Abstract
Background/Objectives
Resilience, the ability to resist or recover from adverse effects of a stressor, is of widespread interest in social, psychologic, biologic, and medical research and particularly salient as the capacity to respond to stressors becomes diminished with aging. To date, research on human resilience responses to and factors influencing these responses has been limited.
Methods
The National Institute on Aging convened a workshop in August 2015 on needs for research to improve measures to predict and assess resilience in human aging. Effects of aging-related factors in impairing homeostatic responses were developed from examples illustrating multiple determinants of clinical resilience outcomes. Research directions were identified by workshop participants.
Results
Research needs identified included expanded uses of clinical data and specimens in predicting or assessing resilience, and contributions from epidemiological studies in identifying long-term predictors. Better measures, including simulation tests, are needed to assess resilience and its determinants. Mechanistic studies should include exploration of influences of biologic aging processes on human resiliencies. Important resource and infrastructure needs include consensus phenotype definitions of specific resiliencies, capacity to link epidemiological and clinical resilience data, sensor technology to capture responses to stressors, better laboratory animal models of human resiliencies, and new analytic methods to understand the effects of multiple determinants of stress responses.
Conclusions
Extending the focus of care and research to improving the capacity to respond to stressors could benefit older adults in promoting a healthier life span.
Keywords: Physiologic resilience, Human aging, Epidemiology, Biologic aging
Resilience, the ability to resist or recover from adverse effects of a stressor, has been of widespread interest in numerous areas of social, psychologic, biologic, and medical research (1,2) and may be considered as the opposite of vulnerability (3). Resilience is especially important as it is diminished with age just as the risk of many stressors is increasing. Low levels of resilience confer vulnerability to stressors, leading to adverse outcomes. Thus, higher levels of resilience can result in desirable clinical or functional outcomes, and can thus serve as goals for health maintenance or therapeutic strategies. Research on the determinants of resilience of stress responses and the effects of interventions that modulate resilience can further these goals. Advanced age is accompanied by an increasing exposure to varied psychosocial and medical stressors, for example, bereavement, loss of one’s home, surgery, hip fracture, or myocardial infarction. Correspondingly, increasing age is also accompanied by an increasing likelihood of impairments in the ability to respond effectively to such challenges. A considerable amount of research has focused on the influence of psychosocial factors on resilience to age-related social and behavioral stressors and (to a lesser degree) health-related stressors (4,5). However, there has been only limited research in humans on resilience of responses to physiologic or pathological stressors, and clinical and physiologic factors influencing these responses (Table 1).
Table 1.
Examples of Stressors, Resilient Responses, and Regulatory Factors
| Stressor | Potential Adverse Consequences of Stressor | Resilient Clinical or Functional Response | Example Systems Influencing Level of Resilience |
|---|---|---|---|
| Exposure to infectious agent | • Septicemia | • Avoidance of infection | • Immune |
| • Restricted activity | • Rapid recovery from infection | • Pulmonary | |
| • Mortality | • Genitourinary | ||
| • Accelerated LBM loss | • Dermatologic | ||
| Hip fracture | • Persistent mobility disability | • Recovery of ambulation within 30 days | • Circulatory |
| • Fracture nonunion | • Fracture union | • Musculoskeletal | |
| • Neurologic | |||
| Exposure to anticholinergic drugs | • Delirium | • Maintain cognitive function | • Neurologic |
| • Sensory | |||
| • Renal hepatic |
Better methods to predict and assess resilience and vulnerability in older persons may contribute to progress in treating and preventing disease and disability across several domains:
• Therapeutic decision-making (eg, on options for surgery or chemotherapy) taking into account individualized risks for specific adverse outcomes.
• “Pre-habilitation” strategies to lessen risks of adverse outcomes from surgery or other risky procedures.
• Acute care management strategies incorporating better or quicker detection of incident risks for complications or sequelae, for example, delirium.
• Preventive strategies to diminish risk for adverse events such as falls and infections in healthy older persons.
• Rehabilitation strategies that improve the rate and degree of recovery from stressors.
• Assessment of possible adverse medication effects on vulnerability to stressors.
• Improved resilience biomarkers for assessing potential benefit and risks of new interventions that can improve health span.
The National Institute on Aging (NIA) convened a workshop on August 26–27, 2015 to identify the needs and opportunities for research to improve measures to predict and assess resilience. The meeting was organized as part of an effort to better conceptualize and study the dynamic nature of physiologic responses to stressors and their role in maintaining or regaining normal homeostasis and function. Thus, an overarching conceptual premise of the workshop was that specific resilient responses differ depending on both the stressor being exerted on the individual and the clinical or physiologic property of interest to be maintained or restored. Hence the workshop considered specific aspects of “resiliencies” as a group of differing responses, dependent on the type of stressor applied. Thus, when we refer to “resilience” we specify the specific aspects critical to a specific stress. We considered the range of varied responses in multiple different systems and their interactions rather than considering “resilience” as a single global construct. Nevertheless, we recognize that some properties and mechanisms may be shared by different categories of resilience. Thus, the potential influence of common underlying factors, including aging mechanisms, on multiple types of resilience was noted as an important topic for future research.
This workshop followed a 2014 NIA-sponsored workshop on resilience in laboratory animals (6,7), which focused principally on identifying tests of key aspects of resilience that could serve as predictive markers for assessing candidate interventions that target fundamental aging processes with potential human relevance. Resilience tests reflective of the status of multiple physiologic systems rather than a single system were a priority. The 2015 workshop focused principally on aging-related aspects of human resiliencies in response to clinically or functionally meaningful “real-world” stressors. A shared premise of both workshops is that resilience measures of responses to stressors have the potential to provide increased sensitivity to effects of aging-related mechanisms that influence longevity and health span, but might not be detectable under basal unstressed conditions until very advanced age.
Research domains addressed by the workshop include as follows:
Concepts Related to Resilience
The concept of resilience has been applied to a wide range of systems ranging from cells to individuals to communities, and to psychological, behavioral, physiologic, clinical, and social outcomes. Recently, physical resilience at the whole person level has been defined as a characteristic which determines one’s ability to resist or recover from functional decline following a health stressor(s) (2). The concept of resilience can also be applied to physiologic and cellular responses to stressors, which can influence whole-person resilience.
