Abstract
Background:
Olfactory impairment is common among older adults; however, data are largely limited to whites.
Methods:
We conducted pooled analyses of two community-based studies: the Atherosclerosis Risk in Communities study (ARIC, 1,398 blacks and 4,665 whites), and the Health, Aging, and Body Composition study (Health ABC, 958 blacks and 1,536 whites) to determine the prevalence of anosmia and associated factors for black and white older adults in the United States.
Results:
The overall prevalence of anosmia was 22.3% among blacks and 10.4% among whites. Blacks had a markedly higher odds of anosmia compared to whites in age and sex adjusted analyses (odds ratio [OR] 2.96, 95% confidence interval [CI] = 2.59–3.38). In both blacks and whites, higher anosmia prevalence was associated with older age and male sex. The highest prevalence was found in black men 85 years or older (58.3%), and the lowest in white women aged 65–69 years (2.4%). Higher education level, lower cognitive score, ApoE ε4, daytime sleepiness, poorer general health status, lower body mass index, and Parkinson disease were associated with higher prevalence of anosmia in one or both races. However, the racial difference in anosmia remained statistically significant after adjusting for these factors (fully adjusted OR = 1.76, 95%CI: 1.50–2.07). Results were comparable between the two cohorts.
Discussion:
Anosmia is common in older adults, particularly among blacks. Further studies are needed to identify risk factors for anosmia and to investigate racial disparities in this sensory deficit.
Keywords: Prevalence, Anosmia, Racial disparity
Olfactory impairment is common among older adults, and may represent a substantial, yet under-recognized, public health problem. The human sense of smell decreases with age. Olfactory impairment affects about a quarter of U.S. adults 50 years or older, and the prevalence increases to over 60% for those 80 years or older (1). A poor sense of smell adversely affects safety, nutrition, and quality of life, and predicts both short-term (2–4) and long-term mortalities (5,6) in older adults, even after accounting for dementia and a number of chronic diseases and subclinical conditions. Further, accumulating evidence suggests that olfactory impairment is a prodromal symptom for several major neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) (7–9). A few cross-sectional studies have investigated the factors that are associated with poor sense of smell and consistently identified older age and male sex (1,10–15). However, data on other potential risk factors (eg, smoking) are limited and inconsistent (1,10–12). More importantly, most of these studies were conducted among predominantly white populations, and their findings may not be readily extrapolated to other races.
A recent study reported that blacks had markedly poorer sense of smell than whites (13). The study included 2,365 U.S. whites and 289 blacks and used the short 5-item Sniffin’ Sticks test. Subgroup analysis was not possible in this study due to sample size nor was testing whether these findings were related to genetic susceptibility. Given the increasingly recognized importance of the sense of smell in healthy aging (3,4,16), this racial disparity needs to be independently confirmed and investigated. Hereby, using data from two large, well-characterized U.S. cohorts of older adults and the 12-item smell identification tests, we reported the prevalence of anomia among 6,201 whites and 2,356 blacks, and independently examined the potential racial difference in anosmia and associated factors.
Methods
Study Population
We analyzed data from the Atherosclerosis Risk in Communities study (ARIC) and the Health, Aging, and Body Composition study (Health ABC). Details of these two cohorts have been described previously (17,18). Briefly, ARIC is an ongoing community-based longitudinal study established in 1987–1989 to investigate risk factors for cardiovascular diseases (17). At baseline, the study recruited 15,792 participants aged 45–64 years from four U.S. communities (Forsyth County, NC; Jackson, MI; suburban Minneapolis, MN; and Washington County, MD). Nearly all black participants were recruited in Jackson, Mississippi. In 2011–2013, the study tested the sense of smell of 6,523 participants of the ARIC Neuro-Cognitive Study (NCS). Of these, 6,063 participants completed the odor identification test, including 1,398 blacks and 4,665 whites. The Health ABC is a prospective study designed to investigate if changes in body composition act as a common pathway through which multiple diseases affect morbidity, disability, and mortality in community-dwelling older adults (19). In 1997–1998, the study enrolled 3,075 well-functioning community-dwelling individuals (48.4% men and 41.6% blacks) aged 70–79 years in the metropolitan areas of Pittsburgh, PA, and Memphis, TN. Whites were recruited from a random sample of Medicare beneficiaries, and blacks were recruited from Medicare beneficiaries and all age-eligible residents in these areas. Eligibility criteria included self-report of no difficulty walking one quarter of a mile or climbing 10 steps without resting, no difficulty performing mobility-related activities of daily living, no life-threatening cancers with active treatment within the past 3 years, and no plans to move out of the area for at least 3 years. Olfaction was evaluated among 2,494 participants at the year 3 clinical visit in 1999–2000, including 958 blacks and 1,536 whites. Individual study protocols were approved by relevant Institutional Review Boards and all study participants provided written consent.
