Abstract
Background
Hospitalization is a major risk factor for functional decline, disability, loss of independence, and mortality in older adults. Evidence-based interventions to improve functional recovery from hospitalization are difficult to evaluate and implement in geriatric patients. The goal of this pilot study was to test the feasibility of recruiting geriatric inpatients and implementing pragmatic interventions to improve physical function following hospitalization.
Methods
Enrolled subjects were randomized to one of five 30-day posthospitalization interventions: isocaloric placebo (P), whey protein supplement (W), in-home rehabilitation+placebo (R+P), rehabilitation+whey protein (R+W), or testosterone (T). Data were collected from a single-site university hospital to determine: (i) institution-based feasibility (nonmodifiable factors including number of patients screened, eligible, contacted) and (ii) patient-based feasibility (modifiable factors including number of patients refusing, enrolled, randomized, intervention adherence, and withdrawal).
Results
From January 2014 to July 2016, 4,533 patients were chart screened; 594 (13.1%) were eligible to participate; 384 eligible subjects were contacted; 113 were enrolled; and 100 were randomized. Supplement adherence was 75% and was not different by age, education, level of independence, depression, supplement type, or dual intervention, but was significantly higher in subjects who completed the intervention (p < .01). Rehabilitation session adherence was 77% and did not vary significantly by age, education, level of independence, depression, or supplement type, but was significantly higher for sessions directly supervised (p < .01). Adherence was 100% in the testosterone arm with 94.7% of injections given within 24 hours of discharge.
Conclusions
Findings from this clinical trial indicate that posthospitalization interventions in geriatric patients are feasible at both the institution and patient level.
Keywords: Protein, Rehabilitation, Testosterone, Hospitalization
Older adults admitted to the hospital experience a significant decline in physical function (1,2). The posthospital syndrome is a period of increased vulnerability with high risk of adverse events in geriatric patients (3). Acute hospitalization can have catastrophic and long-lasting consequences for the physical function and independence of geriatric patients (4,5). Consequently, there is a need to identify and develop practical, feasible, and cost-effective interventions to accelerate recovery of physical function in geriatric patients following hospitalization.
Hospitalized older adults are profoundly inactive (6,7) and often malnourished (8,9), which can contribute to muscle dysfunction, increased falls, and loss of independence (4,10,11). The inability to regain physical function following a hospital stay is a strong predictor of rehospitalization and mortality in geriatric patients (4,12). In the United States, patients aged 65 years and older make up more than one-third of all acute care discharges, yet account for only 13% of the overall population (13). These patients are also at high risk for early readmission with estimates indicating that approximately 1 in 5 will be readmitted within 30 days of discharge (14). Readmissions are deleterious not only for patients but also for hospitals, since the Centers for Medicare and Medicaid Services penalize hospitals with 30-day readmission rates higher than benchmarked peers (15).
There is a lack of evidence-based strategies to improve recovery after hospital discharge. Our group and others have identified protein supplementation, exercise, and testosterone as potential means to improve physical functioning in healthy older adults (16–18). However, the feasibility of translating these interventions to the posthospital setting is unclear and there is a need for identifying barriers or facilitators to their implementation.
The goal of this Phase I pilot clinical trial was to test the feasibility of protein supplementation, in-home rehabilitation, and testosterone interventions to accelerate recovery and improve physical function in older adults following acute hospitalization. We tested the feasibility of implementing this pilot study at two levels: (i) institution-based feasibility (nonmodifiable factors) and (ii) patient-based feasibility (modifiable factors).
Methods
The methods for this randomized controlled pilot trial have been previously published in detail (19). This study was approved by the University of Texas Medical Branch (UTMB) Institutional Review Board and is registered at ClinicalTrials.gov (NCT02203656). Written informed consent was obtained from each subject prior to any study procedures. All data were collected from a single university hospital.
Inclusion/Exclusion Criteria
To be eligible for inclusion, participants had to meet the following general criteria: (i) admitted to the hospital with an acute onset of disease or condition; (ii) residing at home before and after hospitalization; (iii) able to consent to participate in the study; (iv) self-reported ability to walk across a small room (with or without an assistive device) 2 weeks prior to hospitalization; (v) ability to stand independently at baseline testing; (vi) no medical contraindication to wearing the loose fitting velcro strap of a step activity monitor on one ankle; (vii) living within 30 miles of the hospital; and (viii) aged 65 years or older. If a patient’s planned discharge location changed after enrollment from home to placement into skilled nursing or rehabilitation facility, the subject was withdrawn from the study prior to randomization (see below).