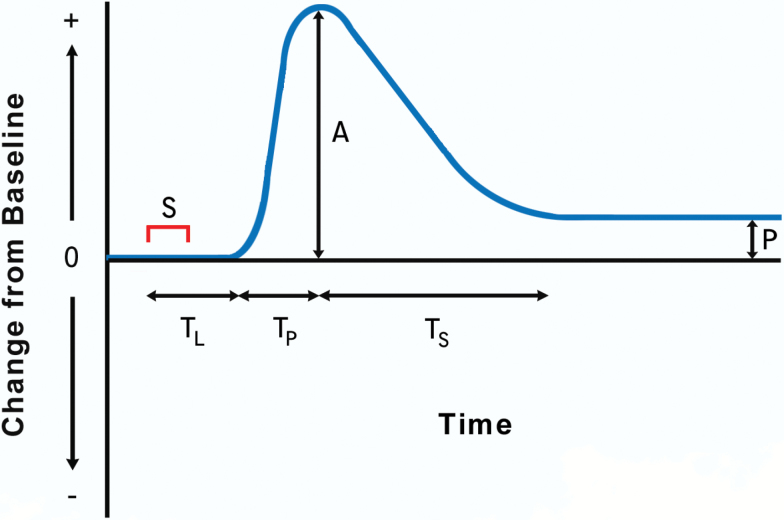
Depending on the outcome of interest, resilience can be evaluated as a dichotomous trait (nonoccurrence vs occurrence of an adverse event following a stressor), or as a continuous quantitative trait (degree of perturbation of a function following a stressor). Resilience in response to a stressor of given magnitude and duration may be conceptualized as homeostatic response with sequential components:
• prestress basal levels of the property of interest (eg, blood pressure, body temperature, circulating glucose levels, balance, cognition, strength)
• rate of increase and peak magnitude of the perturbation (if any) in response to the stress. (The term resistance has been used to characterize the extent to which this initial perturbation is minimized)
• time course of recovery (if present) toward baseline conditions after perturbation
• level at which the property stabilizes (if this occurs) after the recovery phase
These response components are illustrated in Figure 1, with each potentially exerting differing effects on clinical or physiologic outcomes following a given stressor. Thus, quantifying each (and relationships among them), rather than generating a single summary measure, can be of value in resilience studies.
Figure 1.
Trajectory of change in a physiologic property or function from baseline levels after exposure to a stressor (S). L: lag time before perturbation begins; TP: interval from initial to maximum perturbation; A: maximum perturbation: TS: interval from maximum perturbation to stabilization; P: persisting difference from baseline level. Adapted from figure in Kirkland and colleagues (6).
Alternative Trajectories of Stress Responses: Role of Physiologic Reserve and Threshold Effects
In many cases, the trajectory of response to a stressor does not follow the classic homeostasis pattern of perturbation followed by return to basal conditions. In some cases, the stress is insufficient to detectably perturb the property of interest, that is, the property is fully resistant to the stressor. In others, the degree of perturbation never diminishes (ie, recovery never begins) and may continue to increase until terminated by an adverse event. In still other cases, recovery occurs, but the property stabilizes at a level higher or lower than its prestress level.
The degree of physiologic reserve can influence the type of response trajectory. Reserve may be defined as the difference between the basal level of a system and its maximal capacity to respond. The amount of reserve can determine threshold stress levels above which the system becomes perturbed or, if perturbed, cannot return to its prestress state. The threshold level of stress for dichotomous outcomes (eg, fracture/nonfracture) may thus be determined by the level of reserve (eg, bone maximum capacity to withstand shear stress). Reserve may be influenced by aging, disease, and prior exposure to stressors. Prior stress exposures may enhance reserve and resilience through an anamnestic immune response as occurs following vaccination or via hormesis, a term used to refer to the ability of substances normally viewed as toxins to generate beneficial effects at very low concentrations (8). Conversely, the concept of allostatic load (9), suggests that repeated or chronic stress may result in “wear and tear” on neural and endocrine systems over time, with potentially negative effects on resilience.
Homeostatic Mechanism Responses
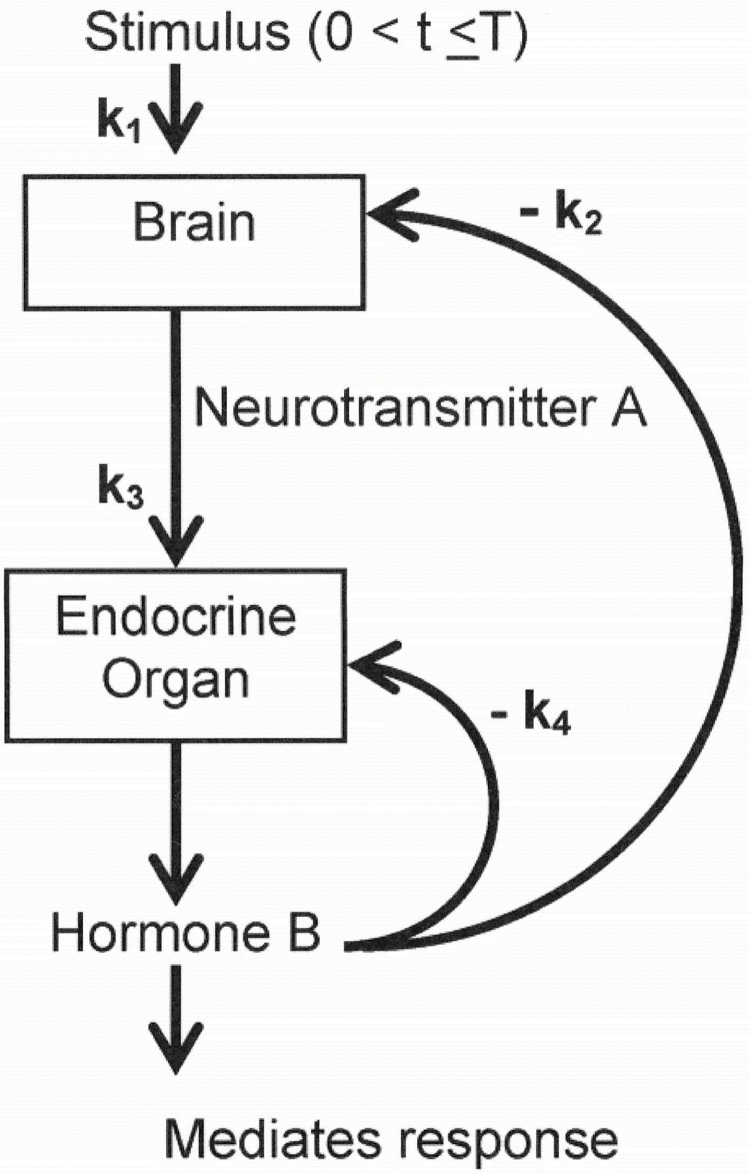
Besides characterizing the series of components of a physiologic property’s resilience, concepts illustrated in Figure 1 can also be used to characterize the series of responses by the cellular and physiologic systems that are activated when that property is perturbed (eg, changes in circulating norepinephrine levels in response to cold exposure), and serve as mechanisms for minimizing perturbations and/or recovering from them. These components of homeostatic responses to an acute stressor, and their interactions, can be elucidated by dynamical systems modeling approaches, using the stimulus-response experimental paradigm (10). The use of such approaches is shown in Figure 2, which illustrates relationships between a stressor and brain and endocrine responses operating in feedback loops is determined by rate constants K1 − K4 that control the rate and magnitude of these responses. Using experimental manipulations, this approach can be used to estimate the parameters for a specific system and to predict how alterations in them would affect physiologic responses to the stressor.
