Abstract
Skeletal muscle, in addition to being comprised of a heterogeneous muscle fiber population, also includes extracellular components that do not contribute to positive tensional force production. Here we test segmental bioelectrical impedance spectroscopy (S-BIS) to assess muscle intracellular mass and composition. S-BIS can evaluate electrical properties that may be related to muscle force production. Muscle fiber membranes separate the intracellular components from the extracellular environment and consist of lipid bilayers which act as an electrical capacitor. We found that S-BIS measures accounted for ~85% of the age-related decrease in appendicular muscle power compared with only ~49% for dual-energy x-ray absorptiometry (DXA) measures. Indices of extracellular (noncontractile) and cellular (contractile) compartments in skeletal muscle tissues were determined using the Cole–Cole plot from S-BIS measures. Characteristic frequency, membrane capacitance, and phase angle determined by Cole–Cole analysis together presented a S-BIS complex model that explained ~79% of interindividual variance of leg muscle power. This finding underscores the value of S-BIS to measure muscle composition rather than lean mass as measured by DXA and suggests that S-BIS should be highly informative in skeletal muscle physiology.
Keywords: Bioelectrical impedance spectroscopy, Contractile muscle tissue, Membrane capacitance
Skeletal muscle, the largest organ in the reference human body, is vital for any physical activity and also plays a role in maintaining whole-body metabolic homeostasis (1,2). Loss of skeletal muscle mass and function increases risk for impaired mobility, falls, fractures, metabolic dysfunction, and mortality and is a major concern in human health, acute and chronic diseases, immobility, and aging (3–6).
It has traditionally been hypothesized that the age-related reduction of muscle force and power results mainly from skeletal muscle mass loss. However, the age-related decrease in skeletal muscle mass estimated by computerized axial tomography (CT), magnetic resonance imaging (MRI), or dual-energy x-ray absorptiometry (DXA) is insufficient to explain the decline in muscle strength (7,8). Skeletal muscle tissue includes extracellular tissue and bundles of metabolically heterogeneous fiber types. Fibers atrophy with age and levels of fibrosis and intramuscular fat increase within and around the bundles (9). This age-related increase in noncontractile content of muscle is related to loss in muscle strength and power (10,11), however, age-related changes in muscle composition are not accessible using routine clinical measures (1). Current sarcopenia consensus definitions include DXA-measured appendicular lean mass (12–15). A major drawback of this technique is that fat-free mass (FFM) is largely the measurement of water and, moreover, it cannot inform about changes in skeletal muscle composition. In contrast, segmental bioelectrical impedance spectroscopy (S-BIS) has the ability to distinguish intracellular water (ICW) from extracellular water (ECW), the ratio of ICW/ECW (16–18), and additionally may evaluate other changes in muscle composition. As such, we tested if the electrical properties of S-BIS could be reasonable metrics to estimate functional muscle mass and whether bioelectrical characteristics could provide insights into interindividual variance of maximum muscle power production that are not explained by DXA-measured lean mass.
Methods
Participants
Fifty-seven community-dwelling volunteers aged 26–76 years (13 men and 44 women) who participated in the national survey study “Midlife in the United States” (MIDUS), a study designed to evaluate health and well-being in midlife (19), were studied. MIDUS participants were identified through a nationally representative random-digit-dial sample of noninstitutionalized, English-speaking adults, aged 26–76 years, in the continental United States. This analysis uses a subsample of participants from the Refresher cohort who participated in biomarker data collection at the University of Wisconsin, Madison. Measurements were conducted between June 5, 2013 and October 19, 2013. This study was approved by the University of Wisconsin Health Sciences Institutional Review Board and conducted in compliance with global, national, and local regulations.
Bioelectrical Impedance Spectroscopy
The basic theory of BIS is described elsewhere (16,18) and the detailed method is provided in the Supplementary Material. Briefly, bioelectrical impedance was measured using a logarithmic distribution of 256 frequencies, ranging from 4 to 1,000 kHz (SFB7, ImpediMed, Pinkenba, QLD, Australia), using disposable tab-type monitoring electrodes (2 cm × 2 cm, Medtronic, Minneapolis, MN). The R0 and R∞ for the whole-body and leg were determined by extrapolation after fitting the spectrum of bioimpedance data to the Cole–Cole model (Bioimp software, ImpediMed; Figure 1B). For BIS, the RI was calculated using 1/[(1/R∞) − (1/R0)]. Segment length (L; cm) was measured from the most lateral aspect of the lateral greater trochanter of the femur to lateral tibial malleolus. Intracellular impedance index was calculated as L2/RI, and extracellular resistance index was calculated as L2/R0. L2/RI reflects segmental ICW, and L2/R0 reflects segmental ECW in the leg. The resistance ratio, calculated as R0/RI, is the index of the ratio of ICW/ECW (16,17) (Figure 1C). The membrane capacitance (Cm), characteristics frequency (fc), and phase angle (φ) were also obtained from the Cole–Cole model (20) (Figure 1B,D).
