Abstract
Background
Positive affect (PA) and negative affect (NA) reflect subjective emotional experiences. Although related to depression and anxiety, these dimensions are distinct constructs representing affective states and patterns. Prior studies suggest that elevated depressive symptoms are associated with risk of mild cognitive impairment (MCI) and probable dementia, but whether affective states are associated with cognitive impairment is still unknown. The present study examined relationships between baseline affective states and cognitive impairment (MCI, probable dementia) in nondepressed women.
Method
Baseline PA and NA were assessed in postmenopausal women (N = 2,137; mean age = 73.8 years) from the Women’s Health Initiative Study of Cognitive Aging (WHISCA) using the Positive and Negative Affect Schedule (PANAS). Women were followed annually for an average of 11.3 years; those with elevated depressive symptoms at baseline were excluded.
Results
Higher NA was associated with a higher risk of MCI and probable dementia, even after adjusting for important covariates including age, education, sociodemographic, lifestyle, and cardiovascular risk factors, global cognition, and hormone therapy assignment at baseline. PA was not significantly associated with either outcome.
Conclusions
We present the first evidence to date that greater NA, even in the absence of elevated depressive symptoms, is associated with higher risk of MCI and dementia. This suggests that NA may be an important, measureable and potentially modifiable risk factor for age-related cognitive decline.
Keywords: State affect, Probable dementia, Mild cognitive impairment, PANAS
Affect reflects the subjective experience of positive or negative emotional states in response to environmental stimuli. It is commonly conceptualized as consisting of two distinct dimensions: positive and negative (1). These dimensions may reflect states or trait affective patterns. Negative affect (NA) includes feelings of subjective distress and aversive mood states, such as guilt, fear, hostility, contempt, disgust, and nervousness (2). Positive affect (PA) includes feelings such as enthusiasm, determination, confidence, excitement, inspiration, and alertness.
State affect varies throughout the day in healthy individuals (3), although people with mood disorders are more vulnerable to specific affective states. For example, both depression and anxiety are characterized by high NA, reflecting the experience of subjective distress. However, depression, but not anxiety, is also associated with low PA (4). Furthermore, the state dimensions of PA and NA are closely related to trait positive and negative emotionality, which roughly correspond to the personality factors of extraversion and neuroticism, respectively (1).
Previous research in large studies of aging has found that baseline depressive symptoms (5) and anxiety (6) are associated with increased risk of incident cognitive impairment, including mild cognitive impairment (MCI) and dementia. However, there has been considerably less investigation into the relationship between state affect and cognitive impairment.
In healthy younger individuals, higher PA is associated with better performance across a range of cognitive tasks, including recall memory, problem solving, and decision making (7). This association may persist during brain aging in older populations. For example, higher PA as assessed by the Positive and Negative Affect Schedule (2) was associated with stronger episodic memory performance on a list-learning task in older adults from the Maastricht Aging Study (8). In contrast, higher NA was associated with increased subjective memory complaints in healthy older adults (9). Although several studies have found that an absence of PA (eg, anhedonia) was associated with increased risk of cognitive decline (10,11), the purpose of these latter studies was to assess the relationship between depressed mood and cognitive outcomes. PA was measured as a subscale on a depression questionnaire, and the studies did not control for other clinical symptoms of depression that contributed to cognition independently of affective states.
Personality traits such as neuroticism and extraversion are associated with a person’s propensity to experience NA or PA, respectively (1). These personality factors have also been studied in relation to dementia risk. Allen and colleagues found that older adults with high extraversion scores, a proxy for PA, performed better on a test of long-term episodic memory than those with lower extraversion (12). However, a recent study using data from participants in the Health and Retirement Study failed to find an association between extraversion and risk for cognitive impairment (13). In the Baltimore Longitudinal Study of Aging, older adults with lower neuroticism (NA) scores were more likely to maintain normal cognition even in the presence of AD neuropathology confirmed at autopsy (14). In the Religious Orders Study, individuals with high neuroticism were twice as likely to develop Alzheimer’s disease as those with low neuroticism (15). In a meta-analysis from five prospective observational studies, a standard deviation unit (SD) increase in neuroticism was associated with 30% higher risk of AD (16). Neuroticism and extraversion may also interact to impact dementia risk, with those exhibiting both low neuroticism and high extraversion having lower risk of cognitive impairment (17), though another study failed to replicate this effect (13).
