Abstract
Background
Multimorbidity is an important health outcome but is difficult to quantify. We recently developed a multimorbidity-weighted index (MWI) and herein assess its performance in an independent nationally-representative cohort.
Methods
Health and Retirement Study (HRS) participants completed an interview on physician-diagnosed chronic conditions and physical functioning. We determined the relationship of chronic conditions on physical functioning and validated these weights with the original, independently-derived MWI. We then determined the association between MWI with physical functioning, grip strength, gait speed, basic and instrumental activities of daily living (ADL/IADL) limitations, and the modified Telephone Interview for Cognitive Status (TICS-m) in adjusted models.
Results
Among 20,509 adults, associations between chronic conditions and physical functioning varied several-fold. MWI values based on weightings in the HRS and original cohorts correlated strongly (Pearson’s r = .92) and had high classification agreement (κ statistic = .80, p < .0001). Participants in the highest versus lowest MWI quartiles had weaker grip strength (−2.91 kg, 95% confidence interval [CI]: −3.51, −2.30), slower gait speed (−0.29 m/s, 95% CI: −0.35, −0.23), more ADL (0.79, 95% CI: 0.71, 0.87) and IADL (0.49, 95% CI: 0.44, 0.55) limitations, and lower TICS-m (−0.59, 95% CI: −0.77, −0.41) (all p < .001). We observed monotonic graded relationships for all outcomes with increasing MWI quartiles.
Conclusion
A multimorbidity index weighted to physical functioning performed nearly identically in a nationally-representative cohort as it did in its development cohorts, confirming broad generalizability. MWI was strongly associated with subjective and objective physical and cognitive performance. Thus, MWI serves as a valid patient-centered measure of multimorbidity, an important construct in research and clinical practice.
Keywords: Multiple chronic conditions, Comorbidity, Chronic disease, Physical performance, Disability
As adults survive longer with multimorbidity, patient-oriented outcomes beyond mortality are vital. A fundamental goal among many adults is maintaining functional independence to age in place (1,2). Those with functional impairment are at increased risk for poor health outcomes such as hospital readmission, institutionalization, and mortality (3–5). Despite the high value patients and families place on maintaining good function, clinicians and investigators lack validated tools that capture the burden of multimorbidity on function. A measure that focuses on functional status as the outcome is particularly important for those with multimorbidity. Such a tool can be used to better identify individuals at risk for functional decline, tailor clinical decision-making, and guide interventions to maximize and preserve physical functioning.
Current methods to measure multimorbidity have been limited to counting or weighting diseases to less patient-centered outcomes such as mortality, healthcare cost, and utilization. Although common, disease count fails to capture the full impact of these conditions on current and future functional status (6–8). Comorbidity measures that weight disease severity by mortality risk, such as the Charlson–Deyo (9,10) index and Elixhauser (11) measure, or by cost, such as the Centers for Medicare and Medicaid-Hierarchical Condition Categories (12) are often used for risk-adjustment but are less valuable to patients and families. Overall, these indices have not progressed alongside the increasing need for comprehensive patient-centered approaches to capture disease burden.
We recently developed and internally validated a multimorbidity-weighted index (MWI) (6) that weights 81 chronic diseases and conditions by their impact on the Short Form-36 physical functioning scale, a universally valued patient-centered outcome (13), in three large cohorts of community-dwelling adults (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study). The calculation of the MWI and its disease weightings are shown in Supplementary Appendix 1 and Supplementary Table 1. The MWI better captured the high and low extremes of multimorbidity than other methods and was more strongly associated with mortality and future physical functioning (7). However, the index was derived from three large but occupationally and racially homogeneous samples, raising questions about whether it appropriately captures multimorbidity in the general U.S. population.
To determine the extent to which the original MWI may be applied validly across diverse populations, and its impact on varied measures of health, we identified the associations of chronic diseases and conditions with physical functioning among participants in an independent, nationally-representative cohort of adults from the Health and Retirement Study (HRS) and compared these weightings and rank ordering of diseases with the original development cohorts. We then determined the associations between MWI with physical functioning, grip strength, gait speed, limitations in basic and instrumental activities of daily living (ADLs, IADLs) (14), and cognitive performance in HRS. We aimed to produce and validate an informative, scalable, easy to use index that captures the full burden of multimorbidity solely based on self-reported diseases for broad sectors of the population.
