Abstract
Background
Adiposity depots may differentially affect cognition. African Americans (AA) have higher rates of obesity and dementia but lower visceral adipose tissue (VAT) than whites, yet are underrepresented in studies of adiposity and cognition. Our study compared relations of cognitive function to clinical adiposity measures and computed tomography (CT)-imaged abdominal adiposity in AA.
Methods
CT-imaged subcutaneous adipose tissue (SAT) and VAT measurements were obtained in the AA cohort of the Genetic Epidemiology Network of Arteriopathy Study (N = 652, mean age 68 ± 8.4 years, 74% females, 59% obese, 82% hypertensive). Clinical adiposity measures included waist circumference (WC) and body mass index (BMI). Global cognition was operationalized as a global cognitive z-score generated from the average of four cognitive domain z-scores. Generalized estimating equations were used to examine cross-sectional associations between individual standardized adiposity measures and cognition, accounting for age, sex, education, smoking status, and familial clustering. A collective model was constructed including multiple supported adiposity measures and age-by-adiposity interactions.
Results
In the collective model, higher WC was associated with worse global cognition, β = −0.12 (95%CI: −0.21, −0.03); higher SAT was associated with better cognition, β = 0.09 (0.01, 0.18); higher BMI was associated with worse cognition at younger ages with attenuation at older ages (BMI-by-age-interaction p = .004). VAT was not significantly associated with global cognition, β = −0.03 (−0.07, 0.02).
Conclusions
WC may be the simplest and most efficient measure of adiposity to assess with respect to cognition in clinical settings, although studies to determine mechanistic effects of subcutaneous and other adiposity depots on cognition are warranted.
Keywords: Obesity, Cognitive function, Health disparities, Imaging
An estimated 5.3 million Americans have Alzheimer’s disease, and this number is projected to rise to nearly 14 million by 2050 (1). Total health care costs for dementia are expected to quadruple over the next 30 years (1), burdening families and health care systems. Currently, there is no effective treatment. Prevention through early identification and treatment of modifiable risk factors may be a prudent approach for reducing the public health burden of the disease. We and others have shown associations of adiposity with abnormalities of brain structure (2,3) and with dementia and cognitive decline (3–6). Adipose tissue depots vary in their associations with cardiometabolic diseases and metabolic dysfunction, both of which have been shown to have detrimental effects on the brain and cognitive function (7–10). We have also reported that relations of adiposity to cognition differ by age for overall measures of adiposity, that is, body mass index (BMI) but not for measures of central adiposity, that is, waist circumference (WC) (6). Determining which adipose tissue depots are adversely associated with cognition and which measures of adiposity best reflect those relationships may help direct targeted prevention of cognitive impairment.
Clinical measures of adiposity—BMI and WC—are simple and inexpensive to obtain, yet may not adequately characterize abdominal adiposity. Computed tomography (CT) imaging is considered the optimal method for the quantitative assessment of abdominal adipose tissue (11). It is unclear whether CT imaging complements clinical measures significantly enough for either research or clinical purposes to warrant the added expense and burden. Distinctions between clinical and CT imaging measures may be especially important in African Americans (AA) who have less visceral adipose tissue (VAT) than non-Hispanic whites (NHW) with comparable BMI measures (12,13). Additionally, AA are disproportionately affected by dementia (14) and obesity (15), yet this population has been markedly under-represented in prior studies of adiposity and cognitive function or dementia. Identifying independent and joint relations of clinical and imaging measures of adiposity to cognition may provide a better understanding of the influence of specific adiposity depots on dementia risk. We hypothesized that CT-derived measures of abdominal adiposity, particularly VAT, would be more strongly associated with cognitive function than clinical adiposity measures in AA.
Methods
Study Design
Data are from the AA cohort of the Genetic Epidemiology Network of Arteriopathy (GENOA) Study. GENOA is an observational study of common polymorphic genetic variations to determine individual differences in blood pressure and hypertension in three racial groups.
