Abstract
Humans, as well as their closest ancestors, the higher African primates, exhibit female-biased survival and multiple sex differences in causes of death. However, the effects of sex on aging and longevity in an excellent model of human health, the companion dog, have not been well explored. Using two large independent databases on companion dog longevity and causes of death, we performed the most extensive analysis of sex differences in dog aging to date. Unlike the findings in humans, we observed only a small effect of sex on canine longevity. When broken down by neutering status, we discovered a small male advantage in survival among intact dogs but a clear female survival advantage among neutered dogs. Overall, the effect of neutering on life span was greater than the effect of sex. However, we found few sex differences in causes of death in either intact or neutered dogs. The results of this study suggest limited sex effects on either longevity or causes of death in the companion dog. Our analysis suggests that the majority of apparent sex differences in the wider canine populations may be due to the effects of neutering.
Keywords: Sex differences, Life span, Cause of death, Neutering, VetCompass, VMBD
Women live longer than men in all human populations for which reliable demographic data are available (1). Females also outlive males in our closest relatives—Old World apes and monkeys (2,3). In the wild, females also live longer than males in diverse nonprimate mammalian species including pilot whales, red deer, African lions, pipistrelle bats, and prairie dogs (1,4). Thus, it has become an accepted general rule that female mammals are longer-lived than males with a few unusual exceptions such as in socially monogamous species, for example, the meerkat (4,5). But is this accepted belief valid? A review of the published literature certainly identifies numerous exceptions. For instance, in one particularly well-studied case, nearly 500 banner-tailed kangaroo rats of each sex were followed for 7 years without observing any appreciable sex difference in longevity (6). In other species, such as Brandt’s bat or caribou, without notably unusual mammalian mating systems, males appear to live longer than females (4,7). In fact, there may be no safe generalization we can make about which sex is likely to live longer among mammals and each species may need to be evaluated on its own data.
Answering evolutionary questions such as those about absolute longevity is best done with animals living under evolutionarily relevant conditions, that is, in nature. However, if the research question of interest is less about absolute longevity and more about whether one or the other sex has slower intrinsic aging processes or is generally more physiologically robust than the other, studies of wild populations may not be particularly informative. Longevity in the wild can be affected in sex-specific ways by numerous behavioral and ecological variables unrelated to intrinsic processes. For instance, relative longevity between sexes can be affected by the energetic cost of reproduction, differences in foraging or dispersal patterns, greater or lesser exposure to infectious diseases, or direct male–male interactions. These factors could potentially mask underlying physiological sex differences that may become much more influential under nonwild lifestyles such as those of current pet or farm species or indeed the modern human. For addressing questions of intrinsic robustness or comparative aging rates, it may be preferable to examine captive or domesticated species where the impacts from extrinsic survival factors related to life in the wild are effectively removed or mitigated.
Captive or companion mammalian species are typically fed nutritionally adequate diets. They are largely protected from extrinsic or behavioral hazards and may often receive medical care as needed; therefore, any sex difference in observed longevity is likely to better reflect intrinsic processes than results taken from wild populations. From this perspective, there is no consistent difference in longevity reported overall between the sexes in laboratory mice, although differences in either direction have been reported in individual studies. In addition, no consistent sex differences are seen across studies of the same inbred strain strains of mice. Among laboratory rats, as in humans, females show fairly consistently longer lives than males (1).
Arguably, we know more about the health, physiology, pathophysiology, and general biology of the domestic dog than any other mammal species except humans (8). Human selection for dogs of a variety of different phenotypes has created over 300 currently known “breeds” and the domestic dog is now the most phenotypically variable of all mammal species (9). Dogs are also highly variable in longevity, with smaller breeds generally living longer and aging more slowly than larger breeds (10–16). Indeed, the longest-lived breeds live as much as 50% longer, and experience age-related diseases proportionally later, than the shorter-lived breeds (17,18).
Given how much investigation there has been of dog longevity, there is surprisingly little information on the relative longevity or mortality profile of the sexes. One reason may be because companion dogs have functionally four—rather than two—sexes, confounding simple analyses. Because dogs are often surgically neutered in early life and because neutering can have manifold secondary effects, there are four categories of dogs to consider—intact females and males as well as neutered females and males. Previous research results on the effects of neutering on life span have been conflicting (14,19) but interestingly, these studies have tended to look only at the effects of neutering on longevity within a sex rather than focusing on any differing neutering effects between males and females.
One study of over 3,000 British dogs that did analyze sex differences found that neutered female dogs were the longest-lived sex, intact females the shortest-lived, with males of either neutering status being intermediate in longevity (15). In that analysis, data were controlled for body size and restricted to dogs that died at 3 years of age or older. A second, independent, study of a similar number of dogs—also in Britain—found similar but not identical results. Neutered females were significantly longer-lived than any of the other three sexes, which did not differ statistically from one another (16). This second study, we should note, relied on owners’ recollections of their dogs’ ages rather than actual death records.