Methods
Study Population
The HRS is an ongoing, nationally-representative population-based longitudinal cohort of more than 38,000 U.S. population-based adults aged 51 years and older followed since 1992. The HRS sample uses a multi-stage area probability design that involves geographical stratification, clustering, and oversampling of African-American, Hispanic, and minority households (15). During each 2-year cycle of interviews, more than 20,000 participants who represent a diversity of backgrounds, occupations, family compositions, living arrangements, and other aspects of life, are surveyed. Participants receive biennial questionnaires regarding physician-diagnosed medical conditions, health status, health behaviors, income, employment, and insurance status, described previously (16,17). To maximize the contemporaneous nature of our analyses, we studied participants who completed both an interview in 2010 on physician-diagnosed chronic diseases and conditions and physical functioning, and who were also assessed in-person for objective measures of physical and cognitive performance.
Of 22,032 participants in the 2010 interview, we identified 20,805 (94.4%) aged 51 years and older who completed information on chronic diseases and physical function for this analysis. We then excluded 184 (0.88%) who had one or more missing health condition variables assessed in the multivariate analysis and 112 (0.54%) who had five or more missing physical functioning items, according to the SF-36 protocol on missing data (18). Excluded participants (1.4%) were of similar age and sex but more likely to be Hispanic and on average have a lower grip strength, gait speed, TICS-m, and more ADL and IADL limitations than included participants (Supplementary Table 2). Descriptive and multivariate analyses for the association between chronic diseases and physical functioning and secondary analyses on MWI with subjective outcomes (physical functioning, ADL and IADL limitations were based on a final sample of 20,509 participants. Objective outcomes including grip strength and gait speed were assessed through face-to-face assessments randomly assigned to half the cohort (half-samples alternate every other wave so longitudinal data from face-to-face interviews is obtained from all participants every 4 years) (16). Gait speed was further limited to adults aged 65 years and older. The final sample of participants with in-person assessments and the complete set of covariates of interest in the multivariate models was N = 6,460 for grip strength and N = 3,386 for gait speed. Proxy respondents provided information on cognitive function for 1,268 (6.2%) participants and IADL limitations for 5 (0.02%) participants.
Chronic Disease Assessment
Physician-diagnosed diseases were assessed at each study wave. Participants were asked, “Has a doctor ever told you that you have…?” Sixteen assessed conditions in 2010 included diseases of the heart (myocardial infarction, angina, congestive heart failure, arrhythmia, other heart problems), stroke, hypertension, chronic lung disease, cancer (malignant tumor excluding skin), diabetes, joint disease (arthritis, connective tissue disease), joint replacement (hip, knee), dementia, and glaucoma using a binary present or absent response.
Subjective Physical Functioning Assessment
Nine physical functioning items (18) resembling the 10-item Short Form-36 physical functioning scale (18) that was used in the development cohorts were assessed in the HRS. For the HRS physical functioning measure to be comparable to the SF-36, we used methods described previously (18) to weight the items according to their SF-36 physical functioning equivalents, and then rescale and standardize the score to range 0 to 100, in which 100 corresponds to no difficulty with any physical functioning item while 0 represents difficulty with all items. As one of the ten physical functioning stems in the HRS was imputed, and the response categories were two-item values compared with three-item values in the SF-36, the physical functioning measure was meant to serve as an approximation for the SF-36 physical functioning scale and provide relative between-disease comparisons.
We imputed the tenth physical functioning item of the SF-36, “walking more than a mile,” which was intermediate between responses of “walking several blocks” and “running a mile” assessed in HRS. We assumed that individuals with difficulty walking several blocks would also have difficulty walking more than a mile and assigned them with this response. For individuals with other responses, we substituted the average value of the other completed physical functioning items, according to the SF-36 manual protocol for missing data (18). The final HRS physical functioning scale was based on 10 items and ranged continuously from 0 to 100.
Objective Physical Performance Assessment
Objective measures of physical performance including grip strength and gait speed were assessed in half the cohort who received face-to-face interviews. Grip strength was measured continuously in kilograms using a Smedley spring-type hand dynamometer and based on the highest grip strength after two attempts in each hand, that is, best of four attempts, regardless of the documented dominant hand. In participants aged 65 years and older, gait speed was assessed continuously in meters per second through a timed 2.5-m walk test. The best of two attempts was recorded and used in the analysis.
Cognitive Performance Assessment
Cognitive function was assessed using a modified version of the Telephone Interview for Cognitive Status (TICS-m), which included immediate and delayed recall items, serial 7s subtraction, and counting backwards. TICS-m has been used to screen for dementia (the original purpose of TICS) and mild cognitive impairment (19). The total cognitive score was measured continuously using the composite measure, range 0–27, where 0–6 is consistent with dementia, 7–11 is cognitive impairment without dementia, and 12–27 is normal, based on prior validation studies (19).