Exclusion criteria were: (i) nursing home resident or hospice patient; (ii) uncontrolled blood pressure (systolic > 150, or diastolic > 100 mmHg); (iii) history of stroke with motor disability; (iv) estimated glomerular filtration rate < 30 mL/min/1.73 m2 or evidence of kidney failure; (v) liver disease (AST/ALT 2 times above the normal limit, hyperbilirubinemia); (vi) recent (within 3 months) treatment with anabolic steroids; (vii) planned/elective hospitalization within 30 days of discharge; (viii) cognitive dysfunction determined by chart review, reported by nursing staff, or observed by trained research staff (not alert or oriented, dementia, active delirium); (ix) living >30 miles from the hospital; and (x) any other condition or event considered exclusionary by the principal investigator or treating physician. Additional exclusion criteria for the testosterone arm were: (i) history of breast or prostate cancer; (ii) palpable prostate nodule or induration or prostate-specific antigen ≥ 4 ng/mL; (iii) prostate-specific antigen ≥ 3 ng/ml in men at high risk of prostate cancer; (iv) hematocrit ≥ 50%, or (v) decompensated heart failure.
Interventions
Enrolled subjects were randomized immediately prior to hospital discharge to one of five intervention arms: (i) whey protein supplementation (W), (ii) in-home rehabilitation and placebo supplementation (R+P), (iii) in-home rehabilitation and protein supplementation (R+W), (iv) single testosterone injection (t), or (v) isocaloric placebo supplementation (P). Subjects followed their assigned intervention protocol in addition to any standard of care prescribed by their physician (eg, home health, PT/OT, nutritional support, medications). Each intervention was initiated upon discharge and continued uninterrupted for 4 weeks after discharge (27–33 days postdischarge) unless a study endpoint was met prior to that time (ie, readmission, withdrawal from the study). We called all subjects once a week to monitor adherence and answer to any study-related questions they might have had.
Supplementation
Supplements (whey protein or placebo) were provided in single-dose containers, premixed with one packet of sugar free flavored drink mix to mask the taste and color. Subjects were instructed to consume one dose of the supplement twice daily by mixing it with approximately 8oz of water. The whey protein supplement weighed 22 g (BiPro, Eden Prairie, MN). The isocaloric placebo supplement was 20 g of maltodextrin. To confirm adherence, subjects returned all supplement containers (both empty and full).
In-home rehabilitation
The progressive in-home rehabilitation program was performed 3 days per week throughout the intervention period. It was developed and directed by a licensed physical therapist, and added to any physical and/or occupational therapy ordered by the treating physician. Modifications were made to the exercise program to accommodate for medical conditions (eg, fibromyalgia, joint pain, limited range of motion) or for posthospitalization instructions (eg, sternal precautions following coronary artery bypass grafting surgery, shoulder precautions following pacemaker placement) or due to fall concerns (eg, weakness, unsteadiness, blood pressure issues) or pain. Sessions were supervised at the patient’s home by trained research staff once to twice per week, with the remaining exercise session(s) performed without supervision. The program was designed to begin at low or moderate intensity, depending on the patient’s baseline abilities. It included a series of five exercises: chair rises (low intensity: 1 set of 5; moderate intensity: 2 sets of 5; upper extremity use allowed as necessary), toe stands (low intensity: 1 set of 10; moderate intensity: 2 sets of 10), seated knee extensions with Thera-band (low intensity: 1 set of 10 each leg; moderate intensity: 2 sets of 10 each leg), seated row with Thera-band (low intensity: 1 set of 10; moderate intensity: 2 sets of 10), and seated arm extensions with Thera-band (low intensity: 1 set of 10; moderate intensity: 2 sets of 10). The resistance of the Thera-band was changed to keep the rehabilitation program progressive in difficulty. To determine adherence with rehabilitation sessions, subjects were asked to fill out a calendar after each session. A rehabilitation session was counted as completed if the patient did all five exercises in the program.
Testosterone
Subjects randomized to the testosterone intervention arm received a single intramuscular injection of testosterone enanthate (men: 200 mg; women: 100 mg) within 24 hours of discharge. These doses have been previously and safely utilized by UTMB and other investigators in chronic studies involving weekly testosterone injections in older men and women (20).
Outcome Measures
The main outcome measures were institution-based feasibility and patient-based feasibility. Institution-based feasibility describes nonmodifiable factors that investigators cannot control, such as patient census and diagnoses. It is important to determine whether a site has adequate patient volume with the specific characteristics to support similar studies with similar yields. Patient-based feasibility is dependent upon study design and research team skills. It can be modified based upon the information gathered with pilot data, such as those collected in this study.
