered that resistance to multiple forms of stress is not a static property of cells, tissues, or organisms. Indeed, all protective systems thus far studied exhibit great transient plasticity in response to extremely minor changes in, oxygen, oxidants, temperature, acid, alkali, salt, etc. Multiple publications now demonstrate that, a wide variety of small changes that cause no damage at all are still capable of inducing transient protective mechanisms (13,27–32). Because these transient modifications of the homeostatic range are not examples of repair or restoration of damage to produce a stronger organism, they do not qualify as examples of hormesis. Similarly, neither hereostasis nor allostasis (33,34), with their psychological overtones and requirements for overall nervous system control, seem adequate to describe transient variations in the homeostatic range that occur as discrete responses to (nondamaging) changes in the levels of internal or environmental factors. Thus, the term “adaptive homeostasis” was coined to describe processes by which small, nondamaging, changes in external or internal parameters may cause short-term, transient, and reversible expansions or contractions of the homeostatic range for resistance to, or protection against, more severe stresses (35). Operationally, adaptive homeostasis has been defined as follows, “The transient expansion or contraction of the homeostatic range in response to exposure to sub-toxic, nondamaging, signaling molecules or events, or the removal or cessation of such molecules or events.”(35).
In our own work on transient adaptation to oxidative stress, we have found that protein turnover can be radically altered by biochemical modifications to the key proteolytic enzymes, Proteasome and the mitochondrial Lon protease (36,37), which can undergo biochemical alteration to differentially modify the cellular proteome. The nuclear form of Proteasome, for example, undergoes post-translational ADP-ribosylation by poly ADP-ribose polymerase in response to signaling by oxidants such as hydrogen peroxide (H2O2), and this modification increases nuclear Proteasome’s ability to degrade histone proteins (36).
In addition to such very rapid post-translational adaptations, transient adaptive responses in gene expression profiles can allow cells and organisms to cope with a far greater range of conditions. Many such adaptive alterations to the homeostatic range are mediated by discrete signal transduction pathways that transiently alter transcription/translation (38,39). A good example of such pathways is the Keap1-Nrf2 system which regulates the expression of a wide variety of stress-responsive genes (28,30,40,41). Clearly, the Keap1-Nrf2 signal transduction pathway can effect transient but powerful changes in the homeostatic range of cellular defenses against electrophiles and oxidants, yet it is not an example of heterostasis, allostasis, or hormesis; hence, the need for the more accurate and appropriate term, adaptive homeostasis.
Sex-Dependent Adaptive Homeostasis
In 2001, a report released from the Institute of Medicine suggested the importance of sex as a biological variable (42). However, it garnered little attention, and even less implementation within the scientific community. Less than half of all clinical trials report outcomes separated by sex, and only 22%–42% of all studies report the sex of the animals used (43). Moreover, sex-identification in cell culture studies is even less prevalent. Indeed, one study found that less than 20% of cardiology studies reported the sex of the donors from whom cells were derived (44). Additionally, a study surveying findings published in the American Journal of Physiology, found that none of the 2013 publications included the sex of the donors from whom cell lines originated (43). Fortunately, the 2014 mandate, released from the National Institutes of Health, requires researchers to account for cell and animal sex as a biological variable (43), which may hopefully, change this oversight in the future.
Despite growing evidence suggesting the importance of a cell’s donors’ sex, this variable is commonly given little attention in cell culture studies, further contributing to the lack of insight about sex influences on biological responses. Irrespective of donor sex, cells contain the same general architecture, including nuclei, mitochondria, endoplasmic reticulum, etc.; and show similar functional characteristics, including proliferation, metabolic processes, and apoptosis (43). Hence, many may assume that sex has few implications for the mechanics of signaling pathways. However, as mounting research demonstrates, the need to account for a cell’s sex is crucial in understanding pathways that are necessary for sex-based differences in disease presentation and mortality, including those responsible for the day-to-day adaptive responses necessary for cellular homeostasis.
Adaptive Response to Oxidative Stress
Responding to oxidative stresses involves multiple cellular adaptive stress responses that have recently been described as “adaptive homeostasis (35),” as described above. The ability of a cell to transiently and dynamically regulate key antioxidant defenses and stress-responsive enzymes is critical to maintain cellular homeostasis. Activation of key stress-protective signaling pathways, is largely mediated by the Keap1-Nrf2 transcriptionally regulated pathway (30,40), which activates increased expression of various antioxidant defense mechanisms and stress-responsive enzymes.
Some of the earliest measures of adaptation, and one still employed in present-day studies, is the assessment of stimulated growth. Studies as far back as the 1950s, showed that transient exposure of yeast or bacteria to low concentrations of hydrogen peroxide (H2O2) (45,46), or antibiotics (47), resulted in increased cellular growth. Studies in cell culture identified a similar phenomenon, wherein exposure of embryonic chick cells to antibiotics (streptomycin) (48), mouse liver cells treated with multiple pesticides (49), and human lung fibroblasts exposed to small amounts of ginseng and hydrocortisone (50), all promoted increased cell proliferation. However, in many of these early cell culture studies, the sex of a cell was not taken into consideration. As a result, we have limited insight about the extent of a cell’s sex on the adaptive stress response.