In humans, not only are life spans different between the sexes, so are disease frequency and mortality profiles. Women are more likely than men to suffer from autoimmune disorders and more likely to die of Alzheimer’s disease, whereas men are more likely to die from virtually everything else (1). The domestic dog shares many of the same diagnostic possibilities as humans, yet it has not been previously reported whether sex-biased causes of death are also seen in the companion dog.
Here, we focus on describing sex differences in longevity among companion dogs using two large data sets, one from North American veterinary teaching hospitals (VMDB) (The VMDB does not make any implicit or implied opinion on the subject of the paper or study.), the other from primary veterinary clinics in the United Kingdom (VetCompass). These data differ in significant ways. The Veterinary Medical Database (VMDB) reflects animals that were referred to a veterinary teaching hospital and died there. If an animal recovered and went home before dying, it would not be included. Thus, longevities reported in the VMDB reflect the sick and referred subset of the dog population and are considerably shorter than in most other published reports on dogs (15,16,20,21). By contrast, the VetCompass data include the full electronic medical records of dogs seeing their primary family veterinarians and thus are more representative of the general dog population.
Methods
Data
Data from North America were compiled from the VMDB (https://vmdb.org), comprising 80,958 canine deaths that occurred at 24 veterinary teaching hospitals over the 20-year time interval 1984–2004. As noted earlier, this is by no means a random sample of companion animals. Most of these dogs would have been referred to the hospitals from a primary veterinarian likely because of an unusual or complex health problem and would have had to die in the hospital to be included in the data. Animals that recovered, were sent home, and later died elsewhere would not be included. Consequently, longevity reported in this database is substantially less than reported from dogs in the general population (15). Also, in this data set, instead of exact ages, dogs were grouped into 10 age bins, four of which contained animals less than 1 year of age (0–2 weeks, 2 weeks to 2 months, 2–6 months, and 6 months to 1 year). The age bins for adults were 1–2, 2–4, 4–7, 7–10, 10–15, and 15+ years. Thus, exact age of death is unknown for dogs in the VMDB, and there is only a single bin to capture all dogs greater than 15 years of age. In our analyses, median age of each bin was used as the “age” of the animal as described previously, with 17.5 being used for the age bin “15+ years” (14). Cause(s) of death for each dog were assigned based on medical records, sometimes confirmed by necropsy, and grouped into different organ system and pathophysiological process categories of death (22). A limited number of dogs (5.8%) had no cause of death provided in the VMDB other than “euthanasia” or “unknown”; these dogs were excluded from diagnoses-specific analyses described below.
The second and completely independent set of data was extracted from VetCompass, a database compiled from information obtained from primary veterinary practices in the United Kingdom (23). The VetCompass data set included 5,095 dog deaths from January 1, 2009 to December 31, 2011 from a total population of 102,609 dogs in the data set during that same period (15). Exact ages of death are known for the majority of dogs in this data set because precise dates of birth and death are generally entered on the electronic patient record system for most dogs; completeness of age data has been reported at 99.7% for VetCompass data (24). Cause of death was assessed by the practicing veterinarian, typically without necropsy but often with the benefit of many years of personal knowledge of the animal’s health. The VetCompass data additionally benefitted by including both deaths that occurred at the veterinary clinics and also those that occurred away from the clinic and that were reported to the practices by the dogs’ owners. Ethical approval for the use of VetCompass data for the study was granted by the RVC Ethics and Welfare Committee (reference number 2015/1369).
For the current study, we excluded all animals that died at younger than 1 year of age (before which the vast majority of neutering is performed) or if they were euthanized for non-health-related reasons (eg, “healthy” and “Dangerous Dog Act”). This left a remaining sample of 70,148 dogs in the VMDB and 4,828 dogs in VetCompass that were included in our further analyses.
Statistical Analyses
All statistical analyses were completed in the program R (25). We performed standard longevity and survival analyses for the total sample from each data set of each of the four sexes separately. Multivariate Cox proportional hazard models were employed to determine the effects of sex, neutering, and their interaction on longevity. We then applied the same analyses to those breeds that were the most populous in both data sets (arbitrarily, we chose the lower cutoff to be at least 400 total dogs per breed in the VMDB and at least 100 dogs in the smaller VetCompass data set). Those non-health-related causes of death removed from the VetCompass, as described earlier, were censored (rather than removed) for our Cox proportional hazard models.
Age-specific mortality rates were calculated for both the VMDB and VetCompass data sets. Age-specific mortality rates were calculated by dividing the number of dogs that died at a certain age by all dogs that were still alive at that point. As the VMDB is already classified into predefined bins, we used these age bins to calculate our age-specific mortality rates. We grouped the VetCompass data into the same bins but we added a 15–20 years bin and a greater than 20 years bin due to the more accurate life spans of dogs reported in the VetCompass. Ratios of male/female age-specific mortality were calculated for intact and neutered dogs. We first calculated age-specific mortality rates for all dogs and then repeated the calculation after removing extrinsic causes of death (trauma).