Figure 2.
Dynamical responses to a stressor. Relationships between a stressor and responses in two systems with feedback loops controlling the rate and magnitude of the responses. Source: Varadhan and colleagues (10).
Longer-Term Characterization of Resilience
Many stressors are chronic rather than acute and can persist longer, even days or years. Additionally, many acute stressors can occur repeatedly or concurrently with other stressors. Longer-term exposure to chronic or repeated stressors may affect acute stress responses. Such effects could be protective (eg, exposure to a pathogen improving immune responses) or adverse (eg, depletion of a stem cell population needed for tissue regeneration).
Sustained or repeated stress exposure may also contribute to changes over time in “basal” levels of physiologic functions and physiologic reserve, either favorably or adversely. Biologic repair processes are likely to influence the effects of chronic or repeated stressors on changes over time in basal levels of function and responsiveness. Thus, it would be critical to evaluate basal function as well as responsiveness in characterizing resilience to sustained or repeated stressors.
Factors Influencing Resiliencies in Aging
Resilience and Homeostenosis
Diminished resiliencies can often be usefully considered as impairments in homeostasis, often referred to as homeostenosis (11). With increasing age there is a greater likelihood of diminished capacities to maintain homeostasis in response to stressors such as elevated or lowered ambient temperature, elevated or lowered serum glucose, fluid depletion or overload, orthostasis, sepsis, trauma, fractures, bed rest, and chemotherapeutic agents (12). There is a need to better characterize key physiologic features of age-related decrements in homeostasis, and of resilient responses maintaining it. The possibility of shared physiologic mechanisms responsible for decrements in multiple types of resilience is of particular interest (13).
Knowledge of dose–response relationships to the magnitude of stressors is particularly important in understanding resilience because of the potential for threshold effects. In addition, since stressors frequently occur in combinations that affect homeostatic responses (eg, medication and dehydration effects on blood pressure) information on additive or synergic effects of co-occurring stressors is crucial for characterizing “real-world” resilience. Dose–response relationships and combinations of either concurrent or sequential stressors may produce emergent effects not predictable from a single stressor level or an isolated stress.
Shared Features of Age-Related Impairments in Resilience
Many factors associated with aging-related decrements in homeostasis may characterize loss of resilient responses in multiple domains. Features associated with impaired resilience in differing systems include higher basal activity of some regulatory factors (eg, sympathetic nervous system activity, mild pro-inflammatory state), lower end-organ responsiveness to such factors, and loss of negative feedback inhibition (eg, in degree of changes in baroreceptor activation in response to changes in blood pressure). Age-related impairments in feedback loops may also be related to exaggerated stimulus responses of some regulatory factors and delays in return to baseline levels, for example, attenuated and delayed glucose recovery post-insulin challenge (14) or dysregulated cortisol patterns (10). These features are shared in age-related changes in responses to differing SNS stressors (eg, orthostasis and glucose load), and responses of other homeostatic regulatory systems, for example, the hypothalamic-pituitary axis (6,11).
Failure to return at any point to prestress levels of function is common in older persons. Alternatively, some stressors, for example, antigen exposure, may induce sustained stable poststress improvements in function. For analyses of the determinants of such shifts among stable states, the toggle switch or “tipping point” concept can be useful. In this approach, resilience can be considered to be categorically present with either maintenance of prestress state or shift to a more favorable or less favorable state and absent if there is no return to any stable state.
Research on determinants of specific types of resilience is needed for development of better diagnostic tests that might identify presently treatable contributors to impaired resiliencies. It is also essential for development of more effective interventions to improve resilience. The mechanisms influencing physiologic resilience can be considered in a variety of dimensions, for example, molecular, cellular, tissue, and system dynamics, and (although not a primary focus of the workshop) psychosocial factors. Understanding how these dimensions interact could lead to finding better targets for intervention.
The value of establishing “performance requirements” of different biologic systems (eg, various forms of resilience biomarkers) for specific types of resilience was noted. Since most types of resilience likely involve multiple interacting physiologic components, requirements for one component may be affected by performance of another component. This consideration is especially important in the presence of multimorbidity. Such interactions pose challenges and opportunities for tests and analytic methods to assess these interacting factors.
Physiologic Influences on Resiliencies
Genetic, environmental, physiologic, cellular, and molecular functions, and their interactions, can influence resiliencies. Studies on frailty in older persons, which has been associated with impairments in some types of resilience (eg, postsurgical outcomes) (15) suggest that at a minimum five major, interconnected physiologic domains may influence degree of resilience: autonomic nervous system, hypothalamic-pituitary axis, innate immunity, renin-angiotensin system, and insulin/growth hormone/IGF-1 pathways. Interactions among these systems in responses to specific stressors can markedly influence resiliencies. For example, exaggerated production of inflammatory mediators or cortisol may interfere with return to basal conditions.
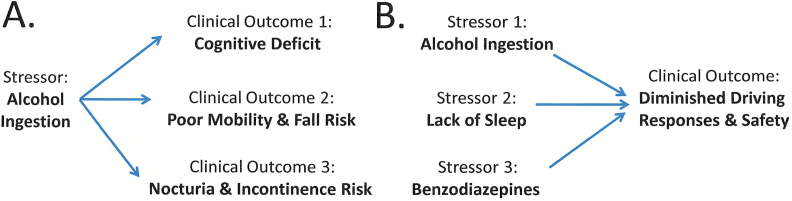
The complexity of interactions suggests that simultaneous assessment of responses in multiple physiologic domains in response to a given stressor will be crucial for understanding the determinants of resilience of responses to it (14) spanning multiple systems. This need is augmented by the fact that many real-world stressors are complex, placing simultaneous demands on multiple functions, and at times interacting with other stressors, for example, cognitive and motor challenges in avoiding an unexpected obstacle when driving or walking. Thus, for example, a single stressor (eg, evening glass of alcohol or “nightcap”) may at the same time adversely influence multiple different functional domains resulting in function deficits involving cognition, falls or nocturia, and incontinence (Figure 3A). Conversely, the temporal convergence of different stressors (eg, alcohol ingestion, lack of sleep, benzodiazepines) in one individual may result in lack of overall resilience when dealing with complex tasks such as driving or even walking (Figure 3B).