The Smell Identification Tests
The ARIC study used the 12-item Sniffin’ Sticks screening test (SS, Burghart, Wedel, Germany) (20) to evaluate the sense of smell, and the Health ABC used the 12-item Brief Smell Identification Test (B-SIT, also known as the cross-cultural smell identification test, Sensonics, Haddon Heights, NJ) (21). Both are brief screening tests for olfactory deficit that have been widely used in clinical and epidemiological studies (20,21). Both tests ask participants to smell 12 common odorants, one at a time, and to identify the correct odorant from four possible answers in a forced multiple-choice format. One point was given for each correct answer with a total score ranging from 0 to 12. The test platforms and exact odorants in these two tests are different. The SS conceals each of the following odorants in a felt-tip pen: orange, leather, cinnamon, peppermint, banana, lemon, licorice, coffee, cloves, pineapple, rose, and fish (20). The B-SIT uses a scratch and smell format and have the following odorants: banana, chocolate, cinnamon, gasoline, lemon, onion, paint thinner, pineapple, rose, soap, smoke, and turpentine (21). In this study, we chose to use anosmia as the main analytic outcome because it could be more reliably assessed with such screening tests than the less severe hyposmia (20). We defined anosmia as a smell identification score ≤ 6, a threshold that has been validated using SS with clinically confirmed anosmia patients (20). In both study populations, this cutoff corresponds to approximately the 10th percentile of the general populations of older adults (20,21).
Assessment of Covariates
Unless otherwise stated, covariates were collected at the same clinical visit of the individual cohorts that the sense of smell test was conducted (17,18). The exact questions asked in these two cohorts were slightly different for some variables, and we harmonized variable definitions prior to statistical analyses.
Information on demographics (eg, age, sex, race, and education), lifestyle (eg, smoking and alcohol drinking), and general medical history was collected via structured interview. Education level was categorized as “less than high school,” “high school,” and “above high school.” Smoking and alcohol drinking behaviors were defined as current, former, or never. Body mass index (BMI) was calculated as measured weight in kilograms divided by height in meters squared and categorized as normal (<25 kg/m2), overweight (25–29.9 kg/m2) and obese (≥ 30 kg/m2). Self-reported general health status was defined as “excellent or very good,” “good,” and “fair or poor.” The occurrence of head injury was only asked once in ARIC in the 1996–1998 clinical visit as “have you ever had a major head injury? That is, one that resulted in your losing consciousness, no matter how briefly, or that led you to see a physician or seek hospital care.” A similar question was asked at year 3 in Health ABC: “Have you ever been hit in the head hard enough to make you faint?” Affirmative answers to these questions were defined as ever head injury. For daytime sleepiness, ARIC asked a yes-or-no question that is “are you sleepy most of the day?” In contrast, Health ABC asked how often participants “feel excessively (overly) sleepy during the day” with five categorical choices [never, rarely (once per month or less), sometimes (2–4 times/month), often (5–15 times/month), almost always (16–30 times/month)]. We defined “often” or “almost always” as daytime sleepiness to be consistent with the ARIC definition.
As part of the smell identification test, ARIC participants were asked “Do you suffer from smell loss or a significantly decreased sense of smell” and “Have you had a stuffy nose in the past 2 weeks, for example, from a cold or allergies.” Slightly different questions were also asked in Health ABC: “Do you suffer from smell and/or taste problems” and “Have you had a cold in the past week?” Based on these questions, we defined self-reported smell deficits and recent cold/allergy episodes (runny nose in the past 1–2 weeks). Both cohorts also evaluated cognitive status at the same time of the sense of smell test: the Mini-Mental State Examination (MMSE, maximal score 30) in ARIC and the Modified Mini Mental Status Examination (3MS, maximal score 100) in Health ABC. In the analyses, we standardized cognitive score into a Z-score according to the distribution of test scores in corresponding cohorts. The ARIC study used the 10-item Center for Epidemiologic Studies Depressive symptoms (CES-D) to assess depressive symptoms while the Health ABC used the 15-item CES-D. In the analyses, we defined depressive symptoms as CESD-10 score ≥ 8 in ARIC (22) and CESD-15 score ≥ 10 in Health ABC (23). In both cohorts, we defined potential PD as either a self-reported physician diagnosis of PD or use of PD medications based on medication inventory assessment. Finally, we determined ApoE genotypes by genotyping two ApoE variants at codons 130 and 176 (formerly 112 and 158). ApoE ε4 carrier was defined as at least one copy of the Apo ε4 allele.