While existing evidence suggests that clinical states such as depression are risk factors for cognitive impairment, little is known about whether global affective tone or states are. As low PA and high NA are highly correlated with depressive symptoms (4), it is important to assess these subjective mood states in healthy individuals, independently of a clinical condition such as syndromal or subsyndromal depression. Thus, we restricted our analyses to state affect in the absence of elevated symptoms of depressed mood. The objective of this study was to explore the associations between positive and negative state affect and subsequently adjudicated cognitive impairment in nondepressed postmenopausal women participating in the Women’s Health Initiative Study of Cognitive Aging (WHISCA). Focusing on postmenopausal women has particular public health relevance, as this group is disproportionately affected by Alzheimer’s disease and other dementias (18).
Method
Participants
The Women’s Health Initiative (WHI) included randomized controlled trials evaluating the effects of postmenopausal estrogen supplementation versus placebo on multiple women’s health outcomes. The Women’s Health Initiative Memory Study (WHIMS) added dementia as an outcome in a subset (N = 7,479) of WHI Hormone Trial participants between 65 and 79 years of age. WHISCA was an ancillary study to WHI and was designed to explore the effects of hormone therapy on cognition and affect in women without dementia. Enrollment procedures and baseline sample characteristics for WHISCA have been described elsewhere (19). WHISCA enrollment began an average of three years after WHIMS enrollment. Of the 2,302 postmenopausal women recruited into WHISCA, 2,264 were included in the present analysis. Exclusion criteria for the present analyses included a diagnosis of MCI at baseline (N = 27) or missing PANAS data (N = 11). The mean follow-up interval was 11.3 years (SD = 4.8 years; range: 0.97 years–20.1 years). This includes both the RCT and the post-RCT follow-up periods, when the trial medications were no longer administered. Medications in the conjugated equine estrogen (CEE) combined with medroxyprogesterone acetate (MPA) trial were terminated in 2002 due to an unsatisfactory risk-to-benefit ratio, while medications in the CEE-alone trial were terminated in 2004 due to an increased risk of stroke and lack of evidence for prevention of cardiovascular disease. Cognitive assessment and adjudication of cognitive outcomes in this cohort is ongoing; the present analyses include available data through May of 2016.
Procedures
PA and NA
State affect was assessed using the PANAS (2) collected at the WHISCA baseline assessment. The PANAS is a 20-item list of adjectives, with 10 words related to pleasant affective states (eg, enthusiastic, inspired, active) and 10 related to negative affective states (eg, distressed, jittery, irritable). Total scores for PA and NA range from 10 to 50; higher scores indicate greater affect. Participants were instructed to rate each descriptor on a five-point scale to indicate to what extent they had experienced that affective state in the past 2 weeks. Thus, PANAS scores can be considered a measure of state affect at the time of baseline assessment.
MCI and probable dementia
A detailed description of the protocol for classification of MCI and probable dementia has been published (20). During annual WHIMS follow-up prior to 2007, all WHISCA participants were administered the Modified Mini-Mental State Examination (3MS) annually (21). Women who scored below education-specific 3MS cutpoints received a thorough neuropsychiatric evaluation. This included a modified Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) battery of neuropsychological tests (22); standardized interviews to assess behavioral and cognitive impairment; interviews with a designated informant regarding the participant’s degree of impairment; a complete clinical evaluation by a local physician-specialist with experience in dementia diagnosis; and optional lab tests, including computerized tomography and standard blood tests. Women were initially classified by the local dementia specialist into one of three groups: no cognitive impairment, MCI, or probable dementia, using standardized criteria (Diagnostic and Statistical Manual of Mental Disorders, Third Edition [DSM-III]; see ref. (20) for detailed protocol). All clinical and test data were submitted to the WHIMS Clinical Coordinating Center for review and final classification by a central adjudication committee consisting of three board-certified specialists (two neurologists and one geropsychiatrist). After the extension of WHIMS, WHIMS-Epidemiology of Cognitive Health Outcomes (WHIMS-ECHO) began in 2007, in which participants underwent annual telephone-based assessment with a validated telephone cognitive test battery (23) that included the modified Telephone Interview for Cognitive Status (TICSm) (24). All WHIMS-ECHO participants included in these analyses were part of the original WHIMS cohort who provided consent for additional follow-up. When women screened positive for cognitive impairment (TICSm < 31), a reliable and pre-identified informant was interviewed via telephone using the validated Dementia Questionnaire (25). All data including prior WHIMS data were reviewed by the central adjudication panel, with individuals classified as no cognitive impairment, MCI, or probable dementia.
Covariates
Baseline data for this analysis were obtained from the last WHI visit prior to or on the date of WHISCA enrollment. If the covariate data were not available for that visit, data from the WHI baseline visit were used. These included demographics (age, race, education, marital status), lifestyle factors (physical activity, smoking status, drinking status), and medical history (cardiovascular disease, stroke, transient ischemic attack, hypertension, high cholesterol, physical activity, current antidepressant use). Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Physical activity was operationalized as number of exercise sessions per week, where each session was defined as 20 or more minutes of moderate- or vigorous-intensity physical exercise (metabolic equivalent score ≥ 4.0). Depressed mood in WHISCA was assessed using the 15-item short form of the Geriatric Depression Scale (GDS) (26).