Functional Limitations
To further assess the validity of the HRS measure of physical functioning, we included the number of self-reported ADL (range 0–6) and IADL (range 0–5) limitations assessed in the HRS (20).
Statistical Analysis
We used multivariable regression to adjust for age, age squared, and other comorbidities to obtain coefficients for the association between 16 chronic morbidities and physical functioning adjusted for age, age squared, and other comorbidities. We did not adjust for medication use since we considered medication and potential adverse effects to be part of the burden of disease. MWI was created by weighting morbidities by their respective regression coefficients on physical functioning. The absolute value of the weightings were summed to form a MWI for each participant and compared with rankings based upon the original derivation cohorts.
Pearson’s correlation coefficient was used to compare MWI weightings derived from the HRS and development cohorts. We further categorized the HRS and development MWIs into quartiles and used the κ statistic to assess for agreement between the categorical classification of MWI values.
We used multiple linear regression to determine the association between MWI, using values from the original development cohorts applied in the HRS, and continuous measures of physical functioning, grip strength, gait speed, cognitive function, and ADL and IADL limitations. We used fractional polynomials to assess for non-linear associations between MWI and all outcomes. For nonlinear associations, we assessed overall and piece-wise linear regression coefficients at the MWI value of nonlinearity. Regression models included age, sex, race/ethnicity (White, Black, Hispanic, and other), body mass index (<18.5, 18.5–24.9, 25–30, and ≥30 kg/m2), smoking status (never, past, and current), education (<12, 12, 13–15, and ≥16 years), and household net worth (quartiles). All analyses were additionally adjusted for the HRS complex study design to obtain a nationally-representative sample. All analyses were conducted using STATA, version 14.0 (StataCorp, College Station, TX, 2015).
Results
Participant Characteristics
The final sample included 20,509 participants, nationally- representative of 94.9 million Americans aged 51 years and older, with mean age of 64.7 (SD 10.7) years and physical functioning score of 68 (SD 31) (Table 1; Supplementary Table 3 for comparison between original development and HRS cohorts). The mean grip strength and gait speed were within normative means and similar to those in prior HRS studies (21–24). Participants had an average of 0.48 (SD 1.2) ADL limitations and 0.30 (SD 0.9) IADL limitations. The mean TICS-m score of 16 (SD 4.3) was within the range of normal cognitive performance (25).
Table 1.
Participant Characteristics in the Health and Retirement Study, 2010
| Characteristic, Range | Health and Retirement Survey, N = 20,509 | ||
|---|---|---|---|
| Number (%) | Mean | SD | |
| Age, years | 64.7 | 10.7 | |
| Sex, female | 11,716 (54.1) | ||
| Race | |||
| White | 13,416 (78.4) | ||
| Black | 3,859 (10.1) | ||
| Hispanic | 2,566 (8.1) | ||
| Other | 625 (3.3) | ||
| Body mass index, kg/m2 | 28.4 | 6.0 | |
| <18.5 | 112 (2.1) | ||
| 18.5–24.9 | 1,301 (23.6) | ||
| 25–29.9 | 1,687 (32.0) | ||
| ≥ 30 | 2,087 (42.3) | ||
| Smoking status | |||
| Never smoker | 8,841 (43.5) | ||
| Past smoker | 49.6 (41.0) | ||
| Current smoker | 14.8 (15.5) | ||
| Education, years | 13.5 | 6.3 | |
| <12 | 4,474 (16.3) | ||
| 12 | 6,575 (30.9) | ||
| 13–15 | 4,802 (24.5) | ||
| ≥16 | 4,654 (28.3) | ||
| Household net worth, $ | 388,585 | 1,038,730 | |
| ≤14,000 | 5,120 (20.5) | ||
| 14,001–113,000 | 5,118 (24.1) | ||
| 113,001–323,700 | 5,116 (26.4) | ||
| ≥323,701 | 5,115 (29.0) | ||
| Multimorbidity-Weighted Index, 0–36.8 | 4.7 | 4.5 | |
| Chronic conditions, number, 0–12 | 2.0 | 1.5 | |
| Physical functioning scale, 0–100 | 68.1 | 31.4 | |
| Grip strength, kg | 33.6 | 11.8 | |
| Gait speed, m/s | 0.89 | 3.8 | |
| Telephone Interview for Cognitive Status-modified, 0–27 | 15.7 | 4.3 | |
| Activities of Daily Living limitations, number, 0–6 | 0.48 | 1.2 | |
| Instrumental Activities of Daily Living limitations, number, 0–5 | 0.30 | 0.89 | |
Chronic Diseases and Physical Functioning
The associations between chronic health conditions and the HRS physical functioning scale varied several-fold (median coefficient 7.0, range 2.0–25.5) (Supplementary Table 4). Similar to the original development cohorts, late and end-stage organ diseases were associated with the lowest physical functioning (Table 2). The conditions with the worst MWI scores included dementia, congestive heart failure, lung disease, stroke, arthritis, and knee replacement.