Approximately 5% of the human cellular genome is located on the sex chromosomes (51), of which, 1000–2000 genes are located on the X chromosome (51), and less than 50 genes are located on the Y chromosome (52). As females have two X chromosomes, whereas males have only one, increases the likelihood that sex-linked proteins and their biochemical function, may be biased towards males or females. In an attempt to catalog transcriptional differences based on a cell donors’ sex, 115 female-derived and 118 male-derived lymphoblastoid cell lines were studied, and 10 autosomal genes were identified as having a sex-specific expression pattern (53). These 10 targets were found to participate in an array of cellular processes, including cell adhesion, zinc ion binding, apoptosis, transcription, and structural support (53). Additionally, genes that are homologous between the sex chromosomes, may encode slightly different protein products, as is the case for the ribosomal protein S4, a component of the 40S subunit (54). Taken together, these findings suggest potential differences in cellular responses which may favor one sex over the other, depending on the environment.
Oxidative Stress From Cells to Model Organisms
Mammalian Cell Culture
One of the major threats to cellular homeostasis is oxidative stress. Inability to block the accumulation of oxidized proteins, can decrease cellular function. Endogenous metabolic processes can increase intracellular levels of free radicals and related oxidants, including superoxide (O2°−)(55) and hydrogen peroxide (H2O2) (56). Additionally, extracellular sources such as air pollution, pesticides, UV, and ionizing radiation, all contribute to protein oxidation within cells, and in extracellular fluids and compartments (57). Initial in vitro cellular studies found that although high concentrations of H2O2 are damaging to cells, lower, nontoxic amounts were capable of stimulating cell proliferation (45,58). Key initial studies provided one of the first cellular models to demonstrate that low concentrations of H2O2 (nM–µM) do not damage cells, but activate the stress-responsive pathway to increase protection. In these investigations, HA-1 cells (45,58) of the Chinese hamster ovary fibroblasts line (female), were pretreated with various low concentrations of H2O2 [100 µM–200 µM] and, when challenged with a much higher, normally lethal H2O2 concentration exhibited much improved viability (25%–45%).
Studies using mouse embryonic fibroblasts (of unknown sex origin) and the immortalized leukemia K562 cell line (female origin) to assess protein turnover, identified the ability of low signaling levels of H2O2 to activate key stress-protective enzymes such as the 20S proteasome (27,28,59). Further studies using primary human dermal fibroblasts (sex origin unknown) showed that pretreatment of early-passage cells with low signaling concentrations of H2O2, elicited a robust increase in cellular proliferation and clearance of oxidized proteins, which was lost in late passage cells (60).
Additional cell culture studies demonstrated that increased synthesis of both the regulator of calcinuerin 1 (RCAN1) protein and the mitochondrial Lon protease, in response to low (nM–µM) signaling levels of extracellular H2O2 both contribute to protective adaptive responses; these studies were all conducted in female-derived mammalian cell lines. The first identification of the short-term inducibility of RCAN1 in response to H2O2 (61), was conducted using HA-1 cells. Later identification found that upregulation of RCAN1, in turn, stimulated increased superoxide dismutase production (62). Other cell culture studies investigated the inducibility of the mitochondrial ATP-dependent Lon protease in response to low (nM–µM) signaling levels of extracellular H2O2. A rhabdomyosarcoma cell line (female) was used for the first demonstration of the robust cellular inducibility of the Lon protease to low-stimulatory doses of H2O2 (37). Follow-up studies found that treatment with low signaling levels of H2O2 achieved strong induction of Lon protease expression in early-passage human lung fibroblasts (female) and prevented the accumulation of oxidized proteins if these cells were subsequently exposed to (normally) toxic levels of H2O2; importantly, both Lon induction and protection against protein oxidative damage were lost on cellular senescence (63).
Cumulatively, the majority of cell culture studies assessing the inducibility of adaptive homeostatic responses by extracellular hydrogen peroxide employ female-derived cell lines (with some studies in cells from donors of unknown sex origin). Thus, although we can say with some confidence that H2O2 adaptation is a generalized property of mammalian cells derived from female donors, we cannot yet make the same assertion for cells derived from males and we cannot make any meaningful male/female comparisons.
Model Organisms
Hydrogen peroxide adaptation
When first identified within the cell, H2O2 was aptly considered a noxious molecule, due to the fact that high amounts cause cellular havoc if left unchecked. However, the paradigm has shifted, and at low, physiologically relevant, concentrations H2O2 is now recognized as a crucial cellular signaling molecule. At low concentrations, H2O2 can act as an insulin mimetic (64), trigger cell proliferation (45), and activate various transcription factors involved in the adaptive stress response (65).