Population
GENOA is part of the Family Blood Pressure Program that recruited sibships (1995–2000), of whom at least two siblings had a diagnosis of hypertension before age 60 (16). All other siblings in a sibship were invited to join the study regardless of their hypertensive status. GENOA used a network of three-field centers to recruit NHW from Rochester, MN; AA from Jackson, MS; and Hispanics from Starr County, TX. The current study focuses on the AA cohort, who were invited to participate in an ancillary study of coronary artery calcification after the second GENOA visit. The GENOA Coronary Calcium Study conducted CT imaging of the heart and abdomen and a cognitive assessment at the Jackson site in 752 AA. Of these, 1 participant was missing BMI and WC measurements and 99 were missing imaging measurements, leaving 751 with clinical adiposity measures and 652 with clinical and imaging adiposity measurements. The study was approved by institutional review boards and followed ethical rules as stated in the Declaration of Helsinki, including written informed consent from all participants.
Clinical Assessment
WC, height, and weight were assessed with participants wearing lightweight clothing. Participants were instructed to stand erect with weight equally distributed on both feet. A wall-mounted stadiometer was used to measure height, and an electronic balance was used to measure weight. BMI was calculated as weight (kg)/height2 (m2). WC was measured to the nearest 0.1 cm at the umbilicus at the end of exhalation.
CT-imaged Adiposity
VAT and subcutaneous adipose tissue (SAT) measures were measured from axial images of the abdomen. Exclusion criteria for the CT exam included pregnancy, weight greater than 350 pounds, prior heart surgery, prior angioplasty, or CT scan in the past year. Women of childbearing age were screened by a urine pregnancy test. Imaging was conducted with a calibration phantom positioned beneath each participant in a supine position. A 16-channel multidetector CT scanner (GE Healthcare LightSpeed 16 Pro, Waukesha, WI) was used to image the lower abdomen from L3-S1. A 50 cm display field of view CT scan series was taken covering 60 mm of the lower abdomen. The images consisted of 24 contiguous 2.5-mm-thick slices centered on L4-5 (12 before and 12 after) with no interslice gaps (17). The abdominal muscular wall was manually traced, and a semiautomatic segmentation technique measured the fat volumes in different abdominal compartments. Volume analysis software (Advantage Windows; GE Healthcare, Waukesha, WI) segmented and categorized each individual voxel as a tissue attenuation of fat. The threshold range used was −190 to −30 Hounsfield Units (HU). The voxels from the 24 slices were then characterized and used to quantify VAT and SAT volumes. Quality control and image analysis were performed at the GENOA core CT reading center at Wake Forest University School of Medicine in Winston-Salem, NC. Interclass correlations for inter-reader comparisons for this protocol were 0.95 for VAT and SAT (18).
Cognitive Testing
Cognitive testing followed standardized protocols. Measures of speed were multiplied by −1 so that higher scores represented better function across all cognitive tests. A standardized z-score was created for individual tests and averaged within a domain when more than one test was included in a domain (19,20). A global cognitive z-score (hereafter called global cognition) was constructed from the average of domain-specific z-scores and was the primary cognitive outcome of interest.
Overall cognition
The Mini-Mental State Examination (range 0–30) (21) was administered following the protocol established by the Consortium for the Establishment of a Registry for Alzheimer’s disease (22).
Executive function
Timed tests were used to assess processing speed and executive function domains, hereafter termed executive function. The Wechsler Adult Intelligence Scale Revised Digit Symbol Substitution Task (DSST) requires participants to match numbers to symbols using a key (23). DSST is scored as the number of correct translations completed in 90 seconds. In the Trail-Making Test (TMT)-A, one must draw a line connecting randomly placed letters in ascending order. The TMT-B presents letters and numbers on a page; the examinee must draw a line connecting the letters and numbers in ascending order, alternating letters and numbers. Errors on TMT-A or -B resulted in a score of the maximum time. Times were measured to the nearest second for a maximum of 4 minutes (24).
Memory
The Rey Auditory Verbal Learning Test (RAVLT, range 0–15) was used to assess learning and memory. The RAVLT utilizes multiple learning trials and a 30-minute delayed recall of 15 items on a list (24). The WAIS-III Incidental Learning Task was used to allow for the continuation of the DSST until the third row of the test had been completed (23,25). The symbol pairs with free-recall task (24) was repeated after a 5-minute delay.