Figure 1.
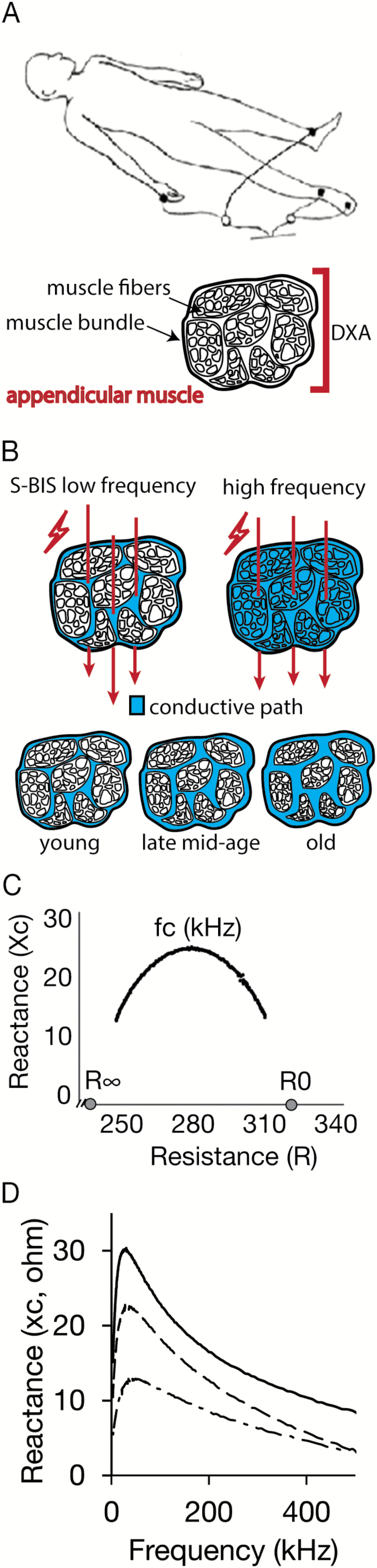
(A) Upper panel: Electrode placements of segmental bioelectrical impedance spectroscopy (S-BIS) measurement for a single leg. Lower panel: Schematic representation showing muscle mass detection by dual-energy x-ray absorptiometry (DXA) and S-BIS. DXA measures appendicular lean mass and cannot inform about lean mass composition. (B) S-BIS takes advantage of the partitioning of contents in appendicular skeletal muscle between intracellular and extracellular pools. (C) Representative Cole–Cole plot of resistance versus reactance measures obtained by leg S-BIS from one individual from the study cohort. The intracellular resistance (RI) was calculated using 1/[(1/R∞) − (1/R0)]. (D) Representative frequency versus reactance measures obtained by leg S-BIS from 29, 56, and 76-year-old female adults (solid line, dashed line, and chain line, respectively). Older adults tended to have lower reactance.
Dual-Energy X-Ray Absorptiometry
A Lunar iDXA (GE Healthcare, Madison, WI) densitometer was used for whole-body and leg composition assessment. Routine densitometry quality assurance procedures were conducted and no instrument drift or shift was detected during the study period. All scans were acquired and analyzed with enCORE software; version 13.31 in accordance with International Society for Clinical Densitometry (ISCD) recommendations (21). All measurements were performed by ISCD-certified technologists. Participant tissue composition, including fat, bone, and lean mass, was obtained as described previously for the whole-body and leg regions (22,23).
Jumping Mechanography
Jumping mechanography has been described elsewhere (24,25), and the detailed method is in the Supplementary Material. Briefly, countermovement jumps were performed on a ground reaction force platform (Leonardo Mechanograph; Novotec Medical, Pforzheim, Germany). Leonardo software version 4.2 (Novotec Medical) was used to record voltage and carry out performance calculations. Volunteers were instructed to jump as high as possible, making effort to touch the ceiling with their head. Two trained staff members were present during jumping to explain the procedure and help the participant with regaining balance if necessary. Jump power (Pmax) was calculated by the software using a model previously described (24,25).