Figure 3.
The manner by which resilience factors may interact across different systems. (A) A single stressor (eg, alcohol ingestion) may result in multiple different adverse clinical outcomes (eg, cognitive deficit; poor mobility, and enhanced fall risk; nocturia and enhanced incontinence risk). (B) Multiple different stressors (eg, alcohol ingestion; lack of sleep; benzodiazepine ingestion) may also both individually and collectively enhance the risk of a relevant clinical outcome (eg, diminished driving responses and safety). In both cases, declines in resilience mechanisms shared across different systems might contribute to enhanced vulnerability in the face of multiple varying stressors or outcomes.
Integration of Molecular, Cellular, Tissue, Organ, and Systemic Processes in Resilience
Systematic understanding of an individual’s resilience requires an appreciation of the continuum of the determinants of resilience spanning cell to organ, to whole organism, and to family and society. Responses at any level generally have multiple determinants, with relative roles directly altered under stress, and relative importance depending on the level of stress. Multiple contributors to resiliencies may also serve as redundant systems, with some components becoming more active in the case of deficiency in others (16).
Physiologic resilient responses entail the integration of processes occurring over differing time scales ranging from seconds (eg, cellular signaling) to months (eg, recovery of muscle strength after injury). For example, the rate at which cellular factors regulating NF-kappa-beta shuttle between the cytoplasm and nucleus over short time scales may determine changes in levels of circulating inflammatory factors over longer periods. Similarly, rates of short pulsatile beta-cell insulin release influence longer-term trajectories of glucose levels in response to glycemic loads.
The contribution of these processes and their degree of integration can be assessed by time-series measures showing short- and long-term fluctuations in levels of a given function (eg, heart rate, balance, or gait). These measures provide an indicator of the complexity of regulation of a function, that is, the extent to which multiple inputs interact over different time or spatial scales to produce a given function. Time series analyses can distinguish patterns that reflect the integration and correlation of multiple inputs over different scales in time or space (eg, fractal patterns) from those that are random, lacking any functional connection among them (eg, white noise) (17). With aging, many physiologic control systems lose complexity. Such diminutions have been associated with some types of decreased resilience (eg, frailty, myocardial infarction, lethal arrhythmias, poor balance, and elevated risk of falling).
Some types of resilience require a specific sequence of processes, in which completion of one is critical for ability to conduct the next successfully. One example is wound healing in which an inflammatory phase is followed by a proliferative phase involving fibro blast and vascular cells, which in turn is followed by matrix deposition and remodeling. Aging-related alterations in the completion, timing, and the magnitude of components of such co-ordinated sequences may influence subsequent steps in the sequence, adversely affecting resiliencies. For example, with advancing age, both the rise and diminution of factors such IL-6 and CRP in responses to stimuli can be delayed, resulting in persistent elevation of these factors. Nevertheless, in spite of the key role of circulating IL-6 levels as a systematic marker of inflammation, as well as predictor of frailty, disability, and death, IL-6 is at the same time required for vaccine-mediated responses to influenza vaccination and exercise-mediated improvements in muscle performance.
Age-related changes in resilience responses may reflect pathological and/or compensatory responses. For example, it has been suggested that age-related insulin resistance can be compensatory, to reduce glucose metabolism so as to reduce oxidative stress generated during oxidative phosphorylation.
The above considerations imply that understanding of the causes and consequences of impaired resilience will require testing using varying levels of stress, evaluation of responses over multiple time scales, and assessment of cellular and physiological effects over multiple domains.
Influence of Biologic Aging Mechanisms on Resilience
Given the increasing frequency of impairments in many types of resilience with aging, there is a prima facie case for research on possible influences of aging mechanisms on levels of resiliencies. Aging-related changes in cellular and molecular functions, for example, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, or dysfunction (18,19) could influence changes in a variety of types of resilience. Several involve diminution or inadequacy of processes to regulate cell functions or survival (eg, maintain macromolecular integrity, remove defective molecules, maintain replicative capacity, or make ATP). Progress on understanding effects of aging-related changes in stem cell function on muscle injury repair and the influence of aging changes in inflammation and bone cell progenitor populations on fracture repair illustrate the potential insights from studies on such aging mechanisms.
These aging mechanisms could contribute to progressive impairments in clinical and physiologic resiliencies with age. Chronic inadequacies of protective/regulatory processes could lead to increasing malfunction, disruption, or loss of body “machinery” (eg, cells, receptors, signaling pathways, mitochondria) needed for resilient responses. Moreover, even with intact machinery, age-related impairments in the regulation of protective/regulatory stress responses (eg, impaired induction and/or subsequent diminution of these responses) may exist. In cases where laboratory animal resilience tests can provide a measure of impairments in such mechanisms, there may be value in developing analogous human tests.
Considerations for Human Studies
Types of Human Resilience Outcomes
In the clinical setting, resilient responses can be characterized in terms of outcomes. These include clinical (eg, postmyocardial infarction recovery of myocardial function), physiologic (eg, postinfarction changes in enzyme levels), and functional (eg, mobility, cognition, or composite scales such as Instrumental Activities of Daily Living). In clinical studies on resilience, it is important to consider patient preferences, “ability outcomes” as well as disabilities, since many performance measures have ceiling effects and are therefore cannot characterize recovery to high levels of function.
Cascades of Stressors Influencing Outcomes
A prominent feature of several types of clinical resiliencies is the need to respond to stressors that are triggered by either preceding or co-existing stressors or by responses to those stressors. A dramatic example is provided by elective surgery, where the stress of surgery might be followed by a cascade of complicating stressors such as bleeding from inadequate coagulation, followed by a myocardial infarction that requires additional procedures. These in turn will stress respiratory and renal function, with combined effects on risk of mortality. Similarly, inadequate ability to respond to an initial stressor, such as a balance perturbation, can lead to a fall with mechanical stress sufficient to fracture an osteoporotic hip, incurring the need for resilient healing responses, and resilience in recovery of physical function after restricted mobility. Also, failure of immune defenses against influenza infection places secondary stressors on pulmonary and cardiac function.
In such cases, succeeding stressors place differing demands on differing systems, and affect differing clinical outcomes. Each occurs under physiologic conditions influenced by the preceding stressor, and responses to it, including interventions against it. Over time, a series of stressors and responses may deplete physiologic reserves prior to exposure to the next stressor. This may be illustrated by the eventual deconditioning after a series of major stressors. Clinical resilience to a stressor under such conditions where reserve has been compromised may differ from resilience when the stressor occurs during healthy equilibrium.
