Abstract
Background
Frailty is an age-related clinical syndrome of decreased resilience to stressors and is associated with numerous adverse outcomes. Although there is preponderance of literature on frailty in developed countries, limited investigations have been conducted in less developed regions including China—a country that has the world’s largest aging population. We examined frailty prevalence in China by sociodemographics and geographic region, and investigated correlates of frailty.
Methods
Participants were 5,301 adults aged ≥60 years from the China Health and Retirement Longitudinal Study. Frailty was identified by the validated physical frailty phenotype (PFP) scale. We estimated frailty prevalence in the overall sample and by sociodemographics. We identified age-adjusted frailty prevalence by geographical region. Bivariate associations of frailty with health and function measures were evaluated by chi-squared test and analysis of variance.
Results
We found 7.0% of adults aged 60 years or older were frail. Frailty is more prevalent at advanced ages, among women, and persons with low education. Age-adjusted frailty prevalence ranged from 3.3% in the Southeast and the Northeast to 9.1% in the Northwest, and was more than 1.5 times higher in rural versus urban areas. Frail versus nonfrail persons had higher prevalence of comorbidities, falls, disability, and functional limitation.
Conclusions
We demonstrated the utility of the PFP scale in identifying frail Chinese elders, and found substantial sociodemographic and regional disparities in frailty prevalence. The PFP scale may be incorporated into clinical practice in China to identify the most vulnerable elders to reduce morbidity, prevent disability, and enable more efficient use of health care resources.
Keywords: Frailty, Aging, China, Disparities
Frailty is commonly defined as an age-related clinical syndrome of decreased resilience to internal and external stressors and is associated with a wide range of adverse outcomes including hospitalization, falls, fractures, disability, and death (1–3). Frailty is prevalent among older adults, and the prevalence is higher at advanced age and among women and socioeconomically disadvantaged individuals (4,5). Although there is preponderance of literature on frailty in developed countries, limited investigations have been conducted in less developed regions (6). Population aging is a global phenomenon. In 2015, over two-thirds of the world’s population aged 60 years or older resided in developing countries and the growth rate is accelerating (7).
China is the most populous country with the world’s largest aging population. In 2013, over 202 million people were over the age of 60 years in China and the number is projected to nearly double by 2040 (402 million) (7,8). In addition, the number of Chinese people aged 80 years or older is estimated to quadruple over the next 35 years, from 22.6 million in 2013 to 90.4 million in 2050 (9). China’s population has been aging rapidly and has profound implications for its health care system that is facing enormous challenges including escalation of health care costs, underutilization and inefficient allocation of resources, and inequalities in health and health care services (10,11). The aging of the population will increase the number of frail elders. Frailty screening and assessment may provide an opportunity for early detection, intervention, and monitoring of the most vulnerable Chinese elders to reduce morbidity, prevent disability, and enable more effective use of health care resources. A comprehensive picture of epidemiology of frailty in China would identify subgroups of Chinese older adults with high frailty prevalence, provide insights into potential sociodemographic and geographic disparities in frailty, and enhance our understanding of potential risk factors for, and consequences of, frailty among the Chinese elders.
In this study, we used the China Health and Retirement Longitudinal Study (CHARLS), a nationally representative prospective study of community-dwelling Chinese population, to determine the prevalence of frailty in China by sociodemographics and geographic region, and to examine health, function, and biomarker correlates of frailty. Frailty was operationalized using the most widely used and validated physical frailty phenotype (PFP) scale developed by Fried et al. (2) in the Cardiovascular Health Study (CHS).
Method
Participants
Data are from the baseline survey (2011–2012) of the CHARLS, an ongoing longitudinal cohort study of a nationally representative sample of community-dwelling adults from 28 provinces in China. The response rate of 80.5% produced a total of 17,708 Chinese residents aged 45 years or older enrolled at baseline. All participants gave informed consent; the protocol was approved by the Ethical Review Committee at the Peking University. Further details about the recruitment strategy, design, and sampling approaches of the CHARLS have been previously documented (12). A total of 7,681 participants were aged 60 years or older, of which 5,301 had data on four or more frailty components (analytic sample). Physical activity was assessed in a random sample of half of all participants and represented 53% of missing frailty component, compared to 1.4% for grip strength, 2.4% for gait speed, 0.7% for exhaustion, and 2.7% for weight loss.
Frailty
Frailty was measured by the PFP scale in which five elements are included: weakness, slowness, exhaustion, inactivity, and shrinking (2).