Statistical Analysis
Primary analyses were conducted separately by race. In descriptive analyses, we presented means ± standard deviations (SD) for continuous variables, and counts and percentages (%) for categorical variables. We calculated age and sex specific prevalence of anosmia and estimated 95% confidence intervals (CIs) using normal approximation or the exact method when appropriate. We further used these prevalence data and the age and sex distribution data from the 2014 U.S. census (http://www.census.gov) to estimate the race specific anosmia prevalence among U.S. adults aged 65 years or older. We also examined the performance of self-reported poor sense of smell as a surrogate for anosmia with the smell identification test as the gold standard. For this purpose, we calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
We conducted multivariate logistic regression analyses to examine the cross-sectional relationships of anosmia with age, race, sex, educational level, self-reported poor sense of smell, runny nose, ApoE 4 status, cognition Z score, depressive symptoms, daytime sleepiness, smoking, alcohol drinking, BMI, self-reported general health status, history of head injury, and PD, and presented odds ratios (ORs) and 95% CIs. We further examined whether these factors could explain the racial difference of anosmia prevalence, by adding factors that showed statistically significant associations with anosmia in either race-specific or race-combined analyses into a series of logistic regression models.
To examine the robustness of main results, we conducted stratified analyses by sex or cohort and sensitivity analyses by excluding potential PD patients or individuals with the lowest 15% of cognitive score. To investigate whether the observed racial difference in anosmia prevalence could be explained by differences in racial familiarity of particular odorants, we compared the proportion of correct answers for each individual odorant by race and cohort. Such data were entered for all eligible Health ABC participants, all blacks in ARIC and a randomly sample of 1,135 whites in ARIC. Finally, we repeated the main analyses by using the natural log of the sense of smell score plus 1 as a continuous outcome variable. All analyses were performed using SAS version 9.3 (SAS Systems, Inc. Cary NC). Statistical tests were two sided with α = 0.05.
Results
The analyses included 6,063 participants from the ARIC study and 2,494 participants from the Health ABC study. The mean age was 75.6 years for both cohorts (Supplementary Table 1). The ARIC study included 58.7% women and 23.1% blacks and the corresponding percentages were 51.6% and 38.4% in Health ABC. Although the two studies used different screening tests of smell identification, distributions of test scores were comparable (Supplementary Figure 1) with an average of 9.2 ± 2.4 in ARIC and 9.2 ± 2.3 in Health ABC. Using a cutoff score of 6 or less, 14.0% of ARIC participants and 13.0% of Health ABC participants were classified as anosmic.
Supplementary Table 2 presents population characteristics by race in the pooled analytic sample. Compared to whites, blacks had lower levels of education, lower cognitive test scores, and higher BMI, and reported worse general health status. They were also more likely to carry ApoE ε4 allele but less likely to report a diagnosis of PD or use of PD medications.
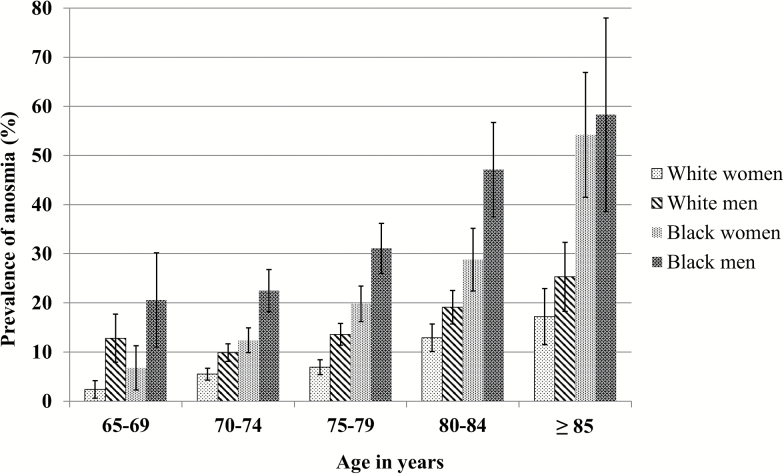
The overall prevalence of anosmia was 22.3% among blacks and 10.4% among whites (Figure 1). In both groups, the prevalence of anosmia increased with age and was higher in men than in women. In each age- and sex-specific group, blacks had higher prevalence of anosmia than whites. Similar racial differences and patterns were also observed in cohort specific analyses (Supplementary Figure 2). Using 2014 U.S. census data, we estimated that 3.5 million (9.6%) U.S. whites and 0.8 million (19.9%) blacks aged 65 years or older suffer from anosmia.
