Abstract
The escalating increase in retirees living beyond their eighth decade brings increased prevalence of aging-related impairments, including locomotor impairment (Parkinsonism) that may affect ~50% of those reaching age 80, but has no confirmed neurobiological mechanism. Lifestyle strategies that attenuate motor decline, and its allied mechanisms, must be identified. Aging studies report little to moderate loss of striatal dopamine (DA) or tyrosine hydroxylase (TH) in nigrostriatal terminals, in contrast to ~70%–80% loss associated with bradykinesia onset in Parkinson’s disease. These studies evaluated the effect of ~6 months 30% calorie restriction (CR) on nigrostriatal DA regulation and aging-related locomotor decline initiated at 12 months of age in Brown-Norway Fischer F1 hybrid rats. The aging-related decline in locomotor activity was prevented by CR. However, striatal DA or TH expression was decreased in the CR group, but increased in substantia nigra versus the ad libitum group or 12-month-old cohort. In a 4- to 6-month-old cohort, pharmacological TH inhibition reduced striatal DA ~30%, comparable with decreases reported in aged rats and the CR group, without affecting locomotor activity. The dissociation of moderate striatal DA reduction from locomotor activity seen in both studies suggests that aging-related decreases in striatal DA are dissociated from locomotor decline.
Keywords: Substantia nigra, Parkinsonism, Bradykinesia, Parkinson’s disease, Striatum
Aging-related Parkinsonism shares the cardinal symptoms of Parkinson’s disease (PD) and has been well documented in the elderly population, affecting up to 50% of those reaching age 80 (1–6). Although there is evidence of pathology in the central nervous system (CNS) associated with aging-related Parkinsonism (7,8), a specific molecular deficit associated with the motor decline has not been identified despite substantial investment in such efforts. Given the strong similarity of clinical symptoms of PD with aging-related Parkinsonism, including non-motor effects (6), studies of motor decline in established models of aging have centered upon possible striatal dopamine (DA) deficits. However, no aging study to date has reported that striatal DA or tyrosine hydroxylase (TH) loss is on par with the magnitude of loss observed in PD (9,10). During the human life span, loss of TH protein is ~15%, comparing old age versus mature adult putamen or caudate (11,12). Conversely, TH protein loss associated with onset of one of the cardinal signs of PD, bradykinesia, in a PD model is ~80% (10). Even 60% striatal TH loss is not associated with bradykinesia onset (10), and the most loss of striatal DA reported in any aging study is also ~60% (11,13,14). In fact, many aging studies have reported far less DA or TH loss, from near 0% to 30% loss (12,15–19).
If we are to arrest, prevent, or at least attenuate the severity of motor impairment due to aging, a noninvasive approach is likely necessary, given its prevalence in the elderly adults. Furthermore, if we are to understand the neurobiological basis for how such noninvasive measures taken may be effective at reducing locomotor impairment, the continuing focus only on striatal DA regulation must be widened to include the possibility that changes in DA regulation in the substantia nigra (SN) are not only affected by aging, but could also be partially restored with successful noninvasive strategies that attenuate, prevent, or reverse locomotor decline. Nigral DA release was first characterized 40 years ago (20), and there is evidence that perturbations in DA neurotransmission that occur only in the SN can affect locomotor activity (21–23). Furthermore, transient restoration of nigral DA to levels above that in aged rats may increase locomotor activity in conjunction with increased nigral, but not striatal, TH protein expression (24).
The biological basis for aging-related decline in locomotor activity in humans can be addressed in established models of aging in rodent and nonhuman primates (25). Lifelong caloric restriction (CR) attenuates locomotor decline associated with aging (26,27). Intervention with 30% CR at 12 months old in male Brown-Norway Fischer 344 F1 hybrid (BNF) rats also attenuates locomotor activity decline (28), with parameters pertaining to locomotor activity (movement number and horizontal activity), independent of movement speed, most affected by CR intervention. Furthermore, the response to CR intervention was reported to be greatest in rats that were least active at the 12-month-old baseline (28). The CR effect was not associated with an increase in striatal DA or expression of TH or dopamine transporter compared with the ad libitum (AL) group. In the current study, we expand the scope of determining the neurobiological impact of CR. A 12-month-old control group, which reflects dopaminergic neurochemistry during the life span at the time of CR initiation, was included. We also evaluated nigral DA regulation, to determine if CR impact could affect TH regulation in the SN. In a separate cohort of young BNF rats, we also evaluated if experimental reduction of striatal DA alone, by direct striatal infusion of the TH inhibitor, α-methyl-p-tyrosine (AMPT), affected locomotor activity. Together, these studies were designed to evaluate the role of nigrostriatal DA regulation in aging-related motor decline and mechanisms associated with a nutritional intervention hypothesized to attenuate aging-related motor decline.
Methods
Test Subjects and Weight Determinations
BNF rats were obtained from NIA-contract facilities and used in the CR and striatal TH inhibition studies under approved protocols at LSU Health Sciences Center-Shreveport, University of North Texas Health Science Center, and Pennington Biomedical Research Center (PBRC) Institutional Animal Care and Use Committees. The BNF hybrids exhibit aging-related changes in nigrostriatal DA tissue content and locomotor decline comparable with nonhuman primates and outbred Sprague-Dawley rat (15,18,24,29).
For the striatal TH inhibition study, male BNF rats aged 4–6 months were used. Surgical procedures used for assessment of DA reduction specificity from TH inhibition and related locomotor effects are further described in the Striatal Tyrosine Hydroxylase Inhibition Study section.
CR Study
Calorie restriction regimen
A total of 42 male BNF rats arrived at PBRC having been maintained on an ad libitum diet (NIH-31) since weaning. To provide careful control over food intake, rats were singly housed in plastic cages in an SPF vivarium located at PBRC maintained at 22 ± 2°C, 70% ± 10% humidity. The light cycle was controlled automatically with lights on at 5:00 and off at 17:00. Rats were provided fortified NIH-31 diet obtained from Harlan Teklad (catalog no 7017; Madison, WI) to assure that CR rats were not deprived of essential vitamins and minerals.
At 12 months of age, the rats were assessed for locomotor activity in an open field (see below) and assigned into two groups such that the group mean for rats placed into the AL (n = 15) or CR group (n = 15) did not have a significant difference in locomotor activity. The CR group had 70% of food intake, based on the consumption amount of the AL group, which was adjusted, as necessary, on a weekly basis as the study progressed. The 30% CR was gradually introduced, with restriction at 90%, 80%, and then 70% of AL group consumption, at Weeks 1, 2, and 3 into the study. Daily chow was given to CR rats ~1 hour before lights were turned off in the vivarium. Body weights were determined weekly. All locomotor testing was conducted between 08:30 and 15:00.
Assessment of locomotor activity
At baseline, and at 6, 12, 18, and 24 weeks after initiation of CR or AL, spontaneous locomotor activity was assessed in five separate trials, for 1 hour using automated activity chambers equipped with a matrix of infrared beams (VersaMax Animal Activity Monitoring System, Columbus, OH). Beam breaks were used by the software to produce the CR study locomotor measures. The sum total for movement number, horizontal activity (a unitless measure), total distance (in centimeter), time spent moving (in second), and vertical activity were recorded in the hour-long session. Movement speed was calculated, taken by dividing total distance by the associated time spent moving (centimeter per second).
Striatal Tyrosine Hydroxylase Inhibition Study
Surgical and infusion procedures
Two surgeries were performed (a) to implant guide cannula used in the locomotor component of the study or (b) to determine the extent of DA reduction by striatal infusion of the TH inhibitor AMPT. Rats were anesthetized by either sodium pentobarbital (50 mg/kg) or isoflurane anesthesia. Double guide cannula (PlasticsOne, Roanoke, VA) were implanted for repeated bilateral infusions of sterile saline or AMPT via infusion cannula to evaluate the impact on locomotor function. Stainless steel cannula on the guide cannula were 5.0 mm apart, giving medial–lateral coordinates of ±2.5 ML and placed +1.0 anterior–posterior to Bregma at a depth of −4.5 dorsal–ventral to bilaterally target dorsolateral striatum. The infusion cannula (28 gauge), used in ensemble with guide cannula on locomotor testing days, had +1.0 mm projection beyond the termination of the guide cannula. This “stepped” configuration reduces backflow and maximizes the volume of distribution (30). Two small screws implanted into the skull served as anchors for dental cement applied to secure guide cannula. Rats recovered for at least 5 days prior to locomotor testing.