Statistical Analysis
The IBM SPSS Statistics, Version 22, was used for all statistical analyses. Participants’ characteristics are given as mean (SD) in Table 1. Normal distribution was tested using a Kolmogorov–Smirnov test and variables showed statistically normal distribution. Regression analysis was applied to examine the relationship between age and Pmax, DXA and S-BIS variables for men and women separately, and the relative decrease rate per decade was calculated from the regression equation using a 30-year-old normative value as reference. Relationships between Pmax and DXA and S-BIS variables were examined using correlation analysis. Correlation coefficients were compared using Meng’s statistical method (26). The partial correlation analysis was also conducted to examine the relationship between Pmax and the intracellular impedance index, impedance differential, Cm, fc, and φ with age, sex, height, and weight as control variables. Stepwise multivariate linear regression analysis was performed using intracellular resistance index (L2/RI), resistance ratio (R0/RI), membrane capacitance (Cm), characteristics frequency (fc), phase angle (φ) of S-BIS as independent variables and Pmax was dependent variable with 0.05 entry and 0.10 removal probability of F.
Table 1.
Physical and Electrical Characteristics of Study Participants
| Men | Women | p Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (y) | 48.8 (12.4) | 52.0 (14.8) | .482 |
| Height (cm) | 178.3 (6.5) | 164.5 (7.4) | <.001 |
| Weight (kg) | 98.4 (31.9) | 88.3 (29.5) | .289 |
| BMI (kg m−2) | 30.6 (8.4) | 32.6 (11.1) | .559 |
| P max (kW) | 3.42 (0.94) | 2.03 (0.64) | <.001 |
| DXA measurements | |||
| Percent body fat (%) | 29.2 (10.2) | 40.4 (9.3) | <.001 |
| FFM (kg) | 68.8 (13.3) | 49.1 (8.8) | <.001 |
| ALST (kg) | 30.6 (6.5) | 21.3 (4.9) | <.001 |
| Leg lean mass (kg) | 21.9 (4.5) | 16.2 (3.7) | <.001 |
| S-BIS measurements in the leg | |||
| Intracellular resistance index (cm2 Ω−1) | 11.5 (3.5) | 7.9 (2.5) | <.001 |
| Extracellular resistance index (cm2 Ω−1) | 28.6 (6.5) | 22.9 (7.1) | .011 |
| Resistance ratio | 0.40 (0.08) | 0.35 (0.08) | .069 |
| Cm (nF) | 6.0 (2.4) | 4.6 (1.7) | .067 |
| fc (kHz) | 32.6 (8.6) | 35.0 (6.0) | .258 |
| Phase angle (deg) | 4.2 (0.9) | 3.7 (0.8) | .052 |
Note: Cohort N = 57; men n = 13, women n = 44.
ALST = appendicular lean soft tissue; BMI = body mass index; Cm = membrane capacitance; DXA = dual-energy x-ray absorptiometry; Extracellular resistance index was calculated as L2/R0; fc = characteristic frequency; FFM = fat-free mass; Intracellular resistance index was calculated as L2/RI; Pmax = maximum leg muscle power; Resistance ratio was calculated as (R0/RI); S-BIS = segmental bioelectrical impedance spectroscopy. Bold values are statistically different between men and women (p < .05).
Results
Impact of Age on Muscle Power, DXA, and S-BIS
We examined the impact of age on leg maximum muscle power output, DXA leg lean mass, whole-body impedance, and S-BIS in the leg. Regression analysis revealed that the correlation between leg maximum power outputs and age was strong and negative (Men: r = −0.817, p < .001; Women: r = −0.700, p < .001) (Figure 2A). Men had a steeper negative slope for maximum jump power output as a function of age than women (0.6 and 0.3 kW less per decade, respectively), although the relative decrement was similar for men and women (13.1% and 11.1% per decade, respectively). A weaker correlation was detected between age and leg lean mass obtained by DXA (LLMDXA) (Men: r = −0.501, p = .08; Women: r = −0.337, p = .03) (Figure 2B). The relative decrement (percent slope) of leg lean mass obtained using DXA (LLMDXA) (7.2% and 4.8% per decade, in men and women, respectively) was only ~49% of the relative decrements of muscle functional leg muscle power against age.
Figure 2.