Weakness
Weakness was defined, using maximum handgrip strength of either hand (two trials for each; measured in a standing position), as ≤20th percentile of the weighted population distribution, adjusting for sex and body mass index (BMI). Linear models regressing grip strength on BMI for males and females separately were fitted. Residuals were computed, representing sex- and BMI-adjusted grip strength. Grip strength of participants whose measuring position was unknown or lying down and who did not appear to give full effort was coded missing.
Slowness
Slowness was defined, using the average of two-timed walk tests over a 2.5-meter course, as being ≤20th percentile of the weighted population distribution, adjusting for sex and height via the residual approach described previously.
Exhaustion
Participants met criteria for exhaustion if they answered “A moderate amount of time; 3–4 days” or “Most of the time” to either of two questions from the modified Center for Epidemiological Studies-Depression (CES-D) scale (13): “I could not get going” and “I felt everything I did was an effort”.
Inactivity
Participants met criteria for inactivity if they self-reported that they did not walk 10 or more minutes continuously during a usual week.
Shrinking
Shrinking was defined as self-reporting loss of 5 or more kilograms in the previous year or having a BMI of 18.5 kg/m2 or less.
Frailty level was identified by the number of criteria met. Individuals with none were considered “robust/nonfrail”; those meeting one or two criteria were considered “prefrail”; and those with three to five criteria were defined as “frail”.
Demographics
Demographic characteristics included age (60–64, 65–69, 70–74, 75–79, 80–84, and 85+ years), sex, education (no formal education/illiterate, can read but did not finish elementary school, elementary school/traditional Chinese school, middle school, and high school or above), marital status (married /living together, widowed, and others), current residence location (urban vs rural), and geographical region (Northeast, Northern, Central, Southwest, South, Northeast, East, South Central, and Southeast).
Medical Conditions
Participants reported whether they have been diagnosed with the following conditions: hypertension, diabetes, cancer (excluding minor skin cancers), cardiac disease (including myocardial infarction, coronary heart disease, angina, heart failure, or other heart problems), stroke, chronic lung diseases, liver disease, kidney disease, stomach or other digestive disease, and arthritis/rheumatism. Falls in previous year were self-reported. Depression was assessed using the modified 10-item CES-D scale (13) excluding two items used for identifying exhaustion (a frailty component). Participants with a total score of 12 or higher, as suggested by Cheng et al. (14), were considered depressed.
Disability
Participants were assessed for disability in five activities of daily living (ADL) tasks (dressing, bathing, eating, getting out of bed, and toileting) and five instrumental ADL (IADL) tasks (preparing hot meals, doing household chores, shopping, managing assets, and taking medications). For each ADL/IADL task, participants were asked, “Do you have difficulty in” performing the task? Those participants who responded, “I have difficulty but can still do it”, “Yes, I have difficulty and need help”, or “I cannot do it” to one or more of the ADL/IADL tasks were considered having ADL/IADL disability.
Self-Reported Functional Limitation
Participants were classified as having lower extremity functional limitation if they had difficulty performing any of the following tasks on a regular basis: getting up from a chair after sitting for a long period, climbing several flights of stairs without resting, or stooping, kneeling, or crouching. Participants were considered having upper extremity functional limitation if they reported having difficulty in any of the following tasks: reaching or extending arms above shoulder level, lifting or carrying weights more than 5 kg, or picking up a small coin from a table. For each task, participants were asked, “Do you have difficulty in” performing the task? Those participants who responded, “I have difficulty but can still do it”, “Yes, I have difficulty and need help”, or “I cannot do it” were considered having difficulty.
Clinical Measures
Blood pressure (BP; mmHg) was measured by an automatic BP monitor in the seated position. Three measurements, 45 seconds apart, were conducted, and the average was used. Fasting blood samples were collected by trained nurses in township hospital or a local office of the China Center for Disease Prevention and Control (CDC). Blood-based biomarkers included white blood cell count (103/cm), hematocrit (%), platelets, (103/cm), hemoglobin (g/dL), cystatin C (mg/L), C reactive protein (CRP; mg/L), fasting glucose (mg/dL), low-density lipoprotein cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), total cholesterol (mg/dL), and triglycerides (mg/dL).
Statistical Analysis
We estimated prevalence of frailty in the overall sample and by demographics including age, sex, education, marital status, and residence location. We used a χ2 test to examine the association of each demographic characteristic with frailty. We identified the prevalence of frailty by geographical region, adjusting for age using multinomial logistic regression.