Further evidence suggests that signaling amounts of H2O2 work to induce transcription factors that are necessary for key adaptive cellular responses, in part due to activation of the insulin-like receptor (InR) (66). Moreover, the same downstream targets appear activated on low concentrations of H2O2, including the AKT pathway (64) and Nrf2 activation (29). For example, in response to H2O2 pretreatment, studies using C. elegans and D. melanogaster showed that both stress tolerance (41,67) and adaption (29,41) were reliant on Nrf2 transcriptional regulation. Additionally, knock-down of the Nrf2 homolog in D. melanogaster (CnC) (29) and C. elegans (Skn-1) (41) caused a corresponding loss of the adaptive stress response.
Recent findings in D. melanogaster implicate sex differences in H2O2-mediated adaptation. Females pretreated with low, signaling (and nondamaging) amounts of H2O2 and subsequently challenged with higher, toxic levels of H2O2, showed increased survival compared to females that had not been pretreated; importantly, male flies exhibited no adaptive response to H2O2 (13,29). Additionally, two crucial proteases, the 20S proteasome and the mitochondrial Lon protease, exhibited a female-specific adaptive response to H2O2 (13,29). Females showed elevated expression and activity of both enzymes, whereas males showed no change, irrespective of H2O2 pretreatment. Thus, male and female Drosophila differ significantly in adaptive response pathways for oxidative stress.
One possible explanation for this physiological adaptive difference between males and females may be attributed to disparities within the insulin-like signaling pathway, as female D. melanogaster have higher insulin-like receptor expression compared to males (68). Indeed, mutant female D. melanogaster, lacking the insulin receptor, show increased lifespan, whereas males are not affected (68). Together, these findings may suggest why females are more sensitive (and potentially more responsive) to low, nondamaging amounts of the insulin-mimetic, H2O2. Nor is the lack of male adaptive response due to insufficient H2O2 dosage, as higher concentrations resulted only in toxicity (29).
Moreover, recent work suggests the female-favored adaptive response may be caused by specific tissues. Female D. melanogaster typically have a larger body size and show a higher rate of turnover of their intestinal epithelium, both during homeostasis and periods of stress, compared to males (69). Removal of the female-specific form of transformer, limited to the fat bodies, resulted in the neuronal synthesis of the insulin-like peptide, and the resulting limited growth of peripheral tissues in females (70). Similarly, female-favored intestinal growth enables a robust response to tissue damage and improved metabolic modulation, compared to the male intestinal epithelium (71). Indicating the impact individual tissues can have on growth, and potentially, the adaptive response.
Paraquat adaptation
Paraquat is a redox-cycling agent that has frequently been used to test stress resistance in D. melanogaster (72). However, a recent finding showed that males pretreated with micromolar adaptive amounts of paraquat showed improved survival when challenged with a subsequent toxic dose, whereas females were unaffected (13). This sex-dependent difference may be mediated by differences in paraquat sensitivity. Indeed, paraquat has been found to activate the JNK pathway (73), as overexpression of JNK signaling, in male dopaminergic neurons, improved survival in male D. melanogaster (74).
Although the mechanism of male-specific paraquat adaptation is presently unknown, there are some potential pathways that it may activate. Paraquat reacts with NADPH oxidase enzymes and molecular oxygen in the mitochondria and the cell membrane to generate the paraquat radical and the superoxide anion radical (O2˚−) (75). In turn, superoxide is rapidly dismutated into H2O2 by superoxide dismutases. Thus, paraquat generates both O2˚− and H2O2 within cells, in contrast to exposure to H2O2 alone, as discussed in the prior section. Paraquat has been shown to increase the expression of key antioxidant enzymes, including superoxide dismutase and catalase, which can catalyze the breakdown of superoxide (76). Moreover, within the hippocampal region, male rats showed higher catalase and superoxide dismutase activity compared to females (77).
Additionally, unlike females, male D. melanogaster show greater sensitivity to paraquat-induced behavioral disruptions. Increased dopamine levels, conferred male-favored paraquat resistance, and vice versa (78). Notably, increased expression of the D1-like dopamine receptor (DAMB) increased paraquat sensitivity in a male-specific manner (78). Moreover, DAMB is expressed at higher levels in adult males relative to adult females. Indicating, that males potentially have a greater basal expression of O2˚− sensitive pathways, such as DAMB, compared to females.