Global cognitive function was measured with the Modified Mini-Mental State Examination (3MS) (21). Scores range from 0 to 100, with higher scores reflecting better functioning. The 3MS has demonstrated high sensitivity (87%) and specificity (85%) for detecting cognitive impairment (27). It assesses temporal and spatial orientation, immediate and delayed recall, naming, executive functioning, verbal fluency, abstract reasoning, and praxis. A trained and certified technician administered the 3MS.
Statistical analysis
Frequencies and percentages were calculated for categorical variables, while means and standard errors were calculated for continuous variables in the overall cohort. Associations between the descriptive variables and PANAS positive and negative scales were assessed by fitting simple linear regression models, and parameter estimates and standard errors were calculated. Cox proportional hazards regression was used for unadjusted (single predictor) and adjusted (multiple predictors) analyses. Time to event was based on years from the last 3MS score prior to or on the date of the participant’s first WHISCA visit. Four models were fitted: an unadjusted model (Model 1); adjustment for demographic factors (age, race, education), and randomization arm (Model 2); adjustment for all Model 2 factors plus lifestyle factors (marital status, smoking status, alcohol consumption, exercise) (Model 3); and a fully adjusted model with all Model 3 factors plus health variables (BMI, systolic and diastolic blood pressure, current use of antidepressant medications, high blood pressure, history of cardiovascular disease/stroke/transient ischemic attack, diabetes, high cholesterol, 3MS score) (Model 4). Separate Cox models were fitted for time until first incidence of MCI, probable dementia, and any cognitive impairment (composite outcome of MCI and probable dementia), with separate models for PA and NA. Additionally, a model was fit to determine whether the interaction between PA and NA was associated with incident cognitive impairment. For consistency with prior analysis of WHIMS outcomes, we also examined the combined outcome of any cognitive impairment (MCI + probable dementia). Estimates are presented as unadjusted and adjusted hazard ratios (HRs) with confidence intervals (CI), representing increases in relative risk of MCI and probable dementia per each 10-unit increment on the PANAS scale. For those with study-based cognitive impairment, follow-up time was censored at the date of the cognitive testing, which triggered its adjudication. For those without study-based MCI or probable dementia, follow-up was censored at the participant’s last cognitive assessment. Statistical significance was assessed at the two-sided 0.05 α level; no adjustments for multiple testing were completed. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Participants eligible for WHISCA were 66–84 years of age at baseline. Of the 2,302 postmenopausal women recruited into WHISCA, 2,264 were included in the present analyses. Of these women, 127 (5.6%) reported baseline scores > 5 on the GDS indicating elevated depressive symptoms and were excluded from all subsequent analyses to minimize the influence of syndromal depression (Figure 1). Overall baseline sample characteristics and their association with the PANAS subscales are reported in Table 1. Over a follow-up period of 11.3 years, 185 developed MCI, 177 developed probable dementia, and 1,830 remained cognitively unimpaired. Baseline PANAS scores for these groups are presented in Table 2.
Figure 1.
Flowchart of participants who met inclusion/exclusion criteria. GDS = Geriatric Depression Scale; MCI = Mild Cognitive Impairment; PANAS = Positive and Negative Affect Schedule; WHISCA = Women’s Health Initiative Study of Cognitive Aging.
Table 1.