We then looked for sex effects on individual causes of death. We compared deaths due to cancer, diabetes mellitus, and trauma across data sets as these were felt to be consistently identifiable diagnoses. Diagnoses recorded as euthanasia or unknown in the VDMB were removed from this analysis. We then ran a generalized linear model with a binomial distribution for each individual cause of death to see if sex and neutering influenced either a discrete (diabetes mellitus) or categorical (cancer, trauma) cause of death when controlling for the effects of age. We then looked across pathophysiological processes and organ system causes of death in the VMDB to determine if sex effects existed for these grouped causes of death. Categorization of deaths by pathophysiologic process and organ system was not available for VetCompass data at the time of this study.
Results
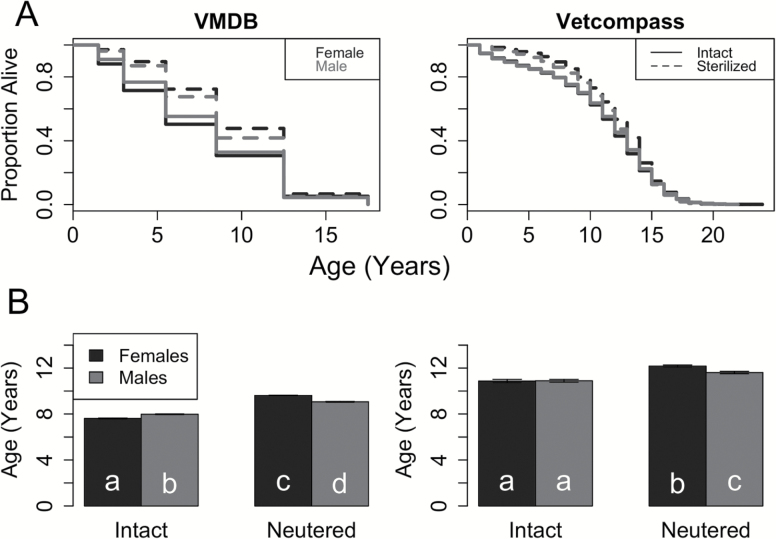
Ignoring neutering status, females overall were longer lived than males in both the Veterinary Medical Database (VMBD) and VetCompass data sets by about half a year (Figure 1; p < .002 for both data sets). However, this result is confounded by the clear impact of neutering and the proportion of each sex that is neutered. Females were much more likely than males to be neutered in both data sets (VMDB: 71% females neutered vs 42% of males; VetCompass: 58% of females neutered vs 47% of males, p < .0001 for both from χ2 test). Our Cox proportional hazard model indicated that neutering had a larger effect than sex on survival in both data sets (Table 2). Indeed, in the larger VMDB data, intact males were significantly longer-lived than intact females but neutered females were longer-lived than neutered males, or in fact any other group (Figure 1). The same general pattern—neutered females living longer than any other group—exists in the VetCompass data as well. In fact, after accounting for neutering status, sex had no statistical impact on longevity in the smaller VetCompass data set (Table 2). There was, however, a significant sex-by-neutering interaction in both sets of data. We should note that despite the fact that the life spans are highly different between the two data sets due to the special nature of the VMDB dog population, the longevity patterns among the sexes are remarkably similar in both sets, suggesting that the underlying intrinsic aging processes are similar across study populations.
Figure 1.
(A) Kaplan–Meier plots and (B) mean longevities for the four dog sexes. Females are represented in black, males in gray. Solid lines represent neutered dogs while dashed lines indicate neutered dogs. Our Cox Proportional hazard model suggests significant effects of neutering and sex-by-neutering interaction in both data sets (Table 1, p < .05 for all). Sex was only significant in the VMDB. Error bars indicate ± SEM. Letters represent significant differences at p less than .05 by Student’s t test. VMDB = Veterinary Medical Database.
Table 2.
Cox Proportional Hazard Model for Sex, Neutering, and Their Interaction in Both the VMDB and VetCompass Data set
| VMDB | |||
|---|---|---|---|
| Variable | Coefficient | Z | p Value |
| Sex (male) | −0.03785 | −3.132 | .00173 |
| Neutered | −0.31882 | −27.265 | <.00001 |
| Sex × Neutered | 0.14529 | 9.11 | <.00001 |
| VetCompass | |||
| Variable | Coefficient | Z | p Value |
| Sex (male) | −0.00495 | −0.198 | .905189 |
| Neutered | −0.15254 | −3.467 | .000317 |
| Sex × Neutered | 0.132247 | 2.334 | .022213 |
Note: VMDB = Veterinary Medical Database.