Figure 1.
Prevalence of anosmia by age, race, and sex.
In the pooled sample, 16% of blacks and 18% of whites reported that they had a substantially reduced or loss of the sense of smell. Using smell identification tests as the gold standard, self-reports of olfactory deficit had low sensitivities but reasonable specificities in identifying anosmia, the overall sensitivity was 39.7% for whites and 25% for blacks, and the corresponding specificity was 83.9% and 87.1% respectively (Supplementary Table 3).
In both blacks and whites, older age, male sex, self-reported reduced or absent sense of smell, poor cognition, and possible PD were associated with a significantly higher prevalence of anosmia (Table 1). Higher prevalence of anosmia was also associated with higher education level and ApoE ε4 allele in only whites and with daytime sleepiness in only blacks. In blacks, higher BMI was associated with a lower prevalence of anosmia. When blacks and whites were analyzed together, fair/poor general health status was associated with a higher prevalence of anosmia.
Table 1.
Cross-Sectional Associations of Population Characteristics With Anosmia
| Characteristics | White | Black | All |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sex | |||
| Women | ref | ref | ref |
| Men | 1.56 (1.29–1.88) | 1.72 (1.35–2.20) | 1.52 (1.32–1.76) |
| Age | |||
| 65–69 | 0.91 (0.60–1.38) | 0.60 (0.35–1.02) | 0.79 (0.57–1.09) |
| 70–74 | ref | ref | ref |
| 75–79 | 1.26 (1.01–1.58) | 1.54 (1.18–2.00) | 1.28 (1.09–1.52) |
| 80–84 | 1.78 (1.39–2.28) | 2.01 (1.44–2.79) | 1.66 (1.37–2.01) |
| ≥ 85 | 2.15 (1.50–3.06) | 2.92 (1.69–5.03) | 1.99 (1.50–2.66) |
| Study | |||
| Health ABC | ref | ref | ref |
| ARIC | 0.84 (0.67–1.06) | 1.51 (1.16–1.97) | 0.99 (0.83–1.17) |
| Education | |||
| Below high school | ref | ref | ref |
| High school | 1.23 (0.91–1.66) | 1.13 (0.85–1.52) | 1.06 (0.86–1.29) |
| Post high school | 1.45 (1.05–1.98) | 1.12 (0.82–1.53) | 1.29 (1.04–1.59) |
| Self-reported poor sense of smell | |||
| No | ref | ref | ref |
| Yes | 3.51 (2.91–4.23) | 2.65 (2.01–3.49) | 3.10 (2.66–3.62) |
| Runny nose in the past 1–2 weeks | |||
| No | ref | ref | ref |
| Yes | 0.95 (0.79–1.15) | 0.96 (0.75–1.22) | 0.95 (0.82–1.10) |
| ApoE ε4 carrier | |||
| No | ref | ref | ref |
| Yes | 1.38 (1.13–1.68) | 0.92 (0.73–1.16) | 1.24 (1.07–1.44) |
| Cognitive test z-score | 0.53 (0.47–0.60) | 0.48 (0.43–0.55) | 0.46 (0.42–0.49) |
| Depressive symptoms | |||
| No | ref | ref | ref |
| Yes | 0.83 (0.60–1.15) | 0.82 (0.60–1.14) | 0.82 (0.65–1.03) |
| Daytime sleepiness | |||
| No | ref | ref | ref |
| Yes | 1.32 (0.94–1.83) | 1.66 (1.06–2.59) | 1.34 (1.03–1.74) |
| Smoking status | |||
| Never | ref | ref | ref |
| Past | 1.00 (0.82–1.23) | 0.81 (0.62–1.06) | 0.94 (0.80–1.10) |
| Current | 0.78 (0.51–1.20) | 0.72 (0.47–1.12) | 0.79 (0.58–1.06) |
| Alcohol drinking | |||
| Never | ref | ref | ref |
| Past | 1.21 (0.91–1.62) | 1.40 (1.06–1.85) | 1.21 (0.91–1.62) |
| Current | 1.13 (0.86–1.46) | 1.12 (0.81–1.55) | 1.13 (0.86–1.46) |
| Body mass index (kg/m2) | |||