Baseline Characteristics for WHISCA Cohort and Associations With Positive and Negative Affect
| PA | NA | ||||
|---|---|---|---|---|---|
| Overall Cohorta | Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Age (years) | 73.9 (0.08) | −0.14 (0.04) | <.001 | 0.03 (0.03) | .31 |
| Race, N (%) | .79 | .72 | |||
| Black | 115 (5.4%) | 0.55 (0.59) | −0.46 (0.52) | ||
| White | 1,936 (91.1%) | Reference | Reference | ||
| Hispanic | 24 (1.1%) | −0.11 (1.27) | −0.38 (1.12) | ||
| Other | 50 (2.4%) | 0.39 (0.88) | −0.55 (0.78) | ||
| Education, N (%) | .007 | .12 | |||
| < 12 y | 102 (4.8%) | Reference | Reference | ||
| > 12 y | 2,019 (95.2%) | 1.69 (0.63) | −0.86 (0.55) | ||
| Marital Status, N (%) | .14 | <.001 | |||
| Never married | 66 (3.1%) | Reference | Reference | ||
| Divorced/Separated/Widowed | 898 (42.1%) | .27 (0.79) | −1.89 (0.69) | ||
| Married | 1,169 (54.8%) | 0.78 (0.78) | −1.12 (0.69) | ||
| Smoking Status, N (%) | .33 | .05 | |||
| Never smoked | 1,158 (54.8%) | Reference | Reference | ||
| Past smoker | 831 (39.3%) | 0.06 (0.28) | 0.41 (0.25) | ||
| Current smoker | 124 (5.9%) | −0.82 (0.58) | −0.71 (0.51) | ||
| Alcohol Status, N (%) | .01 | .48 | |||
| Nondrinker | 261 (12.3%) | Reference | Reference | ||
| Past drinker | 680 (31.9%) | −0.47 (0.45) | 0.40 (0.40) | ||
| Current drinker | 1,188 (55.8%) | 0.40 (0.42) | 0.13 (0.37) | ||
| Frequency of Exercise, N (%) | .006 | .05 | |||
| No activity | 358 (16.8%) | Reference | Reference | ||
| Some activity of limited duration | 957 (44.8%) | 0.65 (0.38) | −0.27 (0.34) | ||
| 2–3 episodes per week | 367 (17.2%) | 1.01 (.46) | −0.83 (0.40) | ||
| >4 episodes per week | 454 (21.2%) | 1.47 (0.44) | −0.86 (0.38) | ||
| BMI (kg/m2) | 28.6 (0.1) | −0.01 (0.02) | .82 | 0.01 (0.02) | .66 |
| Systolic BP (mmHg) | 131.2 (0.4) | 0 (0.01) | .74 | 0.01 (0.01) | .12 |
| Diastolic BP (mmHg) | 73.3 (0.2) | 0 (0.01) | .95 | 0 (0.01) | .78 |
| Current Antidepressant Use, N (%) | 140 (6.6%) | −0.38 (0.54) | .49 | 1.34 (0.48) | .005 |
| Hypertension, N (%) | 1,173 (54.9%) | −0.48 (0.27) | .07 | 0.05 (0.24) | .84 |
| CVD, Stroke, or TIA, N (%) | 382 (17.9%) | −0.56 (0.35) | .11 | 0.51 (0.31) | .10 |
| Diabetes, N (%) | 190 (8.9%) | −0.57 (0.47) | .22 | 0.46 (0.41) | .27 |
| High Cholesterol, N (%) | 373 (17.7%) | −0.61 (0.35) | .08 | 0.63 (0.31) | .04 |
| 3MS Score | 96.9 (0.1) | 0.01 (0.05) | .81 | −0.17 (0.04) | <.001 |
| GDS Total Score | 1.12 (0.03) | −1.75 (0.09) | <.001 | 1.67 (0.08) | <.001 |
| Treatment Arm, N (%) | .51 | .66 | |||
| Placebo | 1,087 (50.9%) | Reference | Reference | ||
| Hormone Therapy | 1,050 (49.1%) | −0.18 (0.27) | 0.10 (0.24) | ||
Note: Associations between the descriptive variables and PANAS positive and negative scales were assessed using simple linear regression, with parameter estimates and standard errors provided here. Reference groups for categorical variables are denoted. BMI = Body mass index; CVD = Cardiovascular disease; GDS = Geriatric Depression Scale; 3MS = Modified Mini-Mental State Examination; NA = Negative affect; PA = Positive affect; TIA = Transient ischemic attack; WHISCA = Women’s Health Initiative Study of Cognitive Aging.
a N = 2,137.
Table 2.
Baseline Positive and Negative Affect and Incident Cognitive Impairment
| Not Impaireda | Mild Cognitive Impairmentb | Probable Dementiac | ||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Positive Affect | 36.5 (6.2) | 12–50 | 36.5 (6.6) | 15–49 | 36.6 (6.0) | 21–49 |
| Negative Affect | 15.4 (5.4) | 10–42 | 16.6 (5.8) | 10–43 | 16.0 (5.7) | 10–41 |
Note: aN = 1,830; bN = 185; cN = 177.
