Abstract
Background
Previous studies have shown that chronic kidney disease (CKD) is associated with accelerated loss of skeletal muscle in patients on dialysis. However, the relationships of sarcopenia with albuminuria and early-stage CKD in patients with type 2 diabetes have not been examined.
Methods
We analyzed diabetic subgroup data from 409 patients with type 2 diabetes from the Korean Sarcopenic Obesity Study (KSOS). Sarcopenia was defined as a skeletal muscle mass index (SMI; SMI [%] = total skeletal muscle mass [kg]/weight [kg] × 100) less than 2 SD below the sex-specific mean for a younger reference group. The estimated glomerular filtration rates and urinary albumin-to-creatinine ratios were used to assess renal function and albuminuria.
Results
The prevalence of sarcopenia was significantly increased in the albuminuria group compared with the normo-albuminuria group (26.7% vs 12.6%, p = .001), as well as in CKD 3 group compared with the CKD 1–2 group (46.7% vs 15.1%, p = .005). After adjusting for age, SMI was negatively correlated with urinary albumin-to-creatinine ratios and positively correlated with aspartate aminotransferase, alanine aminotransferase, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels. Multiple logistic regression analysis revealed that the odds ratio for albuminuria association was 3.02 (95% CI 1.37–6.67) in the lowest tertile of SMI compared with the highest tertile after adjusting for various confounding factors.
Conclusions
Sarcopenia is more prevalent in individuals with albuminuria than in those without albuminuria. Furthermore, increased albuminuria is independently associated with low muscle mass in patients with type 2 diabetes.
Keywords: Chronic kidney disease, Albuminuria, Sarcopenia, Skeletal muscle mass index, Type 2 diabetes
Like many other countries around the world, Korea is facing the challenge of a rapidly increasing aging population. One conspicuous change in the human body composition related to aging is a progressive loss of muscle mass and strength, called sarcopenia (1). Sarcopenia causes physical disability, functional impairment, metabolic derangement, decreased survival, and increased health care costs (2).
Moreover, the prevalence of chronic kidney disease (CKD) is steadily rising, particularly in the elderly population (3). Diabetic kidney disease is the leading cause of end-stage renal disease (ESRD) and is also linked with cardiovascular and all-cause mortality (4,5). A low creatinine excretion rate has also been associated with increased all-cause mortality in patients with type 2 diabetes with nephropathy (6). In particular, albuminuria is regarded as an independent risk factor for progression to ESRD and for cardiovascular disease (7). Interestingly, albuminuria shares certain pathophysiological mechanisms, such as insulin resistance, inflammation, endothelial dysfunction, and oxidative stress, with sarcopenia (8,9). Recently, we and other groups observed that sarcopenia is independently associated with type 2 diabetes, which is a critical risk factor for CKD and cardiovascular disease (10,11).
Several studies have examined the relationship between muscle mass and kidney function. Muscle wasting has been increasingly reported in patients on dialysis (12). Moreover, patients with ESRD exhibit more severe and earlier loss of muscle mass than control patients (13). However, to the best of our knowledge, no study has yet explored the relationships of sarcopenia with albuminuria and early-stage CKD in patients with type 2 diabetes mellitus after comprehensive adjustment for confounding factors including medications. Therefore, here, we examined the association between albuminuria and skeletal muscle mass index (SMI) in patients with type 2 diabetes mellitus. Furthermore, we compared the SMI with estimated glomerular filtration rate (eGFR) values calculated by the CKD-Epidemiology Collaboration (CKD-EPI) equation.
Methods
Study Subjects
We analyzed baseline cross-sectional data of the diabetic subgroup from the Korean Sarcopenic Obesity Study (diabetic KSOS), an ongoing epidemiological study. This prospective observational cohort was designed to examine the prevalence of sarcopenia and sarcopenic obesity in Korean adults with or without diabetes and to evaluate the effects of these conditions on metabolic disorders and diabetic microvascular and macrovascular complications. Details of this study have been previously published (10,14). Participants were enrolled in the KSOS cohort between September 2007 and August 2009. The study participants consisted of 428 patients with diabetes who were treated at the Diabetes Center of Korea University Guro Hospital. We excluded the participants who had a history of cardiovascular disease (myocardial infarction, unstable angina, stroke, or cardiovascular revascularization), stage 2 hypertension (resting blood pressure ≥ 160/100 mmHg), acute infectious disease, malignant disease, or severe renal or hepatic disease. A total of 409 subjects for whom complete data regarding body composition were available were included in the final analysis. We investigated smoking status, alcohol status, and medication history including the use of insulin, statins, fibrate, angiotensin receptor blockers, and angiotensin-converting enzyme (ACE) inhibitors. Physical activity was classified into two categories, none or regular, with regular exercise defined as exercising at least three times a week for a minimum of 30 minutes each. Written informed consent was obtained from all parents and the Korea University Institutional Review Board approved this study protocol in accordance with the Declaration of Helsinki of the World Medical Association.