Heat Shock From Cells to Model Organisms
Mammalian Cell Culture
The heat shock response was one of the earliest subtypes of the cellular stress response identified. Heat shock proteins (HSPs) are ubiquitous chaperones, and one of the first lines of defense against proteotoxic stress (79). A highly evolutionarily conserved process, the heat shock response is primarily activated by the heat shock factor (HSF), in response to slight temperature elevation above the homeostatic threshold (80), as well as to other forms of stress (81). Cells pretreated with mild heat shock, show improved thermotolerance, against a subsequent, much more severe, heat exposure which would otherwise be lethal. This phenomenon was originally reported in mouse embryonic fibroblasts (82) and with a chicken lymphoma cell line (83). However, in both instances, the sex of the cell donor was unknown. Further studies to understand the adaptive increase in the heat shock response have largely relied on mouse embryonic fibroblasts (cell type unknown or unreported) (84,85), human embryonic kidney cell line HEK293 (female) (85,86), and the adenocarcinoma HeLa cell line (female) (85,87,88). These results suggest that the majority of our understanding of the adaptive cellular heat shock response in vertebrate cells is limited to females.
Model Organisms
A mild heat stress applied early in life can produce increased resistance to subsequent heat stress in Drosophila (heat stress adaptation), and can also yield modest increases in life span (89,90); the life span increase is often referred to as “hormesis.” Similarly, repeated mild heat stress has beneficial effects on growth and function of cultured human cells undergoing aging in vitro (91). Heat stress in Drosophila causes rapid induction of the heat shock proteins (hsps), including the highly conserved cytoplasmic Hsp70 (12). Consistent with an important role for the hsps in heat stress resistance, deletion of all 5 of the genes encoding Hsp70 causes reduced resistance to heat stress in Drosophila (92). Moreover, consistent with a role for Hsp70 in hormesis, flies transgenic for extra copies of Hsp70 exhibited a greater life span extension on mild heat stress than did controls (90). When males and females have been compared, males show a greater increase in life span in response to mild heat stress than do females (90).
The Age-Dependent Loss of Adaptive Homeostasis
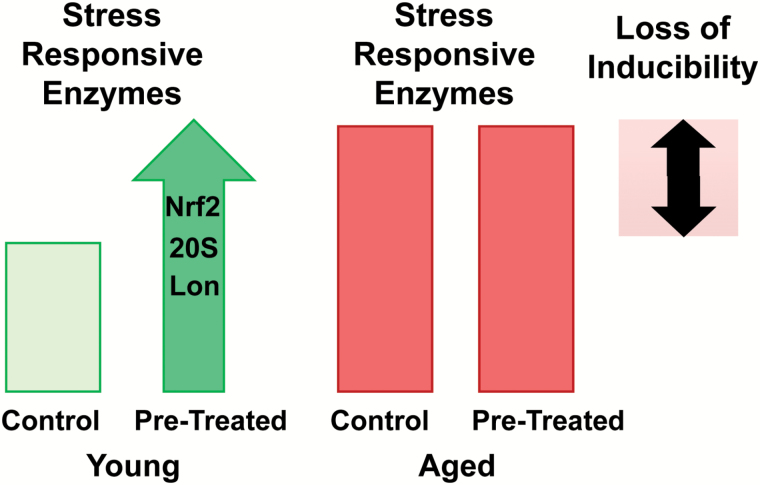
With age, the adaptive response diminishes in the nematode worm and fruit-fly. In the case of the 20S proteasome, 60 day old female D. melanogaster not only lose their adaptive increase in 20S proteasome expression and activity, but the loss is accompanied by a parallel rise in basal expression, suggesting a compensatory mechanism (93). Similarly, 10-day-old C. elegans showed increased basal expression of the 20S proteasome, yet inability to induce an adaptive response (41). Importantly, induction of the Lon protease with H2O2 exposure in D. melanogaster is also lost with age, beginning at 35 days (13). Together, these results suggesting the rise in basal levels of stress-responsive markers may be due to an organism’s attempt to combat chronic, low-grade oxidative stress throughout the lifespan (Figure 2).
Figure 2.
Age-associated loss of adaptive homeostasis. Young organisms, pretreated with a nondamaging amount of an oxidant or stimulant, are capable of a robust increase in multiple stress-responsive enzymes (Nrf2, mitochondrial Lon protease, the 20S proteasome, and heat shock proteins). Yet, with age, although steady-state basal concentrations of these proteins increase, their induction, following pretreatment, is no longer possible. Hence the compression of adaptive homeostasis with age.
Simply forcing the expression of Nrf2 or its regulators is not sufficient to restore the adaptive response. Specifically, chronic overexpression of the C. elegans homologue, Skn-1, improved stress resistance with age, yet did not restore the adaptive response (41). Conversely, chronic knockdown of the Nrf2 cytosolic inhibitor, Keap1, increased stress resistance in 60-day-old D. melanogaster, but was unable to produce a female-specific adaptive response (93). These results imply that simply forcing the overexpression of Nrf2 is not enough to restore the dynamic and highly attuned processes of the adaptive response.