Table 2.
Rank Order of Conditions With Greatest Impact on Physical Functioning in the Development and Validation Cohorts
|
Development Cohorts: Nurses’ Health Study I and II, Health Professionals Follow-up Study, 1992–2008 (N = 216,890 contributing 612,592 observations) |
Validation Cohort: Health and Retirement Study, 2010 (N = 20,509) |
|---|---|
| Knee replacement | Dementia |
| Dementia | Lung disease |
| Congestive heart failure | Arthritis |
| Lung disease | Congestive heart failure |
| Stroke | Stroke |
| Hip replacement | Knee replacement |
| Arthritis | Diabetes |
| Connective tissue disease | Connective tissue disease |
| Diabetes | Arrhythmia |
| Angina | Angina |
| Myocardial infarction | Hypertension |
| Arrhythmia | Glaucoma |
| Cancer excluding minor skin | Hip replacement |
| Other heart condition | Myocardial infarction |
| Glaucoma | Other heart condition |
| Hypertension | Cancer excluding minor skin |
The rank ordering of chronic diseases from greatest to least impact on physical functioning was similar between the original development and independent validation cohorts (Table 2). Diseases had the same ranking or differed by 1–3 positions with the exception of arthritis and hypertension, which ranked higher in the HRS, and knee and hip replacement that ranked lower in HRS.
MWI
The mean MWI in the HRS, using the original development cohort weights, was 4.7 (SD 4.5, range 0–36.8), and the mean disease count was 2.0 (SD 1.5, range 0–12) (Table 1). The mean disease count was lower in the HRS than in the original development cohorts (Supplementary Table 3), likely due to the fewer diseases assessed.
Values for MWI based upon weightings observed in the HRS and development cohorts (Nurses’ Health Study I and II, Health Professionals Follow-up Study) were highly correlated (Pearson’s r = 0.92, p < .0001). After categorizing the development and validation MWIs into quartiles, we found that 85% of the classification agreed, and the κ statistic of measure of agreement was .80 (95% CI: 0.79, 0.80; p < .0001).
MWI and Physical and Cognitive Performance
MWI was associated with subjective and objective physical performance assessed through measured grip strength and gait speed and self-reported physical functioning and difficulty performing ADLs and IADLs. Participants in the highest quartile MWI had worse physical functioning, weaker grip strength, slower gait speed, worse cognitive performance, and more ADL and IADL limitations compared with participants in the lowest quartile. We observed a monotonic graded relationship for all outcomes with increasing MWI quartiles (Table 3). These associations were attenuated but persisted after adjusting for age, sex, race/ethnicity, body mass index, smoking status, education, and household net worth.
Table 3.