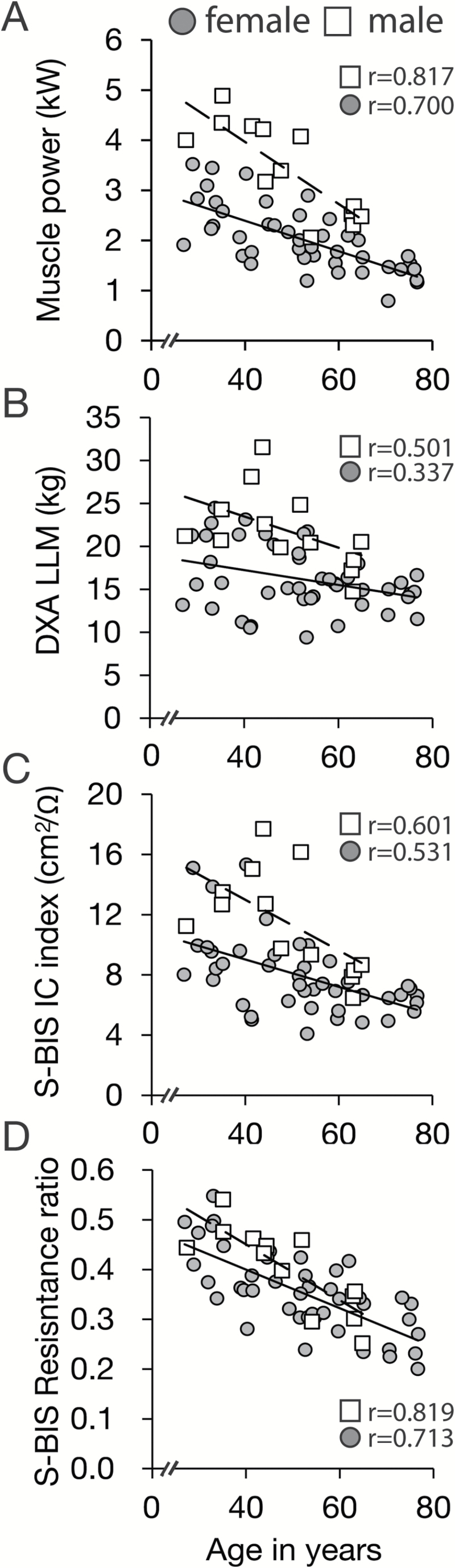
Age-related loss in leg muscle power correlates strongly with segmental bioelectrical impedance spectroscopy (S-BIS). Correlation plots against age for (A) maximum leg muscle power, (B) leg lean mass assessed by dual-energy x-ray absorptiometry (DXA LLM), (C) intracellular resistance (IC) index obtained by S-BIS, and (D) the resistance ratio (R0/RI) by S-BIS in n = 13 men (□) and n = 44 women (●).
Intracellular resistance index (L2/RI) in the leg was calculated for each individual in the cohort using the Cole–Cole plot of resistance against reactance (Figure 2C). L2/RI correlated strongly or moderately with age for both men and women (Men: r = −0.601, p = .03; Women: r = −0.531, p < .001) and the correlation coefficients were significantly greater than with LLMDXA (p < .01) (Figure 2B). The relative decrement of intracellular resistance index was 11.5% and 9.2% per decade and reached 87.8% and 82.9% of the relative decrements of leg muscle power against age in men and women, respectively. In contrast, the extracellular resistance index (L2/R0) in the leg was not related to age. The resistance ratio, calculated as the R0 divided by RI (R0/RI), in the leg was strongly and negatively correlated with age (Men: r = −0.819, p < .001; Women: r = −0.713, p < .001; Figure 2D).
Traditional approaches to estimating skeletal muscle mass by bioelectrical impedance analysis (BIA) typically use a 50-kHz signal and this partially penetrates the intracellular space (27–30). This traditional BIA does not differentiate between intracellular and extracellular compartments. Moreover, the gold standard for using the BIA approach requires regression against total lean tissue or FFM as measured by DXA or MRI (28–30). Not surprisingly, the ability of standard BIA to detect the age-related decline in muscle power was similar to that of appendicular lean soft tissue of DXA (Supplementary Figure 1). These data demonstrate that S-BIS is a more sensitive method for evaluation of age-related loss in muscle power, outperforming traditional BIA and DXA.