Even within a clinical scenario, there is great heterogeneity. In the cancer field, differing types of cancer and their associated therapies can impose differing stressors, as can differing infectious agents. In addition, there is great heterogeneity of severity of stressors, for example, cancer stages, extent of pathogen exposures, and magnitude of balance perturbations. More severe stress levels may trigger threshold effects not caused by milder levels, for example, by placing demands exceeding physiologic reserve for a given response.
Research Opportunities and Needs
Uses of Clinical Data in Predicting or Assessing Resilience
Clinical data can provide information on specific aspects of resilience prior to the onset of a stressor. For example, pre-operative testing assesses reserve in kidney function and an ECG may expose signs of previously unrecognized myocardial ischemia. Outpatient, inpatient, rehabilitation and long term care records will need to be combined to capture the baseline, stressors, and recovery over time.
Analyses of health records can be used to assess specific patient characteristics that predict vulnerabilities to events such as falls or infections. Metrics based on this approach could be used by health care systems to identify particularly vulnerable patients and to identify specific treatable sources of vulnerability in advance. However, this approach depends on current clinical practice for risk and outcome assessments. A common challenge noted for acute events such as a fall or symptomatic infection is the frequent lack of sufficient information in clinical records on pre-existing factors that may have influenced susceptibility to a stressor that led to the event. Nevertheless, new methods such as natural language processing can capture clinical information in useful ways that will enhance the utility of clinical research to identify both exposures and outcomes (20).
There are several challenges for ascertaining outcomes based on clinical data, including electronic health records. These were noted particularly for outpatient events, such as influenza infection, for which medical records may only capture patients with worse outcomes from infections, but not all infected patients. Conversely, those who are more resilient may not seek medical attention.
Current measures of patients’ prestress clinical status for predicting resilience or vulnerability to stressors are of value but very limited. Though substantial deficits in health or function have been shown to be related to likelihood of adverse outcomes after surgery, hospitalization, or infection, risk of falls or fractures, and recovery of ambulation after hip fracture, predictive ability remains limited.
A particular challenge was noted for use of clinical information in identifying vulnerabilities in more healthy functionally independent older persons to diminish risk of adverse events or incomplete recovery from them. Greater use of measures of functional status and physical performance (particularly those with sufficient sensitivity to modest decrements that may markedly influence risk) in “prevention” patient visits may be valuable for this purpose. The incorporation of such measures into electronic health records could facilitate analyses in large populations that could enhance health care systems’ ability to target preventive interventions cost-effectively, improve accessibility of data, and enhance research.
Opportunities for Acquiring Additional Point-of-Care Data and Specimens
Encounters with patients experiencing stress or receiving stressful treatments (eg, surgery or chemotherapy) provide unique opportunities to obtain crucial information on determinants of resilience. Assays on surgically excised specimens could allow mechanistic studies on tissues not available otherwise, such as models to obtain visceral neuronal responses from colon, appendix, and mesenteric specimens. Frequent serial measurements of circulating factors before and after treatments in hospitalized patients or chemotherapy patients can provide information on the timing and sequence of stress responses that may determine the degree of resiliencies. For example, increasing levels of inflammatory cytokines could be useful in predicting coronary events, mortality after sepsis, organ dysfunction in mechanically ventilated patients, and readmissions after heart failure. The likelihood for needing autologous blood transfusions following elective surgical procedures can be predicted using an individual’s baseline characteristics associated with risk of procedure. Specimens collected from healthy cosmetic surgery patients can be particularly valuable for comparisons with those from less healthy patients for assessing factors that distinguish high levels of resilience from impaired resiliencies such as wound healing.
In many instances, there are needs to develop or adapt resilience measures in order to make them feasible in point-of-care studies. Such measures need to be not only valid and reliable, but also feasible and acceptable in conditions when patients may be acutely ill, and in care settings with limitations on opportunities for patient testing.
Point-of-care specimens and other information in healthy outpatients collected over a series of routine checkups or “wellness visits” can be valuable for tests to detect trends in factors whose effects on resiliencies may ultimately reach clinical significance, for example, changes of circulating factors within normal limits.
Contributions From Epidemiological Studies
Longitudinal epidemiological studies have the capacity to measure wide-ranging variables in individuals before and after exposure to acute or chronic stressors. They provide the ability to obtain extensive phenotyping and self-reported information with substantially greater depth and breadth than that found in health care records. Longitudinal studies also often include substantial representation of healthy persons with high levels of resilience, a group with limited relevant information in health care records.
Although population studies rarely match laboratory studies’ ability to control or quantify acute stress exposures and physiologic responses to them, they can provide crucial data from the real world of multiple stressors and responses to them over time. Analytic methods developed to assess multiple risk factors and their interactions can be applied to identify “resilience factors” that are associated with decreased vulnerability to stressors, and assess their influence on stress responses.
Population heterogeneity in such studies provides opportunities to increase the specificity of understanding of resilience factors, for example, to identify which components of physical activity may confer greater resilience to a given stressor (21). Heterogeneity within populations in various combinations of resilience factors also can provide insights into their interactive effects. However, these approaches often require very large population sizes and/or depth and breadth of phenotype data to provide adequate statistical power.
Longitudinal studies can assess fluctuations in expression of resilience factors, their determinants and their effects. For resilience studies on older persons, particularly the oldest old (85+), there is a need to collect data on functional and other outcomes frequently enough to allow adequate ascertainment of the fluctuations in status that characterize this vulnerable population. Such studies can examine how expression of factors influencing resilience is influenced by life-course events such as early life environment, stressful exposures, and diseases. They could also aid in determining whether effects of such exposures accumulate over time, remain constant, or decline. Long-term studies starting relatively early in life are particularly advantageous for exploring the antecedents and progression over the life course of factors influencing changes in resiliencies that may not become clinically important until old age. Longitudinal studies can also measure the relationships of differing levels of resilience to long-term clinical outcomes and rate of progression of physiological and pathological aging changes.
Incorporation of provocative resilience tests, such as exercise stress tests or frequent blood pressure monitoring with controlled physical or cognitive activity into longitudinal studies may enhance ability to predict long-term disease, disability, and mortality. Such measures may in many cases be more sensitive to pathological processes than are static measures. They thus could improve the ability to assess long-term risk for a variety of specific aging-related outcomes that affect life span and health span. Suggestive evidence for the potential role of resilience as long-term predictors comes from studies on the relationship of alterations in stability of blood pressure and gait to risk of future adverse clinical and functional outcomes, and studies on heart-rate recovery following a 400 m walk as a predictor of long-term mortality (22). Given the challenges of assessing many resilience traits in such studies, it will be important to understand how much predictive value they add to “static” measures, which in many cases may be more easily assessed.
Developing or Improving Measures to Assess or Predict Resilience and Understand Their Determinants
Table 2 shows examples of tests suggested for assessing resiliencies and vulnerabilities in the discussions of the clinical scenarios. They include tests and laboratory findings in current clinical use, tests used in research protocols, and new measures.