| <25 | ref | ref | ref |
| 25–29.9 | 0.88 (0.71–1.09) | 0.86 (0.65–1.15) | 0.90 (0.76–1.06) |
| ≥30 | 0.89 (0.70–1.13) | 0.53 (0.39–0.72) | 0.76 (0.63–0.91) |
| General health status | |||
| Excellent/very good | ref | ref | ref |
| Good | 1.04 (0.86–1.27) | 1.03 (0.78–1.35) | 1.13 (0.97–1.32) |
| Fair/poor | 1.33 (0.98–1.79) | 0.99 (0.73–1.34) | 1.34 (1.09–1.64) |
| History of head injury | |||
| No | ref | ref | ref |
| Yes | 0.98 (0.76–1.26) | 0.86 (0.55–1.34) | 0.88 (0.71–1.10) |
| Parkinson’s disease | |||
| No | ref | ref | Ref |
| Yes | 3.30 (2.12–5.13) | 3.08 (1.08–8.81) | 2.90 (1.94–4.34) |
Note: ABC = Aging, and Body Composition; ARIC = Atherosclerosis Risk in Communities study. Odds ratios (OR) and 95% confidence intervals (CI) were obtained from logistic regression models that included all covariates listed above.
Blacks had a higher prevalence of anosmia than whites with an age and sex adjusted OR of 2.96 (95%CI: 2.59–3.38) (Table 2). This association was moderately attenuated after further adjustment for cognitive Z score (OR = 1.66, 95%CI: 1.43–1.94); however, further adjusting for other factors that were associated with anosmia in either race made little difference to the association estimate. For example, the OR that adjusted for all of these factors was 1.76 (95%CI: 1.50–2.07).
Table 2.
Odds Ratios of Anosmia for Race, Adjusting for Various Set of Covariates
| Overall sample | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Race (Black vs. White) | 2.96 (2.59–3.38) | 1.66 (1.43–1.94) | 2.87 (2.51–3.28) | 1.64 (1.41–1.91) | 1.76 (1.50–2.07) |
| Age (vs. 70–74) | |||||
| 65–69 | 0.80 (0.59–1.09) | 0.83 (0.60–1.14) | 0.79 (0.58–1.08) | 0.83 (0.60–1.13) | 0.81 (0.59–1.12) |
| 75–79 | 1.49 (1.27–1.75) | 1.33 (1.13–1.56) | 1.50 (1.28–1.76) | 1.33 (1.13–1.57) | 1.32 (1.12–1.56) |
| 80–84 | 2.55 (2.13–3.05) | 1.97 (1.64–2.38) | 2.56 (2.14–3.07) | 1.98 (1.64–2.39) | 1.92 (1.58–2.32) |
| ≥ 85 | 4.19 (3.24–5.40) | 2.74 (2.09–3.59) | 4.25 (3.29–5.48) | 2.78 (2.12–3.65) | 2.51 (1.90–3.32) |
| Sex (Male vs. Female) | 1.95 (1.71–2.22) | 1.71 (1.49–1.95) | 1.94 (1.71–2.21) | 1.71 (1.49–1.95) | 1.69 (1.47–1.93) |
| Cognitive test z-score | 0.53 (0.49–0.57) | 0.53 (0.50–0.57) | 0.52 (0.48–0.57) | ||
| ApoE (e4 vs. no e4) | 1.31 (1.13–1.50) | 1.18 (1.02–1.37) | 1.17 (1.01–1.36) | ||
| Body mass index (kg/m2, vs. <25) | |||||
| 25–29.9 | 0.91 (0.78–1.08) | ||||
| ≥30 | 0.75 (0.63–0.90) | ||||
| Education (vs. Below high school) | |||||
| High school | 1.16 (0.95–1.41) | ||||
| Post high school | 1.36 (1.10–1.66) | ||||
| General Health Status (vs. Excellent/very good) | |||||
| Good | 1.08 (0.93–1.25) | ||||
| Fair/poor | 1.15 (0.94–1.40) | ||||
| Parkinson’s disease (Yes vs. No) | 3.06 (2.07–4.54) | ||||
| Daytime sleepiness (Yes vs. No) | 1.43 (1.11–1.85) | ||||
Note: Model 1: Adjusted for age and sex. Model 2: Model 1 + cognitive Z score. Model 3: Model 1 + ApoE status. Model 4: Model 1 + cognitive Z score + ApoE status. Model 5: Model 1 + cognitive Z score + ApoE status + all other covariates.