Muscle Power Output and S-BIS Versus DXA
To determine if bioelectrical impedance measures could explain differences in leg maximum muscle power output independent of age, we conducted a regression analysis. Maximum power output of the leg significantly correlated with LLMDXA (Men: r = 0.691, p = .009; Women: r = 0.727, p < .001; Supplementary Figure 2A). A very strong positive correlation was detected between maximum power output and the intracellular resistance index (Men: r = 0.808, p < .001; Women: r = 0.881, p < .001; Supplementary Figure 2B). In agreement with the previous analysis, a strong or moderate positive correlation between maximum power output and the resistance ratio was also detected (Men: r = 0.873, p < .001; Females: r = 0.582, p < .001).
S-BIS Parameters and Muscle Power
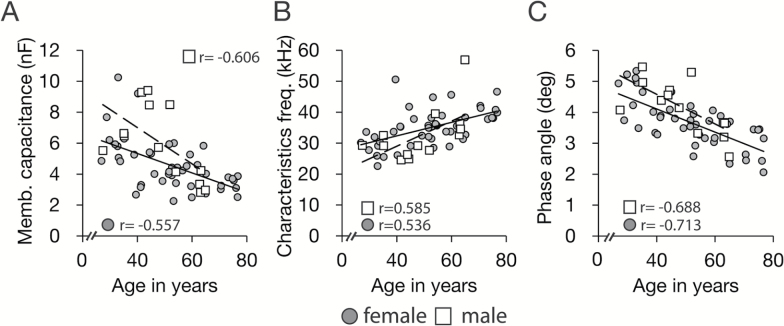
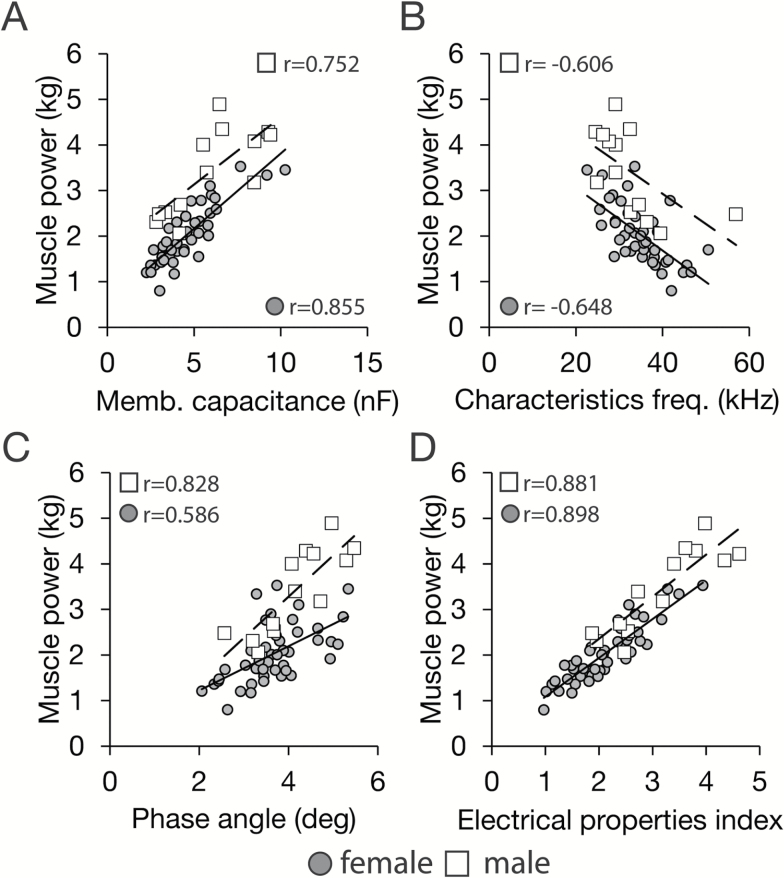
We next investigated if other parameters derived from S-BIS measures would be informative in defining the impact of age on maximum power output. The Cole–Cole model fits the measured resistance with parallel circuits and calculates the electrical capacitance introduced by the nonconducting membrane. The membrane capacitance (Cm) is indicative of the retention capacity of membrane potential gradient and the depolarization reactivity of the muscle cell membranes. The characteristics frequency (fc) is determined by the energy required to supply a constant current though the tissue and reflects heterogeneity in the density of the tissue. The phase angle (φ) has been linked to adiposity in whole-body measures and is thought to positively reflect cell membrane content (31). In this study, Cm and φ both decreased with aging, whereas fc increased with aging (Figure 3). Perhaps not surprisingly, Cm and φ correlated positively with maximum power output, and fc correlated negatively with maximum power output (Figure 4A–C). These data are indicative of broad differences in muscle composition. Correlations between Cole–Cole parameters and maximum power output remained significant even after controlling for age, sex, height, and weight, suggesting that the physiological properties reflected in these measures are fundamentally linked to muscle power production. Stepwise regression analysis of aggregate S-BIS measures (Supplementary Table 1) identified a statistical model where the intracellular impedance index, resistance ratio, Cm, and fc in combination were significant predictors for maximum power output and explained 77.6% and 80.7% of individual variance of leg muscle power in men and women respectively (Figure 4D).