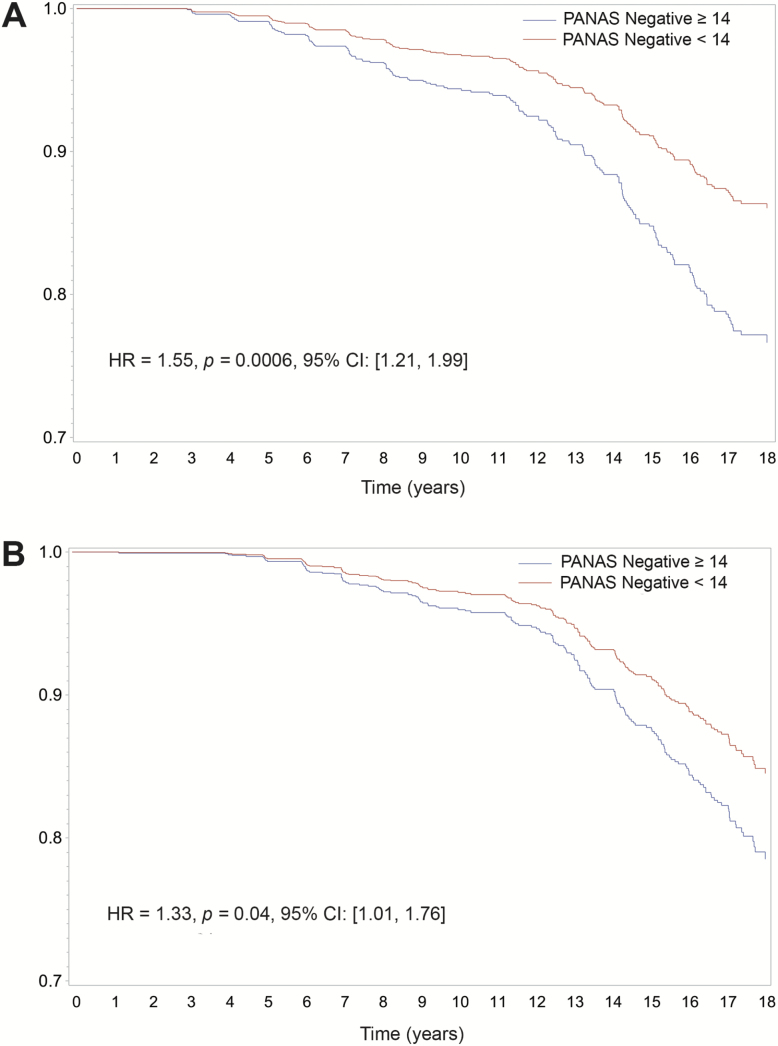
Associations of PA and NA with incident MCI or probable dementia are presented in Table 3. As the PANAS is a continuous variable, hazard ratios represent relative risk associated with each 10-point increase in PA or NA. In the unadjusted analysis (Model 1), NA was associated with higher risk of MCI (HR = 1.54, p = .0002, 95% CI [1.23, 1.94]) and probable dementia (HR = 1.34, p = .02, 95% CI [1.04, 1.72]). In the fully-adjusted model containing all relevant covariates (Model 4), NA was associated with higher risk of MCI (HR = 1.55, p = .0006, 95% CI [1.21, 1.99]) and with probable dementia (HR = 1.33, p = .04, 95% CI [1.01, 1.76]). Survival curves for high and low NA based on a median split are presented in Figure 2.
Table 3.
Association of Negative Affect With Incidence of Probable Dementia and Cognitive Impairment
| Positive Affect | Negative Affect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCI | Probable Dementia | MCI | Probable Dementia | |||||||||
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
| Model 1 | 0.90 | (.71, 1.14) | .4 | 0.94 | (.74, 1.19) | .6 | 1.54 | (1.23, 1.94) | .0002 | 1.34 | (1.04, 1.72) | .02 |
| Model 2 | 0.99 | (.78, 1.26) | .95 | 1.05 | (.82, 1.35) | .68 | 1.64 | (1.30, 2.08) | <.0001 | 1.44 | (1.11, 1.86) | .005 |
| Model 3 | 0.99 | (.78, 1.26) | .92 | 1.06 | (.83, 1.37) | .63 | 1.70 | (1.35, 2.15) | <.0001 | 1.38 | (1.06, 1.80) | .02 |
| Model 4 | 0.99 | (.78, 1.25) | .93 | 1.03 | (.81, 1.32) | .8 | 1.55 | (1.21, 1.99) | .0006 | 1.33 | (1.01, 1.76) | .04 |
Note: Model 1 is the unadjusted model showing the hazard ratios associated with PA and NA (per 10-unit increment on PANAS). Model 2 adjusted for demographic factors (age, race, education), and randomization arm. Model 3 adjusted for all Model 2 variables as well as lifestyle factors (marital status, smoking status, alcohol consumption, exercise). Model 4 is the fully adjusted model that includes all demographic, lifestyle, and health variables listed in Table 1. MCI = Mild cognitive impairment.
Figure 2.
Cumulative hazard estimates for the incidence of (A) mild cognitive impairment and (B) probable dementia based on a median split of PANAS negative affect (NA). HR = Hazard ratio; CI = Confidence interval. Survival curves are unadjusted for demographic and lifestyle factors, while reported hazard ratios are from the fully adjusted models.