Indeed, more is not always better. Several studies show that overexpression of stress-responsive proteins such as the hsps (12,94) and SOD (95,96) can extend life span in invertebrates. In contrast, several studies show that chronic overexpression of various antioxidant enzymes is not sufficient to extend rodent lifespan (97); and in some studies overexpression of stress response enzymes was detrimental (13,98). Chronic overexpression of stress response enzymes was not sufficient to restore adaptation in aged animals (41). Indeed, aging, itself, is associated with basal elevation in various stress-responsive proteins, including hsps (12), the 20S proteasome, and inhibitors of Nrf2, such as Bach1 and c-Myc (23,41,93), potentially to counteract the age-associated rise in reactive oxygen species. Surprisingly, findings from long-lived species showed lower basal levels of reactive oxygen species compared to short-lived species (99). Hence, longer-lived organisms may be more efficient at limiting oxidative damage throughout the lifespan. This may be an example of an energy-efficient approach: it may be energetically less costly to lower endogenous oxidant levels compared to the high cost of protein synthesis as would be necessary in elevating the expression level of protective enzymes and/or replacing damaged proteins.
Thus, one approach to prevent loss of adaptive homeostasis with age (13,41) could be to focus on lowering the accumulation of damage, rather than simply forcing the chronic overexpression of stress-responsive defenses, especially because our attempts so far have demonstrated a “ceiling effect” in the activation of adaptive stress responses.
Conclusions
In the majority of studies seeking to discover the underlying mechanisms behind the age-related decline in adaptive homeostasis, sex has been largely overlooked. This is evident in many cell culture studies, wherein the majority of cell lines studied are female-derived. However, as we and others have reported, sex does impact the stress response. Thus, as we seek approaches to find successful drug and therapeutic solutions for age-associated diseases, accounting for differences in sex may be a potential lynchpin for success.
Additionally, the adaptive differences between the sexes afford us a unique model to understand the mechanisms not only by which females live longer, but why males live shorter lives. Future mechanistic studies reliant on cell culture should strive to incorporate both male- and female-derived cell lines to fill this gap. As well, this work serves to highlight the benefits model organisms, especially D. melanogaster, offer the scientific community in further solving this puzzle. The ability to achieve adaptation in one sex, without affecting the other is crucial. Moreover, the observation that both sexes lose the adaptive response with age implies that these pathways converge over time. Additionally, the relative ease of genetic manipulation of fly sex determination may further help to increase our understanding behind adaptive differences that are highly sexually dimorphic and age dependent. Lastly, as human beings are living longer, but not necessarily healthier lives, it is paramount that we develop approaches that are beneficial to both sexes.
Funding
This work was supported by National Science Foundation grant DGE-1418060 [LCDP], National Institute of Health grant from the National Institute on Aging AG011833 and R56AG049629 [JT], National Institute of Health grant from the National Institute on Aging AG052374 [KJAD], and National Institute of Health grant from the National Institute of Environmental Health ES003598 [KJAD].
Conflict of Interest
The authors have no conflict of interest to report.
References
- 1. Tower J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch Biochem Biophys. 2015;576:17–31. doi:10.1016/j.abb.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birky CW., Jr Uniparental inheritance of organelle genes. Curr Biol. 2008;18:R692–R695. doi:10.1016/j.cub.2008.06.049 [DOI] [PubMed] [Google Scholar]
- 3. Tower J. Sex-specific regulation of aging and apoptosis. Mech Ageing Dev. 2006;127:705–718. doi:10.1016/j.mad.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 4. Innocenti P, Morrow EH, Dowling DK. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science. 2011;332:845–848. doi:10.1126/science.1201157 [DOI] [PubMed] [Google Scholar]
- 5. Camus MF, Clancy DJ, Dowling DK. Mitochondria, maternal inheritance, and male aging. Curr Biol. 2012;22:1717–1721. doi:10.1016/j.cub.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 6. Ostadal B, Ostadal P. Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. Br J Pharmacol. 2014;171:541–554. doi:10.1111/bph.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32:2100–2107. doi:10.1038/jcbfm.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasconsuelo A, Pronsato L, Ronda AC, Boland R, Milanesi L. Role of 17β-estradiol and testosterone in apoptosis. Steroids. 2011;76:1223–1231. doi:10.1016/j.steroids.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Pérez‐Crespo M, Ramírez MA, Fernández-González R, et al. Differential sensitivity of male and female mouse embryos to oxidative induced heat‐stress is mediated by glucose‐6‐phosphate dehydrogenase gene expression. Mol Reprod Dev. 2005;72:502–510. [DOI] [PubMed] [Google Scholar]