Laboratory Measurements
All blood and random spot urine samples were obtained in the morning after a 12-hour overnight fast. Blood samples were immediately stored at −80°C for subsequent assays. Serum total cholesterol, triglyceride, and high-density lipoprotein cholesterol levels were determined enzymatically using a chemistry analyzer (Hitachi 747; Tokyo, Japan). Aspartate aminotransferase and alanine aminotransferase levels were determined enzymatically with the aid of an auto-analyzer (TBA-200FR; Toshiba, Tokyo, Japan). The glucose oxidase method was used to measure fasting plasma glucose. HbA1C levels were measured using high performance liquid chromatography on a Bio-Rad Variant II instrument.
Definition of Urinary Albumin and Kidney Function
Urinary albumin and creatinine levels were used to calculate the urine albumin: creatinine ratio (ACR, expressed in μg/mg). Albuminuria was defined as a urinary ACR concentration ≥ 30 μg/mg. Kidney function was assessed by estimating the eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (15). eGFR levels were classified according to the system recommended by the Kidney Disease Outcome Quality Initiative (K-DOQI) (16). No patient had an eGFR under 30 ml/min/1.73 m2 in the present study.
Assessment of Body Composition
Body mass index (BMI) was computed as weight (kg)/height (m). Waist circumference was measured at the midpoint between the lower border of the rib cage and the top of the lateral border of the iliac crest. A whole-body dual-energy x-ray absorptiometry scan was performed on each patient to measure total and regional lean mass (kg) using fan-beam technology (Discovery A, Hologic, Bedford, MA). The appendicular skeletal muscle mass (ASM [kg]) and SMI (SMI [%] = total skeletal muscle mass [kg]/weight [kg] × 100) were obtained as previously described (10). Sarcopenia was defined as an SMI of 2 SD below the sex-specific mean value for a younger reference group (14). The cutoff point for sarcopenia was 35.9% in men and 30.6% in women. Bioelectrical impedance analysis (MC 780MA, Tanita Corporation, Tokyo, Japan) was performed to determine percent body fat. Fat mass (kg) was calculated as body weight (kg) × percent body fat (%).
Statistical Analysis
Continuous variables with normal distributions are expressed as means ± SD, whereas continuous variables with skewed distributions are expressed as medians and interquartile ranges. All the continuous variables were evaluated using the Shapiro–Wilk test for normality. Selection of parametric and nonparametric analysis was performed according to the distribution and characteristic of variables. Baseline demographic and biochemical characteristics of the study population were compared by albuminuria or chronic renal disease status using an independent t test or the Mann–Whitney U test for continuous variables. Pearson’s Chi-square test or Fisher’s exact test was used to test for differences in the distribution of categorical variables. Analysis of covariance was used to compare urinary ACR and eGFR values between the sarcopenia and nonsarcopenia groups before and after adjusting for sex and age. All statistical results were based on two-sided tests. To evaluate correlations between SMI and metabolic variables, Spearman partial correlation analysis was used after adjusting for age. Odds ratios (ORs) and 95% confidence intervals (CIs) for the prediction of CKD based on eGFR and albuminuria values were obtained from logistic regression models after controlling for potential covariates such as sex and age. Data were analyzed by a professional statistician (S.Y. Hwang) using SAS 9.2 (SAS Institute, Cary, NC); a p value less than .05 was assumed to indicate statistical significance.
