Abstract
Background:
Elevated plasma soluble vascular cell adhesion molecule-1 (sVCAM-1) is a presumed marker of endothelial dysfunction, both in the brain and systemic circulation. Impairments in memory and cognition have been associated with cardiovascular diseases, but little is known about their relationships to abnormal cerebral endothelial function.
Methods:
We studied the cross-sectional association between sVCAM-1 and markers of cerebrovascular hemodynamics and cognitive function in 680 community-dwelling participants in the MOBILIZE Boston Study, aged 65 years and older. Cognitive function was assessed using the Hopkins Verbal Learning Memory Test and Trail Making Tests (TMTs) A and B. Global cognitive impairment was defined as Mini-Mental State Examination (MMSE) score less than 24. sVCAM-1 was measured by ELISA assay. Beat-to-beat blood flow velocity (BFV) and cerebrovascular resistance (CVR = mean arterial pressure / BFV) in the middle cerebral artery were assessed at rest by transcranial Doppler ultrasound.
Results:
sVCAM-1 concentrations were higher among participants with an MMSE score <24 versus ≥24 (1,201±417 vs 1,122±494ng/mL). In regression models adjusted for sociodemographic characteristics and health conditions, increasing levels of sVCAM-1 were linearly associated with higher resting CVR (p = .006) and lower performance on the Hopkins Verbal Learning Memory (immediate recall and delayed recall) and adjusted TMT B tests (p < .05). Higher levels of sVCAM-1 were also associated with global cognitive impairment on the MMSE (odds ratio = 3.9; 95% confidence interval: 1.4–10.9; p = .011).
Conclusions:
In this cohort of elderly participants, we observed a cross-sectional association between elevated sVCAM-1 levels and both cognitive impairment and increased cerebrovascular resistance. Longitudinal studies are needed to determine whether elevated sVCAM-1 is a cause or consequence of cerebrovascular damage.
Keywords: Cerebrovascular resistance, Cognitive impairment, Endothelium, Executive function, sVCAM-1
Cognitive impairments and vascular diseases are increasingly prevalent worldwide in older adults, conferring a major burden on health and health care costs (1,2). Many studies suggest that these conditions may be linked through the development of cerebral endothelial dysfunction and associated cerebral microvascular disease. Previous studies suggest that hypertension may impair cerebrovascular reactivity (3). Hypertension has also been implicated in vascular cognitive impairment (4,5) and Alzheimer’s disease (6,7), and both of these conditions have a significant vascular pathology. Cerebral endothelial dysfunction may be due to a number of conditions associated with aging, including hypertension and type 2 diabetes (8,9). It may also impair cerebral blood flow regulation and cerebral vasoreactivity, and ultimately lead to mobility impairments, including slowing of gait and falls (10,11).
Soluble vascular cell adhesion molecule-1 (sVCAM-1) is a well-known biomarker of endothelial dysfunction that is associated with hypertension and atherosclerosis (12,13). It plays an important role in accelerating atherosclerosis by facilitating the attachment of inflammatory cells to the vascular endothelial wall and promoting their subsequent migration through the endothelium (1,14). The resulting inflammatory response, injury, and stiffening of the vascular wall may result in impaired cerebral blood flow regulation, especially endothelium-dependent vasodilatation in response to changes in blood pCO2, and ischemic damage to the cerebral microvasculature.
We have shown in a previous study that elevated plasma levels of sVCAM-1 may be a marker of chronic cerebral blood flow dysregulation due to cerebral endothelial damage from hypertension and may also signal the clinical consequences of cerebral microvascular disease, including slow gait speed and injurious falls among elderly people (10).
We hypothesized that an elevated plasma concentration of sVCAM-1 may be a marker of cerebral microvascular disease, characterized by increased cerebrovascular resistance and lower cognitive performance in elderly people. We therefore used transcranial Doppler ultrasonography, plasma biomarkers, and cognitive data from the MOBILIZE Boston Study to explore the relationships between plasma levels of sVCAM-1, cerebrovascular resistance, executive dysfunction, and cognitive impairment in a community-based population of older adults.
Materials and Methods
Participants
The study sample consisted of 680 community-dwelling older adults living in the Boston area who participated in the MOBILIZE Boston Study. The design and methodology for this study have been previously described in detail (15,16). To be included, individuals had to be 70 years or older (or age > 65 years if living with a participant), able to understand and communicate in English, and able to walk 20 feet without personal assistance. Exclusion criteria included terminal disease, severe vision or hearing deficits, and a Mini-Mental State Examination (MMSE) score less than 18 (15,16). All participants underwent a complete home and laboratory assessment of demographic characteristics, medical history, medications, functional status, gait speed, smoking status, alcohol use, blood pressure (BP), and cerebral hemodynamics at baseline. Only a subset of 419 participants had an adequate temporal acoustic window to obtain reliable Doppler measures of cerebral blood flow velocity (BFV) and were able to complete the cerebral hemodynamic assessments. Only those participants with complete data for the variables of interest were included in the analyses.