In terminal surgeries for verification of AMPT reduction on DA tissue content, 26 gauge beveled needles infused sterile saline into one hemisphere and AMPT (prepared in sterile saline) at the same coordinates in the other hemisphere. Needles remained in the targeted tissue for 10 minutes following infusion. Striatal DA tissue content was evaluated at two time points (90 or 180 minutes) following striatal infusions. Other DA regions were collected to confirm specificity for the AMPT effect in striatum.
Validation of AMPT-Mediated DA reduction
AMPT (as methyl ester HCl, Sigma–Aldrich [St Louis, MO, catalog no M3281] or TCI-EP [Tokyo, catalog no M1373]) was dissolved in sterile saline prior to striatal infusion. The rationale for the AMPT quantity of 2.1 nmole (delivered in 3 µL at 1 µL/min) was based on previous work showing efficacy to reduce DA and extracellular DA levels locally in striatum after CNS delivery (31,32). This AMPT-mediated decrease in DA is in range of striatal DA loss in rodent and nonhuman primate aging studies, 0%–40% in striatum (15–19,32,33) and in the BNF strain (15,18,31). Increasing the quantity of AMPT by 10- or 30-fold did not produce further DA reduction greater than previously reported at 90 minutes, or as seen at 180 minutes with 2.1 nmole, as now reported here. A total of seven rats for each AMPT quantity were used.
Locomotor activity assessment
To account for variability in baseline locomotor activity among rats and variance in daily locomotor activity (15), the within-subject design compared locomotor activity within each test subject following the bilateral infusion of sterile saline against locomotor activity following AMPT at equivalent time intervals. The locomotor activity following saline infusion is presumed to control for any impact on the volume of saline alone upon the extracellular DA concentrations and represent baseline locomotor activity for each rat. To account for daily variance in activity, saline or AMPT infusions (3 µL total volume bilaterally to each hemisphere) were repeated five times for each test subject, once daily, thereby requiring a total of 10 locomotor testing sessions, alternating the days of bilateral saline or AMPT infusion. Additional details of the infusion protocol and verification of striatal targeting are presented in Supplementary Material.
Target validation after locomotor testing
We verified the guide cannula successfully targeted the striatum and did not cause damage that could impede the introduction of the saline or AMPT after locomotor assessment. Images of coronal slices containing striatum were evaluated with the following two criteria: (a) evidence of track marks dorsal to striatum and (b) no evidence of hematoma or scar tissue within the striatum in either hemisphere. Having passed these criteria, locomotor activity data obtained from passing test subjects were further evaluated by the described (below) statistical analysis.
Both Studies
Analysis of dopamine tissue content and Western blotting assessments
Rats were rendered unconscious with isoflurane and immediately decapitated to rapidly remove the entire brain to chill at 4°C on a rat brain coronal matrix for dissection of striatum and SN (34). For both striatum and SN, DA D1 receptor, ser31 TH phosphorylation, TH protein, and DA tissue content were determined. DA transporter and TH phosphorylation at ser19 and ser40 were analyzed in SN. Details of sample processing, HPLC methods, and Western blotting procedures have been reported (34) and are also presented in Supplementary Material.
Statistics
CR study
Three statistical approaches for data analysis were employed. First, to evaluate possible CR interaction with aging and overall group differences, a repeated measures two-way analysis of variance (ANOVA) was used followed by Fisher’s Least Significant Difference comparing results at each 6-week time interval. Second, to evaluate performance within AL or CR groups at each 6-week interval against individual rats baseline performance, a repeated measures one-way ANOVA followed by Dunnett’s multiple comparison test was used. Third, to determine if baseline performance in individual rats affected either the trajectory of aging-related locomotor decline (as inferred from results in the AL group) or the response to CR, a Pearson’s correlation evaluated the mean baseline value for each locomotor parameter versus the percent change in that parameter after 12 or 24 weeks.
To determine if differences in DA tissue content and DA-regulating proteins were observed as a function of aging and CR intervention, comparisons of the AL and CR groups included a 12-month-old control group (n = 12). The 12-month-old control group served as a representative of dopaminergic neurochemistry at this point in the life span to compare against aging-related effects occurring between 12 and 18 months of age in the AL group and CR impact. The statistical analysis utilized a one-way ANOVA followed by an uncorrected Fisher’s Least Significant Difference post hoc test.
Striatal tyrosine hydroxylase inhibition study
Neurochemistry assessment.
The studies were designed such that one hemisphere of the striatum received AMPT and the contralateral hemisphere received saline. DA tissue content levels were compared in the AMPT-infused hemisphere, the adjacent DA neuron center (nucleus accumbens), and the SN and ventral tegmental area (VTA), the cognate somatodendritic compartments of striatum and nucleus accumbens, respectively. A two-tailed t test determined if significant differences were evident in all four DA regions.
Locomotor assessment.
A repeated measures two-way ANOVA was used to determine if significant differences in locomotor activity (ambulatory counts as characterized by software updated from Columbus Instruments) occurred in each individual rat following AMPT against saline, matching the sum or mean of ambulatory counts over the five assessments done following each infusion within specific time intervals following infusion.
We also evaluated the sums of activity by combining the 20-minute time intervals to determine total locomotor activity at 90 ± 30 minutes of the verified neurochemistry result. A paired t test, comparing the sum of activity following saline versus AMPT for each individual rat was then conducted.
The Grubb’s test identified one outlier in the neurochemistry sets with alpha set to <.05. Significance for all statistical tests was set at p < .05.
Results
Body Weight Change and Food Consumption
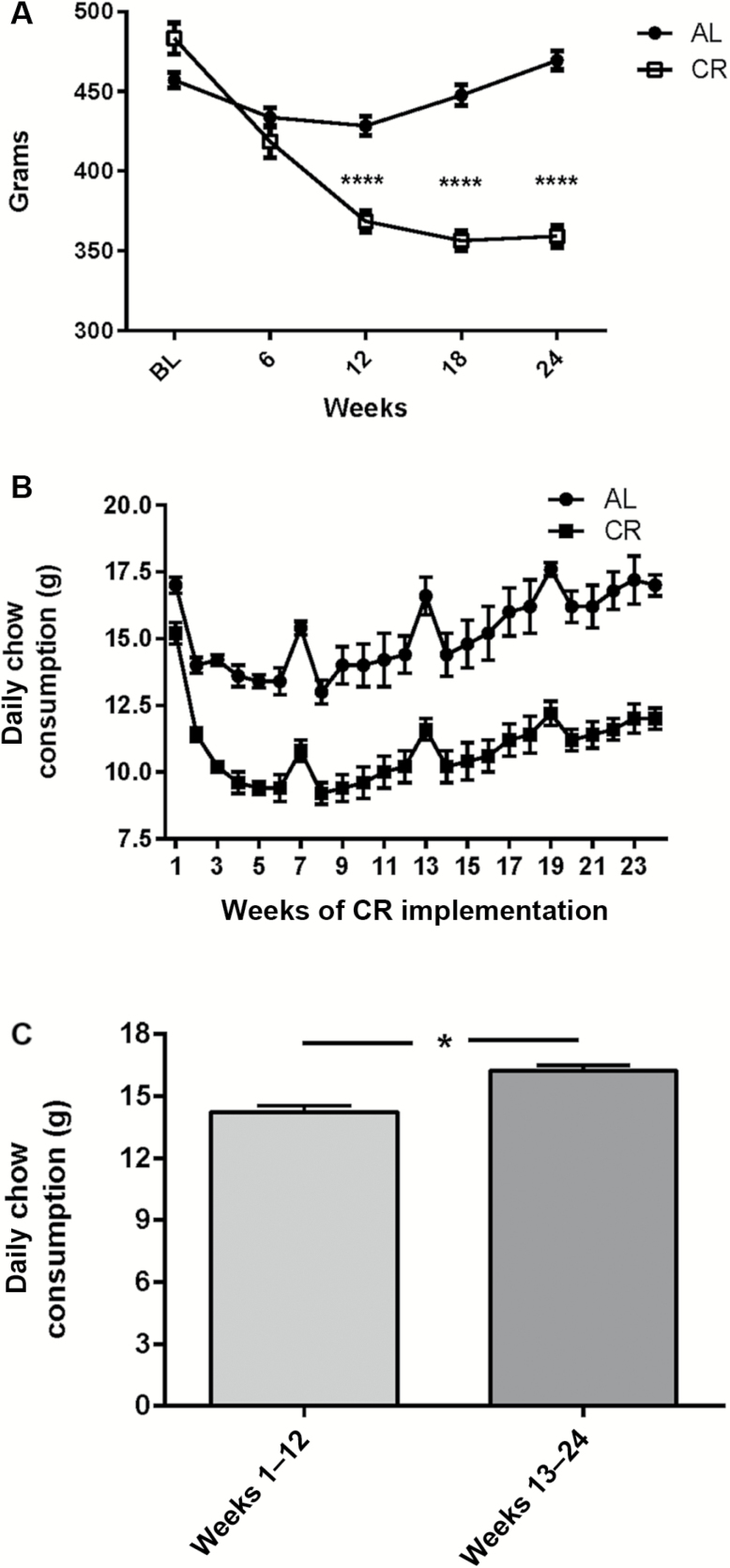
Body weight decreased in the CR group to ~25% of baseline within the first 12 weeks, leveling out over the next 12 weeks to approximate the 30% target level of restriction. A modest, but significant, degree of body weight loss occurred in the AL group over the first 12 weeks but recovered to baseline by 18 weeks (Figure 1A). This weight loss in the AL group was likely due to isolation housing imposed.