Results
Study Subject Characteristics
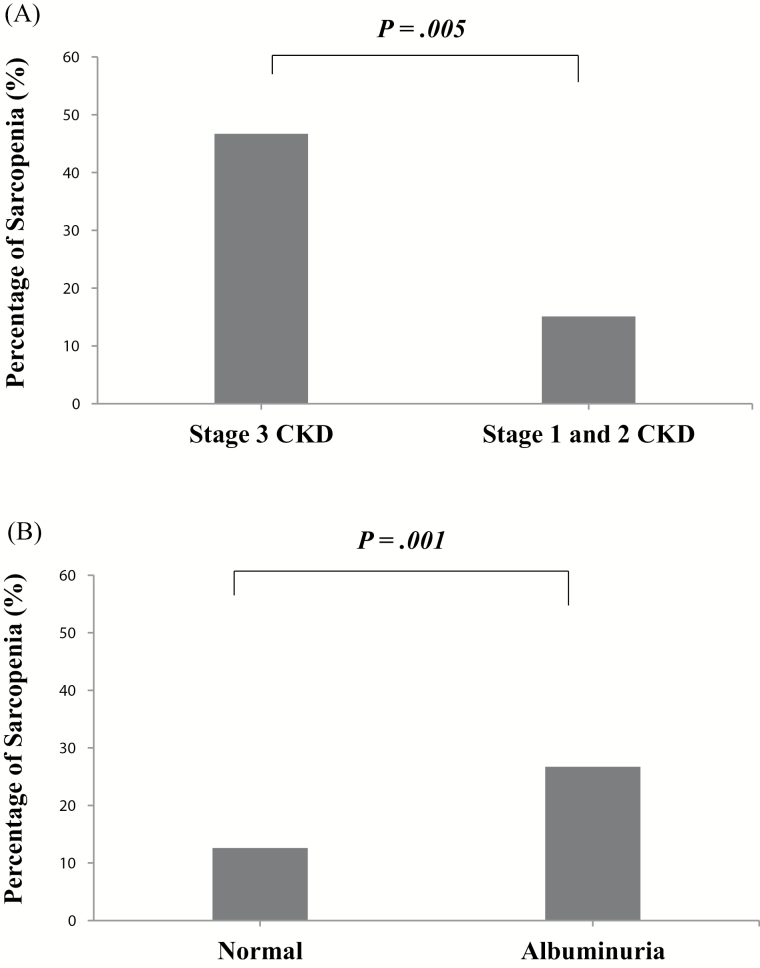
The characteristics of the study subjects with or without albuminuria are presented in Table 1. The subjects in the albuminuria group were significantly older than those in the normo-albuminuria group. The two groups also exhibited significant differences in creatinine and eGFR levels, two markers for kidney function. However, blood pressure, body mass index, physical activity, and laboratory measurements (lipid profile, fasting plasma glucose, and HbA1c level) were similar between the two groups, with the exception of triglycerides. The subjects in CKD 3 group were significantly older than those in CKD 1–2 group (Supplementary Table 1). In particular, the SMI values were significantly lower in the albuminuria group than in the normo-albuminuria group (36.2 [32.8, 39.2] vs 37.5 [33.5, 41.0] %, p = .015), as well as in the CKD 3 group compared to the CKD 1–2 group (35.4 [29.8, 37.7] vs 37.3 [33.4, 40.8] %, p = .046). Furthermore, the prevalence of sarcopenia was significantly higher in the albuminuria group than in the normo-albuminuria group (26.7% vs 12.6%, p = .001), as well as in the CKD 3 group compared to the CKD 1–2 group (46.7% vs 15.1%, p = .005; Figure 1). Also, as the CKD stage increased, the prevalence of sarcopenia increased (CKD stage 1, 2, and 3: 14.4%, 20.0%, and 46.7%, p = .003). In addition, the urinary ACR (p = .013) and eGFR (p = .003) values were significantly different between the sarcopenia group and the nonsarcopenia group. Specifically, the log-transformed urinary ACR level was significantly higher in the sarcopenia group than in the nonsarcopenia group (p = .003) and the log-transformed eGFR level of the sarcopenia group was significantly lower than that of the nonsarcopenia group (p = .014).
Table 1.
Anthropometric and Clinical Characteristics of the Study Subjects With or Without Albuminuria
| Normal (n = 304) | Albuminuria (n = 105) | p | |
|---|---|---|---|
| Male (%) | 52.6 | 54.3 | .770 |
| Age (years) | 58 (52, 65) | 62 (53, 69) | .015 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 | .699 |
| Body weight (kg) | 64.0 (57.6, 70.2) | 67.0 (59.0, 73.2) | .078 |
| Body mass index (kg/m2) | 24.8 (22.9, 27.0) | 24.4 (22.1, 26.7) | .164 |
| Waist circumference (cm) | 86 (82, 91) | 85 (80, 91) | .088 |
| SBP (mmHg) | 127 (118, 136) | 126 (114, 134) | .163 |
| DBP (mmHg) | 79.7 ± 10.2 | 80.2 ± 11.1 | .683 |
| Total cholesterol (mmol/L) | 3.4 (2.7, 4.1) | 3.4 (2.8, 4.1) | .972 |
| LDL cholesterol (mmol/L) | 1.7 (1.2, 2.3) | 1.7 (1.2, 2.3) | .872 |
| HDL cholesterol (mmol/L) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.3) | .192 |
| Triglyceride (mmol/L) | 1.3 (0.9, 1.9) | 1.0 (0.8, 1.6) | .014 |
| AST (U/L) | 15 (10, 21) | 13 (8, 20) | .099 |
| ALT (U/L) | 18 (15, 23) | 18 (14, 23) | .806 |
| Urinary ACR (µg/mg) | 6.5 (4.2, 11.4) | 101.2 (55.1, 239.7) | <.001 |
| Creatinine (µmol/l) | 51.3 (40.7, 63.6) | 56.6 (47.7, 75.1) | <.001 |
| eGFR (ml/min/1.73 m2) | 106.9 (99.0, 117.8) | 98.7 (86.3, 112.2) | <.001 |
| FPG (mmol/L) | 5.9 (5.0, 7.2) | 5.7 (4.6, 6.8) | .205 |
| HbA1C (% [mmol/mol]) | 7.1 (6.6, 7.7) | 7.1 (6.6, 7.7) | .930 |