Neuropsychological Measures
We administered neuropsychological tests to each participant during the home interview, as previously described (17,18). The primary outcome was cognitive impairment assessed according to the MMSE score (19,20). Study participants were categorized into two groups according to baseline MMSE score: those with or without significant global cognitive impairment (MMSE score < 24 and ≥ 24, respectively).
The Hopkins Verbal Learning Test-Revised (HVLT-R) (21) is a 12-item word list learning test in which individuals are presented three learning and recall trials followed by a delayed recall trail and a 24-item word recognition test. The HVLT-R produces three scores: the sum of correct responses in each of three learning trials (HVLT-R learning), the number of items correctly recalled after the delay (HVLT-R delayed recall), and the number of recognition items correctly identified (HVLT-R recognition). In older adults, the encoding procedure relies on working and verbal memory processes and executive function (22).
The Trail Making Test (TMT) Part A consists of number targets to be connected in order, providing an estimate of attention and psychomotor speed. TMT Part B includes number and letter targets that are to be connected in alternating sequence, providing an estimate of set shifting and executive function (23). As in previous studies (17,18), to control for the effect of motor function and information processing speed, we calculated the “adjusted” TMT as the time to perform part B minus the time to perform part A.
Activity of Daily Living score (ADL): The index of ADLs counts the number of ADLs for which a person needs help and is the classic measure of the severity of the need for personal assistance services and other long-term services and supports (24). Instrumental Activity of Daily Living score (IADL): This index measures a participant’s ability to maintain independence in household chores, cooking, shopping, finances, and transportation (25).
Biomarker Measures
Concentrations of soluble intercellular adhesion molecule-1 (sICAM-1), sVCAM-1, and interleukin-6 (IL-6) were measured by ELISA assay (R&D Systems, Minneapolis, MN). For sICAM-1, this assay has a sensitivity of 0.35ng/mL and the day-to-day variability of the assay at concentrations of 64.2, 117, 290, and 453ng/mL are 10.1, 7.4, 6.0, and 6.1%, respectively. For sVCAM-1, the assay has a sensitivity of 2.0ng/mL and the day-to-day variability of the assay at concentrations of 9.8, 24.9, and 49.6ng/mL are 10.2, 8.5, and 8.9%, respectively. For IL-6, the assay has a sensitivity of 0.094 pg/mL and the day-to-day variability of the assay at concentrations of 0.49, 2.78, and 5.65 pg/mL are 9.6, 7.2, and 6.5%, respectively. The concentration of high-sensitivity C-reactive protein was determined using an immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). This high-sensitivity assay has a limit of detection of 0.03mg/L. The day-to-day variability of the assay at concentrations of 0.91, 3.07, and 13.38mg/L are 2.81, 1.61, and 1.1%, respectively. All assays were performed by Dr. Nader Rifai’s group at Boston Children’s Hospital. The biomarker measurement methodology has been previously described in detail (10).
Transcranial Doppler Ultrasound Measures
Participants reported to the Cerebrovascular Laboratory at the Hebrew SeniorLife Institute for Aging Research and were instrumented for heart rate (ECG) and beat-to-beat arterial pressure monitoring (ABP, Finapres, Ohmeda Monitoring Systems, Englewood, CO). Transcranial Doppler (TCD) ultrasonography (MultiDop X4, DWL-Transcranial Doppler Systems, Sterling, VA) was used to measure middle cerebral artery mean BFV at rest (26). The middle cerebral artery signal was identified according to standard criteria and recorded at a depth of 50–60mm (26). Resting BFV was quantified as the average BFV over each beat of the heart during the last minute of a 5-minute period while participants sat quietly in a chair. Cerebrovascular resistance (CVR) was calculated for each beat during this period as the mean arterial BP divided by the BFV, then averaged. CVR represents the resistance of downstream arterioles beyond the middle cerebral artery, which normally constrict or dilate to help regulate blood flow. A higher CVR reduces blood flow to regions of the brain.