Figure 3.
Relationship between age and electrical properties measured by segmental bioelectrical impedance spectroscopy (S-BIS). Relationship between age and (A) membrane capacitance (Cm), (B) characteristics frequency (fc), (C) phase angle (θ), measured by S-BIS in n = 13 men (□) and n = 44 women (●).
Figure 4.
Segmental bioelectrical impedance spectroscopy (S-BIS) parameters correlate with leg muscle power. Relationship between leg muscle power and (A) membrane capacitance (Cm), (B) characteristics frequency (fc), (C) phase angle (φ), and (D) S-BIS electrical properties index model. The S-BIS model was calculated by stepwise multiple regression analysis with following equation: (0.362 × IC index) + (2.498 × Resistance differential) + (−0.319 × Cm) + (−0.034 × fc) + 0.997 (Supplementary Table 1). This model explained 77.6% and 80.7% of variance of leg muscle power in men and women, respectively.
Discussion
The stronger correlations between S-BIS measures and power and functional capacity reported herein suggest that S-BIS may be sensitive to differences in muscle composition, including fiber shrinkage, fibrosis, and adipose infiltration. Previous studies have reported changes in muscle composition with aging in vivo, including changes in intramuscular adipose tissue and muscle density. Intramuscular fat estimated by MRI in the tibialis anterior muscle increases with aging (10), and diffusion tensor MRI (DT-MRI) signal intensity decreases significantly in the muscles of the lower leg with aging (32). More recently, water T2 mean values assessed by MRI and its heterogeneity indices were significantly higher in the elderly participants when compared with young adults (33). These methods require complex and/or expensive measurement techniques and cannot be conducted in routine clinical care. In contrast, S-BIS is an affordable alternative to CT or MRI, noninvasive, easy-to-operate, portable, and fast. We suggest that S-BIS offers a highly effective method for detection of age-related declines in muscle function and as such may be a superior method for clinical assessment of sarcopenia as well as basic medical research for skeletal muscle assessment.
Skeletal muscle undergoes profound changes in composition with age including fiber atrophy and fibrosis that may not be detected by simple measures of muscle girth. S-BIS is an alternative strategy to DXA that takes advantage of the partitioning of water in skeletal muscle between intracellular and extracellular spaces (16,17,34) (Figure 2A). Muscle fiber cell membranes are phospholipid bilayers and act as capacitors that insulate the intracellular compartment from electrical current applied at low frequencies. At higher frequencies, in contrast, the membranes are permeable to the current so that ICW and ECW both contribute to electrical conductance. A series of frequencies applied across appendicular muscle groups allows for separate detection of resistance along the intracellular and extracellular conductivity pathways (35). In addition, S-BIS can also evaluate other electrical properties of muscle tissues and membrane which may be related to tensional force production. Recent studies confirm that the upper leg ECW to ICW ratio obtained by BIS is related to muscle function, but a direct comparison of BIS to other methods of body composition measurement has not been previously made (18). The present study compared BIS and DXA measurements directly with muscle function and muscle aging and found segmental BIS to be more informative in evaluating skeletal muscle than traditional DXA.