Because grouping all dogs together could mask breed-specific sex effects, we also inspected differences within the individual dog breeds that were most numerous in our two data sets. Given the much smaller numbers in specific breeds, statistically significant findings were rare even in the much larger VMDB (Supplementary Table 1). Sex was only a significant factor (p < .05) in 3 of 25 breeds and even in those cases the significance disappeared when correcting for multiple comparisons with a Bonferroni correction. However, among intact animals, 20 of 25 breeds showed a trend to greater male longevity. By contrast, among neutered animals, in 21 of 25 breeds the trend was for females to live longer. Neutering status still dominated, as it was a significant factor affecting longevity in more than half of individual breeds. Not surprisingly, given the much smaller sample sizes per breed, there were no significant sex effects within breeds in the VetCompass data (Supplementary Table 2). However, in concurrence with the VMDB findings, the trends were for greater male longevity in 6 of 13 breeds among intact animals but for female longevity to be greater in 8 of 13 breeds among neutered dogs.
Survival curves, because they record cumulative deaths from birth or some other arbitrary age, (1 year in this study), can obscure age-specific mortality differences; therefore, we also examined age-specific mortality for intact and neutered dogs from both the VMBD and VetCompass data sets (Table 1). These results were reported as mortality ratios comparing male to female mortality and this metric could highlight any sex-by-neutering interaction. Specifically, age-specific mortality rates early in life are considerably more male-biased among neutered dogs in both sets of data compared with intact animals but that bias gradually wanes and, from about age 10, sex-biased mortality virtually disappears. Conversely, age-specific mortality rates are higher in intact females compared to intact males in the VMDB only. Interestingly, removing extrinsic (trauma) causes of death from our age-specific mortality analyses has almost no effect on results in the VMDB data but reveals higher rates of mortality at young ages in males compared to females for both intact and neutered dogs in the VetCompass data (Supplementary Table 3). The mechanisms underlying this early life male survival disadvantage in neutered but potentially not intact animals merits further investigation.
Table 1.
Age-Specific Mortality Ratios (males/females) for Intact and Neutered Dogs From the VetComapss and VMDB
| Age group (years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Data set | Group | 1–2 | 2–4 | 4–7 | 7–10 | 10–15 | 15–20 | 20+ |
| VetCompass | Intact | 1.07 | 1.00 | 0.97 | 0.93 | 1.01 | 0.99 | 1.00 |
| Neutered | 1.82 | 1.15 | 1.30 | 1.56 | 1.02 | 1.01 | 1.00 | |
| VMDB | Intact | 0.76 | 0.83 | 0.94 | 1.04 | 1.04 | 1.00 | N/A |
| Neutered | 1.28 | 1.24 | 1.16 | 1.12 | 1.02 | 1.00 | N/A | |
Note: Mortality rates were calculated by determining the fraction of dogs that died at any time point compared to all dogs alive at that time point. Ratios of males to females were then calculated and displayed below. VMDB = Veterinary Medical Database.
What about causes of death? Do they differ by sex?
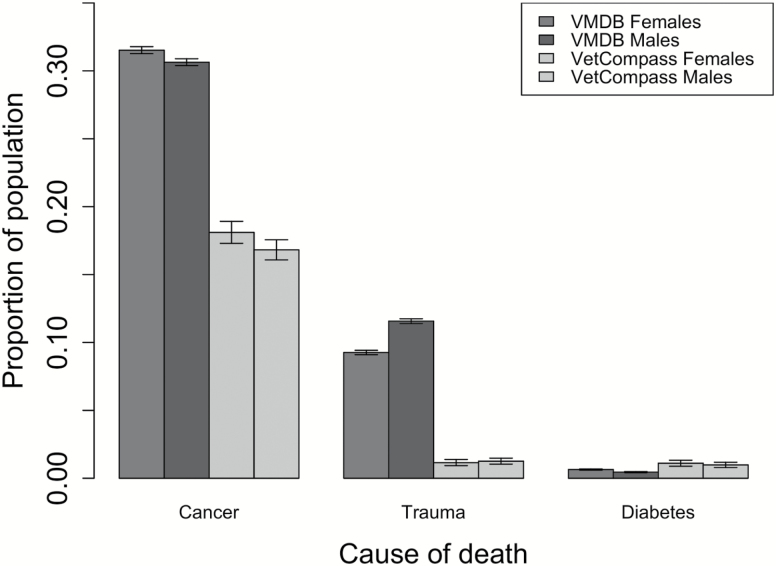
There are hazards in comparing causes of death across data sets, particularly when one (VetCompass) represents the best opinion of general veterinary practitioners almost always without the benefit of necropsy albeit on a more representative dog population, whereas the other (VMDB) has all the expertise and technical resources of veterinary teaching hospitals but emphasizes the biased subset of the general population that gets referred (21). To minimize this problem, we selected three representative diagnoses or diagnostic categories that were likely to be accurately recorded in either setting for analysis. Overall, causes of death—ignoring whether the animals were neutered or not—showed small differences across the two data sets for sex (Figure 2). In both data sets, deaths from cancer and diabetes mellitus were more common in females (though only significant in the VMDB data set, p < .02 for both), whereas trauma as a cause of death appeared more frequent in males; however, again, the results were only statistically significant in the VMDB with its larger sample sizes (p < .0001 VMDB only). Focusing exclusively on this larger VMDB data, causes of death had previously been organized according to the pathophysiological process or organ system involved (22). Inspecting these data according to sex while considering neutering status (eg, intact males vs intact females and neutered males vs neutered females), differences were also small (Table 3). Notable differences were seen in deaths from infectious causes, where intact female incidence (9.6%) was greater than male incidence (8.5%, p = .002). Intact females were more likely than intact males to die of endocrine disorders (F vs M, 5.9% vs 2.2%, p < .0001), whereas the reverse is true of cardiovascular (8.7% vs 10.3%, p < .0001) and hematopoietic disorders (6.4% vs 7.6%, p = .0002).