Language
The F-A-S Test measures letter fluency. Participants have 60 seconds to spontaneously produce words beginning with a specific letter (F, A, S); the score is the sum of number of allowable words (24).
Covariates
Age, sex, and education were self-reported. Clinical measures included blood pressure that was measured three times with an electronic blood pressure machine while the participant was seated. We used the average of the second and third measurements. Hypertension was defined as a blood pressure ≥ 140/90 mmHg or report of prior diagnosis of hypertension and current treatment with antihypertensive medications. Diabetes was defined by use of insulin or oral glucose-lowering medications or fasting glucose level ≥ 126 mg/dL. Smoking was defined as ever having smoked more than 100 cigarettes. Alcohol use was queried by asking participants “Would you describe yourself as a person who never drinks alcoholic beverages?”
Statistical Analysis
Participant characteristics were summarized using means with standard deviations for continuous variables and counts with percentages for categorical variables. Associations between adiposity measures and each cognitive domain were estimated using linear models fit with generalized estimating equations (GEE) to account for familial clustering and Huber-White robust standard error estimates. Because adiposity measures had different measurement units, the measures were standardized to facilitate examinations and interpretations across models. All models were adjusted for age, sex, education, and smoking status. Differential relationships of adiposity depots with cognition across age have not been established, so we first examined relationships of each adiposity measure to global cognition and domain-specific cognition separately in clinically feasible models without considering the modifying effects of age. Next, we included adiposity-by-age interaction terms in corresponding individual adiposity models to examine potential age modifying effects for the different adiposity depots. For these and subsequent analyses, we used only the global cognition measure outcome for brevity. We next examined a saturated model that included all adiposity measures and all adiposity-by-age interaction terms. Finally, we derived a parsimonious “collective” model that included statistically supported adiposity measures and age-interactions along with all a priori specified adjustors. We additionally examined the best fit across interim adjustment models using Akaike information criterion and Bayesian information criterion. The age-interaction models provide differential adiposity effect estimates for any specified age; we illustrated and compared results for participants at age 65 years and 75 years as translational examples. Relationships of cognition to WC and BMI were reported in all participants with these measures to increase precision where available, which included an additional 99 participants who had BMI and WC but not CT measures. Finally, we constructed additional models adjusting for hypertension and diabetes to examine potential mediation effects through these conditions. All analyses were done using Stata v14.1 (Statacorp, College Station, TX).
Results
There were 751 participants with clinical adiposity measurements and 652 with both clinical and CT imaging data. Participants were middle-aged to older AA adults, and the majority (74%) were women. Most (82%) had hypertension, and vascular risk factors including obesity, diabetes, and smoking history were common (Table 1). Compared to those with imaging data, participants without CT-imaging were older, had more hypertension, and generally performed worse on tests of cognition (Supplementary Table 1).
Table 1.
Characteristics of 652 Participants with Clinical and Abdominal Computed Tomography-imaged Adiposity Measurements
| Women | 480 (74%) |
| Age (years) | 68.5 ± 8.4 (40.1–98.1) |
| Education <12 y | 190 (29%) |
| Obese (BMI ≥30 kg/m2) | 384 (59%) |
| Diabetes | 206 (32%) |
| Hypertension | 537 (82%) |
| Alcohol Use | 24 (4%) |
| Ever Smoker | 260 (40%) |
| BMI (kg/m2) | 32.5 ± 7.1 (14.7–61.0) |
| Waist Circumference (cm) | 100.8 ± 15.3 (59.0–151.4) |
| Subcutaneous Adipose Tissue (cm3) | 2,313.4 ± 970.0 (71.8–4,890.5) |
| Visceral Adipose Tissue (cm3) | 901.1 ± 406.6 (54.3–2,766.0) |
| Total Adipose Tissue (cm3) | 3,352.2 ± 1,211.8 (268.2–6,902.2) |
| MMSE (0–30) | 25.8 ± 3.2 |
| DSST (0–93) | 32.5 ± 13.3 |
| TMT-A, seconds (0–240) | 70.0 ± 39.2 |
| TMT-B, seconds (0–240) | 145.8 ± 57.0 |
| Incidental Learning (0–9) | 3.3 ± 2.4 |
| RAVLT (0–15) | 6.2 ± 3.1 |
| FAS (number of words) | 28.3 ± 12.6 |
Note: N = 652. Data column shows N (%) for categorical and mean ± SD (range) for continuous variables. Possible ranges for cognitive scores are shown. BMI = Body mass index; DSST = Wechsler Adult Intelligence Scale Revised Digit Symbol Substitution Task; FAS = F-A-S Test; MMSE = Mini-Mental State Examination; RAVLT = Rey Auditory Verbal Learning Test; TMT-A = Trail-Making Test A; TMT-B = Trail-Making Test B; Incidental Learning: WAIS-III Incidental Learning Task .