| 54 (49, 61) | 54 (49, 61) | ||
| Duration of diabetes (years) | 7.0 (3.0, 11.5) | 7.0 (3.0, 13.0) | .886 |
| Body composition | |||
| ASM (kg) | 21.2 (17.6, 24.6) | 20.4 (17.8, 23.9) | .670 |
| ASM/height2 (kg/m2) | 8.0 (7.3, 8.9) | 8.0 (7.4, 8.7) | .619 |
| SMI (%) | 37.5 (33.5, 41.0) | 36.2 (32.8, 39.2) | .015 |
| BIA FM (kg) | 17.1 (13.8, 21.3) | 15.9 (12.5, 20.0) | .102 |
| BIA PBF (%) | 25.9 (21.3, 31.7) | 25.2 (19.5, 30.8) | .129 |
| Physical activity (%) | 50.0 | 48.6 | .801 |
| Current smokers (%) | 22.0 | 20.0 | .661 |
| Alcohol users (%) | 52.0 | 53.3 | .810 |
Note: Data are expressed as means ± SD, medians (inter-quartile ranges), or n (%). p Values were calculated using an independent two-sample t test, the Mann–Whitney U test, or the χ2 test. Albuminuria was defined as a urinary ACR ≥ 30 μg/mg Cr. ACR = albumin to creatinine ratio; ALT = alanine aminotransferase; ASM = appendicular skeletal muscle; AST = aspartate aminotransferase; BIA FM = bioelectrical impedance analysis fat mass; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; FPG = fasting plasma glucose; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PBF = percent body fat; SMI = skeletal muscle mass index; SBP = systolic blood pressure.
Figure 1.
Prevalence of sarcopenia according to (A) chronic kidney disease (CKD) (stage 3 CKD vs stage 1 and 2 CKD; 46.7% vs 15.1%, p = .005) and (B) albuminuria (normal vs albuminuria; 12.6% vs 26.7%, p = .001) status.
Correlation of SMI With Cardiovascular Risk Factors
Table 2 shows the results of partial correlation analysis of SMI with other major metabolic variables in the study populations. After adjusting for age, SMI was negatively correlated with urinary ACR (p = .004) and positively correlated with aspartate aminotransferase (p = .031), alanine aminotransferase (p = .017), total cholesterol (p = .006), high-density lipoprotein cholesterol (p = .039), and low-density lipoprotein cholesterol (p = .048) in the overall group. In the male subgroup, SMI was negatively correlated with urinary ACR (p = .001).
Table 2.
Spearman Partial Correlation Analysis of SMI and Various Parameters After Adjusting for Age
| Total | Female | Male | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| eGFR | −.001 | .991 | .028 | .699 | .032 | .643 |
| Urinary ACR | −.141 | .004 | −.070 | .338 | −.234 | .001 |
| SBP | .062 | .213 | .080 | .271 | −.008 | .909 |
| DBP | .016 | .745 | .006 | .929 | −.053 | .444 |
| AST | .107 | .031 | −.001 | .994 | −.049 | .476 |
| ALT | .118 | .017 | −.013 | .857 | .024 | .730 |
| Total cholesterol | .136 | .006 | .031 | .675 | .062 | .366 |
| HDL cholesterol | .103 | .039 | −.030 | .680 | .035 | .614 |
| Triglyceride | .093 | .061 | .018 | .802 | .106 | .121 |
| LDL cholesterol | .099 | .048 | .016 | .824 | .028 | .686 |
| FPG | .071 | .156 | −.072 | .322 | −.045 | .509 |
| HbA1C | −.001 | .988 | .131 | .072 | −.069 | .315 |
Note: ACR = albumin to creatinine ratio; ALT = alanine aminotransferase; AST = aspartate aminotransferase; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; FPG = fasting plasma glucose; HDL = high-density lipoprotein; SBP = systolic blood pressure; SMI = skeletal muscle mass index.