Table 2.
Examples of Measures for Assessing Selected Resiliencies
| Type of Resilience | Functions Required | Current or Possible Predictive or Diagnostic Measures or Tests |
|---|---|---|
| • Avoiding fall on uneven surface | • Maintaining balance | • Balance perturbation test |
| • Obstacle course performance | ||
| • The complexity of continuous postural sway measures on a balance platform | ||
| • The complexity of step-to-step gait variability | ||
| • Recovering physical function after bedrest | • Muscle power | • Dynamometry |
| • Cardiopulmonary capacity | • Muscle mass | |
| • Perceived fatigability at given physical task intensity | ||
| • Maximum or submaximal VO2 | ||
| • Avoiding postsurgical MI | • Control of thrombotic and thrombolytic mechanisms | • Cardiac stress test |
| • Electrocardiogram | ||
| • Circulating inflammatory markers | ||
| • The complexity of beat-to-beat sinus rhythm heart rate variability |
Though the examples in the table are focused on individual types of resilience, they fall into general categories of opportunities to improve prediction or assessment of resilience, and understanding the mechanisms that influence them:
Assessing Predictive Value of Current Tests or Clinical Information
Several of the tests identified measure clinical features or physiologic functions that are plausibly related to specific resiliencies, but whose predictive value has not been specifically assessed. It is likely that their predictive value will vary depending on factors such as specific underlying chronic conditions, functional status and medication use. For example, the role of nonimmune factors in resistance to infection in patients who are immunocompromised by diseases (eg, HIV) or medications (eg, glucocorticoids) may be more prominent than in those with less compromised immune function.
Adding Measures or Time Points to Existing Tests or Outcome Measures
There are several opportunities to gain information by adding measurements to existing tests. For example, measurement of continuous heart rate, blood pressure, postural sway, or other physiologic functions will permit the calculation of the complexity of these signals, thereby providing a measure of the integrity and integration of multiple systems controlling these functions. Also, measurement of recovery to baseline status for certain physiologic functions (eg, during cardiac stress tests), could provide insights into cardiac, respiratory, or skeletal muscle abilities to respond to a variety of stressors. Collection of more detailed information on cellular and other aspects of vaccine responses (eg, temperature, injection site reactivity, systemic symptoms) could allow better gradation of resilience to pathogen exposures, as could more detailed outcome measures added to clinical records, for example, characterizing infection duration, activity days lost, and level of fever. The range of outcomes assessed in lipopolysaccharide challenge tests might be extended to assess aging-related factors affecting muscle wasting, impairments in cognition, and propensity to thrombosis associated with infections. Measures of hematologic responses to cancer chemotherapy could be enhanced by studies on cellular and circulating factors influencing these responses.
Further Development and Validation of Simulation Tests
Prediction of resiliencies to “real world” stressors, and understanding of the mechanisms that determine them, can be greatly enhanced by simulation tests under controlled conditions that allow simultaneous measurement of multiple responses to a stressor of known intensity. Such tests can be particularly valuable for understanding interactions of multiple factors that affect resiliencies. However, since laboratory settings for testing responses can differ meaningfully from the conditions in which the real-world stressor being modeled occur, there is a need for validation studies using technologies to monitor both stressors and responses that could be used in daily life or hospital settings. If validated, measures using such technologies would allow assessment of resiliency responses in population studies.
Mechanistic Studies
There are both opportunities and challenges for mechanistic studies on resiliencies in humans, including studies on proposed aging mechanisms. Studies on circulating factors are subject to the caveat that circulating levels may not correlate well with levels in the tissue of interest, but can nonetheless provide insights. Circulating factors that influence tissue repair and whose levels change with age in laboratory animals have been identified through heterochronic parabiosis (23).
Ex vivo stimulation testing of cells from differing individuals can assess mechanisms influencing responses to stressors that cannot be ethically administered to humans in vivo, for example, DNA repair after exposure to carcinogens. When obtaining specimens or measurements for mechanistic measures in the tissue of interest is not feasible or ethical, there are also opportunities to validate such measurements in other tissues for their correlation with specific resiliencies. For example, imaging of the retinal microvasculature is potentially valuable for assessing factors related to wound healing in other tissues.
Selected examples of aging-related mechanisms whose influences on resilience were noted as needing further research are shown in Table 3. A striking feature of the table is that most of the mechanisms listed relate to multiple types of resilience, consistent with the concept that such processes influence a wide-variety of aging related outcomes, including types of resilience. Effects of these mechanisms on differing types of resilience may be complex, including both favorable and unfavorable influences. In addition, there is a need for studies on the influence of signaling pathways that have been implicated in the regulation of health span in experimental models (eg, growth hormone/IGF-1 axis) on aging changes in resilience, including interactions among circulating factors and the central nervous system. In addition to these illustrative examples, it is likely that many other basic mechanisms implicated in the biology of aging (18,19), may individually or collectively influence physiologic resilience.
Table 3.
Selected Examples of Potential Influences of Biologic Aging-Related Mechanisms on Types of Resilience
| Mechanisms | Type of Resilience Affected |
|---|---|
| Cell senescence | Immune responses, cancer chemotherapy tolerance, wound healing |
| Impaired stem/progenitor cell function | Resistance to infections, wound healing, cancer chemotherapy tolerance |
| Dysregulation of inflammatory factors | Infection resistance and recovery, wound healing, avoiding postsurgical MI, cancer chemotherapy intolerance |
| Inadequate DNA repair | Cancer chemotherapy tolerance |
| Impaired mitochondrial function | Ability to meet bioenergetic demands posed by stressors (eg, for averting falls, mounting febrile responses) |
Prediction of Multiple Resiliencies
Beyond identifying predictors of specific types of resilience, it would be useful to assess their predictive value for multiple resiliencies, for example, the degree to which resistance to influenza infection is associated with lowered risk of falls or quicker recovery from trauma, and the potential predictive value of wound healing responses for tolerance of chemotherapy or recovery from damage during lung infections. Studies on the impact of stress on one physiologic system (eg, immune function) on stress responses of other systems (eg, maintenance of muscle mass during bed rest), and of age-related changes in the ability of one system to maintain normal function in response to changes in another, would be valuable for elucidating the pathways contributing to multiple stressor effects and their relationship of aging changes.
There is a need to explore the potential to develop composite measures of resilience and physiologic reserve with sufficient range of sensitivity to identify persons at both very low and very high risk for any of a wide range of outcomes related to poor resilience. Such measures could be useful in identifying additional phenotypes contributing to exceptional health span, as well as very high-risk persons in particular need of targeted interventions.