Physical and Cognitive Performance Outcomes by Multimorbidity-Weighted Index Quartiles in the Health and Retirement Study, 2010
| Outcome, Number of Participants | Q2 (0.3–3.9) | Q3 (4.0–7.8) | Q4 (7.9–37.0) | p-trend** | |||
|---|---|---|---|---|---|---|---|
| β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | β coefficient (95% CI) | p value | ||
| Physical functioning, N = 20,006 | |||||||
| Unadjusted | −11.57 (−12.77, −10.37) | .001 | −25.61 (−26.73, −24.49) | <.001 | −44.37 (−45.86, −42.88) | <.001 | <.001 |
| Adjusted* | −8.36 (−9.47, −7.24) | <.001 | −18.03 (−19.13, −16.94) | <.001 | −32.86 (−34.42, −31.29) | <.001 | <.001 |
| Grip strength, kg, N = 6,460 | |||||||
| Unadjusted | −3.63 (−4.57, −2.69) | <.001 | −6.12 (−6.98, −5.25) | <.001 | −8.17 (−9.08, −7.26) | <.001 | <.001 |
| Adjusted* | −0.95 (−1.54, −0.36) | .002 | −1.80 (−2.31, −1.28) | <.001 | −2.91 (−3.51, −2.30) | <.001 | <.001 |
| Gait speed, m/s, N = 3,386 | |||||||
| Unadjusted | −0.10 (−1.11, 0.92) | .852 | −0.36 (−1.17, 0.46) | .383 | −0.53 (−1.32, 0.25) | .176 | .075 |
| Adjusted* | −0.10 (−1.09, 0.89) | .837 | −0.34 (−1.14, 0.45) | .390 | −0.51 (−1.29, 0.27) | .195 | .108 |
| TICS-modified score (0–27), N = 18,775 | |||||||
| Unadjusted | −0.56 (−0.80, −0.33) | <.001 | −1.46 (−1.66, −1.26) | <.001 | −2.36 (−2.59, −2.15) | <.001 | <.001 |
| Adjusted* | −0.07 (−0.28, 0.13) | .497 | −0.21 (−0.41, −0.01) | .038 | −0.59 (−0.77, −0.41) | <.001 | <.001 |
| Number of ADL limitations (0–6), N = 20,002 | |||||||
| Unadjusted | 0.15 (0.12, 0.19) | <.001 | 0.43 (0.38, 0.47) | <.001 | 1.09 (1.02, 1.16) | <.001 | <.001 |
| Adjusted* | 0.07 (0.04, 0.10) | <.001 | 0.24 (0.19, 0.29) | <.001 | 0.79 (0.71, 0.87) | <.001 | <.001 |
| Number of IADL limitations (0–5), N = 20,002 | |||||||
| Unadjusted | 0.06 (0.04, 0.09) | <.001 | 0.24 (0.21, 0.26) | <.001 | 0.70 (0.65, 0.74) | <.001 | <.001 |
| Adjusted* | 0.01 (−0.02, 0.04) | .502 | 0.11 (0.08, 0.14) | <.001 | 0.49 (0.44, 0.55) | <.001 | <.001 |
Note: MWI Quartile 1 (range 0–0.29) serves as Reference. ADL = Activities of daily living; CI = Confidence interval; IADL = Instrumental activity of daily living; MWI = Multimorbidity-weighted index; N = Number; Q = Quartile; TICS = Telephone Interview for Cognitive Status.
*Adjusted for age, sex, race/ethnicity, body mass index, smoking status, education, household net worth, and the Health and Retirement Study complex study design. **P-trend for increasing MWI quartiles, from Q1 (reference) to Q4
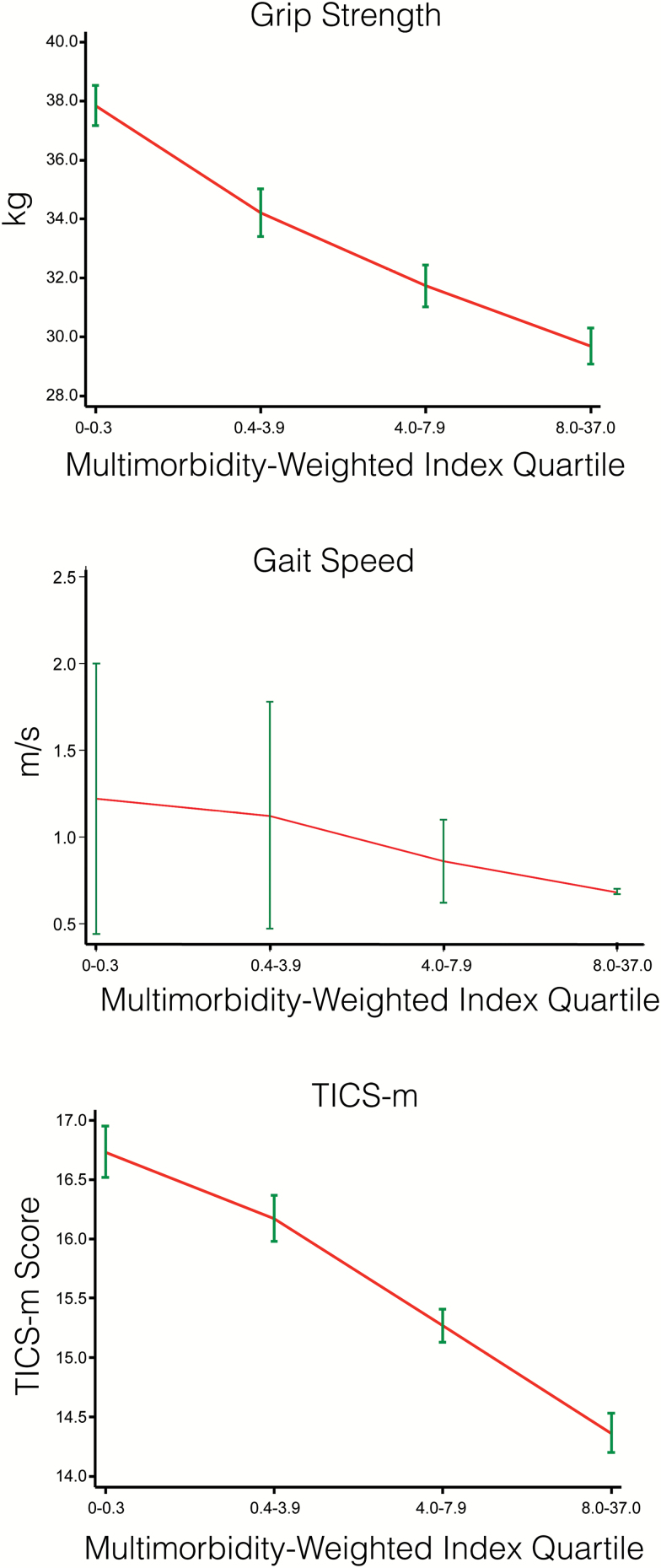
Associations between MWI with physical functioning, gait speed, grip strength, and the TICS-m were essentially linear (Figure 1). Formal testing by adding fractional polynomials of MWI to the model were nonsignificant. Each point increase in MWI was associated with worse physical functioning (−3.73, 95% CI: −3.84, −3.62, p < .001), weaker grip strength (−0.27 kg, 95% CI: −0.32, −0.22, p < .001), slower gait speed (−0.29 m/s, 95% CI: −0.35, −0.23), and worse TICS-m score (−0.06, 95% CI: −0.07, −0.04, p < .001) in fully adjusted models. The monotonic graded relationship with increasing MWI quartiles was significant for all outcomes except gait speed (Table 3).