We identified the prevalence of each chronic condition, falls, depression, disability, and functional limitation across frailty spectrum (robust, prefrail, frail). We used a χ2 test to determine whether the prevalence of each disease and health event differed by frailty status. We estimated the mean (median if the distribution was highly skewed) level of each biomarker by frailty status and used analysis of variance or nonparametric equivalent to determine whether the levels differed by frailty status. We also dichotomized biomarkers using the highest or the lowest quintile (whichever indicated a harmful level) of the sample distribution or clinically relevant cut-points. We utilized a χ2 test to identify whether the proportions differed by frailty status.
We conducted several sensitivity analyses. First, we repeated frailty prevalence analyses including participants with data on all five frailty components (n = 2,061) and compared baseline characteristics between persons with four or more frailty components and those with five. Additionally, because calculation of percentile-based cutoffs requires a reference population, which is not always feasible, we identified cut-points for identifying weakness (stratified by sex and BMI quartiles) and slowness (stratified by sex and median height) to facilitate the assessment of frailty in clinical practice. Percentile-based cutoffs were derived from the analytic sample (n = 5,301).
Multistage probability sampling design of the CHARLS was accounted for by specifying the sampling weight and primary sampling unit parameters. All tests were two-sided with a significance level of p <.05. Analyses were performed using Stata 13.1 and R 3.3.2.
Results
Prevalence of Frailty by Sociodemographics
Among Chinese adults aged 60 years or older, the prevalence of frailty in 2011 was 7.0% (95% CI: 5.9%–8.1%), 51.2% (95% CI: 49.3%–53.3%) were prefrail, and 41.8% (95% CI: 39.4%–44.2%) were robust (Table 1). The prevalence of frailty increased steeply with advancing age. Only 2.9% of persons aged 60 to 64 years were considered frail, whereas about one-third of those aged 85 years or older were classified as frail. Frailty prevalence also differed by sex; 5.9% of men and 8.0% of women were identified as frail, respectively. In addition, higher frailty prevalence was observed in persons who had lower level of education, were not married, and currently lived in rural areas. Approximately 11% of persons who had no formal education were frail, whereas only 0.2% of persons with at least a high school diploma were frail. Frailty prevalence was more than two times higher among widowed persons than those who were married (13.1% vs 5.2%). Frailty prevalence was more than 1.5 times higher in rural versus urban areas (8.1% vs 5.3%).
Table 1.
Prevalence of Frailty Status by Demographic Subgroups Among 5,301 Adults Aged ≥60 Years From the China Health and Retirement Longitudinal Study, 2011; Weighted Estimates
| Demographic characteristics of the sample (%) | Prevalence within subgroup (%) | |||
|---|---|---|---|---|
| Robust | Prefrail | Frail | ||
| All sample | 41.8 | 51.2 | 7.0 | |
| Age (years)*** | ||||
| 60–64 | 37.3 | 50.4 | 46.6 | 2.9 |
| 65–69 | 25.6 | 45.5 | 49.3 | 5.1 |
| 70–74 | 17.9 | 38.5 | 54.0 | 7.5 |
| 75–79 | 11.9 | 26.1 | 62.6 | 11.3 |
| 80–84 | 5.3 | 20.4 | 58.0 | 21.6 |
| 85+ | 2.1 | 12.5 | 55.0 | 32.5 |
| Sex* | ||||
| Male | 50.6 | 43.7 | 50.4 | 5.9 |
| Female | 49.4 | 39.9 | 52.1 | 8.0 |
| Education*** | ||||
| No formal education or illiterate | 34.4 | 31.8 | 56.7 | 11.4 |
| Did not finish elementary schoola | 20.2 | 40.8 | 53.2 | 6.0 |
| Elementary schoolb | 25.6 | 47.1 | 47.9 | 5.1 |
| Middle school | 12.3 | 51.6 | 44.4 | 4.0 |
| High school or abovec | 7.4 | 56.5 | 43.3 | 0.2 |
| Marital status*** | ||||
| Married/living together | 77.2 | 44.7 | 50.1 | 5.2 |
| Widowed | 20.6 | 32.9 | 54.1 | 13.1 |
| Othersd | 2.2 | 22.5 | 66.4 | 11.1 |
| Current residence*** | ||||
| Urban | 42.5 | 46.5 | 48.2 | 5.3 |
| Rural | 57.5 | 38.3 | 53.6 | 8.1 |
aBut capable of reading or writing.
bIncluding traditional Chinese school (i.e. Sishu).
cIncluding graduate from high school, vocational school, college, or post-graduate.
dIncluding separated, divorced, and never married.
***p < .001, **p < .01, *p < .05 for comparison in frailty status (robust, prefrail, frail) within each variable (weighted proportions).