In clinically feasible models with individual adiposity measures that did not include age-by-adiposity interaction terms (Table 2), higher WC was associated only with poorer executive function, β = −0.083, (95% CI: −0.133, −0.033). Similarly, higher BMI was associated with poorer executive function, β = −0.067, (−0.121, −0.014). Relationships of cognition with VAT and SAT were not statistically significant in these models (Table 2). Results were the same in the larger sample of all participants with clinical adiposity measurements (Supplementary Table 2).
Table 2.
Adjusted Associations of Adiposity Measures with Global and Domain-specific Cognition
| Standardized Predictors | ||||
|---|---|---|---|---|
| Composite Outcomes | Waist Circumference | Body Mass Index | Subcutaneous Adipose Tissue | Visceral Adipose Tissue |
| Global Cognition | −0.041 p = .080 | −0.017 p = .476 | 0.006 p = .819 | −0.029 p = .192 |
| (−0.088, 0.005) | (−0.065, 0.030) | (−0.045, 0.057) | (−0.073, 0.015) | |
| Executive Function | −0.083 p = .001 | −0.067 p = .013 | −0.020 p = .477 | −0.042 p = .086 |
| (−0.133, −0.033) | (−0.121, −0.014) | (−0.076, 0.035) | (−0.090, 0.006) | |
| Memory | 0.011 p = .737 | 0.054 p = .135 | 0.065 p = .086 | −0.010 p = .751 |
| (−0.054, 0.077) | (−0.017, 0.124) | (−0.009, 0.139) | (−0.074, 0.053) | |
| Language | −0.055 p = .132 | −0.039 p = .289 | −0.056 p = .153 | −0.027 p = .438 |
| (−0.126, 0.016) | (−0.112, 0.033) | (−0.133, 0.021) | (−0.094, 0.041) | |
Note: N = 652. β, p value, 95% CI.
Adjusted for age, sex, education, and smoking status.
Global Cognition: Derived z-score from all cognitive measures.
Executive Function: Wechsler Adult Intelligence Scale Revised Digit Symbol Substitution Task, Trail-Making Test A, Trail-Making Test B.
Memory: WAIS-III Incidental Learning Task, Rey Auditory Verbal Learning Test.
Language: F-A-S Test.