The age-related impairment in muscle force transmission is thought to be partly associated with increased fibrosis including increased thickness of the extracellular matrix (36). An increase in fibrotic material including associated water plus electrolytes between muscle bundles and among muscle fibers, and relative expansion of extracellular volume against intracellular volume, would be predicted to impact resistance ratio due to an increase in conductive area at low frequency current (18). The reduction in membrane capacitance (Cm) could be linked to fiber shrinkage and fiber loss, both of which would lead to a relative reduction in membrane content. Age-related increase of characteristic frequencies (fc) may be related to fat infiltration or increased fibrosis in the muscle. Age-related changes of phase angle (φ) have been reported previously in the context of whole-body measures of body composition (31,37). Previous studies suggest that φ is linked to ECW/ICW ratio and FFM, but not fat mass (38), and a previous study in patients with cancer has shown that whole-body φ is linked to handgrip strength and peak expiratory flow (39). Increased heterogeneity within leg muscles has previously been reported in MRI studies of older participants (33). Post mortem measures of vastus lateralis suggest that whole-muscle cross-sectional area decreases by ~5% per decade (40). Similar declines in skeletal muscle mass have been reported in vivo using DXA or MRI (41). In addition to overall muscle shrinkage, within the muscle bundles total number of fibers and mean fiber size decrease ~10% per decade (40). The reason for the discrepancy is likely that whole-muscle mass measures include extracellular spaces between muscle fibers but do not account for the ratio of intracellular and extracellular volume that decreases with aging. Data shown here emphasize the importance of muscle tissue quantity and quality in muscle power generation during aging. A recent study (42) reported that the metabolic profiles assessed by liquid chromatography-tandem mass spectrometry are related to muscle quality, and thus, it is interesting to examine the relationship between the heterogeneities of skeletal muscle composition and those factors using S-BIS.
As a limitation, we have not presented the mechanisms linking out BIS data with the functional observations. The observations of others, however, have begun to provide support for a linkage. One possible explanation is that BIS detects changes in muscle density, a property that has been shown to be reduced with aging (as estimated by CT) and related to muscle function (11). Another possibility is detection of changes in intramuscular fat. Prior MRI studies have shown that intramuscular fat increases with age and that the ratio between contractile and noncontractile tissue also changes with aging (10). Studies using diffusion tensor MRI showed that the water diffusivity and heterogeneity are changed with aging (32,33). These hypotheses may be tested using stable isotope infusion techniques (43) or biopsy analysis (44) to delineate age-related changes in muscle composition and how those parameters relate to change in muscle function.
In conclusion, the results of the present study indicate the value of S-BIS to measure muscle composition rather than appendicular lean mass as measured by DXA and suggest that S-BIS should be highly informative in biological and medical researches of skeletal muscle physiology.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The MIDUS research is supported by a grant from the National Institute on Aging (P01-AG020166). The research was further supported by the General Clinical Research Centers Program and UL1TR000427 (UW) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health. This study was supported by JSPS KAKENHI with a research grant provided to Y.Y. (15H05363) and by NIH/NIA grant to R.A. (AG047358).
Supplementary Material
References
- 1. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015:1–12. doi:10.1017/S0029665115000129 [DOI] [PubMed] [Google Scholar]
- 2. Geisler C, Braun W, Pourhassan M, et al. Age-dependent changes in resting energy expenditure (REE): insights from detailed body composition analysis in normal and overweight healthy caucasians. Nutrients. 2016;8. doi:10.3390/nu8060322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. [DOI] [PubMed] [Google Scholar]
- 4. Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203–209. doi:10.1093/ageing/afs194 [DOI] [PubMed] [Google Scholar]
- 5. Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: The health, aging, and body composition study. Journal of Bone and Mineral Research. 2010;25:513–519. doi:10.1359/jbmr.090807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. doi:10.1111/j.1474-9726.2012.00844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi:10.3389/fphys.2012.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi:10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pugh TD, Conklin MW, Evans TD, et al. A shift in energy metabolism anticipates the onset of sarcopenia in rhesus monkeys. Aging Cell. 2013;12:672–681. doi:10.1111/acel.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kent-Braun JA, Ng AV, Young K. Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol (1985). 2000;88:662–668. [DOI] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. [DOI] [PubMed] [Google Scholar]