Figure 1.
Weight change during the study and food consumption. (A) Weight change during the study. The calorie restriction (CR) diet reduced weight (F(1,28) = 40.7, p < .0001) during the study, beginning at 12 weeks, and continuing out to 24 weeks after CR initiation (post hoc results: 6 weeks, t = 1.51, ns; 12 weeks, t = 5.92, p < .0001; 18 weeks, t = 9.05, p < .0001; 24 weeks, t = 10.9, p < .0001). Rats in CR group had slightly greater starting initial weight (t = 2.59, p < .05). This difference was unavoidable due to study design matching rats for equivalent locomotor activity mean and range. (B) Chow consumption between AL versus CR groups. Daily chow consumption varied in the AL group during the study. Data are presented as mean ± SEM. (C) Increased chow consumption in AL group during latter half of study. There was an overall 14% increase in chow consumption in AL group between Weeks 13–14 (16.2 ± 0.3 g) and Weeks 1–12 (14.2 ± 0.3 g; t = 4.84, p < .0001).
Daily food consumption in the AL group varied within 20% throughout the study (Figure 1B). There was a small, but significant increase in food consumption in the AL group from Weeks 13 to 24 versus 1 to 12 during the study (Figure 1C).
Locomotor Activity: Overall Group Performance in Specific Parameters of Locomotor Function
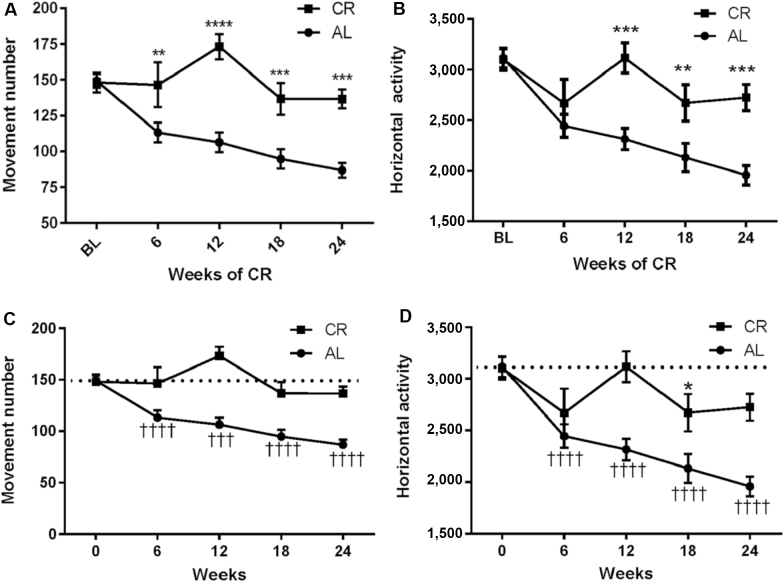
Movement number and horizontal activity are parameters of locomotor function associated with the initiation or generation of locomotor activity, which declines in aging (15,25,28). The implementation of 30% CR at 12 months of age attenuated the aging-related decline in movement number (Figure 2A), with increased movement number versus the AL group mean beginning 6 weeks after CR intervention and at each 6-week interval thereafter. The implementation of 30% CR at 12 months of age also attenuated the aging-related decline in horizontal activity (Figure 2B), as revealed by significant interaction between CR and aging and overall effect of the CR diet, beginning 12 weeks and for the study duration.
Figure 2.
Impact of calorie restriction (CR) versus AL diet on movement number and horizontal activity from 12 to 18 months of age. (A) Interaction of diet impact and aging on movement number. A significant interaction between aging and diet on was observed (F4,112 = 7.85, p < .0001) and diet effect on aging between AL and CR groups (F1,28 = 18.30, p = .002). Post hoc analysis indicated significant differences in between the AL and CR groups beginning at 12 weeks; 6 weeks (t = 2.77, **p = .006), 12 weeks (t = 5.56, ****p < .0001), 18 weeks (t = 3.49, ***p = .0006), and 24 weeks (t = 4.15, ****p < .0001). (B) Interaction of diet impact and aging on horizontal activity. A significant interaction between aging and diet was observed (F4,112 = 6.01, p = .0002) and diet effect on aging between AL and CR groups (F1,28 = 9.09, p = .0054). Post hoc analysis indicated significant differences between the AL and CR groups at 12, 18, and 24 weeks; 6 weeks (t = 1.16, ns), 12 weeks (t = 4.00, ***p = .0001), 18 weeks (t = 2.70, **p = .008), and 24 weeks (t = 3.82, ***p = .0002). (C) Movement number against baseline in AL and CR groups. In the AL group, movement number declined against individual baseline values beginning at 6 weeks (repeated measures one-way analysis of variance [ANOVA; p < .0001, F = 30.01]). Post hoc results (Dunnett’s Multiple Comparison test); baseline versus 6 weeks (q = 6.97, **** p < .0001), baseline versus 12 weeks (q = 5.74, ***p < .001), baseline versus 18 weeks (q = 7.56, ****p < .0001), baseline versus 24 weeks (q = 89.60, ****p < .0001). In the CR group, no significant difference in movement number was observed at any 6-week interval time point out to 24 weeks (repeated measures one-way ANOVA [p < .05, F = 3.64]). Post hoc results (Dunnett’s Multiple Comparison test); baseline versus 6 weeks (q = 0.12, ns), baseline versus 12 weeks (q = 2.17, ns), baseline versus 18 weeks (q = 1.19, ns), baseline versus 24 weeks (q = 1.13, ns). (D) Horizontal activity against baseline in AL and CR groups. In the AL group, horizontal activity declined against individual baseline values beginning at 6 weeks (repeated measures one-way ANOVA [p < .0001, F = 27.73]). Post hoc results (Dunnett’s Multiple Comparison test); baseline versus 6 weeks (q = 6.68, p < .0001), baseline versus 12 weeks (q = 6.32, p < .0001), baseline versus 18 weeks (q = 6.45, p < .0001), baseline versus 24 weeks (q = 8.39, p < .0001). In the CR group, no significant decrease in horizontal activity was observed at 12 weeks after CR intervention, but there were significant decreases (though of lesser magnitude than that in the AL group) at 6, 16, and 24 weeks. Repeated measures one-way ANOVA (p = .012, F = 3.92), post hoc results (Dunnett’s Multiple Comparison test), baseline versus 6 weeks (q = 2.30, ns), baseline versus 12 weeks (q = 0.10, ns), baseline versus 18 weeks (q = 2.84, p < .05), baseline versus 24 weeks (q = 2.54, ns).