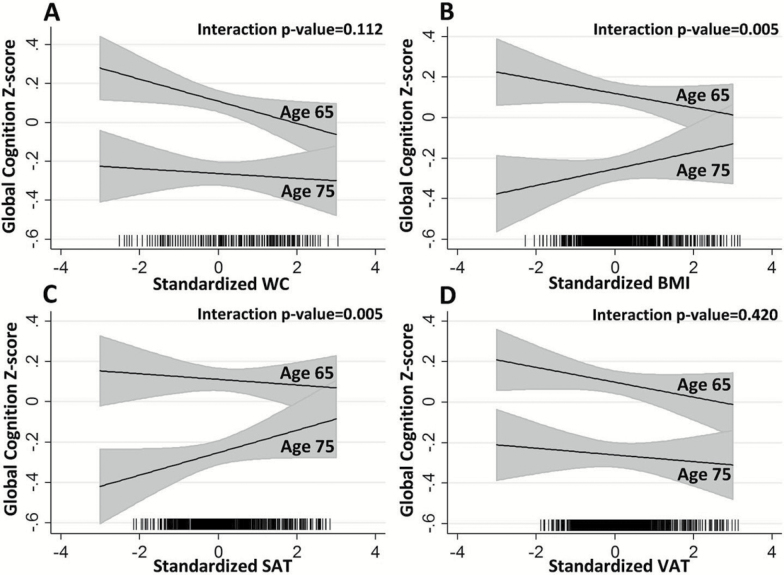
In individual adiposity models (Model 1) that included age-interactions, age modified the relationship of global cognition with BMI (age-by-BMI interaction p value = .005) and SAT (age-by-SAT interaction p value = .005), but not WC or VAT (p values for interaction >.11) (Supplementary Table 3 and Figure 1). Supplementary Table 4 shows stepwise model reduction results (interim models) starting with a saturated model of all adiposity measures, age-by-adiposity interactions, and covariates, and ending with the parsimonious collective multivariable model containing all statistically supported terms (Model 2). In the saturated model, BMI (p = .06) and age-by-BMI (p = .06) were of borderline statistical significance (Supplementary Table 4). Only WC, BMI, SAT, and the age-by-BMI interaction were statistically supported and retained in the collective model (Model 2), along with age, sex, education, and smoking history. Adjusting for hypertension, diabetes, and self-reported hours per week spent in sedentary, moderate, and heavy activity did not substantially attenuate the relationship of adiposity measures with cognition (Supplementary Tables 5 and 6).
Figure 1.
Adjusted relationships of global cognitive z-scores at ages 65 and 75 years with standardized adiposity measures: (A) waist circumference (WC), (B) body mass index (BMI), (C) subcutaneous adipose tissue (SAT), and (D) visceral adipose tissue (VAT). Panels show results from separate models with single adiposity measures. p Values for age-by-adiposity interactions are shown. Rug plots (x-axis tick marks) denote the distributions of observed adiposity.
Higher WC was associated with poorer global cognition at ages 65 and 75 in Model 2, β = −0.117, (−0.205, −0.030) (Table 3). Age-by-WC interaction terms were not statistically supported, whereas age-by-BMI interaction terms were statistically supported in both Models 1 and 2. While the associations of BMI with cognition at the ages of 65 and 75 years shown in Table 3 were not supported, the associations of cognition with BMI in Model 2 were supported and statistically different at the extremes of age, for example, age 46 for the inverse association (β = −0.150, p = .046), and age 84 for the positive association (β = 0.145, p = .050).
Table 3.
Adjusted Associations Between Adiposity and Global Cognition at ages 65 and 75 years
| Model 1 (Separate Models, Individual Measures) | Model 2 (Parsimonious Collective Multiple Regression) | |||||
|---|---|---|---|---|---|---|
| Age = 65 | Age = 75 | Age Interaction | Age = 65 | Age = 75 | Age Interaction | |
| WC | −0.057 p = .027 | −0.012 p = .669 | p = .112 | −0.117 p = .008 | −0.117 p = .008 | |
| (−0.107, −0.006) | (−0.069, 0.044) | (−0.205, −0.030) | (−0.205, −0.030) | |||
| BMI | −0.035 p = .161 | 0.041 p = .186 | p = .005 | −0.003 p = .960 | 0.075 p = .216 | p = .004 |
| (−0.084, 0.014) | (−0.020, 0.102) | (−0.110, 0.104) | (−0.044, 0.193) | |||
| SAT | −0.014 p = .600 | 0.056 p = .067 | p = .005 | 0.093 p = .036 | 0.093 p = .036 | |
| (−0.066, 0.038) | (−0.004, 0.115) | (0.006, 0.180) | (0.006, 0.180) | |||
| VAT | −0.037 p = .131 | −0.016 p = .549 | p = .420 | |||
| (−0.084, 0.011) | (−0.070, 0.037) | |||||
Note: β, p value, 95% CI. All predictors are standardized. See Supplementary Table 4 for details on all interaction models. VAT = Visceral adipose tissue.