- 10. Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc Natl Acad Sci USA. 2011;108:11662–11667. doi:10.1073/pnas.1102635108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathews KW, Cavegn M, Zwicky M. Sexual dimorphism of body size is controlled by dosage of the X-chromosomal gene Myc and by the sex-determining gene tra in Drosophila. Genetics. 2017;205:1215–1228. doi:10.1534/genetics.116.192260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46:355–362. doi:10.1016/j.exger.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pomatto LC, Carney C, Shen B, et al. The mitochondrial lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr Biol. 2017;27:1–15. doi:10.1016/j.cub.2016.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jang T, Lee KP. The genetic basis for mating-induced sex differences in starvation resistance in Drosophila melanogaster. J Insect Physiol. 2015;82:56–65. doi:10.1016/j.jinsphys.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 15. Birchler JA. Parallel universes for models of X chromosome dosage compensation in Drosophila: a review. Cytogenet Genome Res. 2016;148:52–67. doi:10.1159/000445924 [DOI] [PubMed] [Google Scholar]
- 16. Rideout EJ, Narsaiya MS, Grewal SS. The sex determination gene transformer regulates male-female differences in Drosophila body size. PLoS Genet. 2015;11:e1005683. doi:10.1371/journal.pgen.1005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng H, Jasper H. Sexual dimorphism: how female cells win the race. Curr Biol. 2016;26:R212–R215. doi:10.1016/j.cub.2016.01.062 [DOI] [PubMed] [Google Scholar]
- 18. Balaton BP, Brown CJ. Escape artists of the X chromosome. Trends Genet. 2016;32:348–359. doi:10.1016/j.tig.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 19. Huang S, Ye L, Chen H. Sex determination and maintenance: the role of DMRT1 and FOXL2. Asian J Androl. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webster KA, Schach U, Ordaz A, Steinfeld JS, Draper BW, Siegfried KR. Dmrt1 is necessary for male sexual development in zebrafish. Dev Biol. 2017;422:33–46. doi:10.1016/j.ydbio.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cannon WB. The wisdom of the body. UK: Nature Publishing Group; 1932. [Google Scholar]
- 22. de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nat Rev Genet. 2011;12:833–845. doi:10.1038/nrg3055 [DOI] [PubMed] [Google Scholar]
- 23. Zhang H, Liu H, Davies KJ, et al. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med. 2012;52:2038–2046. doi:10.1016/j.freeradbiomed.2012.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cadet J, Davies KJ. Oxidative DNA damage & repair: an introduction. Free Radic Biol Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Southam CM. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. 1943. [Google Scholar]
- 26. Mattson MP, Calabrese EJ. Hormesis: what it is and why it matters, in Hormesis. New York: Springer; 2010, p. 1–13. [Google Scholar]
- 27. Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi:10.1042/BJ20100878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi:10.1074/jbc.M111.277145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJ. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J Exp Biol. 2013;216:543–553. doi:10.1242/jeb.074757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang H, Davies KJ, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–336. doi:10.1016/j.freeradbiomed.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Demirovic D, Rattan SI. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013;48:94–98. doi:10.1016/j.exger.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 32. Perkins TJ, Swain PS. Strategies for cellular decision-making. Mol Syst Biol. 2009;5:326. doi:10.1038/msb.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selye H. The nature of stress and its relation to cardiovascular disease. In: Fisher K, Reason J, eds. Hearts and Heart-like Organs. New York: Wiley; 1980; p. 302. [Google Scholar]
- 34. Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. 1988. [Google Scholar]
- 35. Davies KJ. Adaptive homeostasis. Mol Aspects Med. 2016;49:1–7. doi:10.1016/j.mam.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJA. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci USA. 1999;96:6223–6228. doi:10.1073/pnas.96.11.6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med. 2009;46:1042–1048. doi:10.1016/j.freeradbiomed.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163:560–569. doi:10.1016/j.cell.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi:10.1038/nrm3801 [DOI] [PubMed] [Google Scholar]
- 40. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi:10.1080/03602530600971974 [DOI] [PubMed] [Google Scholar]