This racial difference was also observed in several stratified and sensitivity analyses: the multivariate OR from the fully adjusted model was 1.96 (95%CI:1.61–2.39) in ARIC, 1.55 (95%CI: 1.15–2.08) in Health ABC, 1.73 (95%CI: 1.39–2.17) in men, 1.74 (95%CI: 1.37–2.21) in women, 1.72 (95%CI:1.46–2.02) after excluding potential PD patients, and 1.72 (95%CI:1.42–2.08) after excluding individuals with poor cognitive performance (Supplementary Table 4).
We obtained similar results when using the log-transformed sense of smell score as the outcome variable. For example, the age and sex adjusted racial difference in the log-transformed score was −0.155 ± 0.007 (β ± s.e., p < .001) between blacks and whites. This racial difference was moderately attenuated after accounting for cognitive Z-score (−0.068 ± 0.008, p < .001), but minimally changed with further adjustment for other covariates as defined in Table 2 (−0.073 ± 0.008, p < .001).
In the analyses of individual odorants (Supplementary Table 5), blacks had poor performance on most of the individual odorants. Further, for the five odorants that were tested in both cohorts, the data are generally comparable between cohorts.
Discussion
Based on the data from two large U.S. cohorts, we estimated that 3.5 million U.S. whites and 0.8 million blacks 65 years or older suffer from anosmia. In addition, we also found a clear racial disparity that anosmia was more prevalent among blacks than whites. We identified several factors that were associated with the prevalence of anosmia, mostly common to both races; however, with the exception of cognitive function to some degree, none of these factors explained the racial difference in the prevalence of anosmia. Analyses by cohort generally showed comparable results, supporting the validity of our findings. Taken together, the data suggest a marked and unexplained racial disparity in anosmia prevalence in U.S. older adults. To the best of our knowledge, this is to date the largest community-based study on anosmia prevalence in the United States that includes both black and white older adults.
Several studies have reported the prevalence of olfactory impairment among older adults mainly in the United States and European countries (1,8–13,23). These studies employed a similar smell identification screening test to our study, although the exact test and cutoff value for olfactory impairment were different across studies. Nevertheless, these studies were consistent in reporting that a substantial portion of older adults suffered from olfactory impairment (1,8–13,23). For example, the Epidemiology of Hearing Loss Study (EHLS, USA), Blue Mountain Eye Study (BMES, Australia), and Beaver Dam Offspring Study (BOSS, USA) used the 8-item San Diego Odor Identification Test (SDOIT) and defined a score < 6 as olfactory impairment (1,12,14), which would include anosmia as well as hyposmia. The prevalence was 24.5% in EHLS (ages 53–97), 27% in BMES (ages ≥ 60), and 13.9% in BOSS (ages ≥ 65) (1,12,14). A similar prevalence (22.3%) was reported in a recent analysis of U.S. population (ages 57–85), using a 5-item odor identification test and a cutoff of < 4, among them, 3.5% were anosmic (score ≤ 1) and 18.8% were hyposmic (1< score < 4) (13). One German study used the 16-item version of the SS test and reported a prevalence of 12% for anosmia (score < 8) among participants older than age 65 (15). Another German study used the 12-item SS and defined a score ≤ 6 as anosmia and ≤ 10 as hyposmia. It reported that 6.0% women and 11% men ages 65–74 had anosmia, and 20% men and 30% women had hyposmia (10). Finally a study in Spain reported that about 61% of participants 60 years or older suffered from olfactory dysfunction (hyposmia or anosmia) which was defined as score < 4 on a 4-item smell identification test (24). However, direct comparison of prevalence across studies is challenging, due to differences in study populations, the tests used, variations in covariate adjustments, and definitions for olfactory impairment.
Compared to previous studies, the current study was substantially larger and more diverse, including both black and white participants. Further, we used two well-accepted screening tests to evaluate the sense of smell identification and chose a cutoff value that had been validated for the diagnosis of anosmia. We found that approximately 14% of the study participants had anosmia, a number that was remarkably consistent between the two cohorts. As expected, this prevalence increased with age, and was higher in men than in women.