Figure 2.
Sex differences in causes of death. Colored bars: black, dark gray, light gray, and white indicate VMDB females, VMDB males, VetCompass females, and Vetcompass males respectively. Error bars are ± 1 SEM. VMDB = Veterinary Medical Database.
Table 3.
Proportion of Deaths due to Lesions in Specific Physiological Systems or Specific Lesion Types in the VMDB Data
| Pathphysiological Processes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Congenital | Degenerative | Infectious | Inflammatory | Metabolic | Neoplastic | Unclassified | Toxic | Traumatic | Vascular | |||
| Female intact | 0.021 | 0.026 | 0.096 | 0.029 | 0.054 | 0.229 | 0.37 | 0.023 | 0.141 | 0.011 | ||
| Male intact | 0.017 | 0.025 | 0.085 | 0.021 | 0.055 | 0.264 | 0.366 | 0.018 | 0.14 | 0.009 | ||
| Female neutered | 0.01 | 0.027 | 0.052 | 0.045 | 0.059 | 0.351 | 0.358 | 0.016 | 0.073 | 0.008 | ||
| Male neutered | 0.011 | 0.024 | 0.05 | 0.034 | 0.054 | 0.365 | 0.358 | 0.015 | 0.082 | 0.006 | ||
| Organ Systems | ||||||||||||
| Cardiovascular | Dermatologic | Endocrine | Gastrointestinal | Hematopoietic | Hepatic | Musculoskeletal | Neurologic | Ophthalmologic | Unclassified | Respiratory | Urogenital | |
| Female intact | 0.087 | 0.016 | 0.059 | 0.101 | 0.064 | 0.034 | 0.122 | 0.13 | 0.01 | 0.207 | 0.073 | 0.097 |
| Male intact | 0.103 | 0.021 | 0.022 | 0.104 | 0.076 | 0.03 | 0.125 | 0.137 | 0.008 | 0.206 | 0.077 | 0.09 |
| Female neutered | 0.086 | 0.018 | 0.046 | 0.11 | 0.108 | 0.051 | 0.098 | 0.12 | 0.007 | 0.176 | 0.088 | 0.091 |
| Male neutered | 0.084 | 0.02 | 0.033 | 0.12 | 0.104 | 0.048 | 0.1 | 0.124 | 0.006 | 0.182 | 0.084 | 0.095 |
Note: Categories in which the proportions differ by at least 0.01 are highlighted in bold and italicized. VMDB = Veterinary Medical Database.
An interesting and cautionary pattern is seen in cancer deaths (Figure 2, Table 3). Notice that among both intact and neutered dogs, the proportion of cancer deaths was higher in males than females (intact: 22.9% females, 26.4% males and neutered: 35.1% females, 36.5% males). Neutering as previously reported (14) is associated with a 10%–13% higher cancer death rate in both sexes. Interestingly, this is not due to the fact that neutered animals are on average 1–2 years older at death (14). Consequently, because a greater proportion of females than males are neutered, combining all deaths actually reverses the sex-specific pattern and deceptively suggests that cancer death rates are higher among females (Figure 2). This emphasizes the importance of incorporating neutering status in any demographic analysis of mortality or disease patterns in dogs and potentially other companion species that may be commonly neutered.
Discussion
We have shown that sex differences in longevity among dogs are critically dependent on neutering status. Among intact animals, there is a pattern for male dogs to live slightly longer than females but among neutered dogs, females clearly live longer. This finding suggests that any analysis of longevity patterns in dogs is incomplete without including details of neutering status. In our current study, we did not have access to information on the age at which neutering was performed but it is reasonable to assume that age at neutering is another important variable for understanding sex differences, especially for specific morbidities and mortalities (26,27). We also note that neutered dogs live longer in both of our data sets, as previously described (14) but inspection of patterns of age-specific mortality suggests that most of these differences are due to the impact of early life deaths. At the oldest ages, very few differences were seen in mortality rates across sex and neutering. Overall, our longevity results are similar to a previous study in British dogs that found neutered females were the longest-lived sex/neuter combination (16).