Model 1: Separate models included each adiposity measure, age, age-by-adiposity interaction, sex, education, and smoking status.
Model 2: Waist circumference (WC) + body mass index (BMI) + age + age-by-BMI interaction + subcutaneous adipose tissue (SAT), sex, education, and smoking status.
The age-interaction for SAT was supported in Model 1, but not in Model 2. Higher SAT was associated with better global cognition in Model 2, β = 0.093, (0.006, 0.180) at both ages, given the lack of interaction with age in Model 2. These unexpected results led us to examine if higher SAT was indicative of more robust older participants who might have better cognition and higher WC and/or BMI (relative to frail older persons, in whom undernourishment and low BMI is considered a frailty component). We found that higher SAT remained statistically associated with better cognition across all BMI and WC levels (BMI-by-SAT interaction p value = .75; WC-by-SAT interaction p value = .92). In contrast, the age-by-VAT interaction in Model 1 was not statistically supported.
Discussion
Contrary to our hypothesis, CT-imaged adiposity measures (eg, visceral adiposity) were not more strongly associated with cognition than the clinical adiposity measure counterpart (eg, waist circumference) in this AA cohort with prevalent cardiovascular risk factors. VAT, which we hypothesized would be the strongest predictor of cognitive function, was not associated with any cognitive domain, yet clinical measures of WC and BMI were. This suggests that these simple clinical measures may be the most efficient adiposity assessments to use in clinical or research settings focusing on cognitive outcomes. WC, in particular, may be the optimal measure as it is associated with cognition similarly across ages, and so would be easier to implement and interpret in practice, whereas BMI relationships to cognition depend on age. However, we also found that subcutaneous adiposity from CT-imaging was associated with better cognition after accounting for BMI and WC. This finding warrants further investigation to elucidate potential mechanistic pathways through which different adiposity depots might be associated with cognition.
Similar to our findings, other cohorts with similar prevalence rates of obesity and other vascular risk factors also reported no association between VAT and cognitive function (26,27). In contrast, studies that showed inverse associations between VAT and cognitive performance included participants with lower BMI and lower rates vascular risk factors (28,29). Potentially, the relationship of VAT to cognition may be attenuated in the presence of other vascular risk factors. Another explanation could be that the relationship of VAT with cognition differs in AA compared to other populations due to different adiposity distributions and metabolic activity. For example, AA have lower VAT (12,13) and lower adiponectin levels than NHW counterparts with the same WC or BMI (30). This could also help explain why clinical diseases associated with obesity, for example, hypertension and diabetes, did not completely account for the relationship of adiposity measures with cognition in the current AA cohort. Differences have also been found in the storage and release of fatty acids and inflammation levels by adiposity depots (31). It is possible that clinical measures of adiposity that include a summation of adiposity, such as WC, are better than individual adiposity depot measures, such as VAT, at offsetting the confounding effects of adipose tissue heterogeneity, and thus may better encompass the full relation with systemic metabolic factors.
The relationship of higher SAT with better cognitive function in this cohort was not explained by older age or by overall low adiposity which might be seen in frail persons or persons developing dementia. In the current cohort, SAT showed strong correlations with BMI (r = .84), moderate correlations with waist circumference (r = .68) but modest associations with VAT (r = .38). SAT likely provides different and complementary information than other adiposity measures. These findings build upon results from cohorts with a lower prevalence of hypertension, in which higher SAT was also associated with better cognitive performance (32) and also with lower triglyceride levels (33). It has been posited that SAT may serve as a protective fat depot by storing triglycerides that would otherwise be harmfully deposited as ectopic fat in other organs, for example, in the liver, which has been associated with metabolic complications (34), or around the heart, which has been associated with impaired cognitive function (35). We extend these findings by replicating associations of higher SAT with better cognition in a cohort of largely hypertensive AA. The proposed role of SAT as a protective fat depot, if true, could provide further insight into the relationships of different types and loci of adiposity with cognition.