More importantly, we confirmed a substantial racial disparity in the sense of smell that blacks have a much higher prevalence of anosmia than whites. In the previous published data from the National Social Life, Health and Aging Project (NSHAP), Pinto and colleagues tested the sense of smell of 2,365 U.S. whites, 289 blacks, 202 Hispanics, and 72 individuals of other races, using a 5-item screening test (13). They reported a substantially lower average score for nonwhites, with a difference equivalent to 9 years of aging. We extended this work with a much larger number of black participants from two well characterized community-based cohorts and 12-item screening tests and account for ApoE status. Our data conclusively confirmed that olfactory impairment was more prevalent in blacks than in whites. While this racial difference was due, in part, to differences in cognition function by race, it could not be explained by any of the other demographic and health factors that were assessed. Furthermore, this racial difference was not explained by differential familiarities of these odorants; for most odorants, blacks performed worse than whites, consistent with prior work showing lack of cultural differences in familiarity explaining such results.
Further investigations into this racial difference in olfactory impairment may have significant public health importance. Compared to whites, blacks may have lower socioeconomic status on average and thus are potentially exposed to more hazardous working conditions and living environments, something we could not assess here (25,26). These detrimental environmental exposures may lead to poor sense of smell via mechanisms such as neuro-inflammation, oxidative stress, and DNA damage (27,28). Conversely, individuals with poor sense of smell may be also more likely to live or work in hazardous environments, and thus may be more likely to suffer from the adverse sequelae of olfactory impairment. In addition to the sense of smell, preliminary data suggest that black older adults are also more likely to suffer from other sensory impairments such as poor vision, taste, and touch than their white counterparts (29). If these findings are confirmed, it may inform common mechanisms that may lead to accelerated sensory aging in blacks and inform appropriate preventive strategies.
In addition to race, we also examined a number of factors that may be associated with anosmia prevalence in the U.S. population. As expected, we found that poor cognitive status and possible PD was strongly associated with anosmia in both blacks and whites. The presence of the ApoE ε4 allele was associated with anosmia in whites, but not in blacks. While a biological explanation for this racial difference is elusive, a similar observation has also been made for AD: ApoE ε4 is the most important genetic risk factor for AD in whites, but the evidence is less evident among blacks (30). Previous epidemiological findings on smoking and poor sense of smell are inconsistent. Some studies found higher prevalence of olfactory impairment among active smokers (10,12,14), while others have not (1,11,13,15,24). In ARIC and Health ABC, we did not identify any association of smoking with anosmia. Given the large sample size and simultaneous adjustment for multiple potential confounders in the current study, cigarette smoking is unlikely to be a major factor that affects anosmia. Previous epidemiological data on head injury and olfactory impairment were also inconsistent, with positive association in some (12), but not in others (1,24). We did not find an association in the current study; however, we could not rule out a role of head trauma in olfactory impairment among the elderly, as in ARIC we asked head injury only once about 15 years prior to the assessment of the sense of smell whereas in Health ABC we only included a simple screening question.
In addition, we found that excessive daytime sleepiness was associated with anosmia. This was somewhat expected because both daytime sleepiness and anosmia are prodromal symptoms of PD and thus are likely to occur together among individuals at higher risk for PD. On the other hand, higher BMI was associated with a lower prevalence of anosmia. A similar observation was made previously in the BMES study that BMI and olfactory impairment was inversely associated (14). Explanations for this association are not straightforward, as reverse causation is possible: older adults with poor sense of smell have poor appetite and therefore are more likely to lose weight over time.
The study has several limitations. First, the analyses were cross-sectional and we therefore were unable to make any causal inference and reverse causation could not be excluded. To date, prospective investigations (31–33) are few and are urgently needed to identify risk factors for incident olfactory impairment. Second, we did not have data on several medical conditions or potential confounders that may affect the sense of smell, such as chronic rhinosinusitis or exposure to environmental chemicals or air pollution. Black adults may be more likely to have sinusitis than whites, though this is controversial, with little supporting evidence (34). Therefore, we could not exclude the possibility of residual confounding. Third, ARIC and Health ABC used slightly different test platforms to evaluate the sense of smell. However, both are validated tests and the test results were comparable between the two cohorts. These issues are not likely to alter the main results found here.