Institution-based feasibility
The number of patients admitted, eligible, excluded, and contacted, was collected and analyzed to determine institution-based feasibility. These feasibility measures were based on percent of total admitted patients, eligible patients, contacted and enrolled patients that initiated each intervention. Reasons for exclusion were recorded.
Patient-based feasibility
The number of patients enrolled, randomized, and retained (at time of discharge), as well as number of study withdrawals, reasons for declining participation and for study withdrawal, adherence and compliance to each intervention were recorded. Dependent upon subject availability at the study endpoint, a postintervention questionnaire focused on subjective patient-centered outcomes was also administered. Questions included: “Did you feel like the intervention aided in your recovery?”; “Did you experience any complications that you felt were related to the intervention?; “Do you feel like the rehabilitation program was progressive in difficulty?”; “Do you have any other comments about the study you would like to make?”
Statistical Analyses
All statistical analyses were performed using SAS (version 9.2, SAS Inst. Inc., Cary, NC). A p value less than .05 was considered statistically significant. The total supplement adherence score for each participant over the study period was calculated as the total number of supplements taken divided by the total number of supplements given. The total rehabilitation adherence score for each participant over the study period was calculated as the total number of rehabilitation sessions completed divided by the total number of rehabilitation sessions offered. Supplement and rehabilitation adherence was examined as a binary variable, defined as whether the study participant took ≥80% of their supplements or not. The total adherence score (continuous) was not normally distributed for either supplement or rehabilitation. Thus, we used nonparametric tests (Wilcoxon rank sum and Kruskal–Wallis chi-square) to assess whether the total adherence score varied according to randomly assigned study arm, supplement type, study completion status, single versus dual intervention, ability to increase intensity, and a range of demographic and clinical variables. Logistic regression was used to assess whether the likelihood of having an adherence score ≥80% varied according to randomly assigned study arm. A signed rank test was used to examine if rehabilitation adherence scores varied according to whether a study coordinator was present or not. Categorical independent variables (measured at baseline, during hospitalization, prior to randomization) were classified as follows: age (65–74, 75–84, ≥85), education years (1–8, 9–12, 13–16, ≥17), Geriatric Depression Scale (GDS) score (0–4, ≥5), activities of daily living score (ADL) (0, ≥1), instrumental activities of daily living score (IADL) (<10, vs ≥10;), Short Physical Performance Battery (SPPB) score (functional impairment: 0–9, vs normal function: 10–12), number of prescriptions at discharge (0–4, ≥5), and days of hospital stay (0–3, ≥4).
Results
Institution-Based Feasibility
Screening
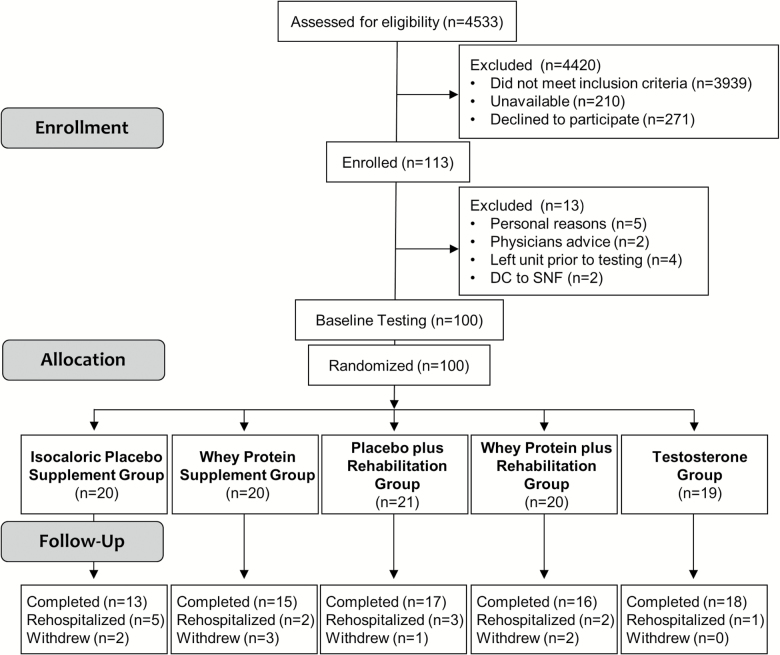
From January 2014 to July 2016, a total of 4533 patients were chart screened and 13.1% were eligible for study participation (see Consort Diagram, Figure 1). Using electronic medical records documentation, we recorded which inclusion/exclusion criteria were met including concurrent incidences. Of those screened: (i) 94% met the inclusion medical diagnosis criteria; (ii) 88% lived at home prior to admission; (iii) 75% were alert and oriented; (iv) 75% were ambulatory; (v) 73% could stand independently; (vi) 95% could wear the monitor; and (vii) 80% lived within 30 miles of UTMB. All screened patients were aged 65 years and older.