Other Covariates
Covariates included sociodemographic characteristics, cardiovascular risk factors, health status, and amount of physical activity. Sociodemographic characteristics included age, sex, race (self-identified), and years of education. We used the validated Physical Activity Scale for the Elderly (PASE) to measure physical activity in the previous week (27). Participants were asked about physician-diagnosed major medical conditions. Diabetes was defined using an algorithm based on self-reported diabetes, use of antidiabetic medications, and laboratory measures, including random glucose (≥200mg/dL) and hemoglobin A1c (≥7%). Body mass index (calculated as weight in kilograms divided by height in meters squared) was calculated from measured height and weight. Comorbidity index was the number of comorbidities or medical conditions. Certain medication use (antihypertensives, antidepressants, and benzodiazepines) was also assessed. Hypertension was defined using three BP categories: Normotension, no history or current evidence of hypertension; controlled hypertension, a history of hypertension and antihypertensive treatment with normal BP (systolic BP < 140 and diastolic BP < 90 during the baseline clinical assessment); and uncontrolled hypertension, a history of hypertension with abnormal BP (systolic BP ≥ 140 or diastolic BP ≥ 90) (28,29).
Smoking and alcohol
Smoking status was determined by asking whether the participant currently smoked cigarettes. Alcohol use was assessed with a question on whether individuals consumed two or more drinks of beer, wine, or liquor each week.
Ethics Statement
The MOBILIZE Boston Study was reviewed and approved by the Hebrew SeniorLife Institutional Review Board. Written informed consent was obtained from each participant. The study was conducted according to the principles of the Helsinki Declaration.
Data Analysis
We compared baseline characteristics of different groups of study participants by using t tests, χ2 tests, or Wilcoxon rank-sum tests. We used multivariate linear regression to examine the cross-sectional relationships between log-transformed sVCAM-1 levels and continuous outcomes (eg, CVR, TMTs A and B) and logistic regression to estimate the relative risk and 95% confidence intervals for quintiles of sVCAM and binary outcomes (eg, cognitive impairment [MMSE score < 24]).
Analyses were adjusted for the following groups of potential confounders: (i) other biomarkers (ICAM-1, IL-6, and C-reactive protein), (ii) sociodemographic conditions (age, gender, White race, education level, body mass index, current smoker, and alcohol use), (iii) health conditions (diabetes, hypertension, heart failure, hyperlipidemia, depression, any cardiovascular medications, coronary artery disease, and previous stroke), and (iv) physical activity level.
Participants with missing data for the main outcomes, sVCAM-1, cognition status, neuropsychological measures, or CVR were excluded from those specific analyses. No imputing methods were used. All analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC). A two-sided p value of less than .05 was considered indicative of statistical significance.
Results
Participants
Table 1 shows the characteristics of participants with and without cognitive impairment. The mean age of participants was 78.1±5.4 years and 62.4% were women. Of the study participants, 361 (53.7%) had controlled hypertension and 162 (24.1%) had uncontrolled hypertension. TCD measures were available for 419 (62%) participants. There was no significant difference in demographics or the number of participants with cognitive impairment between those with and without TCD data.
Table 1.
Characteristics of Participants According to Cognitive Status (MMSE < 24 vs ≥ 24), N = 668
| Baseline Characteristics | Cognitive Status* | P value | ||
|---|---|---|---|---|