Conclusions
A substantial portion of U.S. older adults suffer from anosmia, and blacks appear to face the highest odds of this condition. Future studies should further investigate mechanisms underlying this racial difference, and additional prospective, longitudinal studies are needed to identify risk factors for anosmia in both blacks and whites.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The study was supported by the intramural research program of NIH (Z01 ES101986), the National Institute of Environmental Health Sciences. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C) Neurocognitive data are collected by U01 HL096812, HL096814, HL096899, HL096902, and HL096917 with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. Additional support for this project includes grants R01-HL093029 and R01-NS087541. The Health ABC Study research was supported by in part by the Intramural Research Program of the NIH, National Institute on Aging (NIA) and by NIA contracts N01AG62101, N01AG62103, and N01AG62106.
Conflict of Interest
The authors declare no competing financial interests.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study and Health ABC study for their important contributions.
References
- 1. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. [DOI] [PubMed] [Google Scholar]
- 2. Devanand DP, Lee S, Manly J, et al. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78:401–411. doi:10.1002/ana.24447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9:e107541. doi:10.1371/journal.pone.0107541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chem Senses. 2011;36:63–67. doi:10.1093/chemse/bjq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekstrom I, Sjolund S, Nordin S, et al. Smell loss predicts mortality risk regardless of dementia conversion. Journal of the American Geriatrics Society [Epub ahead of print]. 2017. doi:10.1111/jgs.14770 [DOI] [PubMed] [Google Scholar]
- 6. Schubert CR, Fischer ME, Pinto AA, et al. Sensory impairments and risk of mortality in older adults. J Gerontol A Biol Sci Med Sci. 2017;72:710–715. doi:10.1093/gerona/glw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters ECh, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. doi:10.1002/ana.20160 [DOI] [PubMed] [Google Scholar]
- 8. Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63:167–173. doi:10.1002/ana.21291 [DOI] [PubMed] [Google Scholar]
- 9. Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann N Y Acad Sci. 2009;1170:730–735. doi:10.1111/j.1749-6632.2009.04013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255:1121–1126. doi:10.1007/s00415-008-0807-9 [DOI] [PubMed] [Google Scholar]
- 11. Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: The skövde population-based study. Laryngoscope. 2004;114:733–737. doi:10.1097/00005537-200404000-00026 [DOI] [PubMed] [Google Scholar]
- 12. Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: The Beaver Dam Offspring Study. Chem Senses. 2012;37:325–334. doi:10.1093/chemse/bjr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69:323–329. doi:10.1093/gerona/glt063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karpa MJ, Gopinath B, Rochtchina E, et al. Prevalence and neurodegenerative or other associations with olfactory impairment in an older community. J Aging Health. 2010;22:154–168. doi:10.1177/0898264309353066 [DOI] [PubMed] [Google Scholar]
- 15. Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764–1769. doi:10.1097/00005537-200410000-00017 [DOI] [PubMed] [Google Scholar]
- 16. Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519–528. [DOI] [PubMed] [Google Scholar]
- 17. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 18. Ix JH, Wassel CL, Kanaya AM, et al. ; Health ABC Study Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi:10.1001/jama.300.2.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 20. Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: Reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976–981. doi:10.1177/000348940111001015 [DOI] [PubMed] [Google Scholar]
- 21. Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106(3 Pt 1):353–356. [DOI] [PubMed] [Google Scholar]
- 22. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi:10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 23. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 24. Mullol J, Alobid I, Marino-Sanchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: A population-based survey (OLFACAT study). BMJ open. 2012;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi:10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pais J, Crowder K, Downey L. Unequal trajectories: Racial and class differences in residential exposure to industrial hazard. Soc Forces. 2014;92:1189–1215. doi:10.1093/sf/sot099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calderón-Garcidueñas L, Franco-Lira M, Henríquez-Roldán C, et al. Urban air pollution: Influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62:91–102. doi:10.1016/j.etp.2009.02.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. doi:10.1186/1742-2094-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Correia C, Lopez KJ, Wroblewski KE, et al. Global sensory impairment in older adults in the United States. J Am Geriatr Soc. 2016;64:306–313. doi:10.1111/jgs.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. [DOI] [PubMed] [Google Scholar]
- 31. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The rate of age-related olfactory decline among the general population of older U.S. adults. J Gerontol A Biol Sci Med Sci. 2015;70:1435–1441. doi:10.1093/gerona/glv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. Olfactory function in Wave 2 of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 2):S134–S143. doi:10.1093/geronb/gbu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: Five-year incidence and risk factors. Laryngoscope. 2011;121:873–878. doi:10.1002/lary.21416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: Epidemiology and cost. Allergy Asthma Proc. 2013;34:328–334. doi:10.2500/aap.2013.34.3675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.