The similarities in survival curves (Figure 1A) between the two populations are striking as they are from different geographical regions (VMDB: United States and Canada, VetCompass: United Kingdom), different time frames (VMDB: 1984–2004, VetCompass: 2009–2011), and different veterinary practice types (VMDB: referral institutions, VetCompass: primary practice). The only major difference we observed between the data sets in regards to longevity was the substantially older age at death in the VetCompass database. This is not surprising as VMDB dogs were those who died at veterinary teaching hospitals, whose substantial referral populations comprise dogs with complicated or unusual illnesses. In addition, if these dogs got better, returned home, and died later, they would not have been recorded in the data set. By contrast, VetCompass dogs came from primary veterinary practices which would mostly follow the same dogs throughout their entire lives. Overall, the similarity in the shape of the survival curves between the two very distinct populations suggests that intrinsic aging patterns seen in both data sets may be accurate representations of companion dogs in developed countries.
Across human populations, females significantly outlive males, with age-specific mortality differences lasting throughout life (28). Dogs exhibit no such consistent differences. The differences depend more heavily upon neutering status. In intact animals, the differences are small and one of our data sets (VetCompass) failed to show a statistically significant sex difference in longevity at all (Figure 1B). This is in parallel with most other animal species commonly used to study mechanisms of aging that also show condition-dependent sex differences in longevity as does the dog.
Our breed-based analysis again suggests few sex differences in longevity within individual breeds. Previous research has often analyzed overall breed longevities, not inclusive of sex (eg, 29). Interestingly, among Rottweilers, one of the most studied breeds with respect to longevity, both our data sets showed that neutering increases median and mean life span but the oldest aged dogs were more likely to be intact. No neutered females (and only one neutered male) of this breed in the VMDB data set made it into the oldest age bin in the breed. This is similar to other studies of the Rottweiler that suggest, at least in female dogs, intact individuals are more likely to reach the oldest age (19). In contrast, the oldest Rottweilers in the VetCompass were neutered females suggesting there can be some breed variation across populations in the oldest individuals. While there is some minor variation in breed-by-sex longevity, especially in the oldest aged dogs, overall, a similar pattern emerges with no large sex differences in longevity across breeds.
Similar to longevity, we found some statistically significant, but biologically minor, sex differences in causes of death in the VMDB and similar (though not identical) trends in the same direction in the smaller VetCompass database (Figure 2). Specifically, proportional mortality from cancer and diabetes mellitus were higher for females, whereas deaths from trauma were higher for males. Interestingly, these data which were collapsed across neutering status can be misleading. Breaking out causes of death by both sex and neutering status in the VMDB (Table 3), we see the cancer results are reversed. That is, now cancer deaths are higher for males compared with females in both intact and in neutered animals. This is due to the fact that neutering increases cancer rates, independent of age (14) and females are considerably more likely than males to be neutered. When collapsing the data across neutering status, the neutered female rates bias the results. Not surprisingly, death rates from cancer were greater than for any other cause in both data sets regardless of neutering status (Table 3). Although there were some other differences between sexes found in causes of death (eg, endocrine, infectious diseases, and cardiovascular), these were typically just 1%–2% and the overwhelming impression is how similar causes were between the sexes when taking neutering status into account. These minor differences in causes of death between the sexes contrast with the results we see in humans in which large sex differences in cause of death are often observed.
The absence of substantial sex differences in longevity as well as in causes of death poses some interesting questions about the potential effects of evolution and domestication on sex-related longevity. In domestic cats, neutering increases life span, similar to that seen in dogs; however, a significant female advantage in life span is seen in both intact and neutered cats (30). This suggests that the lack of sex differences seen in the companion dog may not just be a byproduct of domestication. The companion dog has been selected from the gray wolf, Canis lupus, which shows a slight female bias in average longevity (4). However, across carnivore species, there appear to be no consistent sex differences. Combined, although the lack of sex differences in the dog is interesting, the underlying evolutionary determinants remain largely unknown.
Caveats
Although we have presented the most complete work to date that explores sex differences in longevity in the domestic dog, this study is not without its drawbacks. As we have stated earlier, the VMDB consists of animals that were referred to, and died in, veterinary teaching hospitals and is therefore subject to referral and outcome bias. Thus, the VMDB is not likely to be an accurate representation of the entire companion United States/Canadian dog population. In fact, breed longevities reported in the VMDB are much shorter than those reported in other studies of American dog populations (20). The VMDB also groups dogs into age bins of unequal size instead of actual age-at-death, which may have affected our results. In addition, the highest age bin (>15 years) makes it impossible to calculate precise maximum life spans. However, the fact that overall the same patterns were seen in the VMDB and the VetCompass lends more credence to our results as VetCompass records are taken from primary veterinary hospitals and are therefore more representative of the wider dog population.