Figure 1.
Adjusted weighted average (95% CI) of physical and cognitive performance outcomes by multimorbidity-weighted index quartiles. All models adjusted for age, sex, race/ethnicity, body mass index, smoking status, education, household net worth, and the HRS complex study design. TICS-m = Modified Telephone Interview for Cognitive Status.
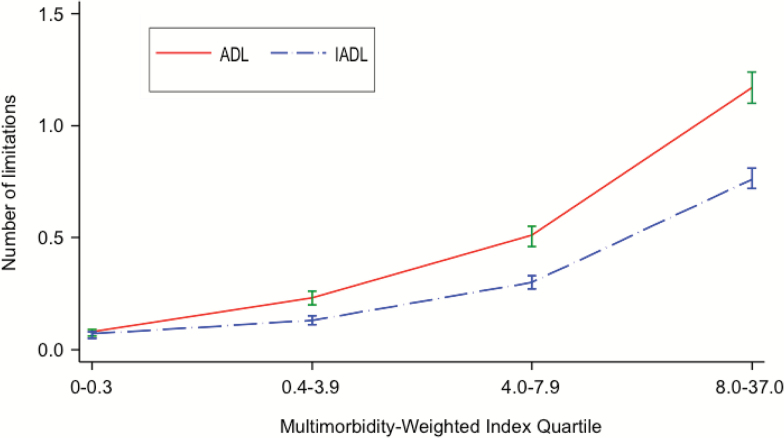
In contrast, the associations between MWI quartiles with ADL and IADL limitations were significantly nonlinear (fractional polynomial models both p < .001) (Figure 2). For each successive quartile MWI, there was more than a doubling in the number of ADL and IADL limitations that persisted after adjustment (Table 3). The overall and piece-wise linear regression coefficients for MWI ≤8 and >8 and number of ADL and IADL limitations are shown in Supplementary Table 5.
Figure 2.
Adjusted weighted average (95% CI) of basic and instrumental activities of daily living limitations by multimorbidity-weighted index quartiles. All models adjusted for age, sex, race/ethnicity, body mass index, smoking status, education, household net worth, and the HRS complex study design. ADL = Activity of daily living; IADL = Instrumental activity of daily living.
Discussion
In an independent cohort nationally-representative of about 95 million U.S. adults aged 51 years and older, we determined the relationship of chronic diseases and conditions on physical functioning to validate an easily-computed MWI that relies solely on self-reported chronic conditions. For the index to be applicable to a broad range of adults and their abilities, we weighted chronic conditions by a modified Short Form-36 physical functioning scale using a full range of functioning, from basic to vigorous activities. Physical functioning is a widely valued patient-centered outcome and also strongly predicts key clinical outcomes such as mortality, hospitalization, and emergency department visits (26,27). Further, identification of early stages of physical decline enables opportunities for early interventions that might slow or prevent further decline.
In the HRS, the rank orderings of participants as well as diseases strongly agreed with those reported in the original cohorts (6), confirming the external validity of the previously-estimated weights. Further, MWI was strongly and consistently related to both subjective and objective measures of physical health, cognition, and disability, further supporting its validity across broad sectors of the U.S. population.