Prevalence of Frailty by Geographic Region
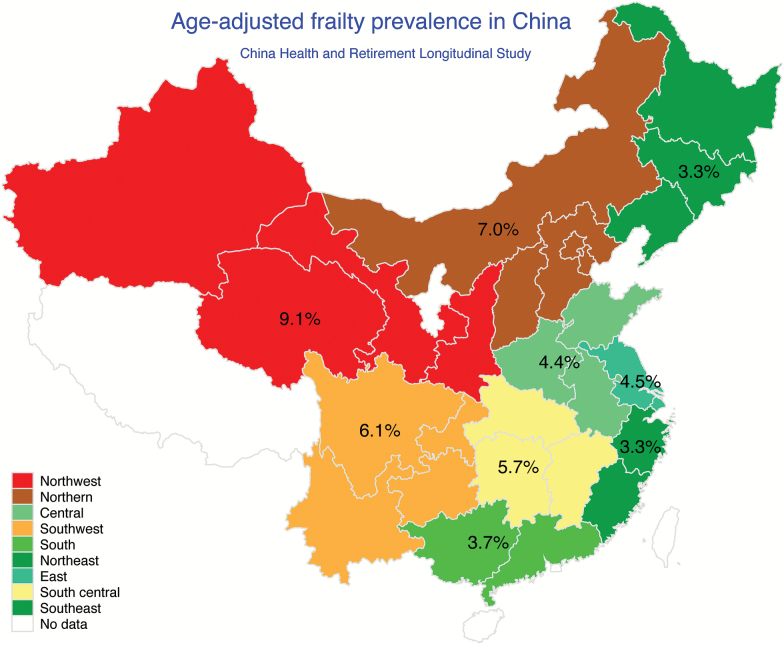
There was substantial geographic variation in frailty prevalence in China. Age-adjusted frailty prevalence estimates ranged approximately threefold from 3.3% in the Southeast and the Northeast to 9.1% in the Northwest (Figure 1). Age-adjusted prefrailty prevalence estimates varied from 38.7% in the Southeast to 58.2% in the Northwest (Supplementary Table S1). In all regions, frailty prevalence was substantially higher in rural than urban areas (Supplementary Table S2). Additionally, there was a decreasing trend in frailty prevalence with lower level of education in all regions of China (Supplementary Table S3).
Figure 1.
Age-adjusted prevalence of frailty among adults aged ≥60 years by districts, China Health and Retirement Longitudinal Study, 2011. Weighted prevalence was estimated at the weighted mean age in each district.
Health and Function Correlates of Frailty
Prevalence of diabetes, cardiac disease, stroke, lung disease, kidney disease, stomach disease, and arthritis was higher in frailer persons (Table 2). The prevalence of having comorbidity (≥2 conditions) was also higher among the frail (54.3%) than the robust (40.8%). Approximately one out of six robust persons reported falls in the previous year, whereas one in four frail persons reported falls. Additionally, the percentage of depression was more than three times higher among the frail than the robust. Moreover, there was a steep gradient in the prevalence of disability and functional limitation from robust to frail. The proportions of persons with ADL and IADL disability were both greater than four times higher among the frail than the robust. The percentage of having lower extremity functional limitation was 86.0% among the frail against 46.9% among the robust. Over 60% of frail persons had upper extremity functional limitation, as opposed to only 11.8% among the robust.
Table 2.
Prevalence of Disease, Health Events, Depression, and Disability by Frailty Status Among 5,301 Adults Aged ≥60 Years From the China Health and Retirement Longitudinal Study, 2011; Weighted Estimates
| Total prevalence for each condition (%) | Prevalence in each frailty status (%) | |||
|---|---|---|---|---|
| Robust | Prefrail | Frail | ||
| n = 2,332 | n = 2,722 | n = 347 | ||
| Self-reported diseases | ||||
| Hypertension | 32.1 | 32.4 | 31.7 | 33.3 |
| Diabetes | 7.2 | 6.5 | 7.4 | 10.0 |
| Cancer | 0.9 | 0.6 | 1.1 | 1.0 |
| Cardiac diseasea* | 16.3 | 14.5 | 17.3 | 20.5 |
| Stroke** | 3.1 | 2.2 | 3.4 | 6.3 |
| Lung disease*** | 14.1 | 9.9 | 16.7 | 19.9 |
| Liver disease* | 4.5 | 3.3 | 5.7 | 2.9 |
| Kidney disease* | 6.0 | 5.0 | 6.6 | 7.8 |
| Stomach disease* | 21.7 | 19.3 | 23.1 | 25.9 |
| Arthritis | 37.2 | 35.3 | 37.9 | 43.1 |
| Having ≥2 diseases*** | 45.1 | 40.8 | 47.3 | 54.3 |
| Falls in previous year*** | 19.1 | 15.6 | 21.0 | 25.9 |
| Depressionb*** | 20.9 | 12.1 | 25.4 | 41.2 |
| ADL disabilityc*** | 18.8 | 9.5 | 22.5 | 46.5 |
| IADL disabilityd*** | 26.1 | 15.1 | 30.1 | 63.2 |
| Lower extremity functional limitatione*** | 59.3 | 46.9 | 65.7 | 86.0 |
| Upper extremity functional limitationf*** | 23.2 | 11.8 | 27.4 | 60.2 |
Note: ADL = activity of daily living; IADL = instrumental activity of daily living.