| Total Sample | Impairment (n = 72, 11%) | No Impairment (n = 596, 89%) | ||
| Demographics | ||||
| Age, mean (±SD), y | 78.1±5.4 | 78.7±5.9 | 78.0±5.3 | .19 |
| Women | 418 (62.6) | 51 (70.8) | 367 (61.6) | .07 |
| White race | 535 (80.1) | 32 (44.4) | 503 (84.4) | <.0001 |
| Educational level, mean (±SD), y | 14.8±6.1 | 13.1±11.9 | 15.2±4.8 | <.0001 |
| Health behaviors | ||||
| Body mass index, kg/m2 † | ||||
| <25 | 210 (31.4) | 15(20.8) | 195 (32.7) | |
| 25–29.9 | 286 (42.8) | 32 (44.4) | 254 (42.6) | .06 |
| ≥30 | 172 (25.7) | 25 (34.7) | 147 (24.7) | |
| Current smoker | 391 (58.5) | 24 (33.33) | 361 (60.6) | <.0001 |
| Alcohol use (≥2 drinks per week) | 174 (26.0) | 6 (8.33) | 165 (27.68) | .0004 |
| Physical activity score‡ | ||||
| 0–66 | 209 (31.3) | 26 (36.11) | 183 (30.70) | |
| 66.01–124 | 226 (33.8) | 30 (41.7) | 196 (32.89) | .069 |
| 124.01–559 | 233 (34.9) | 16 (19.5) | 217 (36.4) | |
| Health conditions | ||||
| Comorbidity index, mean (±SD) | 3.0±1.6 | 3.2±1.6 | 3.0±1.6 | .9 |
| Hypertension | 529 (79.2) | 71 (98.6) | 458 (76.9) | .0001 |
| Hyperlipidemia | 391 (58.5) | 48 (66.7) | 343 (57.6) | .37 |
| Diabetes | 126 (18.9) | 22 (30.6) | 104 (17.5) | .007 |
| Previous stroke | 71 (10.6) | 7 (9.7) | 64 (10.7) | .9 |
| Coronary artery disease | 117 (17.5) | 17 (23.6) | 100 (16.8) | .3 |
| Congestive heart failure | 43 (6.4) | 7 (9.7) | 36 (6.0) | .7 |
| CESD-R score mean (±SD) | 11.3±11.2 | 15.3±15.3 | 10.4±10.4 | .02 |
| Medications | ||||
| Any cardiovascular medication | 460 (68.9) | 61(84.7) | 399 (67.0) | .002 |
| Psychotropic medication | 53 (7.9) | 10 (13.9) | 43 (7.2) | .04 |
| Neuropsychological measures | ||||
| TMT A Score, mean (±SD), s | 56.3±34.2 | 71.4±38.1 | 59.3±32.2 | .041 |
| TMT B Score, mean (±SD), s | 141.1±78.1 | 168.1±88.3 | 121.1±79.0 | .016 |
| HVLT, immediate recall, mean (±SD) | 20.8±5.8 | 29.6±7.5 | 16.8±5.1 | .04 |
| HVLT, delayed recall, mean (±SD) | 6.3±3.5 | 10.1±4.1 | 4.3±3.6 | .03 |
| HVLT, recognition, mean (±SD) | 11.4±2.2 | 14.7±3.2 | 8.1±2.3 | .012 |
| Functional measures | ||||
| IADL score ≥ 1 | 287 (42.9%) | 69 (95.8%) | 218 (36.6%) | .0001 |
| ADL score ≥ 1 | 148 (22.2%) | 58 (80.5%) | 90 (15.1%) | .0001 |
| Neurophysiologic measures§ | ||||
| Cerebral BFV, mean (SD) | 41.0±10.3 | 37.6±10.1 | 41.2±10.3 | .03 |
| Cerebrovascular resistance, mean (SD) | 1.8±0.6 | 2.0±0.6 | 1.7±0.6 | .04 |
| Biomarkers measures | ||||
| C-reactive protein, mean (±SD), mg/L | 4.2±13 | 6.4±12 | 3.9±13 | .002 |
| Interleukin-6, mean (±SD), pg/mL | 4.0±7.3 | 3.9±7.3 | 3.9±3.8 | .2 |
| Soluble ICAM-1, mean (±SD), ng/mL | 262±81 | 263.0±77 | 254.3±108 | .05 |
| Soluble VCAM-1, mean (±SD), ng/mL | 1192±428 | 1201.3±417 | 1122.8±494 | .03 |
Notes: Global test: χ2 or Fisher’s exact test for binary variables; analysis of variance for continuous variables.
BFV = blood flow velocity; CESD-R = Center for Epidemiological Studies Depression Scale Revised; HVLT = Hopkins Verbal Learning Memory Test; ICAM-1 = intercellular adhesion molecule-1; MMSE = Mini-Mental State Examination; TMT = Trail Making Test.
*Cognitive impairment (MMSE < 24).
†Body mass index is calculated as weight in kilograms divided by height in meters squared.
‡Physical activity tertiles measured using the Physical Activity Scale for the Elderly.
§Transcranial Doppler data in cm/s units for cerebral BFV, mmHg.s/cm units for cerebrovascular resistance.
Soluble VCAM-1, BFV, and Cerebrovascular Resistance
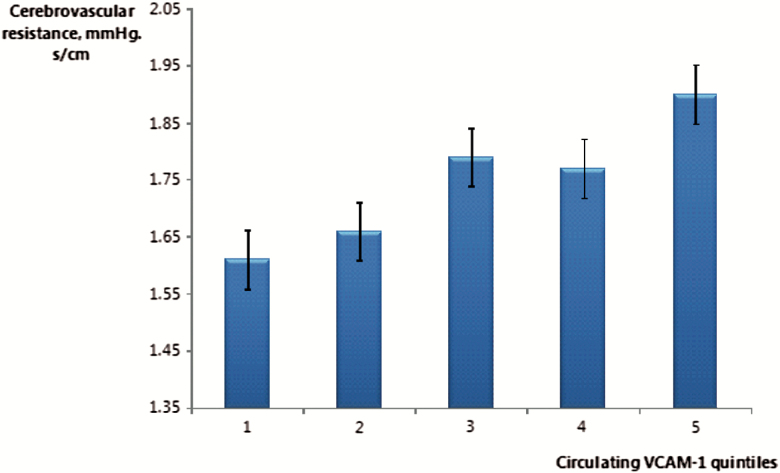
In a cross-sectional analysis, elevated sVCAM-1 levels were associated with higher resting CVR (p = .04; Figure 1). After adjustment for relevant covariates, increasing levels of sVCAM-1 were linearly associated with higher resting CVR (p = .006; Supplementary Table 1). An increase in resting CVR was also correlated with a reduction in cognitive performance (r = .13, p = .015).