Our findings also build on prior studies of adiposity relations to cognitive subdomains. Findings from the Baltimore Longitudinal Study of Aging (BLSA), a primarily white, healthy, better-educated cohort of higher socioeconomic status, demonstrated that higher clinical adiposity measures were associated with poorer language and memory test scores (5,21,36). Relationships of clinical adiposity measures to cognition in our study, however, appeared to be driven mainly by associations with executive function. These discordant findings between BLSA and GENOA could be due to greater prevalence of cardiovascular risk factors in the GENOA cohort. This would be consistent with other studies that found processing speed and executive function were more strongly associated with vascular risk factors than memory or language (37,38).
Some limitations warrant discussion. First, this is a cross-sectional study, so residual confounding cannot be excluded, and causal relationships cannot be assumed. Previous studies have shown sex and race differences in adiposity distributions, with AA women having less VAT and more SAT on imaging than AA men, and less VAT and lower VAT:SAT ratios relative to overall adiposity than NHW (13). The number of men in our study limited our ability to effectively examine sex differences. Furthermore, our findings might not generalize to other populations as our participants were all AA from a single site and had a high prevalence of hypertension. However, these findings could have substantial public health implications as AA are at higher risk of dementia (14) and have higher rates of obesity and hypertension worldwide (15,39). This study fills a gap in the current understanding of adiposity and adiposity depot relations to cognition in an understudied, at-risk population.
In summary, among AA with prevalent hypertension, different depots and distributions of adiposity appear to provide differing information about cognition. This study provides more evidence regarding the potential scientific importance of subcutaneous adiposity. Further studies are warranted to elucidate the mechanistic pathways, including the potential protective direction of associations with subcutaneous depots through which adiposity is associated with cognitive decline and dementia.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging (R01AG045255: B.G.W., S.T.L., M.F., E.J.B., S.T.T., J.J.C., T.H.M.) and the National Institutes of Health (U01-HL054463, HL-81331, and M01 RR00585: T.H.M., S.T.T.; HL085571: L.F.B., P.A.P., J.J.C., J.G.T., S.T.T., T.H.M.).
Supplementary Material
Acknowledgments
Author Contributions: All authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; provided critical revision of the final draft; and provided final approval of the current draft.
Sponsor’s Role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis, or preparation of this article.
Conflict of Interest
None reported.
References
- 1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:1–83. doi:10.1016/j.jalz.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Windham BG, Lirette ST, Fornage M et al. Associations of brain structure with adiposity and changes in adiposity in a middle-aged and older biracial population. J Gerontol A Biol Sci Med Sci. 2017;72:825–831. doi:10.1093/gerona/glw239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debette S, Seshadri S, Beiser A et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi:10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kivipelto M, Ngandu T, Fratiglioni L et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi:10.1001/archneur.62.10.1556 [DOI] [PubMed] [Google Scholar]
- 5. Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi:10.1159/000297742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. West NA, Lirette ST, Cannon VA, Turner ST, Mosley TH Jr, Windham BG. Adiposity, change in adiposity, and cognitive decline in mid- and late life. J Am Geriatr Soc. 2017;65:1282–1288. doi:10.1111/jgs.14786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox CS, Massaro JM, Hoffmann U et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi:10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 8. Hansen T, Ahlström H, Söderberg S et al. Visceral adipose tissue, adiponectin levels and insulin resistance are related to atherosclerosis as assessed by whole-body magnetic resonance angiography in an elderly population. Atherosclerosis. 2009;205:163–167. doi:10.1016/j.atherosclerosis.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 9. Windham BG, Simpson BN, Lirette S et al. Associations between inflammation and cognitive function in African Americans and European Americans. J Am Geriatr Soc. 2014;62:2303–2310. doi:10.1111/jgs.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ. 1994;308:1604–1608. doi:10.1136/bmj.308.6944.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85:1–10. doi:10.1259/bjr/38447238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. [DOI] [PubMed] [Google Scholar]