These differences between the two groups were also evident in comparison with their baseline performance at 12 months of age. In the AL group, significant decreases in movement number were observed at 6 weeks and continued each 6 weeks thereafter; whereas in the CR group, no significant difference in movement number was observed at any of the 6-week intervals (Figure 2C). Aging-related decreases in horizontal activity also occurred in the AL group beginning at 6 weeks and continued out to 24 weeks, whereas in the CR group, no significant differences in horizontal activity were observed at 6-, 12-, or 24-week post-CR intervention (Figure 2D).
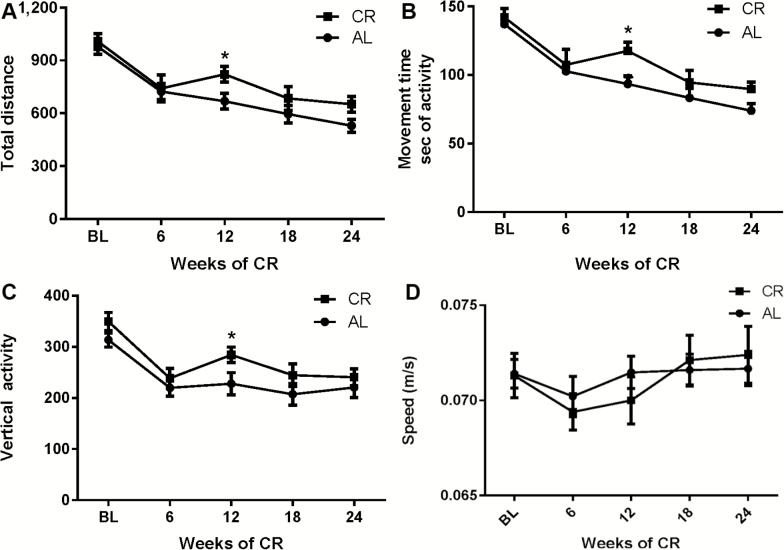
The parameters of total distance, movement time, and vertical activity all declined significantly from 12 to 18 months of age. Each parameter was also affected by CR, but with lesser magnitude and shorter-lived effect compared with movement number and horizontal activity. Significant differences between the CR and AL groups were evident at the 12-week time-point post-CR intervention (Figure 3A–C). These lesser effects of CR on distance and movement time have been previously reported (28). Significant decreases in each parameter were observed against baseline for all three parameters in either group, although the magnitude of decline in the CR group was nearly half of that seen in the AL group at 12 weeks into the study. Although not statistically significant, at the conclusion of the study, the difference in total distance and movement time was still >20% greater in the CR group versus the AL group average. Movement speed was not significantly affected by aging between 12 and 18 months of age and not affected by CR intervention.
Figure 3.
Impact of calorie restriction (CR) versus AL diet on total distance, movement time, vertical activity, and movement speed, 12–18 months of age. (A) Total distance. There was no significant interaction between aging and diet on total distance (F4,112 = 1.43, p = .23) and no significant effect of diet on aging between AL and CR groups across all time points (F1,28 = 2.01, p = .17). There was a significant difference in between the AL and CR groups at 12 weeks; 6 weeks (t = 0.26, ns), 12 weeks (t = 2.1, * p < .05), 18 weeks (t = 1.23, ns), and 24 weeks (t = 1.68, p = .09). (B) Movement time. There was no significant interaction between aging and diet on total distance (F4,112 = 1.47, p = .22) and no significant effect of diet on aging between AL and CR groups across all time points (F1,28 = 2.31, p = .14). There was a significant difference in between the AL and CR groups at 12 weeks; 6 weeks (t = 0.47, ns), 12 weeks (t = 2.41, *p < .05), 18 weeks (t = 1.12, ns), and 24 weeks (t = 1.58, ns). (C) Vertical activity. There was no significant interaction between aging and diet on total distance (F4,112 = 0.78, p = .54) and no significant effect of diet on aging between AL and CR groups across all time points (F1,28 = 2.56, p = 0.12). There was a significant difference in between the AL and CR groups at 12 weeks; 6 weeks (t = 0.71, ns), 12 weeks (t = 2.15, *p < .05), 18 weeks (t = 1.42, ns), and 24 weeks (t = 0.76, ns). (D) Movement speed. There was no significant interaction between aging and diet on total distance (F4,112 = 0.62, p = .65) and no significant effect of diet on aging between AL and CR groups across all time points (F1,28 = 0.041, p = .84). No differences in speed were evident at any 6-week interval; 6 weeks (t = 0.53, ns), 12 weeks (t = 0.96, ns), 18 weeks (t = 0.35, ns), and 24 weeks (t = 0.48, ns).
Relationship of Weight Change Versus Locomotor Function
Body weight at the start of the study did not have a significant correlation to locomotor activity. The weight of rats, designated for the CR and AL groups, was not significantly correlated with either mean movement number or horizontal activity at their 12-month-old baseline (data not shown), consistent with our previous study (28). No significant correlation was observed with individual weight change in the AL group versus the significant declines in movement number or horizontal activity at 24 weeks. The percent decrease in body weight in the CR group did not correlate with the percent change in movement number against the baseline, but did significantly correlate with change in horizontal activity, such that the greater percent decline in body weight, the less decline in locomotor activity (Pearson r = −.589, p = .02). Overall, the percent decline in weight in the CR group was 26% versus a nonsignificant 11% decline in horizontal activity from baseline.
Influence of Baseline Performance on Aging-Related Decline and CR Impact
We evaluated whether locomotor performance at 12 months of age influenced the rate of aging-related locomotor decline in the AL group or the response to CR. The lower the horizontal activity at baseline (12 months old), the greater percentage decline in horizontal activity was observed at 24 weeks (when the rats reached 18 months old; Table 1). Although movement speed was not affected during the 12- to 18-month period of the life span, the lower the movement speed at 12 months old, the greater the decline in movement speed in the AL group (Table 1).
Table 1.
Relationship of Individual Baseline Performance Versus Response to CR
| Locomotor parameter | CR | AL | ||
|---|---|---|---|---|
| Pearson r | p | Pearson r | p | |
| Movement number | ||||
| 12 wk | −.745 | .002** | .070 | ns |
| 24 wk | −.789 | .0005*** | .120 | ns |
| Horizontal activity | ||||
| 12 wk | −.400 | ns | −.490 | .06 |
| 24 wk | −.446 | .096 | −.562 | .03* |
| Total distance | ||||
| 12 wk | −.449 | ns | −.344 | ns |
| 24 wk | −.286 | ns | −.502 | .056 |
| Movement time | ||||
| 12 wk | −.568 | .03* | −.361 | ns |
| 24 wk | −.505 | .055 | −.443 | ns |
| Vertical activity | ||||
| 12 wk | −.622 | .013* | .180 | ns |
| 24 wk | −.677 | .006** | .075 | ns |
| Speed | ||||
| 12 wk | −.685 | .005** | −.462 | .08 |
| 24 wk | −.472 | .076 | −.542 | .04* |
Note: AL = ad libitum; CR = calorie restriction; ns = nonsignificant. *p < 0.05, **p < 0.01, ***p < 0.001.
Individual rats with the least baseline activity responded the most to CR intervention, similar to previous results (28). This was evident for the parameters of movement number and movement time at 12 and 24 weeks during the CR intervention (Table 1). Vertical activity was similarly affected, in that rats with the least amount of vertical activity had greater increases, or decreases of lesser magnitude from baseline, relative to their baseline performance (Table 1).