Figure 1.
Association between cerebrovascular resistance (mmHg. s/cm) and circulating sVCAM-1 quintiles (n = 419).
Soluble VCAM-1 and Neuropsychological Measures
Soluble VCAM-1 and global cognitive impairment (MMSE ≤ 24)
sVCAM-1 concentration was higher among participants with an MMSE score <24 versus ≥24 (1,201±417 vs 1,122±494ng/mL; p = .029; Table 1). This association persisted in multivariate logistic regression analyses, which showed that higher levels of sVCAM-1 were associated with cognitive impairment (odds ratio = 3.9; 95% confidence interval: 1.4–10.9; p = .01; Table 2). After adjustment for relevant covariates, higher levels of sVCAM-1 were cross-sectionally associated with lower MMSE scores (−0.14; −0.18 to −0.09; p = .003; Supplementary Table 2 ).
Table 2.
Odds Ratios (95% CIs) for the Cross-sectional Association Between Quintiles of sVCAM-1 and Concurrent Cognitive Impairment Status (MMSE < 24), N = 668*
| N | Quintiles of sVCAM-1, Median (IQR), ng/mL | p for Trend | ||||
|---|---|---|---|---|---|---|
| 718 (657–809) | 935 (900–971) | 1,118 (1,071–1,169) | 1,333 (1,277–1,390) | 1,850 (1,566–2,011) | ||
| 131 | 133 | 136 | 134 | 134 | ||
| Model 1† | ||||||
| Odds ratio (95% CI) | 1.00 | 0.85 (0.39–1.86) | 0.61 (0.24–1.59) | 0.59 (0.21–1.64) | 2.74 (1.14–6.56) | |
| p Value | Reference | .68 | .31 | .31 | .024 | .054 |
| Model 2‡ | ||||||
| Odds ratio (95% CI) | 1.00 | 0.81 (0.37–1.82) | 0.63 (0.24–1.95) | 0.69 (0.25–1.95) | 3.49 (1.39–8.79) | |
| p Value | Reference | .61 | .34 | .48 | .008 | .0049 |
| Model 3§ | ||||||
| Odds ratio (95% CI) | 1.00 | 0.78 (0.34–1.82) | 0.56 (0.21–1.56) | 0.55 (0.19–1.62) | 3.56 (1.22–9.21) | |
| p Value | Reference | .57 | .27 | .28 | .019 | .0040 |
| Model 4‖ | ||||||
| Odds ratio (95% CI) | 1.00 | 0.73 (0.30–1.77) | 0.56 (0.20–1.59) | 0.66 (0.22–1.97) | 3.87 (1.37–10.93) | |
| p Value | Reference | .49 | .27 | .45 | .011 | .0029 |
Notes: CI = confidence interval; ICAM-1 = intercellular adhesion molecule-1; IQR = interquartile range; sVCAM-1 = soluble vascular cell adhesion molecule-1.
*Missing values = 4 from 672.
†Model 1 = Non adjusted model.
‡Model 2 = Model 1 adjusted for ICAM-1, IL-6, and C-reactive protein.
§Model 3 = Model 2 additionally adjusted for age, gender, White race, education level, body mass index, current smoker, and alcohol use.
||Model 4 = Model 3 additionally adjusted for hypertension, diabetes, stroke, congestive heart failure, coronary artery disease, hyperlipidemia, depression, psychotropic medication, any cardiovascular medication, and physical activities level.
Soluble VCAM-1 and HVLT
The mean scores of HVLT subtests were 20.8±5.8 for the immediate recall component and 6.3±3.5 for the delayed recall component. In multivariate analysis, higher levels of sVCAM-1 were associated with higher risk of having poorer performance on the HVLT test (p < .05; Table 3).
Table 3.