Compared with the original cohorts, the rank ordering of chronic conditions in their impact on physical functioning was similar in this independent, more representative HRS cohort. Late- and end-stage organ diseases were associated with several-fold worse physical functioning compared with other chronic conditions. Diseases that interfere with daily function, such as arthritis, also ranked high in the HRS and original cohorts, while conditions such as hypertension that may impact mortality risk but have a smaller impact on physical functioning, ranked lower. Knee and hip replacement ranked higher than arthritis in the original cohorts, but joint disease overall ranked high in both the HRS and original cohorts.
Our study, along with prior studies, supports the importance of physical functioning as a key outcome to assess burden of disease and weight multimorbidity. A study of early waves of the HRS that focused on adults aged 70 years and older developed a prognostic index for mortality and found physical functioning items to be independent risk factors for mortality, but the index was designed to predict mortality rather than physical function (28). The HRS 1998 survey wave was also used to create a prognostic index for 4-year mortality (29) and incorporated nine comorbidities, in addition to ADLs, IADLs, and three physical functioning items as predictors. Prior studies of the association between multimorbidity and functional decline have reported a positive association and greater physical function decline with an increasing number of conditions but have differed in the number of conditions, such as four (30) or six or more conditions, at which this decline occurs (31). In another HRS analysis, participants with combinations of diseases had greater ADL and IADL limitations compared with those with one or no diseases (32). Our index allows both the type and number of chronic conditions to contribute to the burden of multimorbidity, and with the use of a broad measure of physical functioning, can identify adults earlier in the course of their physical decline, prior to the onset of more advanced ADL and IADL limitations.
Prior indices for comorbidity, which are often extended to multimorbidity, have frequently been limited to hospitalized patients or highly specialized populations and weighted to mortality or non-patient-centered outcomes such as healthcare cost and utilization (9–12). Hence, our multimorbidity index with chronic conditions weighted by physical functioning may be viewed as more patient-centered. While patient-reported outcomes such as physical functioning are important, they are not routinely captured in large administrative studies or clinical settings. This index thus contributes a potentially more practical and patient-valued outcome for capturing chronic disease burden. While MWI was based on self-reported physical functioning, we demonstrated that it was strongly associated with both subjective and objective measures of physical functioning, disability, and cognitive performance.
There are a number of potential limitations of this study. First, only 16 of the final 74 original chronic conditions were assessed in the HRS. However, these conditions spanned several organ systems, were prevalent and highly associated with physical functioning in both the development and validation cohorts, and reflect diseases commonly assessed in other national surveys and risk adjustment indices. Second, self-reported conditions may be under- or over-reported. However, administrative data are also subject to misclassification from incomplete documentation, incomplete or erroneous diagnoses, miscoding, and they present a provider rather than patient-centered view on priority conditions that are further subject to bias in coding due to hospital reimbursement. Third, the physical functioning measure was only an approximation of the SF-36 physical functioning scale after weighting, scaling, and standardizing according to the SF-36 methods. Although direct comparisons to the development cohort disease weightings are limited, this measure similarly spanned the full range of physical functioning, from basic activities of daily living to strenuous aerobic and anaerobic activities, and was thus suitable for comparing conditions to each other within-cohort. Fourth, this study did not include rare diseases, which are not identified in the HRS due to their low prevalence, and were limited to more physical than mental health disorders that were available as a binary yes or no response on the self-reported questionnaire. Finally, the HRS is limited to adults aged 51 years and older, while the original cohorts included younger adults, and hence we cannot yet generalize to younger adults nationwide, although they are least likely to have multimorbidity by any measure.
A MWI that weights chronic conditions to concurrent physical functioning performed nearly identically in a nationally-representative independent cohort of U.S. adults as it did in the development cohorts and thus appears to be a valid measure of multimorbidity in adults. MWI captured a greater range of multimorbidity than did a simple disease count and was linearly or synergistically associated with worse subjective and objective physical and cognitive outcomes, further underscoring the importance of the construct of multimorbidity. This easily computed internally and externally validated index may be used as a measure of multimorbidity in community-dwelling adults. Future studies may further validate MWI for use in administrative studies and electronic health record data.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The HRS is sponsored by the National Institute on Aging (NIA) (U01 AG009740) and conducted at the Institute for Social Research, University of Michigan. K.M.L. received additional support from the NIA grants P30 AG053760 and P30 AG024824. The NIA had no role in the design, methods, participant recruitment, data collection, analysis and preparation of this article. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Conflict of Interest
The authors have no conflict of interest to declare.