There were no significant associations between PA and risk for probable dementia or MCI or probable dementia in the unadjusted or fully-adjusted models (Table 3). The interaction between PA and NA was not significantly associated with risk for MCI or probable dementia (p’s > .05).
Results were similar for the association of state affect with cognitive impairment (combined outcome of MCI/probable dementia), with NA associated with higher risk of cognitive impairment in the fully adjusted model (HR = 1.52, p < .0001, 95% CI [1.25, 1.85]) but no significant association between PA and risk for cognitive impairment.
Discussion
Over an average follow-up interval of 11.3 years, we found that greater state NA, even in the absence of elevated depressive symptoms, is associated with a higher risk of incident cognitive impairment. Each 10-point increase in NA was associated with 55% higher risk of developing MCI and 33% higher risk of developing probable dementia after adjusting for potentially confounding variables, including demographic characteristics, lifestyle variables, cardiovascular risk factors, use of hormone therapy, and baseline cognitive function. PA was not significantly associated with risk of MCI or probable dementia.
Studies exploring the role of state affect on cognitive outcomes in aging are sparse. Our findings are consistent with results from the Cognition in the Study of Tamoxifen and Raloxifene (Co-STAR) sample of nondemented, postmenopausal women, in which higher NA was associated with poorer global cognition, verbal memory, and spatial ability, while higher PA was associated with better verbal fluency (23). This pattern of poorer cognitive performance with higher NA may be an early manifestation of cognitive changes associated with MCI or dementia. The present findings are congruent with this study, although the Co-STAR study reported performance across cognitive domains, rather than diagnosis of MCI or probable dementia, as outcome variables. One limitation of the Co-STAR sample was that it included 4% of women reporting clinically significant depressive symptoms. By excluding participants reporting elevated depressive symptoms, we were able to focus more exclusively on positive and NA in the absence of clinically significant depression.
The present study assessed state rather than trait affect, with state NA reflecting a person’s experience of negative emotions over the prior 2 weeks. NA has been associated with the personality factor of neuroticism, while PA has been associated with extraversion (1). Our finding that higher state NA is associated with increased risk of cognitive impairment is consistent with reports that older adults with high neuroticism have poorer cognitive performance (28), higher rates of cognitive decline (29), higher risk of Alzheimer’s disease (30), and lower likelihood of remaining free of cognitive impairment in the presence of Alzheimer’s disease neuropathology (14). Since an assessment of state affect can also be influenced by personality traits, we cannot be certain which type, state NA or trait NA, accounts for the association we found.
The precise causal mechanisms underlying the association between NA and risk for cognitive impairment are unknown and are likely multifactorial. Greater NA is associated with higher circulating cortisol levels (31), suggesting that negative state affect may influence hypothalamic-pituitary-adrenal (HPA) system activity. Extensive animal (32,33) and human (34,35) literature links high glucocorticoid levels to hippocampal atrophy and age-related cognitive impairment. Postmenopausal women may be particularly vulnerable to the deleterious effects of cortisol on cognition (36), perhaps because cortisol blunts cognition-enhancing effects of estradiol (37).
Higher NA may also be related to lifestyle and health factors that contribute to higher risk of cognitive impairment. For example, higher NA is associated with stress, poor psychological health, self-reported physical health complaints, and poorer immune system activity (38,39). Experiencing negative emotions is also associated with poorer coping strategies and difficulties with stress management (40). Collectively, these factors may negatively impact a person’s resilience to age-related cognitive decline.
We did not observe associations between PA and risk for probable dementia or cognitive impairment in the WHISCA sample. Our findings are consistent with a meta-analysis that reported significant associations between neuroticism and risk for MCI or dementia but not extraversion (41). Other studies, however, have shown that PA (23) and its associated personality construct of extraversion (12) are associated with better performance on memory tasks in aging populations. Indeed, socioemotional selectivity theory (42) predicts that individuals experience more positive emotions as they age and that this age-related positivity effect may be associated with better attention and memory. This provides a potential explanation for reports that individuals with higher PA perform better than those with lower PA on certain cognitive measures (8,23).