Linear Association Between Cognitive Function Tests and sVCAM-1* (change in score on tests with 10% increase sVCAM-1 in linear regression models), N = 668†
| Measures | Change in score (95% CI) | p Value |
|---|---|---|
| Lower scores indicate poorer performance | ||
| MMSE score | −0.14 (−0.2, −0.1) | .003 |
| HVLT immediate recall | −0.2 (−0.3, −0.1) | .04 |
| HVLT delayed recall | −1.6 (−2.3, −0.9) | .03 |
| HVLT recognition | −0.9 (−1.2, −0.6) | .01 |
| Higher scores indicate poorer performance | ||
| TMT A | 0.3 (0.2, 0.4) | .043 |
| TMT B | 0.5 (0.4, 0.6) | .011 |
| Adjusted TMT B* | 0.4 (0.3, 0.5) | .014 |
| IADL score | 0.2 (0.1, 0.3) | .002 |
| ADL score | 0.2 (0.1, 0.3) | <.001 |
Notes: ADL = activity of daily living; HVLT = Hopkins Verbal Learning Memory test; IADL = instrumental activity of daily living; MMSE = Mini-Mental State Examination; sVCAM-1 = soluble vascular cell adhesion molecule-1.
*Adjusted TMT B = the time to perform part B minus the time to perform part A.
†Missing values = 4 from 672; all models are adjusted for ICAM-1, IL-6, C-reactive protein, sociodemographic condition and health condition, psychotropic medication, and any cardiovascular medication.
Soluble VCAM-1 and Adjusted TMT B
The mean scores of TMT were Part A = 56.3±34.2; Part B = 141.1±78.1 and the difference = 87.9±63.7. In multivariate analysis, sVCAM-1 levels were linearly associated with adjusted TMT B test scores (p = .014; Table 3).
Soluble VCAM-1, ADL, and IADL
Of the study participants, 148 (22.2%) had little or lot of difficulty in performing ADLs (ADL ≥ 1) and 287 (42.9%) reported having difficulty in an IADL (IADL ≥ 1). In univariate analysis, elevated levels of sVCAM-1 were linearly associated with lower ADL (p < .0001) and IADL scores (p < .0001). After adjustment for relevant covariates, the association was still statistically significant (p < .0001; Table 3).
Soluble ICAM-1 and cognitive function
Mean ICAM-1 levels were 263±78ng/mL in participants with cognitive impairment and 254±108ng/mL in participants without impairment (p = .05). There were no statistically significant relationships between any of the study outcomes and circulating levels of sICAM-1 in univariate or multivariate analysis.
Discussion
The results of this study show a cross-sectional association between elevated plasma levels of sVCAM-1 and increased cerebrovascular resistance and lower cognitive performance in older adults. To our knowledge, these findings are novel and consistent with the notion that sVCAM-1 is associated with abnormalities in cerebral blood flow regulation and that this may have a clinical or subclinical impact on executive function, cognitive impairment, and functional decline in older people.
VCAM-1 is an endothelial ligand for integrins expressed on leukocytes and platelets, with the function of facilitating endothelial adhesion of circulating leukocytes. The expression of VCAM-1 by endothelial cells is increased in response to inflammatory cytokines (30). The soluble ectodomain of VCAM-1 (sVCAM-1) is proteolytically released from the endothelial cell surface into the circulation upon endothelial activation and injury. Elevated plasma sVCAM-1 levels have been reported in many disease conditions, including coronary and peripheral atherosclerosis, hypertension, and diabetes mellitus (10,31). sVCAM-1 is now a well-established marker for endothelial injury related to inflammatory processes.
The evidence that VCAMs have a role in the progression of atherosclerosis comes from several sources. On histological analysis, human atherosclerotic plaque contains many VCAMs. This may increase peripheral vascular stiffness. It is well known that an increase in peripheral vascular resistance is a hallmark of hypertension. Cellular adhesion molecules increase intimal hyperplasia and the vascular inflammatory response seen after vessel–wall injury (32). Clinical interest in VCAM-1 has grown with the observation that plasma concentrations of VCAM-1 and other inflammatory biomarkers are associated with an increased risk of future vascular events (33).
The results of this study support a vascular mechanism of cognitive impairment. The increase in CVR may be associated with lower blood flow in watershed areas of the brain, resulting in ischemic damage to axons from frontal and prefrontal areas of the brain that control motor function and attentional processes (34,35). Therefore, sVCAM-1 may serve as a physiologic marker, and ultimately a clinical biomarker of cerebral microvascular disease and its clinical consequences such as cognitive and functional decline (36).