Dopamine Tissue Content and Turnover
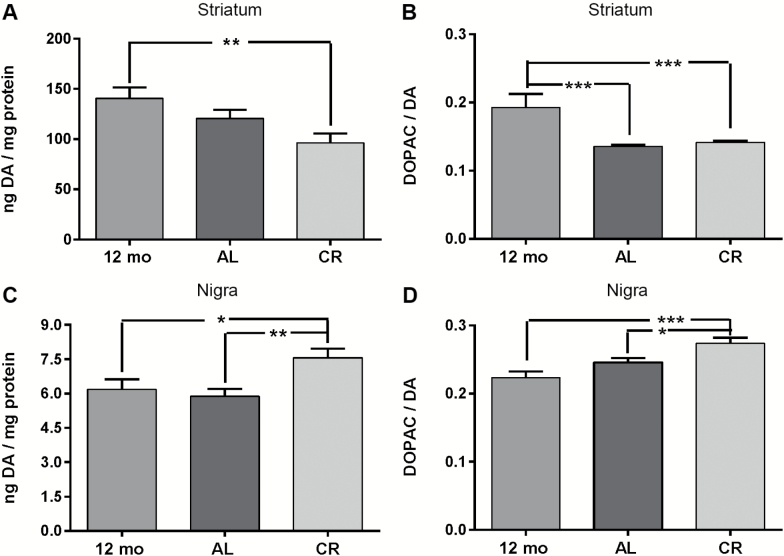
Striatal DA tissue content was significantly reduced ~30% in the CR group against the 12-month-old control group (Figure 4A), with no significant difference in DA in the AL versus 12-month-old control group. There was an overall 20% decrease in striatal DA in the CR group versus the AL group just beyond statistical significance (p = .06). DA turnover was significantly decreased in both the AL and CR groups versus the 12-month control group (Figure 4B). In contrast, in the SN, both DA tissue content and DA turnover were significantly increased in the CR group versus both AL and 12-month-old control groups (Figure 4C and D).
Figure 4.
Dopamine (DA) tissue content and DA turnover in 12-month-old control group and AL and calorie restriction (CR) groups at 18 months old. (A) Striatal DA tissue content. There was a significant difference in DA tissue content across all groups (one-way analysis of variance [ANOVA], F(2,38) = 5.21, p = .01), with a significant decrease in the CR group versus the 12-month-old control group (t = 3.19, **p = .003). No difference between the AL group versus the 12-month-old control group was observed (t = 1.44, ns), but a trend toward a decrease in the AL group versus the CR group (t = 1.91, p = .06). (B) Striatal DA turnover. There was a significant difference in DA turnover across all groups (one-way ANOVA F(2,38) = 10.09, p = .0003), with DA turnover significantly decreased in both the CR group (t = 3.75, ***p = .0006) and AL group (t = 4.19, ***p = .0002) versus the 12-month-old control group. No difference in DA turnover was observed between the CR group and AL group (t = 0.48, ns). (C) Nigral DA tissue content. There was a significant difference in DA tissue content across all groups (one-way ANOVA F(2,38) = 5.62, p = .007), with a significant increase in the CR group versus the 12-month-old control group (t = 2.51, * p < .05) and the AL group (t = 3.12, **p = 0.004). (D) Nigral DA turnover. There was a significant difference in DA turnover across all groups (one-way ANOVA F(2,36) = 9.31, p = .0006), with a significant increase in the CR group versus the 12-month-old control group (t = 4.26, ***p = .0001) and versus the AL group (t = 2.50, *p < .05). A trend toward an increase in DA turnover was observed between the AL group and 12-month-old control group (t = 1.82, p = .07).
Tyrosine Hydroxylase Protein Expression and TH Phosphorylation
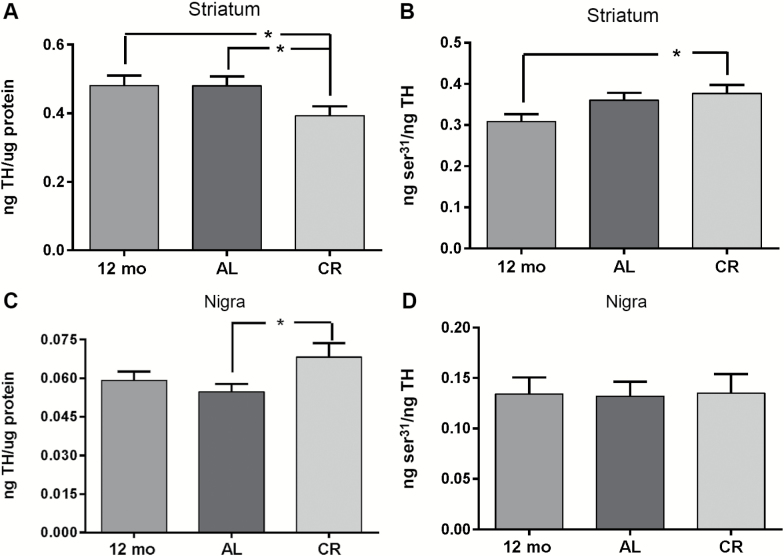
Striatal TH protein expression levels were significantly reduced ~20% in the CR group compared with the AL group (Figure 5A). To the converse, nigral TH protein expression levels were significantly increased by roughly the same margin of difference (~24%) in the CR group versus the AL group (Figure 5C). Within the 12- to 18-month time period, no significant differences in TH expression were observed in the AL group versus the 12-month-old control group in either region.
Figure 5.
Tyrosine hydroxylase (TH) protein expression and ser31 TH phosphorylation. (A) Striatum, total TH. There was a significant difference in TH protein across all groups (one-way ANOVA, F(2,37) = 3.83, p < .05). TH protein expression was significantly less in the calorie restriction (CR) group compared with the AL and 12-month control group. Post hoc (12-month control vs AL, t = 0.04, ns; *12-month control vs CR, t = 2.25, p < .05; *AL vs CR, t = 2.45, p < .05). (B) Striatum, ser31 TH phosphorylation. ser31 phosphorylation TH protein was significantly increased in the CR group versus 12-month-old control group. One-way analysis of variance (ANOVA; F(2,37) = 3.16, p = .054), (post hoc 12-month control vs CR, t = 2.48, p < .05; AL vs 12-month control, t = 1.64, ns; AL vs CR, t = 0.88, ns). (C) Substantia nigra, total TH. TH protein expression was significantly greater in the CR group compared with the AL group. One-way ANOVA (F(2,37) = 2.73, p = .08), (post hoc 12-month control vs AL, t = 0.70, p = .49; 12-month control vs CR, t = 1.44, p = .17; *AL vs CR, t = 2.29, p < .05). (D) Substantia nigra, ser31 TH phosphorylation. No significant differences in ser31 TH phosphorylation were observed among the three groups. One-way ANOVA (F(2,37) = 0.01, p = .99), (post hoc 12-month control vs AL, t = 0.08, ns; 12-month control vs CR, t = 0.03, ns; AL vs CR, t = 0.12, ns).
Increased TH phosphorylation at ser31 may increase TH activity in vivo, and, at ser19 and possibly at ser40, may indicate increased TH proteolysis (35). Our previous analysis of CR effects on ser19 and ser40 in the striatum indicated no significant differences versus the AL group (28). However, there was a small, but significant, increase in ser31 phosphorylation in striatum in the CR group versus the 12-month-old control (Figure 5B). In contrast, in the SN, there were no significant differences in ser31 (Figure 5D), ser19 (12-month control, 0.40 ± 0.03; AL, 0.44 ± 0.05; CR, 0.40 ± 0.04), or ser40 (12-month control, 0.049 ± 0.004; AL, 0.049 ± 0.005; CR, 0.043 ± 0.005 (mean ± SEM, results expressed as ng phosphorylation site per ng TH total protein).
Dopamine Transporter, Dopamine D1 Receptor Expression
CR intervention did not affect striatal DA transporter expression in our previous study (28). Therefore, we evaluated CR effects against aging versus the 12-month control and against the AL group only in the SN. No significant differences in DA transporter expression were noted among the three groups (one-way ANOVA F(2,36) = 0.68, 12-month control vs AL, t = 0.72, ns; 12-month control vs CR, t = 1.16, ns; AL vs CR, t = 0.47, ns).
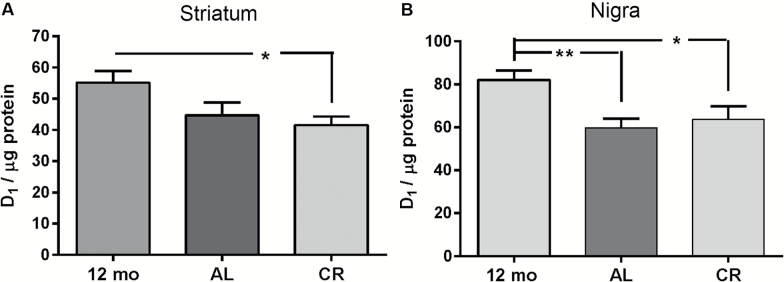
Modulation of the DA D1 receptor in the SN can influence locomotor function (23,36). D1 receptor expression was significantly decreased in both the AL and CR groups versus the 12-month-old control group in both striatum and SN (Figure 6A and B). These data, in contrast to the dichotomous effects on CR on TH expression on the nigrostriatal neuron compartments, show that aging reduces D1 receptor expression in both regions. This indicates that reduced DA neurotransmission by reduced postsynaptic DA receptor expression may contribute to decreased locomotor activity between 12 and 18 months of age in the AL group.