Figure 1.
CONSORT diagram.
The most common reasons for exclusion were: (i) 25% had cognitive dysfunction; (ii) 12% were not expected to return home after discharge; (iii) 20% lived more than 30 miles away from the hospital; (iv) 5% had uncontrolled blood pressure; (v) 8% had a stroke with residual weakness; (vi) 20% had kidney disease; (vii) 4% had liver disease; (viii) 2% had recent treatment with anabolic steroids; (ix) less than 1% had planned/elective hospitalization; and (x) 16% had other reasons including: 1% recent refusal during previous hospitalizations, 5% did not speak English, 3% had swallowing issues, 3% had high adherence risks (drug/alcohol use or homeless), 2% were on hospice or palliative/comfort care measures, and 2% had enrolled in the same study during a prior hospitalization.
Of those who met all general inclusion criteria, 69% also met the testosterone criteria. Reasons for exclusion were: 5% had a history of breast or prostate cancer, 14% had prostate/prostate-specific antigen exclusions, less than 1% had high hematocrit, and 2% had decompensated congestive heart failure. Those who did not meet the additional criteria for the testosterone arm but met all other eligibility criteria were randomized into one of the other four arms.
Of those patients who met the criteria to participate in this study, 35.3% were unavailable at the time of approach due to their treatment plan. Reasons for patient unavailability to research team included: away for diagnostic procedures (eg, x-ray, magnetic resonance imaging, dobutamine stress echocardiogram, colonoscopy) or surgery, meeting with members of their care team (physicians, nurses, physical/occupational therapists, care managers, dieticians), sleeping, in pain, in a mood not conducive to visitors, eating, bathing, talking on the phone, or visiting with family.
After many repeated screenings of patients with numerous readmissions, starting September 2015 we expanded screening from the Acute Care for Elders (ACE) unit to improve recruitment, including all nonsurgical hospital units, except oncology, neurology, and transplant. Following screening expansion 40.2% of the patients screened and 38.5% of those enrolled were from outside the ACE unit.
Patient-Based Feasibility
Enrollment and randomization
Of the 384 approached patients, 70.6% refused to participate in the clinical trial. The most common reasons for refusal included: not interested in participating in any research, felt unable to be compliant with the exercise program, displeasure at the thought of drinking a protein supplement (concern with taste, allergy, etc.), concerns about receiving a testosterone injection, anxiety and uncertainty about their diagnoses, and dissatisfaction with their hospital stay or with the National Institutes of Health/government. Several patients expressed interest in participating, but had to decline because they were being discharged, had travel plans during the intervention period or had medical concerns with other immediate family members for whom they provided care.
Approximately 29% of the approached patients agreed to participate and were enrolled (n = 113). However, 13 subjects were not randomized into an intervention arm for the following reasons: personal reasons (n = 5), treating physician’s advice based on the nature their diagnosis (n = 2), discharged or transferred from the unit prior to completion of testing (n = 4), discharged to skilled nursing facilities (n = 2).
Of those randomized, 76 completed the 30-day intervention, 13 were rehospitalized, and 8 withdrew from the study during the intervention period. Reasons for withdrawal from the study included: extreme weakness/lack of energy to prepare the supplements or perform the rehabilitation program (n = 2), did not wish to continue with the study because they were not willing to drink the supplements (n = 2), one subject had an allergic reaction to the supplement’s artificial sweetener (n = 1), moving out of town to care for an ailing relative (n = 1) or for a need to move in with family for medical care (n = 1). Of those who withdrew from the study, 37.5% were rehospitalized within 30 days of their initial discharge.
Subject characteristics
Subjects (n = 113) enrolled in this study were on average 78.3 years old (SD 7.7), 68.1% female, and 74.3% white (12.4% Hispanic, 11.5% Black, 1.8% other). They were highly educated with 87.6% achieving a high school diploma and 23.9% a college degree. Average SPPB total score was 6.8 (SD 3.8) with 74.1% of the population with a SPPB score less than 10. 17.4% required assistance with at least one ADL and 61.3% required assistance with at least one IADL. Average Charlson Comorbidity Index score was 5.3 (SD 1.5), average length of stay was 3.4 days (SD 3.0), and an average of 11 unique medications were prescribed at discharge (SD 4.8).