- 13. Bray GA, Jablonski KA, Fujimoto WY et al. ; Diabetes Prevention Program Research Group Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr. 2008;87:1212–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi:10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi:10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 16. The Family Blood Pressure Program (FBPP) TFI. Multi-center genetic study of hypertension. The Family Blood Pressure Program (FBPP). 2002;39:3–9. doi:10.1161/hy1201.100415 [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Fox CS, Hickson D et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33:1635–1639. doi:10.2337/dc10-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Fox CS, Hickson DA et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi:10.1210/jc.2010-1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chibnik LB, Shulman JM, Leurgans SE et al. CR1 is associated with amyloid plaque burden and age-related cognitive decline. Ann Neurol. 2011;69:560–569. doi:10.1002/ana.22277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massaro JM, D’Agostino RB Sr, Sullivan LM et al. Managing and analysing data from a large-scale study on Framingham Offspring relating brain structure to cognitive function. Stat Med. 2004;23:351–367. doi:10.1002/sim.1743 [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22. Morris JC, Heyman A, Mohs RC et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi:10.1212/WNL.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 23. Franzen M. Reliability and Validity in Neuropsychological Assessment. 2nd ed New York: Klewer Academic/Plenum Publishers; 2000. [Google Scholar]
- 24. Spreen O, Strauss E. General ability and premorbid intelligence. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd edn. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 25. Kaplan E, Fein D, Delis D, Morris R et al. WAIS-R-NI Manual. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 26. Hsu F-C, Yuan M, Bowden DW et al. Adiposity is inversely associated with hippocampal volume in African Americans and European Americans with diabetes. J Diabetes Complications. 2016;30:1506–12. doi:10.1016/j.jdiacomp.2016.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanaya AM, Lindquist K, Harris TB et al. ; Health ABC Study Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009;66:329–335. doi:10.1001/archneurol.2008.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Aging Neurosci. 2011;3:12. doi:10.3389/fnagi.2011.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoon DH, Choi SH, Yu JH, Ha JH, Ryu SH, Park DH. The relationship between visceral adiposity and cognitive performance in older adults. Age Ageing. 2012;41:456–461. doi:10.1093/ageing/afs018 [DOI] [PubMed] [Google Scholar]
- 30. Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, Saha C, Pratt JH, Considine RV. Lower Serum Adiponectin Levels in African-American Boys. Obesity. 2003;11:1384–1390. doi:10.1038/oby.2003.187 [DOI] [PubMed] [Google Scholar]
- 31. Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi:10.1016/j.mam.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamogawa K, Kohara K, Tabara Y et al. Abdominal fat, adipose-derived hormones and mild cognitive impairment: the J-SHIPP study. Dement Geriatr Cogn Disord. 2010;30:432–439. doi:10.1159/000321985 [DOI] [PubMed] [Google Scholar]
- 33. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot?Diabetes Care. 2009;32:1068–1075. doi:10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fabbrini E, Magkos F, Mohammed BS et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi:10.1073/pnas.0904944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazzoccoli G, Dagostino MP, Vinciguerra M et al. An association study between epicardial fat thickness and cognitive impairment in the elderly. Am J Physiol Heart Circ Physiol. 2014;307:H1269–H1276. doi:10.1152/ajpheart.00175.2014 [DOI] [PubMed] [Google Scholar]
- 36. Roth M, Hopkins B. Psychological test performance in patients over sixty. I. Senile psychosis and the affective disorders of old age. J Ment Sci. 1953;99:439–450. doi:10.1192/bjp.99.416.439 [DOI] [PubMed] [Google Scholar]
- 37. Nishtala A, Preis SR, Beiser A et al. Midlife cardiovascular risk impacts executive function: Framingham offspring study. Alzheimer Dis Assoc Disord. 2014;28:16–22. doi:10.1097/WAD.0b013e3182a715bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiederkehr S, Laurin D, Simard M, Verreault R, Lindsay J. Vascular risk factors and cognitive functions in nondemented elderly individuals. J Geriatr Psychiatry Neurol. 2009;22:196–206. doi:10.1177/0891988709335797 [DOI] [PubMed] [Google Scholar]
- 39. Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi:10.1161/01.HYP.0000252676.46043.18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.