aIncluding myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems.
bAssessed by the modified Center for Epidemiologic Studies Depression scale excluding two items for identifying exhaustion. A total score of ≥12 was considered depressed.
cIncluding dressing, bathing, eating, getting out of bed, and toileting.
dIncluding preparing hot meals, doing household chores, shopping, managing assets, and taking medications.
eHaving difficulty in getting up from a chair after sitting for long periods, climbing several flights of stairs without resting, or stooping, kneeling or crouching.
fHaving difficulty in reaching or extending arms above shoulder level, lifting or carrying weights over 5 kg, or picking up a small coin from a table.
***p < .001, **p < .01, *p < .05 for comparing prevalence of disease, fall, disability, and functional limitation across robust, prefrail, and frail individuals.
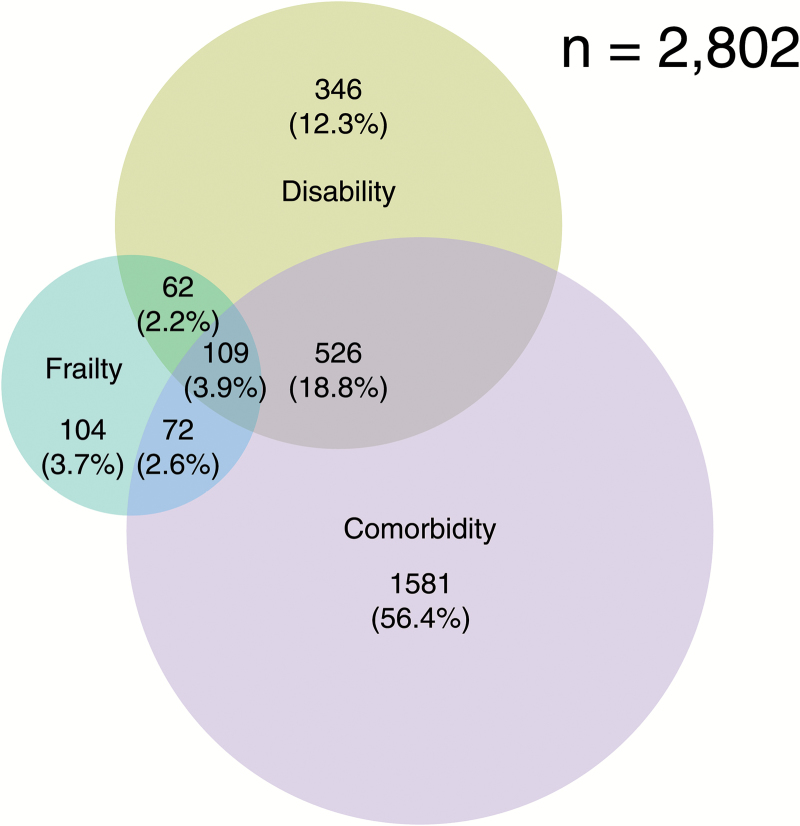
We observed modest overlap between frailty, ADL disability, and comorbidity (Figure 2). Among 2,802 older persons who had frailty and/or disability and/or comorbidity, only 62 (2.2%) had both frailty and disability, 72 (2.6%) had both frailty and comorbidity, and 109 (3.9%) had all three. Of those who were frail, 20.7% had comorbidity, 17.9% had ADL disability, 31.4% had both comorbidity and ADL disability, and 30.0% had neither comorbidity nor ADL disability.
Figure 2.