Effects of Multiple Stressors on Resilience
Many functions are susceptible to multiple stressors, as illustrated by decrements in cognition associated with infection, surgery, and cancer therapies. Such stressors can occur concurrently or sequentially. The presence of multiple contributory stressors implies a need for a broader range of predictive tests that simulate simultaneous or sequential stressor exposures and compare their combined physiologic or functional effects with those of individual stressors (24).
Comparisons of test outcomes using single versus combined stressors have been used in dual-tasking tests assessing cognition while sitting versus walking. These can increase sensitivity to deficits that are not apparent without the added stress of the dual task. In principle, such dual-task tests could provide better prediction of outcomes related to decreased resilience in older person, such as falls. However, results of tests combining cognitive and physical activity stresses to date have not found them clearly superior to single-task tests in predicting falls (25,26) or discriminating between persons with and without cognitive impairment (27,28). Nonetheless, the principle of dual tasking could be extended to assess possible cumulative or synergic clinical and physiologic effects of multiple stressors. This information could be used in development of composite markers that enhance predictive value for resilience outcomes.
Determinants of High Level of Resilience
Particularly because persons with high levels of resilience are less likely to encounter health care systems for events resulting from low resilience, there is a need for strategies to include adequate numbers of such individuals in research designs. Examples include persons with documented high degree of influenza exposure who do not develop clinical infection or those who maintain high level of activities with high fall risk into advanced age, yet do not fall.
Translation of Correlations into Tools for Clinical Decision-Making
It was noted that, although correlations of various factors with the degree of resiliencies may have predictive value and suggest causal relationships, and test responses provide dose–response relationships, they will not in themselves generally provide a strong basis for clinical decisions. Use of causal analytic methods will be important in helping to distinguish factors that actually influence resilience from noncontributory covariates (16). To develop clinical decision rules, methods such as classification and regression tree analysis can be used to develop cut-points that discriminate well between persons with meaningful differences in resiliencies.
Resource and Infrastructure Needs
Development of precise consensus phenotypic definitions of differing aspects or types of physical resilience in human populations, and common protocols for assessing them to enhance comparability and synergy among studies, represents an overarching goal. This would also facilitate the construction of a shared data resource, combining epidemiological, clinical, and biological information that could be used for secondary data analyses by a wide range of investigators. Such a resource could be used for developing and testing models that integrate this range of information. Table 4 summarizes resource and infrastructure needs.
Table 4.
Needs for Research, Resources, and Infrastructure to Increase Understanding of Resilience
| Research: |
| Enhancing use of information in clinical records collected before, during, and after stress exposures |
| Expanding contributions of epidemiologic studies: |
| • Inclusion and evaluation of provocative tests |
| • Linkage with acute care data to capture stressors |
| • Assessing the role of early life-course exposures and response |
| Developing or improving measures to assess or predict specific types of resilience |
| • Assess predictive value of current tests or clinical information |
| • Adding measures or time points to existing tests |
| • Further development and validation of stimulation tests |
| • Development or improvement of tests that can be conducted in clinical settings |
| Studies on influence of biological mechanisms, including aging-related factors, on aging-related changes in resilience |
| Identifying underlying common factors predicting multiple resiliencies |
| Ascertaining effects of multiple coexisting stressors on resilience |
| Determining factors related to high levels of resilience |
| Translation of correlative data on resiliencies into tools for clinical decision-making |
| Resources and infrastructure: |
| Development of precise consensus phenotypic definitions of differing aspects and types of physical resilience, and common protocols for assessing them |
| Capabilities to link longitudinal study data with clinical information collected in stress-related encounters |
| Data linkages with health care system records to identify high-risk populations for events related to impaired resilience |
| Facilities and protocols to conduct stimulation and other tests on stress exposures safely and efficiently |
| Development of wearable sensor technology to assess and report exposures and stress responses in community-dwelling persons in daily life |
| Combined databases on specimens in repositories and tissue banks with linkage to medical records, to facilitate studies on biologic mechanisms influencing resilience |
| Additional or improved laboratory animal models having similar stress responses to those of humans of differing ages |
| New methods to analyze the regulation of stress responses, particularly to address multiple interactions, nonlinear dynamics, and emergent responses |
| Shared data resources combining epidemiologic, clinical, and biologic information for model development and testing |
Ability to link data from longitudinal studies to information collected in clinical settings during acute health care episodes could greatly enhance understanding of the determinants of successful response to acute stressors. For example, levels of inflammatory factors, such as IL-6 and TNF-alpha, collected in healthy persons during longitudinal study visits could be related to outcomes during influenza infection, such as expression of pathogen-binding receptors and immune factors in circulating cells. Longitudinal studies should consider adding novel measures of stress responses through new designs that include more frequent monitoring, perhaps by incorporating periods of more frequent assessment. Current challenges to linking longitudinal studies to clinical data include the diversity of health systems accessed by cohort members and the need for preservation of confidentiality.
Establishing harmonized data linkages with health care system records would enhance the ability to identify subpopulations at high risk for clinical events related to impaired resilience (influenza infections, falls), and recruit them for clinical and physiologic studies. Development of facilities and protocols in which studies on stress exposures in older persons can be conducted safely and efficiently, for example, with capabilities for biopsies, specimen storage, etc., as well as good access to older populations willing to participate in research, would lessen the substantial challenges for conducting such studies.
The development of wearable sensor technology for community-dwelling persons that can continuously measure physiologic factors, their baseline complexity and how they change before and during adverse events could greatly enhance understanding of determinants and predictors of resiliencies. Mobile applications designed for reporting adverse events in real time could also improve ascertainment and characterization of vulnerability-related outcomes.
Combined databases on specimens in the large number of repositories and tissue banks could improve prospects for selecting optimal tissues for a specific resilience study. Tissue resources with linkage to patient medical records, including a national bank of tissue acquired through surgical discard, can be especially valuable.
Development or refinement of additional laboratory animal models whose stress responses simulate those of humans well would facilitate mechanistic stress response studies not feasible in humans. One example is the potential for expanded use of pigs (whose wound healing characteristics resemble those of humans more closely than those of laboratory rodents) in studies of aging-related changes in wound healing. A mouse model of chronic inflammation with a variety of frailty-associated phenotypes (29) could yield insights into the role of these factors in resiliencies. Model systems should use older organisms than has been traditional, although progeroid models can also be useful.