Multiple Logistic Regression Analysis for CKD and Albuminuria
Multiple logistic regression analysis was performed using stage 3 CKD and albuminuria as the dependent variables; the ORs and 95% CIs were calculated for each SMI tertile. As shown in Table 3, the SMI tertile was not significantly associated with stage 3 CKD, although increasing trends of ORs of having stage 3 CKD by SMI tertile were observed. However, multiple logistic regression analysis for albuminuria after adjusting for sex and age revealed that subjects in the second (OR = 2.13, 95% CI = 1.16–3.92) and first (OR = 2.69, 95% CI = 1.26–5.75) SMI tertiles had significantly higher risks compared with subjects in the third tertile (p for trend = .010; Table 4). This relationship persisted even after adjusting for percent body fat, smoking status, alcohol status, physical activity, duration of diabetes, HbA1c, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, and medication history (ie, renin–angiotensin system [RAS] antagonists, statins, fibrates, and insulin) (p for trend = .006).
Table 3.
Unadjusted and Adjusted ORs With 95% CIs of Having stage 3 CKD by SMI Tertile
| SMI Tertile (%) | |||||
|---|---|---|---|---|---|
| Q3 | Q2 | Q1 | p | p for Trend | |
| Unadjusted | 1 | 1.69 (0.40, 7.23) | 2.42 (0.61, 9.58) | .445 | .199 |
| Model 1 | 1 | 1.89 (0.43, 8.37) | 3.65 (0.67, 19.91) | .325 | .134 |
| Model 2 | 1 | 1.99 (0.43,9.16) | 5.10 (0.82,31.56) | .211 | .082 |
| Model 3 | 1 | 1.99 (0.42,9.54) | 5.24 (0.81,34.04) | .216 | .085 |
| Model 4 | 1 | 2.01 (0.41,10.01) | 5.30 (0.77,36.42) | .231 | .092 |
Note: Model 1: adjusted for sex and age. Model 2: adjusted for sex, age, percent body fat, smoking status, alcohol status, and physical activity. Model 3: adjusted for sex, age, percent body fat, smoking status, alcohol status, physical activity, duration of diabetes, HbA1c, SBP, LDL cholesterol, HDL cholesterol, and triglyceride. Model 4: adjusted for sex, age, percent body fat (PBF), smoking status, alcohol status, physical activity, duration of diabetes, HbA1c, SBP, LDL cholesterol, HDL cholesterol, triglyceride, use of RAS blockers, use of statins, use of fibrate, and use of insulin. CI = confidence interval; CKD = chronic kidney disease; OR = odds ratio; HDL = high-density lipoprotein; LDL = low-density lipoprotein; RAS = renin–angiotensin system; SBP = systolic blood pressure; SMI = skeletal muscle mass index.
Table 4.
Unadjusted and Adjusted ORs With 95% CIs of Having Albuminuria by SMI Tertile
| SMI Tertile (%) | |||||
|---|---|---|---|---|---|
| Q3 | Q2 | Q1 | p | p for trend | |
| Unadjusted | 1 | 1.85 (1.05, 3.27) | 1.87 (1.06, 3.31) | .056 | .035 |
| Model 1 | 1 | 2.13 (1.16, 3.92) | 2.69 (1.26, 5.75) | .022 | .010 |
| Model 2 | 1 | 2.29 (1.23,4.26) | 2.92 (1.33,6.39) | .014 | .007 |
| Model 3 | 1 | 2.31 (1.23,4.34) | 2.92 (1.33,6.43) | .014 | .007 |
| Model 4 | 1 | 2.38 (1.26,4.49) | 3.02 (1.37,6.67) | .011 | .006 |
Note: Model 1: adjusted for sex and age. Model 2: adjusted for sex, age, percent body fat, smoking status, alcohol status, and physical activity. Model 3: adjusted for sex, age, percent body fat, smoking status, alcohol status, physical activity, duration of diabetes, HbA1c, SBP, LDL cholesterol, HDL cholesterol, and triglyceride. Model 4: adjusted for sex, age, percent body fat (PBF), smoking status, alcohol status, physical activity, duration of diabetes, HbA1c, SBP, LDL cholesterol, HDL cholesterol, triglyceride, use of RAS blockers, use of statins, use of fibrate, and use of insulin. CI = confidence interval; OR = odds ratio; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SBP = systolic blood pressure; RAS = renin–angiotensin system; SMI = skeletal muscle mass index.
Discussion
This study found a higher prevalence of sarcopenia in patients with type 2 diabetes with albuminuria compared with patients with type 2 diabetes with normo-albuminuria. Similarly, a higher prevalence of sarcopenia was found in the CKD 3 group compared with the CKD 1–2 group. Furthermore, the higher risk of albuminuria in patients with type 2 diabetes mellitus was significantly associated with low muscle mass independent of demographic and clinical adjustment variables. These findings suggest that the possibility of sarcopenia should be considered when albuminuria is detected in patients with type 2 diabetes.