Strengths of the present study include extensive assessments in a large sample of high-functioning, community-dwelling postmenopausal women whose cognitive health was characterized through detailed prospective follow-up. However, this study is not without limitations. The WHISCA sample was not population-based, and all participants were women, thus limiting generalizability. Additionally, as administered in this study, the PANAS assessed state affect over the previous 2 weeks. Thus, the results of the current study cannot address how broader, trait affective patterns may impact risk for cognitive decline. Furthermore, reported hazard ratios reflect relative risk associated with a 10-point increment of PA or NA. While PANAS scores range from 10 to 50 and participants reported scores spanning this range, mean NA scores for each diagnostic group were relatively low and within 1 or 2 points of one another at baseline. This suggests that while results are statistically significant, they may only be clinically meaningful for participants reporting high state NA. Finally, extant research—including the current study—has focused almost exclusively on older adults; less is known about the effects of affective states in middle age on risk for incident cognitive impairment later in life. Although one study including participants aged 50 years or older reported that age did not modify the association between higher neuroticism and risk for cognitive impairment (13), more longitudinal studies investigating associations between midlife state affect and risk for cognitive impairment are needed.
In conclusion, we found that baseline NA was modestly but significantly associated with risk of developing cognitive impairment in postmenopausal women. To the best of our knowledge, this is the first study to report an association between state NA and risk for MCI and probable dementia among nondepressed women, independent of important lifestyle and cardiovascular risk factors. These findings suggest that NA may be an important and potentially modifiable risk factor for cognitive impairment.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), U.S. Department of Health and Human Services through Contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221. Wyeth-Ayerst Research Laboratories, Philadelphia, Pennsylvania supplied the active study drug and placebo. Wyeth Pharmaceuticals funded the WHIMS, in part, as an ancillary study to the WHI. WHISCA was supported by the Department of Health and Human Services and the National Institute on Aging (NIA) N01-AG-1-2106. The study was supported in part by the Intramural Research Program, NIA, NIH.
Conflict of Interest
M.A.E. is on the editorial board of the Journals of Gerontology Series A: Medical Sciences. The other authors have no conflicts of interest to declare.
References
- 1. Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- 2. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 3. Merz EL, Roesch SC. Modeling trait and state variation using multilevel factor analysis with PANAS daily diary data. J Res Pers. 2011;45:2–9. doi:10.1016/j.jrp.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joiner TE Jr, Lonigan CJ. Tripartite model of depression and anxiety in youth psychiatric inpatients: relations with diagnostic status and future symptoms. J Clin Child Psychol. 2000;29:372–382. doi:10.1207/S15374424JCCP2903_8 [DOI] [PubMed] [Google Scholar]
- 5. Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 2011;59:57–66. doi:10.1111/j.1532-5415.2010.03233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Potvin O, Forget H, Grenier S, Préville M, Hudon C. Anxiety, depression, and 1-year incident cognitive impairment in community-dwelling older adults. J Am Geriatr Soc. 2011;59:1421–1428. doi:10.1111/j.1532-5415.2011.03521.x [DOI] [PubMed] [Google Scholar]
- 7. Carpenter SM, Peters E, Västfjäll D, Isen AM. Positive feelings facilitate working memory and complex decision making among older adults. Cogn Emot. 2013;27:184–192. doi:10.1080/02699931.2012.698251 [DOI] [PubMed] [Google Scholar]
- 8. Hill RD, van Boxtel MP, Ponds R, Houx PJ, Jolles J. Positive affect and its relationship to free recall memory performance in a sample of older Dutch adults from the Maastricht Aging Study. Int J Geriatr Psychiatry. 2005;20:429–435. doi:10.1002/gps.1300 [DOI] [PubMed] [Google Scholar]
- 9. Dux MC, Woodard JL, Calamari JE et al. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J Int Neuropsychol Soc. 2008;14:327–336. doi:10.1017/S1355617708080363 [DOI] [PubMed] [Google Scholar]
- 10. Gatz JL, Tyas SL, St John P, Montgomery P. Do depressive symptoms predict Alzheimer’s disease and dementia?J Gerontol A Biol Sci Med Sci. 2005;60:744–747. [DOI] [PubMed] [Google Scholar]
- 11. Turner AD, Capuano AW, Wilson RS, Barnes LL. Depressive symptoms and cognitive decline in older african americans: two scales and their factors. Am J Geriatr Psychiatry. 2015;23:568–578. doi:10.1016/j.jagp.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allen PA, Kaut K, Baena E, Lien M-C, Ruthruff E. Individual differences in positive affect moderate age-related declines in episodic long-term memory. J Cog Psychol. 2011;23:768–779. doi:10.1080/20445911.2011.570254 [Google Scholar]