Figure 6.
Dopamine (DA) D1 receptor expression. (A) Striatum D1 receptor expression was decreased in the calorie restriction (CR) and AL groups versus 12-month-old control group. One-way ANOVA ([F(2,38) = 3.59, p = .037], post hoc 12-month control vs AL, t = 2.01, p = .051; 12-month control vs CR, t = 2.58, *p = .01; AL vs CR, t = 0.64, ns). (B) Substantia nigra D1 receptor expression was significantly decreased (~25%–30%) in the CR and AL groups versus 12-month-old control group. One-way analysis of variance ([F(2,35) = 4.83, p = .01], post hoc 12-month control vs AL, t = 2.94, **p = .006; 12-month control vs CR, t = 2.46, *p = .019; AL vs CR, t = 0.56, ns).
AMPT-Mediated DA Reduction in Striatum and Impact on Locomotor Function
Verification of striatum-specific DA reduction
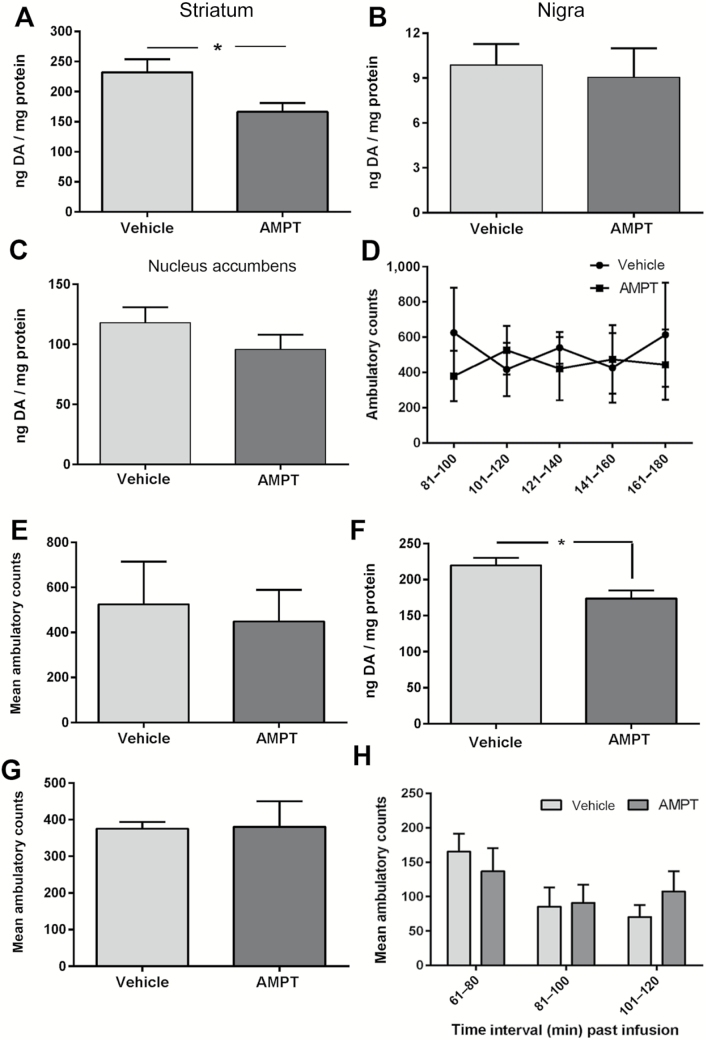
To further evaluate if reduction of striatal DA affected locomotor function, we employed a method previously shown to decrease DA specifically in striatum and not SN (31) in 6-month-old BNF rats. A decrease in striatal DA similar to that previously reported in aging studies in young rats, presumed to be devoid of aging-related impairment to locomotor capabilities, could determine if such striatal DA loss affects locomotor activity. AMPT, at the concentration previously shown effective at 90 minutes (31), also reduced DA in dorsal striatum 3 hours after infusion, averaging ~30% reduction (Figure 7A), without effect in the SN (Figure 7B), the nucleus accumbens (Figure 7C), or VTA (data not shown; ng DA/mg protein, vehicle = 12.8 ± 2.3, AMPT = 14.5 ± 1.8; t = 0.58, ns, df = 10).
Figure 7.
Specificity of striatal dopamine (DA) reduction and impact on locomotor function. α-Methyl-p-tyrosine (AMPT)-mediated reduction of DA tissue content 3 hours following 2.1 nmole AMPT infusion in striatum (A–C) and locomotor impact (D, E), AMPT-mediated reduction of striatal DA tissue content 90 minutes following with 21 nmole AMPT infusion in striatum (F) and locomotor impact (G). (A) Dorsal striatum. DA tissue content decreased 30%, 3 hours following 2.1 AMPT nmole infusion (t = 2.50, * p < .05, df = 12). (B) Substantia nigra. DA tissue content in the substantia nigra (SN) was unaffected by striatal AMPT infusion (t = 0.35, ns, df = 12). (C) Nucleus accumbens. DA tissue content in the nucleus accumbens was unaffected by striatal AMPT infusion (t = 1.24, ns, df = 12). (D) Striatal AMPT (2.1 nmole) impact on locomotor activity. Ambulatory counts in rats 81–180 minutes following AMPT into the striatum were not significantly different versus ambulatory counts in the same time period following vehicle infusions. Data represent the sum of ambulatory counts obtained over five trails for each rat following infusion of either vehicle or AMPT. Repeated measures two-way analysis of variance (ANOVA) results: AMPT effect: (F(4,16) = 0.15, p = .96). Time following infusion: (F(1,4) = 0.32, p = .60). There was no significant interaction of time following infusion × AMPT (F(4,16) = 1.02, p = .43). Post hoc test results: 81–100 minutes, t = 1.67, ns; 101–120 minutes, t = 0.74, p = .47; 121–140 minutes, t = 0.80, ns; 141–160 minutes, t = 0.32, ns; 161–180 minutes, t = 1.15, ns. (E) Total ambulatory counts between 81 and 180 minutes. The mean sum of ambulatory counts during 81–180 minutes following striatal AMPT infusion (2.1 nmole) versus that following vehicle infusion was not significantly different (paired t test matched for test subject [t = 0.57, ns, df = 4; 5 rats]). (F) DA tissue content, dorsal striatum. DA tissue content decreased 30%, 90 minutes following 21 AMPT nmole infusion (t = 2.99, *p < .05, df = 11). (G) Mean of sum total ambulatory counts 61–120 minutes following AMPT (21 nmole) infusion. The mean sum of ambulatory counts 61–120 minutes following striatal AMPT infusion (21 nmole) was not significantly different versus that following vehicle infusion (paired t test matched for sum of each test subject [t = 0.08, ns, df = 4; 5 rats]). (H) Reduced striatal DA impact on ambulatory counts 61–120 minutes. Given similar striatal DA reduction from either 2.1 (31) or 21 nmole of AMPT (Figure 7F) of ~30%, mean ambulatory counts between 61 and 120 minutes following vehicle or AMPT infusion were analyzed by two-way ANOVA. The time past infusion was significant (F(2,18) = 9.82, p = .0013). No significant overall difference in mean ambulatory counts following AMPT infusion versus vehicle infusion was observed (F(1,9) = 0.10, ns).
This AMPT-mediated effect on DA reduction was maximal at 2.1 nmole, as 10-fold greater quantity (21 nmole) did not further reduce striatal DA tissue content at 90 minutes (Figure 7F), versus previously reported decreased DA at this time period (31). DA tissue content in the other DA regions was also unaffected by this AMPT quantity (SN [ng DA/mg protein, vehicle = 6.8 ± 0.6, AMPT = 6.5 ± 0.8; t = 0.29, ns, df = 10], nucleus accumbens [ng DA/mg protein, vehicle = 93 ± 11, AMPT = 91 ± 12; t = 0.10, ns, df = 11], or the VTA [ng DA/mg protein, vehicle = 11.4 ± 2.6, AMPT = 14.4 ± 2.3; t = 0.86, ns, df = 8]). In summary, the experimental reduction of ~30% striatal DA, following either quantity of AMPT, approximates the average loss of striatal DA reported in aged BNF or Sprague-Dawley rats (15,18,29,33), is specific for the striatum alone, and equivalent to the decrease in striatal DA observed in the CR group compared with the AL and 12-month control groups.