The pathophysiological mechanisms of sarcopenia and albuminuria are multifactorial and highly similar to one another. Their shared underlying mechanisms, including insulin resistance, endothelial dysfunction, inflammation, oxidative stress, and activation of the RAS may explain the observed relationship between sarcopenia and albuminuria. Skeletal muscle is the largest organ responsible for insulin-mediated glucose disposal in humans. Moon and colleagues reported that sarcopenia is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population (17). The progressive loss of skeletal muscle might lead to insulin resistance, which could in turn promote albuminuria and cardiovascular disease. Insulin resistance has been shown to induce endothelial dysfunction, glomerular hyperfiltration, and increased vascular permeability, which result in albuminuria (18,19). In patients with type 2 diabetes, insulin resistance is independently associated with microalbuminuria (20). Loss of podocytes by apoptosis is a representative characteristic of the early stages of diabetic kidney disease. One recent study in an animal model reported that podocyte insulin resistance induces susceptibility to cell death, which may contribute to albuminuria (21). Additionally, kidney endothelial dysfunction has been shown to play an important role in the development of albuminuria by reducing vascular relaxation and inflammatory cell infiltration (22). Moreover, endothelial dysfunction and insulin resistance influence the regulation of skeletal muscle protein balance, which may contribute to the development of sarcopenia (23). Decreased nitric oxide and increased endothelin-1 levels lead to impaired endothelium-dependent vasodilatation and potentially induce inflammation through increased leukocyte–endothelium interactions (23). Albuminuria is also known to reflect endothelial dysfunction and subclinical inflammation (24). In addition, an accumulating body of evidence suggests that sarcopenia is an inflammatory state driven by cytokines and oxidative stress (25). The age-related low-grade inflammatory profile (CLIP) is related to decreased muscle mass and strength in elderly persons (26). CLIP disrupts protein kinase B (Akt) signaling and mitochondrial oxidative capacity, in addition to upregulating nuclear factor κB (NF-κB) and heat shock protein (Hsp) expression, all of which are linked to sarcopenia (26). Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are frequently reported as circulating inflammatory parameters that may explain the link between inflammation and sarcopenia (26). In addition, the RAS has been suggested to play a critical role in the connection between sarcopenia and albuminuria. ACE is expressed in the vascular endothelial cells of skeletal muscle (27). Recently, ACE inhibition was suggested to play a major role in counteracting sarcopenia (28). RAS-blocking drugs have been reported to ameliorate endothelial dysfunction, increase skeletal muscle blood flow, and enhance glucose uptake by skeletal muscle, supporting the hypothesis that RAS inhibition may protect against sarcopenia (28). ACE inhibitors (ACEIs) and angiotensin 1 receptor blockers, two major classes of drug that target the RAS, have been established to reduce the risk of renal and cardiovascular events (29). Moreover, RAS blockers have shown a protective effect on the kidney against the development of albuminuria and progression to ESRD (30). Previous studies have identified advanced age, male sex, elevated BP, elevated HbA1c, elevated cholesterol, smoking status (smoker), and ethnicity (Afro-Caribbean, Asian, or Native American) as factors that contribute to the development of diabetic kidney disease (31,32). In this study, multiple logistic regression analysis revealed that low muscle mass has stepwise relationship with albuminuria, even after adjusting for possible risk factors related to medication history, such as use of RAS blockers, statins, fibrates, etc.
Previous studies have reported loss of skeletal muscle mass and strength in patients with ESRD. Moreover, the incidence of sarcopenia in patients undergoing maintenance hemodialysis (MHD) was found to be high and to increase gradually with age (33); similarly, the risk of mortality in patients with sarcopenia was higher than in patients without sarcopenia (33). Noori and colleagues also demonstrated that high mid-arm muscle circumference (MAMC), a surrogate measurement of lean body mass (LBM), is an independent predictor of improved survival and mental health in patients undergoing MHD. In patients with ESRD, the most significant predictor of LBM loss is the presence of diabetes mellitus (34). In nondiabetic patients on chronic hemodialysis, insulin resistance has been shown to be associated with the breakdown of skeletal muscle protein (35). A few recent studies investigated the associations between the early stages of CKD and sarcopenia in the general population without ESRD. Foley and colleagues first reported an association between decreased glomerular filtration rate and increased sarcopenia prevalence in the Third National Health and Nutrition Examination Survey (NHANES III) (36). The prevalences of sarcopenia were reported to be 3.8%, 5.3%, and 9.4% in patients with CKD 1, 2, and 3–5, respectively. In the study by Foley and colleagues, most (76.9%) participants were Caucasian and skeletal muscle mass was calculated using the bioelectrical impedance analysis equation. In a different study using data from the 2008–2011 Korean National Health and Nutrition Examination Surveys (KNHANES), Moon and colleagues showed that CKD stage was significantly related to an increased prevalence of sarcopenia in men but not in women (37). Moon and colleagues proposed that elderly Korean women may retain muscle mass better than elderly Korean men because of household labor. Even more recently, Kim and colleagues demonstrated that the prevalence of albuminuria in the KNHANES 2011 survey was higher in participants with sarcopenia than in patients with a normal SMI (38). In addition, Han and colleagues reported that the overall prevalence of albuminuria was 16.5% in the general population from the KNHANES 2008–2011 (39). They found that increased risk of albuminuria was associated with sarcopenia independent of comorbidities (ORs 1.76–2.88, all p < .05). In our study including patients with type 2 diabetes, age-adjusted partial correlation analysis revealed that SMI values were correlated with albuminuria in men but not in women, which is in agreement with the findings of Moon and colleagues (37).