Adherence to supplementation
The overall mean supplement adherence score (SD) for the entire study sample was 75% (28%) (Table 1). Fifty-six percent of subjects had an adherence score ≥80%. The adherence score did not vary according to number of current prescriptions (p = .86), randomly assigned treatment arm (p = .61), supplement type (p = .91), single versus dual intervention (p = .87), gender (p = .46), age category (p = .62), education (p = .43), GDS score (p = .57), ADL score (p = .11), IADL score (p = .68), SPPB score (p = .91). However, the adherence score did vary significantly according to study completion status (p = .0005); patients who did not complete the study had lower adherence prior to their readmission or withdrawal. Finally, there were no significant differences across the four randomly assigned study arms in the likelihood of having an adherence score ≥80% (p = .45).
Table 1.
Supplement Adherence
| n | Average | SE | |
|---|---|---|---|
| All supplement arms | 81 | 75.1% | 3.1% |
| Whey Protein (W, R+W) | 40 | 70.7% | 4.9% |
| Placebo (P, R+P) | 41 | 79.4% | 3.8% |
| Dual intervention (R+W, R+P) | 41 | 75.4% | 3.7% |
| Single intervention (W, P) | 40 | 74.9% | 5.1% |
| R+W | 20 | 70.7% | 5.6% |
| R+P | 21 | 79.9% | 4.9% |
| W | 20 | 70.7% | 8.2% |
| P | 20 | 79.0% | 5.9% |
| All patients in the supplement arms | |||
| Completed intervention | 61 | 83.2% | 2.5% |
| Rehospitalized/Withdrew | 20 | 50.5% | 7.9% |
Note: This table does not include subjects in the Testosterone arm. P = Placebo; R = Rehabilitation; W = Whey protein.
Adherence to in-home rehabilitation
The overall mean rehabilitation adherence score (SD) for the entire study sample was 77% (23%) (Table 2). Fifty-four percent of the subjects had an adherence score ≥80%. The rehabilitation adherence score did not vary according to intensity increase (p = .39), randomly assigned treatment arm (p = .31), gender (p = .92), age category (p = .50), education (p = .38), ADL score (p = .72), IADL score (p = .40), SPPB score (p = .86), or completion status (p = .72). However, rehabilitation adherence was significantly greater when a coordinator was present than when sessions were performed on their own (mean 82% vs 69%; p = .01). Finally, there were no significant differences between the two rehabilitation study arms in the likelihood of having an adherence score ≥80% (p = .27).
Table 2.
Rehabilitation Adherence
| n | Average | SE | |
|---|---|---|---|
| All rehabilitation arms | 41 | 77.3% | 3.6% |
| R+W | 20 | 73.7% | 5.7% |
| R+P | 21 | 80.8% | 4.5% |
| All patients in the rehabilitation arms | |||
| Sessions with coordinator | 41 | 82.5% | 3.4% |
| Sessions without coordinator | 39 | 69.4% | 5.8% |
| All patients in the rehabilitation arms | |||
| Completed intervention | 33 | 79.1% | 3.4% |
| Rehospitalized/Withdrew | 8 | 69.8% | 12.6% |
Note: This table does not include subjects randomized to supplement only or testosterone arms. P = Placebo; R = Rehabilitation; W = Whey protein.
On the day of the in-home 1-week testing 54% of subjects did not perform their rehabilitation session, which consequently decreased compliance in a large portion of our sample. At the start of the program, 46.3% were unable to do the prescribed program at the lowest intensity level, 48.8% started at low intensity level, 2.4% at mid intensity level, and 2.4% at high intensity level. At the completion of the program, 12.1% were still unable to perform the prescribed program at the lowest level, 42.4% performed it at low intensity, 3.0% at mid intensity, and 42.4% at high intensity. All rehospitalized subjects were unable to increase intensity of the rehabilitation program.
Documented reasons why subjects were not compliant with their prescribed rehabilitation program included: illness, fall risk (weakness, lightheadedness, dizziness), pain, cancellations (inclement weather, ER visit, doctor’s appointment), or having a busy day with family visiting. In addition, if a subject was unable to perform any of the low intensity exercises during the first supervised visit, they were not instructed on the exercises until the next scheduled coordinator visit.
Adherence to testosterone
All subjects randomized to the testosterone intervention received an injection. However, one subject was discharged home during a weekend and was given the injection at home, outside the protocol’s 24-hour postdischarge window.