- 12. Chen LK, Liu LK, Woo J, Assantachai P, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi:10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 13. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi:10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartok C, Schoeller DA. Estimation of segmental muscle volume by bioelectrical impedance spectroscopy. J Appl Physiol (1985). 2004;96:161–166. doi:10.1152/japplphysiol.00686.2002 [DOI] [PubMed] [Google Scholar]
- 17. Yamada Y, Schoeller DA, Nakamura E, Morimoto T, Kimura M, Oda S. Extracellular water may mask actual muscle atrophy during aging. J Gerontol A Biol Sci Med Sci. 2010;65:510–516. doi:10.1093/gerona/glq001 [DOI] [PubMed] [Google Scholar]
- 18. Yamada Y, Yoshida T, Yokoyama K, et al. The extracellular to intracellular water ratio in upper legs is negatively associated with skeletal muscle strength and gait speed in older people. J Gerontol A Biomed Sci Med Sci. In press. doi:10.1093/gerona/glw125 [DOI] [PubMed] [Google Scholar]
- 19. Radler BT. The midlife in the United States (MIDUS) Series: a national longitudinal study of health and well-being. Open Health Data. 2014;2. doi:10.5334/ohd.ai [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole KS, Cole RH. Dispersion and absorption in dielectrics I. Alternating current characteristics. J Chem Phys. 1941;9:341–351. [Google Scholar]
- 21. Hangartner TN, Warner S, Braillon P, Jankowski L, Shepherd J. The Official Positions of the International Society for Clinical Densitometry: acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J Clin Densitom. 2013;16:520–536. doi:10.1016/j.jocd.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 22. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass. Health, Aging, and Body Composition Study—Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol. 1999;87:1513–1520. [DOI] [PubMed] [Google Scholar]
- 23. Buehring B, Krueger D, Libber J, et al. Dual-energy X-ray absorptiometry measured regional body composition least significant change: effect of region of interest and gender in athletes. J Clin Densitom. 2014;17:121–128. doi:10.1016/j.jocd.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 24. Buehring B, Krueger D, Binkley N. Jumping mechanography: a potential tool for sarcopenia evaluation in older individuals. J Clin Densitom. 2010;13:283–291. doi:10.1016/j.jocd.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 25. Buehring B, Krueger D, Fidler E, Gangnon R, Heiderscheit B, Binkley N. Reproducibility of jumping mechanography and traditional measures of physical and muscle function in older adults. Osteoporos Int. 2015;26:819–825. doi:10.1007/s00198-014-2983-z [DOI] [PubMed] [Google Scholar]
- 26. Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation-coefficients. Psychol Bull. 1992;111:172–175. [Google Scholar]
- 27. Organ LW, Bradham GB, Gore DT, Lozier SL. Segmental bioelectrical-impedance analysis—theory and application of a new technique. J Appl Physiol. 1994;77:98–112. [DOI] [PubMed] [Google Scholar]
- 28. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89:465–471. [DOI] [PubMed] [Google Scholar]
- 29. Ward LC. Segmental bioelectrical impedance analysis: an update. Curr Opin Clin Nutr Metab Care. 2012;15:424–429. doi:10.1097/MCO.0b013e328356b944 [DOI] [PubMed] [Google Scholar]
- 30. Miyatani M, Kanehisa H, Masuo Y, Ito M, Fukunaga T. Validity of estimating limb muscle volume by bioelectrical impedance. J Appl Physiol (1985). 2001;91:386–394. [DOI] [PubMed] [Google Scholar]
- 31. Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. [DOI] [PubMed] [Google Scholar]
- 32. Galbán CJ, Maderwald S, Stock F, Ladd ME. Age-related changes in skeletal muscle as detected by diffusion tensor magnetic resonance imaging. J Gerontol A Biol Sci Med Sci. 2007;62:453–458. [DOI] [PubMed] [Google Scholar]
- 33. Azzabou N, Hogrel J-Y, Carlier PG. NMR based biomarkers to study age-related changes in the human quadriceps. Exp Gerontol. 2015;70:54–60. doi:10.1016/j.exger.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 34. De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol. 1997;96:161–166. [DOI] [PubMed] [Google Scholar]
- 35. Yamada Y, Ikenaga M, Takeda N, et al. Estimation of thigh muscle cross-sectional area by single- and multi-frequency segmental bioelectrical impedance analysis in elderly. J Appl Physiol. 2014;116:176–182. doi:10.1152/japplphysiol.00772.2013 [DOI] [PubMed] [Google Scholar]
- 36. Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech. 2014;47:944–948. doi:10.1016/j.jbiomech.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 2002;18:153–167. [DOI] [PubMed] [Google Scholar]
- 38. Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. Am J Clin Nutr. 2016;103:712–716. doi:10.3945/ajcn.115.116772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Norman K, Stobäus N, Zocher D, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92:612–619. doi:10.3945/ajcn.2010.29215 [DOI] [PubMed] [Google Scholar]
- 40. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. [DOI] [PubMed] [Google Scholar]
- 41. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 42. Moaddel R, Fabbri E, Khadeer MA, et al. Plasma biomarkers of poor muscle quality in older men and women from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71:1266–1272. doi:10.1093/gerona/glw046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi:10.1093/gerona/glu103 [DOI] [PubMed] [Google Scholar]
- 44. Choi SJ, Files DC, Zhang T, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:557–564. doi:10.1093/gerona/glv169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.