Venn diagram showing extent of overlap of frailty with activity of daily living (ADL) disability and comorbidity (≥2 chronic conditions). A total of 2,802 adults aged ≥60 years had frailty and/or ADL disability and/or comorbidity. Of these, 347 were frail, 2,290 had ADL disability, and 1,043 had comorbidity. Ten self-reported physician diagnosed chronic conditions were considered: hypertension, diabetes, cancer or militant tumor (excluding minor skin cancer), lung disease, liver disease, kidney disease, stomach or other digestive disease, cardiac disease (including myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems), stroke, and arthritis or rheumatism.
Biomarker Correlates of Frailty
We observed differences in systolic BP, hemoglobin, cystatin C, CRP, and low-density lipoprotein cholesterol between robust, prefrail, and frail persons (Table 3). When biomarkers were dichotomized using the lowest or the highest quintile (whichever indicated a harmful level) or clinically relevant cut-points, frail individuals were more likely to have elevated levels of systolic BP and cystatin C, and low level of hemoglobin than the robust.
Table 3.
Association of Clinical Measures with Frailty Among Adults Aged ≥60 Years From the China Health and Retirement Longitudinal Study, 2011; Weighted Estimates)
| Within each frailty status (%) | ||||
|---|---|---|---|---|
| Robust | Prefrail | Frail | p a | |
| n = 2,332 | n = 2,722 | n = 347 | ||
| Meanb | ||||
| SBP, mmHg | 136.0 | 136.0 | 141.2 | .018 |
| SBP ≥150 mmHg, % | 23.4% | 25.5% | 34.5% | .035 |
| DBP, mmHg | 75.8 | 75.3 | 75.6 | .405 |
| DBP ≥90 mmHg, % | 11.8% | 10.5% | 12.0% | .666 |
| WBC count, 103/cm | 6.2 | 6.2 | 6.5 | .210 |
| WBC count ≥7.6 × 103/ cm, % | 18.8% | 18.3% | 21.8% | .584 |
| Hematocrit, % | 41.2 | 40.9 | 40.3 | .075 |
| Hematocrit <36.4%, % | 19.9% | 19.8% | 17.5% | .721 |
| Platelets, 103/cm | 207.0 | 205.4 | 204.9 | .803 |
| Platelets ≥262 × 103/ cm, % | 18.9% | 20.7% | 19.2% | .559 |
| Hemoglobin, g/dL | 14.3 | 14.1 | 14.0 | .042 |
| Hemoglobin <12 g/ dL, % | 8.8% | 12.7% | 14.3% | .023 |
| Cystatin C, mg/L | 1.1 | 1.1 | 1.2 | <.001 |
| Cystatin C ≥1.2 mg/L | 15.3% | 20.4% | 28.2% | <.001 |
| CRP, mg/L, median | 1.3 | 1.2 | 1.7 | .027 |
| CRP, mg/L, mean | 3.2 | 3.4 | 4.7 | .074 |
| CRP ≥2.5 mg/L, % | 19.7% | 18.2% | 24.7% | .106 |
| Fasting glucose, mg/dL | 112.1 | 112.4 | 107.2 | .112 |
| Fasting glucose ≥125 mg/dL, % | 15.2% | 17.6% | 12.9% | .167 |
| LDL cholesterol, mg/ dL | 118.2 | 114.9 | 113.5 | .036 |
| LDL cholesterol ≥144 mg/dL, % | 21.4% | 18.0% | 18.6% | .153 |
| HDL cholesterol, mg/ dL | 49.9 | 51.1 | 51.2 | .262 |
| HDL cholesterol <38 mg/dL, % | 21.6% | 21.9% | 25.5% | .553 |
| Total cholesterol, mg/ dL | 193.9 | 192.2 | 190.6 | .410 |
| Total cholesterol ≥225 mg/dL, % | 19.9% | 19.8% | 20.4% | .983 |
| Triglycerides, mg/dL, median | 107.1 | 105.3 | 114.2 | .011 |
| Triglycerides ≥169 mg/ dL, % | 21.4% | 20.5% | 18.9% | .792 |
Note: CRP = C reactive protein; DBP = diastolic blood pressure; HDL = high-density lipoproteins; LDL = low-density lipoproteins; SBP = systolic blood pressure; SD = standard deviation; WBC = white blood cell.
a p-values were obtained by analysis of variance or nonparametric equivalent for continuous biomarkers and χ2 for dichotomously modeled biomarkers.
bUnless otherwise stated.