Supplementary Material
References
- 1. Li GK, Phillips C, Weber K. On Lok: a successful approach to aging at home. Healthc Pap. 2009;10:44–49; discussion 79. [DOI] [PubMed] [Google Scholar]
- 2. Office of Policy Development and Research, U.S. Department of Housing and Urban Development. Aging in Place: Facilitating Choice and Independence 2013 [2016 August] https://www.huduser.gov/portal/periodicals/em/fall13/highlight1.html.
- 3. Greysen SR, Stijacic Cenzer I, Auerbach AD, Covinsky KE. Functional impairment and hospital readmission in Medicare seniors. JAMA Intern Med. 2015;175:559–565. doi:10.1001/jamainternmed.2014.7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes DE, Mehta KM, Boscardin WJ et al. . Prediction of recovery, dependence or death in elders who become disabled during hospitalization. J Gen Intern Med. 2013;28:261–268. doi:10.1007/s11606-012-2226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. doi:10.1001/jama.279.15.1187 [DOI] [PubMed] [Google Scholar]
- 6. Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am J Epidemiol. 2016;184:357–365. doi:10.1093/aje/kwv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei MY, Mukamal KJ. Multimorbidity, physical functioning, and long-term mortality in three prospective cohorts of community-dwelling adults. Am J Epidemiol. 2017. doi:101093/aje/kwx198 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greene ME, Rolfson O, Gordon M, Garellick G, Nemes S. Standard comorbidity measures do not predict patient-reported outcomes 1 year after total hip arthroplasty. Clin Orthop Relat Res. 2015;473:3370–3379. doi:10.1007/s11999-015-4195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi:10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi:10.1016/S0025-6196(11)64210-9 [DOI] [PubMed] [Google Scholar]
- 11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12. Pope GC, Kautter J, Ellis RP et al. . Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 13. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi:10.1111/j.1532-5415.2008.01923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi:10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed] [Google Scholar]
- 15. Heeringa SG, Connor JH. Sample design and methods for the health and retirement survey. Technical Report. Statistical Design Group, Survey Research Center, University of Michigan, Ann Arbor, MI; 1995. [Google Scholar]
- 16. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43:576–585. doi:10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institute on Aging. Growing Older in America: The Health and Retirement Study National Institutes of Health; 2007. March. https://www.nia.nih.gov/health/publication/growing-older-america-health-and-retirement-study/preface Accessed May 2016. [Google Scholar]
- 18. Ware JE., Jr SF-36 health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center Hospitals, Inc; 1993. [Google Scholar]
- 19. Cook SE, Marsiske M, McCoy KJ. The use of the modified telephone interview for cognitive status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:103–109. doi:10.1177/0891988708328214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fonda S, Herzog AR. Documentation of Physical Functioning Measured in the Heath and Retirement Study and the Asset and Health Dynamics among the Oldest Old Study 2004 http://hrsonline.isr.umich.edu/sitedocs/userg/dr-008.pdf.
- 21. Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi:10.1186/1756-0500-4-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Batsis JA, Germain CM, Vásquez E, Bartels SJ. Prevalence of weakness and its relationship with limitations based on the foundations for the national institutes for health project: data from the health and retirement study. Eur J Clin Nutr. 2016;70:1168–1173. doi:10.1038/ejcn.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stephan Y, Sutin AR, Terracciano A. "Feeling younger, walking faster”: subjective age and walking speed in older adults. Age (Dordrecht, Netherlands). 2015;37:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Studenski S, Perera S, Patel K et al. . Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i162–i171. doi:10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedmann PD, Jin L, Karrison TG et al. . Early revisit, hospitalization, or death among older persons discharged from the ED. Am J Emerg Med. 2001;19:125–9. doi:10.1053/ajem.2001.21321 [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR et al. . Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med. 2005;165:1274–1279. doi:10.1001/archinte.165.11.1274 [DOI] [PubMed] [Google Scholar]
- 28. Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi:10.1111/j.1525-1497.2004.40016.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi:10.1001/jama.295.7.801 [DOI] [PubMed] [Google Scholar]
- 30. Bayliss EA, Bayliss MS, Ware JE Jr, Steiner JF. Predicting declines in physical function in persons with multiple chronic medical conditions: what we can learn from the medical problem list. Health Qual Life Outcomes. 2004;2:47. doi:10.1186/1477-7525-2-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nikolova R, Demers L, Béland F, Giroux F. Transitions in the functional status of disabled community-living older adults over a 3-year follow-up period. Arch Gerontol Geriatr. 2011;52:12–17. doi:10.1016/j.archger.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 32. Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:823–830. doi:10.1093/gerona/glw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.