Our study had some limitations. First, the cross-sectional design precludes investigating the temporal relation between sVCAM-1 elevation and cerebrovascular resistance, and cognitive impairment. A second limitation is the smaller number of participants with adequate TCD data because of an inadequate temporal bone window to obtain reliable Doppler measures of cerebral BFV. This is a common problem among elderly people, affecting approximately one third of elderly participants in previous studies (37). Therefore, our findings in regard to cerebrovascular hemodynamics may not be generalizable to all older adults. Finally, sVCAM-1 may be related to other processes that impair cognition. By inducing T-cell chemotaxis and inflammatory responses, sVCAM-1 may affect the development of neurodegeneration (38). Inflammatory processes have been linked to the pathogenesis of cognitive impairment (39). We tried to address this by controlling for the inflammatory biomarkers C-reactive protein and IL-6 in the multivariable analysis.
On the other hand, our study has several strengths and potential clinical applications. First, the adhesion molecule (VCAM-1 and ICAM-1) concentrations were comparable with those in previous studies (40). In the future, clinicians may be able to use sVCAM-1 measurements to determine brain endothelial and parenchyma health without the use of expensive imaging studies. This would enable clinicians to intervene early to prevent the clinical consequences of cerebral microvascular disease in elderly people, which include mobility impairments (10), depressive symptoms (41), poor cognitive performance, and functional decline.
Conclusion
In this cohort of community-dwelling elderly participants, we observed a cross-sectional association between soluble VCAM-1 levels and both cognitive function and cerebrovascular resistance. Each of these finding suggests that sVCAM-1 may provide a practical way to detect cerebral endothelial damage and guide future therapeutic interventions. Additional prospective studies are needed to confirm our findings and determine the sequence of events in the pathogenesis of cerebral microvascular disease and its clinical consequences.
Funding
This work was supported by grants P01 AG04390, R01 AG025037, and R01 AG041785 from the National Institute on Aging, NS085002 from the National Institute of Neurological Disorders and Stroke, and R01ES020871 and R00ES015774 from the National Institute for Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.E.T. was supported by the Limoges University, University Hospital Center of Limoges (CHU de Limoges), and Regional Council of Limousin, France. L.A.L. holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife.
Conflict of Interest
G.A.W. has received consulting fees from Environmental Health and Engineering, for work unrelated to this article. The other authors declare no competing financial interests.
Supplementary Material
Acknowledgments
We thank the MOBILIZE Boston research team and study participants for their time, effort, and dedication.
References
- 1. Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi:10.1084/jem.178.2.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorina Y, Hoyert D, Lentzner H, Goulding M. Trends in causes of death among older persons in the United States. Aging Trends. 2005:1–12. [PubMed] [Google Scholar]
- 3. Detre JA, Samuels OB, Alsop DC, Gonzalez-At JB, Kasner SE, Raps EC. Noninvasive magnetic resonance imaging evaluation of cerebral blood flow with acetazolamide challenge in patients with cerebrovascular stenosis. J Magn Reson Imaging. 1999;10:870–875. [DOI] [PubMed] [Google Scholar]
- 4. Sierra C, Domenech M, Camafort M, Coca A. Hypertension and mild cognitive impairment. Curr Hypertens Rep. 2012;14:548–555. doi:10.1007/s11906-012-0315-2 [DOI] [PubMed] [Google Scholar]
- 5. Goveas JS, Rapp SR, Hogan PE, et al. Predictors of optimal cognitive aging in 80+ women: the Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71(suppl 1):S62–S71. doi:10.1093/gerona/glv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelley BJ, Petersen RC. Alzheimer’s disease and mild cognitive impairment. Neurol Clin. 2007;25:577–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28:605–611. [DOI] [PubMed] [Google Scholar]
- 8. Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi:10.1161.JAHA.114.000787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. doi:10.1152/ajpheart.00490.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tchalla AE, Wellenius GA, Travison TG, et al. Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension. 2015;66:340–346. doi:10.1161/HYPERTENSIONAHA.115.05180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. [DOI] [PubMed] [Google Scholar]
- 12. Hajjar I, Quach L, Yang F, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation. 2011;123:858–865. doi:10.1161/CIRCULATIONAHA.110.978114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maeda H, Matsumoto M, Handa N, et al. Reactivity of cerebral blood flow to carbon dioxide in hypertensive patients: evaluation by the transcranial doppler method. J Hypertens. 1994;12:191–197. [PubMed] [Google Scholar]