There is also a need for development of new analytic methods for the regulation of stress responses. The multiplicity of interacting determinants of resiliencies spanning different physiologic systems, molecular, cellular, and tissue organizational levels, and time scales poses significant challenges for dynamical modeling. Complex systems approaches like those used by the Virtual Cell software may be applicable to this problem (30). Evidence that physiologic subsystems may function as modules constructed from a hierarchy of lower-level networks suggests that prediction of responses could often be achieved without knowledge of the molecular mechanisms underlying the modules’ behavior, and could complement reductionist approaches. Determining “tipping points” for stress-induced transitions between stable states will require methods to detect threshold effects and nonlinear responses. These may be valuable for understanding the determinants of full versus partial recovery, a particularly important issue in older populations.
These resource and infrastructure developments will likely be driven by novel questions about the underpinnings and outcomes of resilience. Research on these questions can contribute to interventions to improve the capacity of older adults to tolerate physical stressors, improve therapeutic decisions regarding stressful interventions and their management in the elderly, and may inform a deeper understanding of aging processes themselves.
Supplementary Material
Supplementary data are found at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Conflict of Interest
The authors declare no conflict of interest.
Funding
Funding was provided by the National Institute on Aging Division of Clinical Geriatrics and Gerontology.
Supplementary Material
Acknowledgments
Workshop speakers and participants were Heather G. Allore, PhD (Yale University), Jenna M. Bartley (University of Connecticut), Cindy S. Bergeman (University of Notre Dame), Michael L. Blinov, PhD (University of Connecticut), Cathleen S. Colon-Emeric, MD (Duke University), Firdaus S. Dhabhar, PhD (Stanford University), Laura L. Dugan, MD (Vanderbilt University), Chhanda Dutta, PhD (National Institute on Aging), Basil A. Eldadah, MD, PhD (National Institute on Aging), Luigi Ferrucci, MD, PhD (National Institute on Aging), Evan C. Hadley, MD (National Institute on Aging), James L. Kirkland, MD, PhD (Mayo Clinic), Stephen B. Kritchevsky, PhD (Wake Forest University School of Medicine), George A. Kuchel, MD (University of Connecticut), Lewis A. Lipsitz, MD (Harvard Medical School), Neelesh K. Nadkarni, MD, PhD, FRCPC (University of Pittsburgh) Anne B. Newman, MD, MPH (University of Pittsburgh), May J. Reed, MD (University of Washington), Kenneth E. Schmader, MD (Duke University, Durham Veteran’s Administration Medical Center Geriatric Research, Education and Clinical Center), Felipe Sierra, PhD (National Institute on Aging), Stephanie A. Studenski, MD, MPH (National Institute on Aging), Ravi Varadhan, PhD, PhD (Johns Hopkins University), Jeremy D. Walston, MD (Johns Hopkins University), Heather E. Whitson, MD, MHS (Duke University), and Raymond Yung, MD (University of Michigan).
References
In addition to the references below, further references on specific topics of the article are available in supplementary online material.
- 1. Hicks G, Miller RR. Physiological resilience. In: Resnick B, Gwyther LP, Roberto KA, eds. Resilience in Aging Concepts, Research and Outcomes. New York: Springer; 2011:89–104. [Google Scholar]
- 2. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. doi:10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franco OH, Karnik K, Osborne G, Ordovas JM, Catt M, van der Ouderaa F. Changing course in ageing research: the healthy ageing phenotype. Maturitas. 2009;63:13–19. doi:10.1016/j.maturitas.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 4. Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagnild GM, Collins JA. Assessing resilience. J Psychosoc Nurs Ment Health Serv. 2009;47:28–33. doi:10.3928/02793695-20091103-01 [DOI] [PubMed] [Google Scholar]
- 6. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71:1407–1414. doi:10.1093/gerona/glw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman JC, Milman S, Hashmi SK et al. Strategies and challenges in clinical trials targeting human aging. J Gerontol A Biol Sci Med Sci. 2016;71:1424–1434. doi:10.1093/gerona/glw149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monaghan P, Haussmann MF. The positive and negative consequences of stressors during early life. Early Hum Dev. 2015;91:643–647. doi:10.1016/j.earlhumdev.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–4775. doi:10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev. 2008;129:666–670. doi:10.1016/j.mad.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taffet GE. Normal aging. In: Schmader KE, Sokol HN, eds. Up To Date Alphen aan den Rijn, Netherlands: Wolters Kluwer; http://www.uptodate.com/contents/normal-aging; Accessed November 14, 2016. [Google Scholar]
- 12. Kuchel GA. Aging and homeostatic regulation. In: Halter JB, Ouslander JG, Studenski S. et al. , eds. Hazzard’s Geriatric Medicine and Gerontology. 7th ed. New York: McGraw-Hill Education Medical; 2017:1871–1945. [Google Scholar]
- 13. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi:10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16:679–686. doi:10.1007/s12603-012-0369-5 [DOI] [PubMed] [Google Scholar]
- 15. Makary MA, Segev DL, Pronovost PJ et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi:10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 16. Ferrucci L, Giallauria F, Schlessinger D. Mapping the road to resilience: novel math for the study of frailty. Mech Ageing Dev. 2008;129:677–679. doi:10.1016/j.mad.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:B115–B125. [DOI] [PubMed] [Google Scholar]
- 18. Kennedy BK, Berger SL, Brunet A et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta PK, Sundaram A, Mactaggart JN et al. Preoperative anemia is an independent predictor of postoperative mortality and adverse cardiac events in elderly patients undergoing elective vascular operations. Ann Surg. 2013;258:1096–1102. doi:10.1097/SLA.0b013e318288e957 [DOI] [PubMed] [Google Scholar]
- 21. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4:20140040. doi:10.1098/rsfs.2014.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Newman AB, Simonsick EM, Naydeck BL et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi:10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 23. Baht GS, Silkstone D, Vi L et al. Exposure to a youthful circulaton rejuvenates bone repair through modulation of β-catenin. Nat Commun. 2015;6:7131. doi:10.1038/ncomms8131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldstein DS. Adrenaline and the Inner World: An Introduction to Scientific Integrative Medicine. Baltimore, MD: Johns Hopkins Press; 2006. [Google Scholar]
- 25. Lord SR, Delbaere K, Gandevia SC. Use of a physiological profile to document motor impairment in ageing and in clinical groups. J Physiol. 2016;594:4513–4523. doi:10.1113/JP271108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menant JC, Schoene D, Sarofim M, Lord SR. Single and dual task tests of gait speed are equivalent in the prediction of falls in older people: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:83–104. doi:10.1016/j.arr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 27. Nascimbeni A, Caruso S, Salatino A et al. Dual task-related gait changes in patients with mild cognitive impairment. Funct Neurol. 2015;30:59–65. [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JC. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture. 2013;37:126–130. doi:10.1016/j.gaitpost.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 29. Walston J, Fedarko N, Yang H et al. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaff JC, Slepchenko BM, Loew LM. Physiological modeling with virtual cell framework. Methods Enzymol. 2000;321:1–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.