The binning of dogs into unequal age groups in the VMDB also potentially led to biases in our age-specific mortality analysis. At the older ages, the bins are quite large, encompassing large numbers of years, and thus large proportions of the canine population. However, we wanted to make a definitive comparison between the VMDB and VetCompass data set, so we used the same bins for the VetCompass age-specific mortality analysis. The smaller sample size of the VetCompass also makes it difficult to divide our age-specific mortality analysis into smaller age bins. Although we agree the nature of the data may lead to a bias in discovering underlying sex differences, we believe the consistency seen across data sets and analyses suggests a real absence of sex differences in the companion dog. In addition, we could only calculate relative age-specific mortality rates for the population of dogs that died within the set time frames of the data set. Therefore, our analysis is unable to calculate true age-specific mortality rates, as we do not have information on the entire population at risk.
We also had little overlap in the categorization of causes of death between the VMDB and VetCompass making it difficult to compare all causes of death in the population. The VMDB contains full diagnoses for each dog while the VetCompass has some specific but mostly more general causes of death and these were often for disorders that may not typically be referred. Because we only compared three causes of death that were consistently recorded between the two data sets, we potentially may have missed some striking differences between the sexes for other nonevaluated disorders.
Studies on longevity in companion animals may be confounded by the fact that a large proportion of animals are euthanized. In the VetCompass data set, euthanasia accounted for 86.4% of deaths, while 13.6% of deaths were nonassisted (15) and there was no significant difference in the probability of euthanasia between females and males (probability in females 87.0% vs in males 85.9%, p = .245). The euthanasia option is usually elected at the cost of some quantity of life when the animal’s quality of life is deemed to have declined too far (31). The majority of the dogs in both data sets analyzed here were euthanized which could lead to biases in the lack of sex differences witnessed. However, while euthanasia may produce a lower total life span than would actually occur if the dogs were allowed to die a natural death, we find no bias in overall rates of euthanasia between the sexes and we would not expect to see any sex bias toward when an individual dog is euthanized. Therefore, a high proportion of euthanasia deaths would not be expected to alter the main sex-related conclusions of the current study. Indeed, it could be argued that the option of euthanasia for pets whose lives have deteriorated to an unacceptable quality enables veterinary data sets to provide a better reflection of natural aging processes and healthspan than modern human longevity data that are heavily influenced by access to extreme medical interventions that postpone the end of life beyond a natural expectation.
Finally, looking retrospectively at the effects of neutering can lead to biases in mortality analyses, as not all neutering occurs before 1 year of age. Therefore, some of the dogs that were recorded as neutered in our study may have been neutered later in life. This also leads to the potential for a reverse causality effect whereby neutering itself does not increase life span but as dogs age, they are more likely to have been neutered at some point in their lives (32). Future longitudinal studies that record the precise age at neutering across breeds are necessary to understand the full effect of neutering (and potentially sex) on mortality and longevity within the companion dog.
Conclusions
Here, we have presented the most comprehensive analysis of sex differences in mortality and longevity in companion dogs to date. Overall, we find that female dogs are longer lived on average but there is a sex-by-neutering interaction effect in which neutered females and intact males are the longest lived of their respective sexes. However, this significant sex difference is minor and not nearly as dramatic as is seen in human populations. Additionally, we discovered few sex differences across causes of death that were significant and those that were significant had fairly small effect sizes. Overall, we conclude that sex itself only plays a minor role in contributing to longevity and cause of death in the domestic dog.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This research was conducted while J.M.H. was a Glenn/AFAR Postdoctoral Fellow. In addition, this work was supported by the National Institute of Aging at the National Institutes of Health (P30 AG 050886 to S.N.A), the Glenn Foundation for Medical Research (S.N.A), and the Kennel Club Charitable Trust at the RVC (D.G.O.).
Supplementary Material
Acknowledgments
The authors thank Daniel Promislow for insightful comments on the manuscript. The authors are grateful to The Kennel Club, The Kennel Club Charitable Trust and Dogs Trust for supporting VetCompass in the United Kingdom. The authors also thank the two anonymous reviewers for their insightful comments on the manuscript.