- 14. Mulvihill NT, Foley JB, Crean P, Walsh M. Prediction of cardiovascular risk using soluble cell adhesion molecules. Eur Heart J. 2002;23:1569–1574. [DOI] [PubMed] [Google Scholar]
- 15. Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi:10.1186/1471-2318-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samelson EJ, Kelsey JL, Kiel DP, et al. Issues in conducting epidemiologic research among elders: lessons from the mobilize boston study. Am J Epidemiol. 2008;168:1444–1451. doi:10.1093/aje/kwn277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wellenius GA, Boyle LD, Coull BA, et al. Residential proximity to nearest major roadway and cognitive function in community-dwelling seniors: results from the MOBILIZE Boston Study. J Am Geriatr Soc. 2012;60:2075–2080. doi:10.111/j.1532-5415.2012.04195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eggermont LH, Milberg WP, Lipsitz LA, Scherder EJ, Leveille SG. Physical activity and executive function in aging: the MOBILIZE Boston Study. J Am Geriatr Soc. 2009;57:1750–1756. doi:10.111/j.1532-5415.2009.02441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20. Escobar JI, Burnam A, Karno M, Forsythe A, Landsverk J, Golding JM. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity. Cultural and linguistic artifacts. J Nerv Ment Dis. 1986;174:607–614. [DOI] [PubMed] [Google Scholar]
- 21. Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13:348–358. [DOI] [PubMed] [Google Scholar]
- 22. Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. J Exp Psychol Learn Mem Cogn. 2008;34:809–822. doi:10.1037/0278-7393.34.4.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pugh KG, Kiely DK, Milberg WP, Lipsitz LA. Selective impairment of frontal-executive cognitive function in African Americans with cardiovascular risk factors. J Am Geriatr Soc. 2003;51:1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 25. Barberger-Gateau P, Dartigues JF, Letenneur L. Four Instrumental Activities of Daily Living Score as a predictor of one-year incident dementia. Age Ageing. 1993;22:457–463. [DOI] [PubMed] [Google Scholar]
- 26. Sorond FA, Khavari R, Serrador JM, Lipsitz LA. Regional cerebral autoregulation during orthostatic stress: age-related differences. J Gerontol A Biol Sci Med Sci. 2005;60:1484–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. [DOI] [PubMed] [Google Scholar]
- 28. Wellenius GA, Wilhelm-Benartzi CS, Wilker EH, et al. Ambient particulate matter and the response to orthostatic challenge in the elderly: the maintenance of balance, independent living, intellect, and zest in the elderly (MOBILIZE) of Boston Study. Hypertension. 2012;59:558–563. doi:10.1161/HYPERTENSIONAHA.111.180778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi:10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 30. Constans J, Conri C. Circulating markers of endothelial function in cardiovascular disease. Clin Chim Acta. 2006;368:33–47. [DOI] [PubMed] [Google Scholar]
- 31. Li YH, Teng JK, Tsai WC, Tsai LM, Lin LJ, Chen JH. Elevation of soluble adhesion molecules is associated with the severity of myocardial damage in acute myocardial infarction. Am J Cardiol. 1997;80:1218–1221. [DOI] [PubMed] [Google Scholar]
- 32. Frenette PS, Wagner DD. Adhesion molecules–Part 1. N Engl J Med. 1996;334:1526–1529. [DOI] [PubMed] [Google Scholar]
- 33. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. [DOI] [PubMed] [Google Scholar]
- 34. Scherder E, Eggermont L, Achterberg W, et al. [Pain and physical (in)activity in relation to cognition and behaviour in dementia]. Tijdschr Gerontol Geriatr. 2009;40:270–278. [DOI] [PubMed] [Google Scholar]
- 35. Schneider AL, Jonassaint C, Sharrett AR, et al. Hemoglobin, anemia, and cognitive function: the atherosclerosis risk in communities study. J Gerontol A Biol Sci Med Sci. 2016;71:772–779. doi:10.1093/gerona/glv158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huffman KM, Pieper CF, Kraus VB, Kraus WE, Fillenbaum GG, Cohen HJ. Relations of a marker of endothelial activation (s-VCAM) to function and mortality in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1369–1375. doi:10.1093/gerona/glr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med Biol. 2009;35:21–29. doi:10.1016/j.ultramedbio.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–582. [DOI] [PubMed] [Google Scholar]
- 39. Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi:10.1212/WNL.06013e318225ae07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Novak V, Zhao P, Manor B, et al. Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care. 2011;34:2438–2441. doi:10.2337/dc11-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tchalla AE, Wellenius GA, Sorond FA, Travison TG, Dantoine T, Lipsitz LA. Elevated circulating vascular cell Adhesion Molecule-1 (sVCAM-1) is associated with concurrent depressive symptoms and cerebral white matter Hyperintensities in older adults. BMC Geriatr. 2015;15:62. doi:10.1186/s12877-015-0063-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.