End of study questionnaire
In the whey protein arm, 85% of the participants felt that taking the supplements aided in their recovery; however only 41% of those in the placebo arm felt that taking the supplements helped with recovery. There were a few complications that subjects felt were related to taking supplements. Complaints from subjects in the protein arm included: bloating (n = 1), using the restroom more often (n = 1), constipation (n = 1), feeling more full (n = 1), and diarrhea (n = 2). Complaints from subjects in the placebo arm included: lethargic (n = 1), using the restroom more often (n = 2), constipation (n = 1), feeling more full (n = 2), and higher evening blood pressure measurements (n = 1). Other supplement related comments included: mentioning that they “enjoyed” taking them, felt that they “received benefits” (strength, energy), and felt they decreased cravings for sweets/dessert. A few individuals disliked the amount that they had to consume or preferred a different flavor/form (chocolate/lemonade flavoring, protein bar, etc). Only 50% of subjects felt that the rehabilitation program was progressive in difficulty; however, 94% felt that the rehabilitation program helped with their recovery. Four subjects complained of pain or soreness related to the rehabilitation program. Other comments included: felt that they “received benefits” (strength, energy) and that they felt that the in-home coordinator visits were beneficial to encouraging them to exercise. One subject said “I got in my son’s truck for the first time in years without using a stool. Thanks to this program, I am definitely feeling stronger.” In the testosterone arm, 88% of subjects felt that the testosterone injection aided in their recovery. There were no complaints related to the injection.
Discussion
One of the main goals of this Phase I randomized pilot clinical trial was to test the feasibility of several of promising interventions designed to accelerate recovery and improve physical function in older adults following acute hospitalization. We examined the feasibility of implementing these interventions at two levels: (i) institution-based feasibility and (ii) patient-based feasibility. At the institution-based level, we were able to contact in person 384 acutely ill older adults within the planned 3-year timeframe. At the patient-based level, we were able to recruit 133 patient, 29.4% of those directly contacted. Adherence was 75% for supplements, 77% for rehabilitation sessions, and 94.7% for testosterone injection within 24 hours of discharge. The findings from this study provide foundational information for the design of successful future clinical trials in hospitalized geriatric patients.
Clinical trials in older adults oftentimes fail to meet the recruitment targets (21,22). Our study illustrates the complexity of institutional and patient challenges (patient census and characteristics, varying level of enthusiasm by healthcare professionals, high rates of cognitive and physical debility in this population, cultural bias, polypharmacy, electronic medical records, frequent transitions of care) to be addressed before a successful clinical trial can be completed in recently hospitalized seniors with multiple comorbidities (Table 3). While we were able to meet the recruitment targets we had set, our screening to eligibility ratio was relatively high with eight participants screened for each eligible patient. This suggests that our exclusion criteria might have been too strict. A review of 14 recently published trials found a much lower average 3:1 ratio for screening (23), however the range was broad, from 3% to 49%. While our rate of 13% does fall within the range, we believe that we would have had a better recruitment rate had we relaxed our inclusion and exclusion criteria. For example, 20% of the patients lived more than 30 miles away from the hospital. Expanding the radius of catchment would have increased the patient pool. Reported refusal rates are also variable across published studies, ranging from 8% to 54% (23). Our refusal rate was higher, at 70%, which we attribute to the added burden of hospitalization. Our withdrawal rate of 8% was similar to other published studies, which ranged from 5% to 37% (23).
Table 3.
Summary of Potential Strategies to Overcome the Major Challenges Posed by Recruitment of Hospitalized Older Adults for Clinical Trials on Posthospital Recovery
| Challenges | Potential Solutions |
|---|---|
| Hospital census | Multi-site clinical trial |
| Support from clinical staff | Build rapport with clinical staff |
| Education | |
| Cultural bias | Community outreach and education |
| Few eligible patients | Revise inclusion/exclusion criteria |
| Multi-site clinical trial | |
| Severely ill patients not willing to be involved in clinical research | Recruitment toward the end of admission |
| Simplify description of study | |
| Use of staff-administered questionnaires instead of pen and paper | |
| Patient unavailable during the day due to clinical procedures | Recruit after hours and during the weekend |
| Increase staffing | |
| Mobility limitation/transportation | Home visits |
| Adherence to rehabilitation program | Supervised sessions |
There are unique challenges to recruiting research subjects from a hospitalized population as compared to recruiting healthy subjects from the community using ads, websites, and mailings. Potential subjects in our study were first and foremost seriously ill patients, oftentimes in pain or discomfort and concerned about dying. Thus, communication with health care staff regarding the patient’s eligibility, concerns, and the continuity of care was a key component of our approach. Others have also noted the difficulty of recruiting subjects in acute care hospital settings, particularly in the first day or two after admission, which is a stressful and busy time for testing and procedures (23,24). We found more success when recruiting subjects closer to their discharge, probably because they felt better and were able to concentrate on the consent process and study details more easily. Another barrier is the need for support of patient care staff (nurses, doctors, etc.) (23,24). Over the past decade, we have developed a close working relationship with the geriatric teams on the ACE unit. When we extended recruitment to include other hospital units we found it necessary to establish a rapport with the nursing staff and medical teams of those units by providing repeated presentations about the study. Building these relationships with clinical teams helped ensure that everyone was aware of the eligibility criteria, study design, potential benefits to the patients, and progress of the clinical trial.