The present study did have some limitations. First, because of the inherent limitations of the cross-sectional study design, we could not determine whether a causal relationship exists between sarcopenia and albuminuria. Second, the sample size may not have been sufficiently large to detect a significant relationship between sarcopenia and CKD, although the ORs of CKD risk tended to progressively increase with SMI tertile. Additionally, as we only investigated this relationship in Asian diabetic patients without cardiovascular disease, the results should be confirmed in other ethnic and general diabetic populations. Third, serum creatinine has typically been used to calculate eGFR and urine creatinine has typically been used to calculate urinary ACR. Creatinine levels are known to deviate according to non-GFR factors, such as muscle mass, diet, use of fibrate, age, sex, and ethnicity (40). Current guidelines recommend that initial assessment of albuminuria includes measuring the albumin-to-creatinine ratio in an untimed spot urine collection and reporting the estimated GFR (eGFR) based on serum creatinine calculated using the CKD-EPI equation (40). In contrast, eGFR based on cystatin C might be desirable in cases of extreme muscle mass (40). With regard to eGFR, which uses a variable derived from muscle breakdown (creatinine), this method might tend to underestimate the association between muscle wasting and declining kidney function (36). Lastly, physical activity, which has anti-inflammatory and vascular protective effects, was only assessed based on questionnaire.
However, the present study also has several strengths. First, this study was a well-designed epidemiological study designed to examine the impact of sarcopenia on patients with type 2 diabetes. Second, we assessed muscle mass using dual-energy x-ray absorptiometry, which is regarded as the standard method for defining sarcopenia. Third, we performed extensive adjustment for confounding variables, including age, sex, lifestyle, medication use, and anthropometric and laboratory measurements.
In conclusion, this is the first study to show that low muscle mass is independently associated with increment of albuminuria, a known surrogate marker of adverse renal and cardiovascular outcomes, in patients with type 2 diabetes after adjusting for confounding factors. Further long-term studies in other ethnic groups and with larger study populations are needed to confirm our results.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
K.M.C. was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science and Technology (2015R1D1A1A09057389).
Conflict of Interest
The authors have no conflicts of interest.
Supplementary Material
References
- 1. Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi:10.1038/oby.2004.107 [DOI] [PubMed] [Google Scholar]
- 2. Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–365. [DOI] [PubMed] [Google Scholar]
- 3. Walker SR, Wagner M, Tangri N. Chronic kidney disease, frailty, and unsuccessful aging: a review. J Ren Nutr. 2014;24:364–370. doi:10.1053/j.jrn.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Saver JL, Chang KH, Ovbiagele B. Level of albuminuria and risk of stroke: systematic review and meta-analysis. Cerebrovasc Dis. 2010;30:464–469. doi:10.1159/000317069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svensson MK, Cederholm J, Eliasson B, Zethelius B, Gudbjornsdottir S. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: a nationwide observational study from the Swedish National Diabetes Register. Diab Vasc Dis Res. 2013;10:520–529. doi:10.1177/1479164113500798 [DOI] [PubMed] [Google Scholar]
- 6. Sinkeler SJ, Kwakernaak AJ, Bakker SJ, et al. Creatinine excretion rate and mortality in type 2 diabetes and nephropathy. Diabetes Care. 2013;36:1489–1494. doi:10.2337/dc12-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi:10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116:1171–1178. doi:10.1002/jcb.25077 [DOI] [PubMed] [Google Scholar]
- 9. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013;20:1–10. doi:10.11005/jbm.2013.20.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care. 2010;33:1497–1499. doi:10.2337/dc09-2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int. 2014;14(suppl 1):115–121. doi:10.1111/ggi.12189 [DOI] [PubMed] [Google Scholar]
- 12. Qureshi AR, Alvestrand A, Danielsson A, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int. 1998;53:773–782. doi:10.1046/j.1523-1755.1998.00812.x [DOI] [PubMed] [Google Scholar]