Locomotor impact of striatal AMPT
Having confirmed the extent to which striatal DA was reduced by AMPT, we evaluated the impact of striatal infusion of AMPT on locomotor activity in 6-month-old BNF rats. Neither 2.1 (Figure 7D and E) nor 21 nmole (Figure 7G) of AMPT reduced ambulatory counts. Given that both quantities of AMPT produced similar striatal DA reduction (~30%), the locomotor results were then analyzed together in three 20 minute intervals, 60 to 120 minutes, following striatal infusion. There was no significant overall difference in ambulatory counts (Figure 7H). Taken together, these data indicate that decreased striatal DA and TH in the CR group may be dissociated from CR effects on locomotor function and that previously reported decreases of striatal DA in aging studies up to 30% may not be associated with aging-related locomotor decline.
Discussion
A noninvasive strategy to potentially attenuate aging-related motor decline, CR, is shown to be effective when initiated in middle-aged rats. Parameters associated with locomotor activity, horizontal activity, and movement number were significantly preserved in the CR group versus the AL group at 18 months of age. Furthermore, CR improved all locomotor parameters measured for at least out to 12 weeks after initiation with the exception of movement speed (which was not affected by aging as evident in the AL group). Thus, from the perspective of locomotor function and its decline during aging, CR appears to preserve the capacity to initiate movement over a significant portion of the rat life span beyond middle age. While a longer study would be necessary to determine the true extent to which locomotor function can be preserved when CR is initiated at 12 months old, it seems likely that it would extend significantly beyond the ages tested in the current study. Previous studies of CR maintained throughout life failed to detect significant locomotor decline at ages up to 24–30 months (26,37). CR initiation at middle age can also improve fine motor performance (38). Longer studies of CR initiated at this age would address how CR would concurrently affect DA regulation against the continuing effects of aging, as further decreases in TH expression and phosphorylation occur beyond 18 months of age, notably in the SN (15,24,29,31).
It is conceivable that CR effects on motor function could be related to diminished body mass (39). However, the percentage decline in weight was not correlated to percent change in movement number in the CR rats, a finding that we have previously reported (28). Furthermore, the divergence in slope of movement number and horizontal activity between the AL and CR groups clearly suggest a preservation of these indices of the ability to initiate locomotor activity against the aging-related decline observed in the AL group. There was also no significant increase in activity above baseline in the CR group at any point in the study. Long-term CR studies in mice that addressed this possibility directly also suggested that functional status of AL and CR at 24 months could not be reversed by refeeding or introducing short-term CR (40,41).
The addition of a 12-month-old control group in the current study gave an aging-related reference point to evaluate how nigrostriatal DA regulation may change over the course of ~6-month study (in the AL group), how CR implementation may have affected nigrostriatal DA regulation, and how these changes were related to the locomotor parameters examined. Striatal DA tissue content and TH expression were both significantly decreased in the CR group versus the 12-month-old control group, despite that movement number and horizontal activity at the end of the study (24-week post-CR) were not significantly different from baseline activity. Striatal TH expression was also significantly lower in CR versus the AL group, despite the greater decline in movement number and horizontal activity in the AL group compared with CR group. Total distance and movement time were also decreased, but by around half the decline in movement number and horizontal activity.
We have previously reported no relationship of striatal DA with these locomotor parameters in a longitudinal aging study (15). However, to determine if aging-related decreases in striatal DA alone could affect locomotor activity, we used another direct approach to pharmacologically and specifically inhibit TH activity to decrease DA specifically in the striatum of rats that were much younger (6 months old), representing an age presumably free of aging-related motor impairment. The experimental design allowed for direct comparisons of locomotor activity within each rat following vehicle or AMPT infusion, controlling for differences in innate locomotor activity that existed within the cohort. We also found no significant locomotor effect of striatal DA reduction upon ambulatory counts. Therefore, these results suggest that the reduction of striatal DA in the CR group was not directly involved with the locomotor effects associated with the CR group. Furthermore, as numerous aging studies report striatal DA reduction to be, on average, similar to the loss associated with AMPT or CR (~30%), it may be concluded that moderate striatal DA loss may be dissociated from parameters of movement initiation capabilities and unrelated to aging-related decreases in locomotor activity.
We should note, however, that a postmortem evaluation of DA markers in striatum indicated ~35% loss in a Parkinson’s patient who died ~1 year after diagnosis (42). This finding might suggest that moderate loss of striatal DA, akin to that in aging, could contribute to locomotor impairment. However, a very recent study reported that a Parkinson’s patient who received bilateral fetal mesencephalic grafts in putamen never exhibited improved locomotor function over 16 years, despite evidence of robust TH-positive innervation (43). To the contrary but in the same vein of argument, striatal TH loss of 60% has not been associated with bradykinesia onset in primate PD models (10). Therefore, in light of these findings in more-advanced mammalian species, our findings of dissociation of striatal TH or DA with locomotor functions are consistent. We point out, however, that one limitation to supporting this conclusion is that a more complete profile of evaluating changes in DA neurotransmission versus the motor outcome could be gained by evaluation of potential changes in DA release in the compartments of the nigrostriatal pathway. For example, CR has been reported to decrease DA release in the nucleus accumbens (44,45), which might suggest a similar outcome from CR in the nigrostriatal terminal fields.
CR attenuation of aging-related decreases in locomotor activity between 12 and 18 months of age may be related, at least in part, to increased nigral TH expression and DA tissue content. The 30%–50% loss of TH protein expression or DA neurotransmission in SN is consistently reported in aging studies across mammalian species, including human, and is associated with aging-related Parkinsonism (7,15–19,31,46,47). Similar loss of nigral neurons is reported in cases of incidental Lewy body disease (48). In relation to PD models, nigral TH loss at ~40% is also associated with bradykinesia onset (10). Recovery of aging-related deficits in locomotor function is also related to increased nigral, but not striatal, DA, or TH expression, including aged rats (24). Modulation of nigral DA neurotransmission beyond the DA biosynthesis step at TH can also affect open-field locomotor activity. Postsynaptic DA D1 receptors on the striatonigral terminals increase GABA release upon binding DA, which in turn disinhibits GABAergic output neurons from the SN pars reticulata to modulate basal ganglia outflow (36,49). Blocking these striatonigral D1 receptors in the SN decreases open-field locomotor activity (23). Applied to our findings, the decrease in locomotor activity between 12 and 18 months may be related to decreased DA D1 receptor expression we observed against the 12-month control group. We speculate that the increase in DA tissue content, associated with increased TH expression in SN alone, of the CR group may counteract the aging-related loss of DA D1 receptors. Increased DA tissue content likely would increase DA release capacity, given that this relationship has been shown in vivo (32). Therefore, increased nigral DA tissue content in the CR group may partially compensate for aging-related decreased D1 receptor expression to attenuate locomotor decline. Exercise, also associated with improved locomotor function in PD, is another noninvasive measure that increases TH protein expression only in SN and not striatum (50), similar to the observations from the CR intervention here. Other studies evaluating locomotor function in movement disorders have also implicated nigral, not striatal, DA function in locomotor performance measures (15,21,22,24). Thus, increased nigral TH expression may attenuate locomotor decline by offsetting eventual aging-related declines in nigral TH expression.