- 41. Raynes R, Juarez C, Pomatto LC, Sieburth D, Davies KJ. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J Gerontol A Biol Sci Med Sci. 2017;72:143–151. doi:10.1093/gerona/glw093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wizemann T, Pardue M. Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences. Exploring the biological contributions to human health: does sex matter. 2001, Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- 43. Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells?Am J Physiol Cell Physiol. 2014;306:C3–C18. doi:10.1152/ajpcell.00281.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor KE, Vallejo-Giraldo C, Schaible NS, Zakeri R, Miller VM. Reporting of sex as a variable in cardiovascular studies using cultured cells. Biol Sex Differ. 2011;2:11. doi:10.1186/2042-6410-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davies JM, Lowry CV, Davies KJ. Transient adaptation to oxidative stress in yeast. Arch Biochem Biophys. 1995;317:1–6. doi:10.1006/abbi.1995.1128 [DOI] [PubMed] [Google Scholar]
- 46. Davies KJ, Lin SW. Degradation of oxidatively denatured proteins in Escherichia coli. Free Radic Biol Med. 1988;5:215–223. doi:10.1016/0891-5849(88)90015-9 [DOI] [PubMed] [Google Scholar]
- 47. Spendlove GA, Cummings MM, Fackler WB, Michael M. Enhancement of growth of a strain of M. tuberculosis (Var. hominis) by streptomycin. Public Health Reports. 1948;63:1177–1179. doi:10.2307/4586686 [PubMed] [Google Scholar]
- 48. Stebbing AR. Hormesis–the stimulation of growth by low levels of inhibitors. Sci Total Environ. 1982;22:213–234. doi:10.1016/0048-9697(82)90066-3 [DOI] [PubMed] [Google Scholar]
- 49. Gabliks J, Bantug-Jurilla M, Friedman L. Responses of cell cultures to insecticides. IV. Relative toxicity of several organophosphates in mouse cell cultures. Proc Soc Exp Biol Med. 1967;125:1002–1005. doi:10.3181/00379727-125-32261 [DOI] [PubMed] [Google Scholar]
- 50. Fulder SJ. The growth of cultured human fibroblasts treated with hydrocortisone and extracts of the medicinal plant Pan ax ginseng. Exp Gerontol. 1977;12:125–131. doi:10.1016/0531-5565(77)90020-1 [DOI] [PubMed] [Google Scholar]
- 51. Ross MT, Grafham DV, Coffey AJ, et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–337. doi:10.1038/nature03440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi:10.1038/nature01722 [DOI] [PubMed] [Google Scholar]
- 53. Zhang W, Bleibel WK, Roe CA, Cox NJ, Eileen Dolan M. Gender-specific differences in expression in human lymphoblastoid cell lines. Pharmacogenet Genom. 2007;17:447–450. doi:10.1097/FPC.0b013e3280121ffe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watanabe M,Zinn AR, Page DC, Nishimoto T. Functional equivalence of human X-and Y-encoded isoforms of ribosomal protein 34 consistent. Nature Genet. 1993;4:268–271. doi:10.1038/ng0793-268. [DOI] [PubMed] [Google Scholar]
- 55. Chouchani ET, Pell VR, James AM, et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016;23:254–263. doi:10.1016/j.cmet.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 56. Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem. 2014;289:8735–8741. doi:10.1074/jbc.R113.544635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tomanek L. Proteomic responses to environmentally induced oxidative stress. J Exp Biol. 2015;218:1867–1879. doi:10.1242/jeb.116475 [DOI] [PubMed] [Google Scholar]
- 58. Wiese AG, Pacifici RE, Davies KJ. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi:10.1006/abbi.1995.1225 [DOI] [PubMed] [Google Scholar]
- 59. Grune T, Catalgol B, Licht A, et al. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med. 2011;51:1355–1364. doi:10.1016/j.freeradbiomed.2011.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jung T, Höhn A, Catalgol B, Grune T. Age-related differences in oxidative protein-damage in young and senescent fibroblasts. Arch Biochem Biophys. 2009;483:127–135. doi:10.1016/j.abb.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 61. Crawford DR, Leahy KP, Abramova N, Lan L, Wang Y, Davies KJ. Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch Biochem Biophys. 1997;342:6–12. doi:10.1006/abbi.1997.0109 [DOI] [PubMed] [Google Scholar]
- 62. Ermak G, et al. DSCR1(Adapt78) modulates expression of SOD1. FASEB J. 2004;18:62–69. doi:10.1096/fj.03-0451com [DOI] [PubMed] [Google Scholar]
- 63. Ngo JK, Pomatto LC, Bota DA, Koop AL, Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:1178–1185. doi:10.1093/gerona/glr145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Iwakami S, Misu H, Takeda T, et al. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One. 2011;6:e27401. doi:10.1371/journal.pone.0027401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hayes G, Lockwood D. Role of insulin receptor phosphorylation in the insulinomimetic effects of hydrogen peroxide. Proc Natl Acad Sci USA. 1987;84:8115–8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi:10.1126/scisignal.3112re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Magwere T, Chapman T, Partridge L. Sex differences in the effect of dietary restriction on life span and mortality rates in female and male Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2004;59:B3–B9. doi:10.1093/gerona/59.1.B3 [DOI] [PubMed] [Google Scholar]
- 69. Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. doi:10.1146/annurev-genet-111212-133343 [DOI] [PubMed] [Google Scholar]
- 70. Rideout EJ, Narsaiya MS, Grewal SS. The sex determination gene transformer regulates male-female differences in Drosophila body size. PLOS Genetics. 2016;11:e1005683. doi:10.1371/journal.pgen.1005683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530:344–348. doi:10.1038/nature16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–370. doi:10.1016/j.mad.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 73. Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi:10.1074/jbc.M404596200 [DOI] [PubMed] [Google Scholar]