Another institution-based challenge to recruitment was the limited staffing of this pilot study, with one coordinator working Monday to Friday from 8 AM to 5 PM. As a consequence, we did not screen, enroll, test, or visit subjects at home on weekends or evenings after 5 PM. Some eligible patients were missed because the coordinator was involved with newly enrolled subjects or away from the hospital for in-home rehabilitation sessions or follow-up testing. Additionally, more than one third of the subjects were unavailable to approach from 8 AM to 5 PM during their hospitalization. In future studies, recruitment could be increased by having coverage available after 5 PM and on weekends, when patients typically have more time and fewer interruptions due to their medical care.
At the patient level, we and others have found that patients may wish to participate, but poor health (multiple comorbidities), fatigue, depression, mobility limitations, transportation difficulties, caregiver burden, medical concerns, pain, and vision or hearing problems prevent their enrollment (22,25). Although the rehabilitation program was designed and overseen by a geriatric physical therapist, many subjects were unable to complete all exercises as prescribed for each session. Since we had established a priori that incomplete sessions would not be included in the final count, the result was a suboptimal adherence with the rehabilitation program. This is a limitation that warrants further research and consideration when designing future studies. Other issues included a longer time to obtain consent because older patients often wanted to consult with family or their medical team prior to making a decision (23), and additional time spent by the research team discussing concerns with family and caregivers (26). We found it especially important in recruiting hospitalized patients to explain the study in a simple way. We highlighted the potential benefits and risks of the study. We also carefully designed the study to decrease burden whenever possible, while maintaining rigor. We increased flexibility for timing and location of testing; questionnaires were administered orally by a coordinator rather than asking the patients to provide written answers; and many data, including medical history, were collected from medical records.
The major strength of this study was that we have demonstrated that we can recruit from the hospital setting and retain a large sample size of severely ill geriatric patients. We utilized comprehensive and multicomponent interventions in an attempt to achieve the highest possible rate of acceptance and adherence to the interventions. The supervised rehabilitation sessions ensured that patients used the proper technique, and significantly increased adherence as compared to the unsupervised sessions. We evaluated institution-based and patient-based facilitators and barriers using both subjective and objective measures of feasibility.
This study also has several potential limitations. First, we designed the study to exclude cognitively impaired patients based on chart review, clinical staff report or research staff observation. However, we did not use a specific delirium screening tool. Thus, we cannot exclude that some of the enrolled patients may have had unrecognized delirium. Considering that the research staff was trained in recognizing delirium, we estimate that the prevalence of unrecognized delirium was very low. Exclusion of cognitively impaired patients may have reduced the representativeness of our sample. Approximately one-fourth of the patients are admitted in the hospital with various degrees of cognitive dysfunction/delirium, which may improve with treatment of the primary disease. To increase inclusiveness, consent by proxy should be considered in future studies, particularly in those patients with an acute onset cognitive problem. Second, the study population was not homogeneous. Patients had a variable number and combination of acute diseases and chronic comorbidities. Nonetheless, the comorbidity index was not different across study arms due to successful randomization. However, it is important to underscore that this is a pilot study designed to determine in acutely ill older adults feasibility and acceptability of interventions originally developed in healthy individuals.
To the best of our knowledge, this is the first randomized clinical trial targeting acutely ill older adults that focuses on the feasibility of implementing interventions starting immediately after discharge from the hospital, with the goal of accelerating recovery of muscle mass and function. These data will provide critical information to design larger clinical trials of promising posthospitalization interventions.
Funding
This work was supported by the National Dairy Council grant number 1229; UTMB Claude D. Pepper OAIC (P30 AG024832) from the National Institute on Aging; and the UTMB Clinical and Translational Science Award (UL1 TR001439 and TL1 TR001440) from the National Center for Advancing Translational Sciences. Nutritional whey protein supplements were provided in kind by BiPro, Eden Prairie, MN, USA.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors thank the UTMB Institute for Translational Sciences Clinical Research Center for providing nursing support.