References
- 1. Austad SN. Sex differences in longevity and aging. In: Masoro EJ, Austad SN, eds. The Handbook of the Biology of Aging. 7th ed San Diego: Academic Press; 2011:479–496. [Google Scholar]
- 2. Bronikowski AM, Altmann J, Brockman DK, et al. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science. 2011;331:1325–1328. doi: 10.1126/science.1201571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allman J, Rosin A, Kumar R, Hasenstaub A. Parenting and survival in anthropoid primates: caretakers live longer. Proc Natl Acad Sci USA. 1998;95:6866–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Promislow DEL. Costs of sexual selection in natural populations of mammals. ProcRoySocLondB Biol. 1992;247:203–210. [Google Scholar]
- 5. Clutton-Brock TH, Isvaran K. Sex differences in ageing in natural populations of vertebrates. Proc Biol Sci. 2007;274:3097–3104. doi:10.1098/rspb.2007.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waser PMJ, Wasner WT. Survival and reproductive effort in banner-tailed kangaroo rats. Ecology. 1991;72:771–777. doi: 10.2307/1940579 [Google Scholar]
- 7. Podlutsky AJ, Khritankov AM, Ovodov ND, Austad SN. A new field record for bat longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1366–1368. [DOI] [PubMed] [Google Scholar]
- 8. Ettinger S, Feldman E. Textbook of Veterinary Internal Medicine. 7 ed Saint Louis, MO: Saunders; 2010. [Google Scholar]
- 9. Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338 [DOI] [PubMed] [Google Scholar]
- 10. Albert RE, Benjamin SA, Shukla R. Life span and cancer mortality in the beagle dog and humans. Mech Ageing Dev. 1994;74:149–159. [DOI] [PubMed] [Google Scholar]
- 11. Bonnett BN, Egenvall A, Olson P, Hedhammar A. Mortality in insured Swedish dogs: rates and causes of death in various breeds. Vet Rec. 1997;141:40–44. doi:10.1136/vr.141.2.40 [DOI] [PubMed] [Google Scholar]
- 12. Bronson RT. Variation in age at death of dogs of different sexes and breeds. Am J Vet Res. 1982;43:2057–2059. [PubMed] [Google Scholar]
- 13. Egenvall A, Bonnett BN, Hedhammar A, Olson P. Mortality in over 350,000 insured Swedish dogs from 1995-2000: II. Breed-specific age and survival patterns and relative risk for causes of death. Acta Vet Scand. 2005;46:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffman JM, Creevy KE, Promislow DE. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS One. 2013;8:e61082. doi: 10.1371/journal.pone.0061082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of owned dogs in England. Vet J. 2013;198:638–643. doi:10.1016/j.tvjl.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 16. Michell AR. Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Vet Rec. 1999;145:625–629. [DOI] [PubMed] [Google Scholar]
- 17. Kraus C, Pavard S, Promislow DE. The size-life span trade-off decomposed: why large dogs die young. Am Nat. 2013;181:492–505. doi:10.1086/669665 [DOI] [PubMed] [Google Scholar]
- 18. Selman C, Nussey DH, Monaghan P. Ageing: it’s a dog’s life. Curr Biol. 2013;23:R451–R453. doi:10.1016/j.cub.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 19. Kengeri SS, Maras AH, Suckow CL, Chiang EC, Waters DJ. Exceptional longevity in female Rottweiler dogs is not encumbered by investment in reproduction. Age (Dordr). 2013;35:2503–2513. doi:10.1007/s11357-013-9529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82:208–214. doi: 10.1016/j.rvsc.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 21. Bartlett PC, Van Buren JW, Neterer M, Zhou C. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med. 2010;94:264–271. doi: 10.1016/j.prevetmed.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 22. Fleming JM, Creevy KE, Promislow DE. Mortality in north american dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25:187–198. doi:10.1111/j.1939-1676.2011.0695.x [DOI] [PubMed] [Google Scholar]
- 23. O Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS One. 2014;9:e90501. doi: 10.1371/journal.pone.0090501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Neill DG, Meeson RL, Sheridan A, Church DB, Brodbelt DC. The epidemiology of patellar luxation in dogs attending primary-care veterinary practices in England. Canine Genet Epidemiol. 2016;3:4. doi:10.1186/s40575-016-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core Team. R: A language and environment for statistical computing. Vienna, Austria:R Foundation for Statistical Computing; 2013. [Google Scholar]
- 26. Beauvais W, Cardwell JM, Brodbelt DC. The effect of neutering on the risk of mammary tumours in dogs–a systematic review. J Small Anim Pract. 2012;53:314–322. doi:10.1111/j.1748-5827.2011.01220.x [DOI] [PubMed] [Google Scholar]
- 27. Torres de la Riva G, Hart BL, Farver TB, Oberbauer AM, Messam LL, Willits N, et al. Neutering dogs: effects on joint disorders and cancers in golden retrievers. Plos One. 2013;8:e55937. doi: 10.1371/journal.pone.0055937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi:10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–B178. [DOI] [PubMed] [Google Scholar]
- 30. O’Neill DG, Church DB, McGreevy PD, Thomson PC, Brodbelt DC. Longevity and mortality of cats attending primary care veterinary practices in England. J Feline Med Surg. 2015;17:125–133. doi:10.1177/1098612X14536176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeates J. Ethical aspects of euthanasia of owned animals. In Practice. 2010;32. doi:10.1136/inp.c516 [Google Scholar]
- 32. Flanders WD, Augestad LB. Adjusting for reverse causality in the relationship between obesity and mortality. Int J Obes (Lond). 2008;32(suppl 3):S42–S46. doi:10.1038/ijo.2008.84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.