- 13. Terracciano A, Stephan Y, Luchetti M, Albanese E, Sutin AR. Personality traits and risk of cognitive impairment and dementia. J Psychiatr Res. 2017;89:22–27. doi:10.1016/j.jpsychires.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terracciano A, Iacono D, O’Brien RJ et al. Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiol Aging. 2013;34:1045–1050. doi:10.1016/j.neurobiolaging.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. [DOI] [PubMed] [Google Scholar]
- 16. Terracciano A, Sutin AR, An Y et al. Personality and risk of Alzheimer’s disease: new data and meta-analysis. Alzheimers Dement. 2014;10:179–186. doi:10.1016/j.jalz.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang HX, Karp A, Herlitz A et al. Personality and lifestyle in relation to dementia incidence. Neurology. 2009;72:253–259. doi:10.1212/01.wnl.0000339485.39246.87 [DOI] [PubMed] [Google Scholar]
- 18. Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15:451–452. doi:10.1016/S1474-4422(16)00067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women’s Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1:440–450. doi:10.1191/1740774504cn040oa [DOI] [PubMed] [Google Scholar]
- 20. Shumaker SA, Reboussin BA, Espeland MA et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. [DOI] [PubMed] [Google Scholar]
- 21. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 22. Morris JC, Heyman A, Mohs RC et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 23. Danhauer SC, Legault C, Bandos H et al. Positive and negative affect, depression, and cognitive processes in the Cognition in the Study of Tamoxifen and Raloxifene (Co-STAR) Trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2013;20:532–552. doi:10.1080/13825585.2012.747671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welsh KA, Breitner J, Magruder-Habib KM. Detection of dementia in the elderly using the telephone interview for cognitive status. Neuropsychiatry, Neuropsychol, & Beh Neurology. 1993;6:103–110. [Google Scholar]
- 25. Ellis RJ, Jan K, Kawas C et al. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol. 1998;55:360–365. [DOI] [PubMed] [Google Scholar]
- 26. Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: a comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4:173–178. [DOI] [PubMed] [Google Scholar]
- 27. Tombaugh T, McDowell I, Kristjansson B, Hubley A. Mini-mental state examination (MMSE) and the modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assessment. 1996;8:48. [Google Scholar]
- 28. Luchetti M, Terracciano A, Stephan Y, Sutin AR. Personality and cognitive decline in older adults: data from a longitudinal sample and meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2016;71:591–601. doi:10.1093/geronb/gbu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman B, Duberstein P, Tindle HA et al. ; Gingko Evaluation of Memory Study Investigators Personality predicts cognitive function over 7 years in older persons. Am J Geriatr Psychiatry. 2012;20:612–621. doi:10.1097/JGP.0b013e31822cc9cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duberstein PR, Chapman BP, Tindle HA et al. Personality and risk for Alzheimer’s disease in adults 72 years of age and older: a 6-year follow-up. Psychol Aging. 2011;26:351–362. doi:10.1037/a0021377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polk DE, Cohen S, Doyle WJ, Skoner DP, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi:10.1016/j.psyneuen.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 32. Brureau A, Zussy C, Delair B et al. Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiol Aging. 2013;34:1426–1439. doi:10.1016/j.neurobiolaging.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 33. Montaron MF, Drapeau E, Dupret D et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–654. doi:10.1016/j.neurobiolaging.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 34. Popp J, Wolfsgruber S, Heuser I et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36:601–607. doi:10.1016/j.neurobiolaging.2014.10.031 [DOI] [PubMed] [Google Scholar]
- 35. Greendale GA, Kritz-Silverstein D, Seeman T, Barrett-Connor E. Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo Study. J Am Geriatr Soc. 2000;48:1655–1658. [DOI] [PubMed] [Google Scholar]
- 36. Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. [DOI] [PubMed] [Google Scholar]
- 37. Baker LD, Asthana S, Cholerton BA et al. Cognitive response to estradiol in postmenopausal women is modified by high cortisol. Neurobiol Aging. 2012;33:829.e9–829.20. doi:10.1016/j.neurobiolaging.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen S, Doyle WJ, Skoner DP, Fireman P, Gwaltney JM Jr, Newsom JT. State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. J Pers Soc Psychol. 1995;68:159–169. [DOI] [PubMed] [Google Scholar]
- 39. Watson D. Intraindividual and interindividual analyses of positive and negative affect: their relation to health complaints, perceived stress, and daily activities. J Pers Soc Psychol. 1988;54:1020–1030. [DOI] [PubMed] [Google Scholar]
- 40. Billings DW, Folkman S, Acree M, Moskowitz JT. Coping and physical health during caregiving: the roles of positive and negative affect. J Pers Soc Psychol. 2000;79:131–142. [DOI] [PubMed] [Google Scholar]
- 41. Low LF, Harrison F, Lackersteen SM. Does personality affect risk for dementia? A systematic review and meta-analysis. Am J Geriatr Psychiatry. 2013;21:713–728. doi:10.1016/j.jagp.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 42. Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am Psychol. 1999;54:165–181. [DOI] [PubMed] [Google Scholar]