- 74. Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi:10.1016/S1534-5807(03)00323-X [DOI] [PubMed] [Google Scholar]
- 75. Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi:10.1074/jbc.M708597200 [DOI] [PubMed] [Google Scholar]
- 76. Krůček T, Korandová M, Šerý M, et al. Effect of low doses of herbicide paraquat on antioxidant defense in Drosophila. Arch Insect Biochem Physiol. 2015;88:235–248. doi:10.1002/arch.21222 [DOI] [PubMed] [Google Scholar]
- 77. Mármol F, Rodríguez CA, Sánchez J, Chamizo VD. Anti-oxidative effects produced by environmental enrichment in the hippocampus and cerebral cortex of male and female rats. Brain Res. 2015;1613:120–129. doi:10.1016/j.brainres.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 78. Cassar M, Issa AR, Riemensperger T, et al. A dopamine receptor contributes to paraquat-induced neurotoxicity in Drosophila. Hum Mol Genet. 2015;24:197–212. doi:10.1093/hmg/ddu430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ungelenk S, Moayed F, Ho CT, et al. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun. 2016;7:13673. doi:10.1038/ncomms13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fujimoto M, Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277:4112–4125. doi:10.1111/j.1742-4658.2010.07827.x [DOI] [PubMed] [Google Scholar]
- 81. Bartelt-Kirbach B, Golenhofen N. Reaction of small heat-shock proteins to different kinds of cellular stress in cultured rat hippocampal neurons. Cell Stress Chaperones. 2014;19:145–153. doi:10.1007/s12192-013-0452-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi:10.1074/jbc.273.13.7523 [DOI] [PubMed] [Google Scholar]
- 83. Tanabe M, Kawazoe Y, Takeda S, Morimoto RI, Nagata K, Nakai A. Disruption of the HSF3 gene results in the severe reduction of heat shock gene expression and loss of thermotolerance. EMBO J. 1998;17:1750–1758. doi:10.1093/emboj/17.6.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Beckham JT, Wilmink GJ, Mackanos MA, et al. Role of HSP70 in cellular thermotolerance. Lasers Surg Med. 2008;40:704–715. doi:10.1002/lsm.20713 [DOI] [PubMed] [Google Scholar]
- 85. Bellmann K, Charette SJ, Nadeau PJ, Poirier DJ, Loranger A, Landry J. The mechanism whereby heat shock induces apoptosis depends on the innate sensitivity of cells to stress. Cell Stress Chaperones. 2010;15:101–113. doi:10.1007/s12192-009-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li WH, Lee YM, Kim JY, et al. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J Invest Dermatol. 2007;127:2328–2335. doi:10.1038/sj.jid.5700880 [DOI] [PubMed] [Google Scholar]
- 87. Andrews GK, Harding MA, Calvet JP, Adamson ED. The heat shock response in HeLa cells is accompanied by elevated expression of the c-fos proto-oncogene. Mol Cell Biol. 1987;7:3452–3458. doi:10.1128/MCB.7.10.3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abravaya K, Phillips B, Morimoto RI. Attenuation of the heat shock response in HeLa cells is mediated by the release of bound heat shock transcription factor and is modulated by changes in growth and in heat shock temperatures. Genes Dev. 1991;5:2117–2127. doi:10.1101/gad.5.11.2117 [DOI] [PubMed] [Google Scholar]
- 89. Hercus MJ, Loeschcke V, Rattan SI. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi:10.1023/A:1024197806855 [DOI] [PubMed] [Google Scholar]
- 90. Khazaeli AA, Tatar M, Pletcher SD, Curtsinger JW. Heat-induced longevity extension in Drosophila. I. Heat treatment, mortality, and thermotolerance. J Gerontol A Biol Sci Med Sci. 1997;52:B48–B52. doi:10.1093/gerona/52A.1.B48 [DOI] [PubMed] [Google Scholar]
- 91. Rattan SI, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis, and differentiation. Dose Response. 2009;7:90–103. doi:10.2203/dose-response.08-014.Rattan [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gong WJ, Golic KG. Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics. 2006;172:275–286. doi:10.1534/genetics.105.048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pomatto LCD, Wong S, Carney C, Shen B, Tower J, Davies KJ. The age- and sex-specific decline of the 20S proteasome and Nrf2/CncC signal transduction pathway in adaptation and resistance to oxidative stress in Drosophila melanogaster. Aging. 2017. doi:10.18632/aging.101218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. [DOI] [PubMed] [Google Scholar]
- 95. Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi:10.1128/MCB.19.1.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Curtis C, Landis GN, Folk D, et al. Transcriptional profiling of MnSOD-mediated lifespan extension in Drosophila reveals a species-general network of aging and metabolic genes. Genome Biol. 2007;8:R262. doi:10.1186/gb-2007-8-12-r262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pérez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead?Biochim Biophys Acta. 2009;1790:1005–1014. doi:10.1016/j.bbagen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reis‐Rodrigues P, Czerwieniec G, Peters TWet al. Proteomic analysis of age‐dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–127. doi:10.1111/j.1474-9726.2011.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–3717. doi:10.1210/en.2005-0378 [DOI] [PubMed] [Google Scholar]