References
- 1. Lamont CT, Sampson S, Matthias R, Kane R. The outcome of hospitalization for acute illness in the elderly. J Am Geriatr Soc. 1983;31:282–288. doi:10.1111/j.1532-5415.1983.tb04872.x2. [DOI] [PubMed] [Google Scholar]
- 2. Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med. 1982;16:1033–1038. doi.org/10.1016/0277-9536(82)90175-7 [DOI] [PubMed] [Google Scholar]
- 3. Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi:10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. doi:10.1111/j.1532-5415.2004.52354.x [DOI] [PubMed] [Google Scholar]
- 5. Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Crit Care Med. 2009;37(10 Suppl):S337–S346. doi:10.1097/CCM.0b013e3181b6e974 [DOI] [PubMed] [Google Scholar]
- 6. Fisher SR, Goodwin JS, Protas EJ et al. . Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59:91–95. doi:10.1111/j.1532-5415.2010.03202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. doi:10.1111/j.1532-5415.2009.02393.x [DOI] [PubMed] [Google Scholar]
- 8. Gariballa SE, Parker SG, Taub N, Castleden M. Nutritional status of hospitalized acute stroke patients. Br J Nutr. 1998;79:481–487. doi:10.1079/BJN19980085 [DOI] [PubMed] [Google Scholar]
- 9. Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281:2013–2019. doi:10.1001/jama.281.21.2013 [DOI] [PubMed] [Google Scholar]
- 10. Vellas B, Conceicao J, Lafont C et al. . Malnutrition and falls. Lancet. 1990;336:1447. [DOI] [PubMed] [Google Scholar]
- 11. Johnson CS. The association between nutritional risk and falls among frail elderly. J Nutr Health Aging. 2003;7:247–250. [PubMed] [Google Scholar]
- 12. Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi:10.1378/chest.129.3.536 [DOI] [PubMed] [Google Scholar]
- 13.Wier L, Pfuntner A, Steiner C. Hospital utilization among oldest adults, 2008: Statistical Brief #103. 2010. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. https://www.ncbi.nlm.nih.gov/books/NBK52646/ [PubMed] [Google Scholar]
- 14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi:10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 15. Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361:2637–2645. doi:10.1056/NEJMsa0904859 [DOI] [PubMed] [Google Scholar]
- 16. Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248–253. doi:10.1097/MCO.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paddon-Jones D, Sheffield-Moore M, Zhang XJ et al. . Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi:10.1152/ajpendo.00368.2003 [DOI] [PubMed] [Google Scholar]
- 18. Ferrando AA, Sheffield-Moore M, Yeckel CW et al. . Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi:10.1152/ajpendo.00362.2001 [DOI] [PubMed] [Google Scholar]
- 19. Deer RR, Dickinson JM, Fisher SR, Ju H, Volpi E. Identifying effective and feasible interventions to accelerate functional recovery from hospitalization in older adults: A randomized controlled pilot trial. Contemp Clin Trials. 2016;49:6–14. doi:10.1016/j.cct.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dillon EL, Basra G, Horstman AM et al. . Cancer cachexia and anabolic interventions: a case report. J Cachexia Sarcopenia Muscle. 2012;3:253–263. doi:10.1007/s13539-012-0066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cassidy EL, Baird E, Sheikh JI. Recruitment and retention of elderly patients in clinical trials: issues and strategies. Am J Geriatr Psychiatry. 2001;9:136–140. doi:10.1097/00019442-200105000-00005 [PubMed] [Google Scholar]
- 22. McHenry JC, Insel KC, Einstein GO, Vidrine AN, Koerner KM, Morrow DG. Recruitment of older adults: success may be in the details. Gerontologist. 2015;55:845–853. doi:10.1093/geront/gns079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMurdo ME, Roberts H, Parker S et al. ; Age and Ageing Specialty Group, NIHR, Comprehensive Clinical Research Network. Improving recruitment of older people to research through good practice. Age Ageing. 2011;40:659–665. doi:10.1093/ageing/afr115 [DOI] [PubMed] [Google Scholar]
- 24. Sellors J, Cosby R, Trim K et al. ; Seniors Medication Assessment Research Trial (SMART) Group. Recruiting family physicians and patients for a clinical trial: lessons learned. Fam Pract. 2002;19:99–104. doi:10.1093/fampra/19.1.99 [DOI] [PubMed] [Google Scholar]
- 25. Provencher V, Mortenson WB, Tanguay-Garneau L, Bélanger K, Dagenais M. Challenges and strategies pertaining to recruitment and retention of frail elderly in research studies: a systematic review. Arch Gerontol Geriatr. 2014;59:18–24. doi:10.1016/j.archger.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 26. Hancock K, Chenoweth L, Chang E. Challenges in conducting research with acutely ill hospitalized older patients. Nurs Health Sci. 2003;5:253–259. doi:10.7748/nop.16.1.8.s10 [DOI] [PubMed] [Google Scholar]