In summary, this study has shown that locomotor decline in BNF rats begins during middle age and may be linked to decreased D1 receptor expression. Moreover, we have confirmed that the implementation of CR attenuated locomotor decline associated with aging with decreased TH expression and DA tissue content in striatum, but increased TH and DA in the SN. This dichotomous response of the nigrostriatal pathway to CR has been observed following other noninvasive interventions such as exercise and mechanistic interventions in DA neurotransmission. As such, the outcome provides further evidence of the dichotomy of DA neurotransmission of the nigrostriatal pathway between the striatum and SN. The mounting evidence of the incongruity between striatal DA and locomotor function from other studies is supported by this study, wherein pharmacological inhibition of TH activity that produces decreased striatal DA levels similar to that in aging did not affect locomotor activity. Therefore, the involvement of reduced striatal DA function seen in aging upon locomotor decline must be re-evaluated in light of our finding in conjunction with other literature. The consideration of a role of nigral DA signaling as a mechanism to preserve locomotor function in aging should be at the forefront of efforts to determine strategies to address locomotor impairment in aging.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (AG040261) to M.F.S.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Bennett DA, Beckett LA, Murray AM et al. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi:10.1056/NEJM199601113340202 [DOI] [PubMed] [Google Scholar]
- 2. Murray AM, Bennett DA, Mendes de Leon CF, Beckett LA, Evans DA. A longitudinal study of Parkinsonism and disability in a community population of older people. J Gerontol A Biol Sci Med Sci. 2004;59:864–870. [DOI] [PubMed] [Google Scholar]
- 3. Fleischman DA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Parkinsonian signs and functional disability in old age. Exp Aging Res. 2007;33:59–76. doi:10.1080/03610730601006370 [DOI] [PubMed] [Google Scholar]
- 4. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–19. doi:10.1111/j.1532-5415.2006.01032.x [DOI] [PubMed] [Google Scholar]
- 5. Buchman AS, Wilson RS, Leurgans SE, Bennett DA, Barnes LL. Change in motor function and adverse health outcomes in older African-Americans. Exp Gerontol. 2015;70:71–77. doi:10.1016/j.exger.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchman AS, Leurgans SE, Yu L et al. Incident Parkinsonism in older adults without Parkinson disease. Neurology. 2016;87:1036–1044. doi:10.1212/WNL.0000000000003059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudow G, O’Brien R, Savonenko AV et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–470. doi:10.1007/s00401-008-0352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosano C, Bennett DA, Newman AB et al. Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:957–962. doi:10.1093/gerona/glr262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. [DOI] [PubMed] [Google Scholar]
- 10. Bezard E, Dovero S, Prunier C et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–585. [DOI] [PubMed] [Google Scholar]
- 12. Wolf ME, LeWitt PA, Bannon MJ, Dragovic LJ, Kapatos G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J Neurochem. 1991;56:1191–1200. [DOI] [PubMed] [Google Scholar]
- 13. Kish SJ, Shannak K, Rajput A, Deck JH, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–648. [DOI] [PubMed] [Google Scholar]
- 14. Collier TJ, Lipton J, Daley BF et al. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to Parkinsonism. Neurobiol Dis. 2007;26:56–65. doi:10.1016/j.nbd.2006.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS One. 2009;4:e8466. doi:10.1371/journal.pone.0008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irwin I, DeLanney LE, McNeill T et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- 17. Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. [DOI] [PubMed] [Google Scholar]
- 18. Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. [DOI] [PubMed] [Google Scholar]
- 19. Ponzio F, Calderini G, Lomuscio G, Vantini G, Toffano G, Algeri S. Changes in monoamines and their metabolite levels in some brain regions of aged rats. Neurobiol Aging. 1982;3:23–29. [DOI] [PubMed] [Google Scholar]
- 20. Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. [DOI] [PubMed] [Google Scholar]
- 21. Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur J Neurosci. 2006;24:617–624. doi:10.1111/j.1460-9568.2006.04953.x [DOI] [PubMed] [Google Scholar]
- 22. Bergquist F, Shahabi HN, Nissbrandt H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003;973:81–91. [DOI] [PubMed] [Google Scholar]
- 23. Trevitt JT, Carlson BB, Nowend K, Salamone JD. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology. 2001;156:32–41. [DOI] [PubMed] [Google Scholar]
- 24. Pruett BS, Salvatore MF. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol. 2013;47:988–999. doi:10.1007/s12035-013-8397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. [DOI] [PubMed] [Google Scholar]
- 26. Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. [DOI] [PubMed] [Google Scholar]
- 27. Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 1997;62:97–103. [DOI] [PubMed] [Google Scholar]
- 28. Salvatore MF, Terrebonne J, Fields V et al. Initiation of calorie restriction in middle-aged male rats attenuates aging-related motoric decline and bradykinesia without increased striatal dopamine. Neurobiol Aging. 2016;37:192–207. doi:10.1016/j.neurobiolaging.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cruz-Muros I, Afonso-Oramas D, Abreu P et al. Aging of the rat mesostriatal system: differences between the nigrostriatal and the mesolimbic compartments. Exp Neurol. 2007;204:147–161. doi:10.1016/j.expneurol.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 30. Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J Neurosci Methods. 2010;187:46–51. doi:10.1016/j.jneumeth.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS One. 2012;7:e29867. doi:10.1371/journal.pone.0029867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watanabe S, Fusa K, Takada K et al. Effects of alpha-methyl-p-tyrosine on extracellular dopamine levels in the nucleus accumbens and the dorsal striatum of freely moving rats. J Oral Sci. 2005;47:185–190. [DOI] [PubMed] [Google Scholar]
- 33. Emerich DF, McDermott P, Krueger P et al. Locomotion of aged rats: relationship to neurochemical but not morphological changes in nigrostriatal dopaminergic neurons. Brain Res Bull. 1993;32:477–486. [DOI] [PubMed] [Google Scholar]
- 34. Salvatore MF, Pruett BS, Fields V, Dempsey C. Comprehensive profiling of dopamine regulation in substantia nigra and ventral tegmental area. J Vis Exp. 2012. doi:10.3791/4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salvatore MF. ser31 Tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J Neurochem. 2014;129:548–558. doi:10.1111/jnc.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kliem MA, Maidment NT, Ackerson LC, Chen S, Smith Y, Wichmann T. Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol. 2007;98:1489–1500. doi:10.1152/jn.00171.2007 [DOI] [PubMed] [Google Scholar]
- 37. Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi:10.1006/abbi.1996.0380 [DOI] [PubMed] [Google Scholar]
- 38. Kastman EK, Willette AA, Coe CL et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2010;30:7940–7947. doi:10.1523/JNEUROSCI.0835-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell BA, Gaddy JR. Rate of aging and dietary restriction: sensory and motor function in the Fischer 344 rat. J Gerontol. 1987;42:154–159. [DOI] [PubMed] [Google Scholar]
- 40. Forster MJ, Lal H. Neurobehavioral biomarkers of aging: influence of genotype and dietary restriction. Biomed Environ Sci. 1991;4:144–165. [PubMed] [Google Scholar]
- 41. Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20:167–176. [DOI] [PubMed] [Google Scholar]
- 42. Kordower JH, Olanow CW, Dodiya HB et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136:2419–2431. doi:10.1093/brain/awt192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kordower JH, Goetz CG, Chu Y et al. Robust graft survival and normalized dopaminergic innervation does not obligate recovery in a PD patient. Ann Neurol. 2016;81:46–57. doi:10.1002/ana.24820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carr KD. Nucleus accumbens AMPA receptor trafficking upregulated by food restriction: an unintended target for drugs of abuse and forbidden foods. Curr Opin Behav Sci. 2016;9:32–39. doi:10.1016/j.cobeha.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emborg ME, Ma SY, Mufson EJ et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 47. Ross GW, Petrovitch H, Abbott RD et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi:10.1002/ana.20226 [DOI] [PubMed] [Google Scholar]
- 48. Iacono D, Geraci-Erck M, Rabin ML et al. Parkinson disease and incidental Lewy body disease: just a question of time? Neurology. 2015;85:1670–1679. doi:10.1212/WNL.0000000000002102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Windels F, Kiyatkin EA. Dopamine action in the substantia nigra pars reticulata: iontophoretic studies in awake, unrestrained rats. Eur J Neurosci. 2006;24:1385–1394. doi:10.1111/j.1460-9568.2006.05015.x [DOI] [PubMed] [Google Scholar]
- 50. Arnold JC, Salvatore MF. Exercise-mediated increase in nigral tyrosine hydroxylase is accompanied by increased nigral GFR-α1 and EAAC1 expression in aging rats. ACS Chem Neurosci. 2016;7:227–239. doi:10.1021/acschemneuro.5b00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.