Sensitivity Analyses
Baseline characteristics were virtually the same between persons who had four frailty measures and those who had five (Supplementary Table S4). The estimate of frailty prevalence was less than 1% higher among persons with data on all frailty components (n = 2,061; Supplementary Table S5). Similarly, the frailty prevalence was negligibly different (7.0% to 6.3%) when we used the lowest quintile of grip strength by sex and BMI quartiles to identify weakness and the lowest quintile of gait speed by sex and median height to determine slowness (Supplementary Table S6). Quartiles of BMI and median height were much lower among the CHARLS cohort than the CHS cohort, where the PFP scale was initially developed (Supplementary Table S7). The sex and BMI-specific cut-points for defining weakness were comparable between the CHARLS and the CHS cohort. The sex- and height-specific cutoffs for defining slowness were lower in the CHARLS cohort compared to either the CHS or the NHATS cohort. The prevalence of each frailty component was also similar between the CHARLS and three U.S. cohorts (Supplementary Table S8).
Discussion
In this large, nationally representative sample of Chinese older adults, we found 7% of Chinese adults aged 60 years or older were frail. Frailty prevalence differed substantially across age, sex, education, and marital status. We observed substantial geographic heterogeneity and rural–urban disparities in frailty prevalence in China. We found that frail Chinese older adults had excessive burden of adverse health and functioning outcomes. The PFP scale was developed and mainly utilized in western populations. This work demonstrates its utility for identifying older adults who are frail in China, a country that has the largest aging population in the world. Our findings also contribute to a better understanding of China’s increasingly growing regional disparities in health and health care resources (15) and have implications for public health policy and practice. A standardized evaluation of frailty, which provides a means to improve the detection, treatment, and management of high-risk populations, may be incorporated into China’s existing health care system to reduce morbidity, prevent disability, and curb excessive health care costs.
Our findings are consistent with previous studies, showing that frailty prevalence was higher at older ages, among women, persons with unfavorable socioeconomic status, and those who lived alone or were not married (2,4,16,17). Additionally, we found that frailty prevalence was 50% higher in rural versus urban areas, and coastal regions had a lower frailty prevalence than inland regions. These findings are in line with a large body of literature demonstrating health disparities between rural and urban areas (18,19) and regional health disparities in China (20,21).
We found that frail Chinese elders had higher prevalence of chronic conditions than the robust. Moreover, frail Chinese elders had excessive burden of adverse health events including falls, depression, disability, and functional limitation. These findings were echoed in an earlier study by Chen et al. (17), who found that 55% of frail Chinese adults aged 65 years or older had eating difficulty and nearly 50% had mobility impairments. Moreover, we demonstrated that there is only modest overlap between frailty, disability, and comorbidity among Chinese older adults—supporting the view that these three clinical entities are conceptually distinct (22).
We observed an elevated level of CRP among frail Chinese older adults. The association of inflammatory markers with frailty has been repeatedly reported (23–26). Our findings corroborate the view that inflammation plays an important role in the pathogenesis of frailty. Additionally, we showed that 14.3% frail persons had a hemoglobin concentration below 12 g/dL, a cutoff for defining anemia by World Health Organization (27), as opposed to only 8.8% among the robust. Similar findings have been reported among U.S. older women (26). Furthermore, compared with the robust, proportionally more frail persons had an elevated level of cystatin C, a marker of kidney function (28). Previous studies have demonstrated an association of impaired kidney function and chronic kidney disease with frailty (29).
Our study has several strengths. First, we are among the first to utilize the PFP scale—a widely used and validated frailty assessment—to estimate frailty prevalence in China using a nationally representative sample. Second, we constructed cut-points for defining five PFP criteria in Chinese elders, allowing for standardized screening for frailty in routine clinical practice in China. Third, this study is the first to examine regional variation in frailty in mainland China. Fourth, the association of biomarkers with frailty has not been investigated previously among Chinese elders. Our findings may enhance the understanding of the physiological basis of frailty among Chinese elders and provide insights into whether there exists a general pathogenesis of frailty.
This study has limitations. First, we did not include nursing-homes residents because only community-dwellers were enrolled in the baseline survey of the CHARLS. We may therefore underestimate the prevalence of frailty among the entire Chinese elderly population; however, this is not likely to result in severe bias because only 1.5% of older adults live in nursing homes in China (30). Second, all five frailty components were only measured once; these measures may vary over time. Future research needs to characterize the trajectories of frailty and examine their relation to adverse outcomes. Finally, we were unable to establish a causal association of chronic conditions and disability with frailty because our study is a cross-sectional analysis. Future research utilizing longitudinal design may elucidate a potential dynamic relationship between these related but conceptually distinct clinical entities.