- 13. Domanski M, Ciechanowski K. Sarcopenia: a major challenge in elderly patients with end-stage renal disease. J Aging Res. 2012;2012:754739. doi:10.1155/2012/754739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim TN, Yang SJ, Yoo HJ, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond). 2009;33:885–892. doi:10.1038/ijo.2009.130 [DOI] [PubMed] [Google Scholar]
- 15. Florkowski CM, Chew-Harris JS. Methods of Estimating GFR - Different Equations Including CKD-EPI. Clin Biochem Rev. 2011;32:75–79. [PMC free article] [PubMed] [Google Scholar]
- 16. National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 17. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J. 2014;61:61–70. [DOI] [PubMed] [Google Scholar]
- 18. De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013;28:29–36. doi:10.1093/ndt/gfs290 [DOI] [PubMed] [Google Scholar]
- 19. Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab. 2005;1:100–110. doi:10.1038/ncpendmet0046 [DOI] [PubMed] [Google Scholar]
- 20. Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. [DOI] [PubMed] [Google Scholar]
- 21. Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi:10.1038/ki.2008.109 [DOI] [PubMed] [Google Scholar]
- 22. Satoh M. Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease. Clin Exp Nephrol. 2012;16:518–521. doi:10.1007/s10157-012-0646-y [DOI] [PubMed] [Google Scholar]
- 23. Timmerman KL, Volpi E. Endothelial function and the regulation of muscle protein anabolism in older adults. Nutr Metab Cardiovasc Dis. 2013;23(suppl 1):S44–50. doi:10.1016/j.numecd.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldt-Rasmussen B. Microalbuminuria, endothelial dysfunction and cardiovascular risk. Diabetes Metab. 2000;26(suppl 4):64–66. [PubMed] [Google Scholar]
- 25. Jensen GL. Inflammation: roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008;32:656–659. doi:10.1177/0148607108324585 [DOI] [PubMed] [Google Scholar]
- 26. Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22. doi:10.1097/MCO.0b013e32834dd297 [DOI] [PubMed] [Google Scholar]
- 27. Schaufelberger M, Drexler H, Schieffer E, Swedberg K. Angiotensin-converting enzyme gene expression in skeletal muscle in patients with chronic heart failure. J Card Fail. 1998;4:185–191. [DOI] [PubMed] [Google Scholar]
- 28. Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia--a potential target for Angiotensin-converting enzyme inhibition?Gerontology. 2006;52:237–242. doi:10.1159/000093656 [DOI] [PubMed] [Google Scholar]
- 29. Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014;19:14–33. doi:10.1177/1074248413501018 [DOI] [PubMed] [Google Scholar]
- 30. Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol. 2006;17:S90–97. doi:10.1681/asn.2005121324 [DOI] [PubMed] [Google Scholar]
- 31. Keller CK, Bergis KH, Fliser D, Ritz E. Renal findings in patients with short-term type 2 diabetes. J Am Soc Nephrol. 1996;7:2627–2635. [DOI] [PubMed] [Google Scholar]
- 32. Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998–1004. [DOI] [PubMed] [Google Scholar]
- 33. Ren H, Gong D, Jia F, Xu B, Liu Z. Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk. Ren Fail. 2016;1–8. doi:10.3109/0886022X.2015.1132173 [DOI] [PubMed] [Google Scholar]
- 34. Pupim LB, Heimburger O, Qureshi AR, Ikizler TA, Stenvinkel P. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int. 2005;68:2368–2374. doi:10.1111/j.1523-1755.2005.00699.x [DOI] [PubMed] [Google Scholar]
- 35. O'Sullivan AJ, Kelly JJ. Insulin resistance and protein catabolism in non-diabetic hemodialysis patients. Kidney Int. 2007;71:98–100. doi:10.1038/sj.ki.5002045 [DOI] [PubMed] [Google Scholar]
- 36. Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi:10.1159/000101827 [DOI] [PubMed] [Google Scholar]
- 37. Moon SJ, Kim TH, Yoon SY, Chung JH, Hwang HJ. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008-2011. PLoS One. 2015;10:e0130740. doi:10.1371/journal.pone.0130740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim TN, Lee EJ, Hong JW, et al. Relationship between sarcopenia and albuminuria: the 2011 Korea National Health and Nutrition Examination Survey. Medicine (Baltimore). 2016;95:e2500. doi:10.1097/md.0000000000002500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han E, Lee YH, Kim G, et al. Sarcopenia is associated with albuminuria independently of hypertension and diabetes: KNHANES 2008-2011. Metabolism. 2016;65:1531–1540. doi:10.1016/j.metabol.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 40. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi:10.1001/jama.2015.0602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.