To our knowledge, this is the first study utilizing the PFP scale to examine frailty prevalence in a nationally representative sample of noninstitutionalized Chinese adults aged 60 years or older. We demonstrated the possibility of using the PFP scale to identify frail Chinese older adults and found substantial sociodemographic and regional disparities in frailty prevalence in China. Five PFP measures are relatively inexpensive and can be easily administered in clinical settings, providing a basis for standardized screening for frailty in geriatric practice. Given the paucity of data on frailty among Chinese older adults, which represent the world’s largest aging population, our study may serve as a basis for future research aimed at evaluating the value of frailty in predicting outcomes and identifying physiological, behavioral, and psychosocial risk factors of frailty in China.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging (K01AG039387 and R01AG46206).
Conflict of Interest
The authors have no conflicts of interest and no financial associations to disclose.
Supplementary Material
Reference
- 1. Ensrud KE, Ewing SK, Taylor BC et al. . Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi:10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandeen-Roche K, Seplaki CL, Huang J et al. . Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi:10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Prince M, Thiyagarajan JA et al. . Frailty: an emerging public health priority. J Am Med Dir Assoc 2016;17:188–192. doi:10.1016/j.jamda.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 7. United Nations and Affairs DoEaS. World population prospects: the 2012 revision 2012. http://www.un.org/en/development/desa/population/theme/trends/index.shtml Accessed January 1, 2017.
- 8. Wu Y, Dang J.. Blue Book of Aging: China Report of the Development on Aging Cause (2013). Beijing: Social Sciences Academic Press (China); 2013. [Google Scholar]
- 9. United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables. New York, NY: United Nations; 2015. [Google Scholar]
- 10. Hu S, Tang S, Liu Y, Zhao Y, Escobar ML, de Ferranti D. Reform of how health care is paid for in China: challenges and opportunities. Lancet. 2008;372:1846–1853. doi:10.1016/S0140-6736(08)61368-9 [DOI] [PubMed] [Google Scholar]
- 11. Tang S, Meng Q, Chen L, Bekedam H, Evans T, Whitehead M. Tackling the challenges to health equity in China. Lancet. 2008;372:1493–1501. doi:10.1016/S0140-6736(08)61364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol 2012;43:61–68. doi:10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psych Meas 1977;1:385–401. doi:10.1177/014662167700100306 [Google Scholar]
- 14. Cheng ST, Chan AC. The Center for Epidemiologic Studies Depression Scale in older Chinese: thresholds for long and short forms. Int J Geriatr Psychiatry. 2005;20:465–470. doi:10.1002/gps.1314 [DOI] [PubMed] [Google Scholar]
- 15. Bureau SS. China Statistical Yearbook 2006. Beijing: Chinese Statistics Press; 2006. [Google Scholar]
- 16. Bandeen-Roche K, Xue QL, Ferrucci L et al. . Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. [DOI] [PubMed] [Google Scholar]
- 17. Chen LJ, Chen CY, Lue BH, Tseng MY, Wu SC. Prevalence and associated factors of frailty among elderly people in Taiwan. Int J Gerontol 2014; 8: 114–119. doi:10.1016/j.ijge.2013.12.002 [Google Scholar]
- 18. Fang H, Chen J, Rizzo JA. Explaining urban-rural health disparities in China. Med Care. 2009;47:1209–1216. doi:10.1097/MLR.0b013e3181adcc32 [DOI] [PubMed] [Google Scholar]
- 19. Yang W, Kanavos P. The less healthy urban population: income-related health inequality in China. BMC Public Health 2012;12:804. doi:10.1186/1471-2458-12-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H, Zhang L, Zou HF. Regional Disparity In Health And Health Care In China. China Economics And Management Academy, Central University of Finance and Economics; 2012. [Google Scholar]
- 21. Mu R. Regional disparities in self-reported health: evidence from Chinese older adults. Health Econ. 2014;23:529–549. doi:10.1002/hec.2929 [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 23. Walston J, McBurnie MA, Newman A et al. ; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. [DOI] [PubMed] [Google Scholar]
- 24. Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi:10.1111/j.1532-5415.2007.01186.x [DOI] [PubMed] [Google Scholar]
- 25. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi:10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. Nutritional Anemias: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1968. [Google Scholar]
- 28. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002;40:221–226. doi:http://dx.doi.org/10.1053/ajkd.2002.34487 [DOI] [PubMed] [Google Scholar]
- 29. Shlipak MG, Stehman-Breen C, Fried LF et al. . The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. [DOI] [PubMed] [Google Scholar]
- 30. Chu LW, Chi I. Nursing homes in China. J Am Med Dir Assoc. 2008;9:237–243. doi:10.1016/j.jamda.2008.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.