Abstract
Background
Diabetes has been linked to dementia risk; however, the cognitive trajectories in older adults with diabetes remain unclear. We aimed to investigate the effect of prediabetes and diabetes on cognitive trajectories among cognitively intact older adults in a long-term follow-up study.
Methods
Within the Swedish Adoption/Twin Study of Aging, 793 cognitively intact older adults aged ≥50 were identified at baseline and followed for up to 23 years. Based on standardized scores from 11 cognitive tests, administered at baseline and up to seven follow-ups, four cognitive domains (verbal abilities, spatial/fluid, memory, perceptual speed) were identified by principal-component analysis. Prediabetes was defined according to blood glucose levels in diabetes-free participants. Diabetes was ascertained based on self-report, hypoglycemic medication use and blood glucose levels. Data were analyzed with linear mixed-effect models adjusting for potential confounders.
Results
At baseline, 68 participants (8.6%) had prediabetes and 45 (5.7%) had diabetes. Compared to diabetes-free individuals, people with diabetes had a steeper decline over time in perceptual speed and verbal abilities. The annual declines in these domains were greater than the annual decline in memory. Prediabetes was associated with lower performance in memory in middle-age, but also associated with a less steep memory decline over the follow-up.
Conclusions
Diabetes is associated with a faster decline in perceptual speed and verbal abilities, while prediabetes is associated with lower memory performance in middle-age. However, the detrimental effects of hyperglycemia seem to not affect memory over time.
Keywords: Type 2 diabetes, Cognitive aging, Longitudinal study, Hyperglycemia
To date, 415 million people in the world live with type 2 diabetes (hereafter referred to as diabetes), and 318 million people have prediabetes (1). Meanwhile, over 46 million people live with dementia (2). Older adults with diabetes have a higher risk for dementia than those without diabetes (3). In addition, some studies suggest that diabetes is also associated with faster cognitive decline among individuals without dementia (3). Prediabetes, a high-risk state of developing diabetes (1), is also suggested to be predictive of cognitive impairment and dementia (4). As prevention and treatment strategies for the classical vascular complications of diabetes have significantly improved, people with diabetes are living longer, leading to an increased number of people at risk for cognitive decline and dementia.
Although cognitive impairment and dementia are increasingly recognized as clinically relevant diabetes-related complications, the cognitive trajectories in cognitively intact older adults with diabetes remain unclear. Prospective studies that investigated the relationship between diabetes and cognitive decline in different domains showed mixed results (5–11). Some studies demonstrated a faster cognitive decline in persons with diabetes than those without diabetes in perceptual speed (6), executive function (5–7), and memory (8,11), while other studies did not find such associations (9,10). Other cognitive domains such as verbal and visuospatial abilities have been less explored. Variability in these findings may reflect different methodological discrepancies, such as inclusion of people with cognitive impairment, shorter follow-up duration, or assessment of cognitive function through screening tools or single cognitive tests. Furthermore, the impact of prediabetes on long-term cognitive trajectories among people not diagnosed with dementia has been poorly investigated.
Based on these observations, we hypothesized that diabetes and prediabetes are related to distinct trajectories of cognitive decline in different domains. In this study, we sought to verify this hypothesis using data from the long-term population-based Swedish Adoption/Twin Study of Aging (SATSA).
Methods
Study Population
SATSA is a population-based longitudinal study consisting of a subset of participants from the Swedish Twin Registry (STR) (12). The study design of SATSA has been reported in detail elsewhere (13). Briefly, from the base population of SATSA, all twins who were aged ≥50 and participated in a mailed questionnaire in 1984 were invited to undergo clinical examinations and cognitive assessments by trained nurses starting in 1986 (first in-person testing, IPT1; n = 645). After IPT1, the participants were followed-up every 3 years from 1986 until 2010. Throughout the study period (mean 13.0 years, max 23 years), eight waves of examinations were carried out. During the follow-up, the twins in SATSA were sequentially included in the study when they turned 50 years old (n = 93 at IPT2, n = 21 at IPT3, n = 99 at IPT5, n = 1 at IPT6, and n = 3 at IPT8; Supplementary Figure 1). Because of the open-cohort design, the date at the participants’ entry was considered as baseline. In total, 862 individuals participated in the baseline examination and completed the cognitive protocol during at least one wave. After excluding at baseline participants with cognitive impairment-no dementia (CIND, n = 58), dementia (n = 11), and missing blood glucose concentration (n = 1), 793 relatively cognitively intact participants were retained for the present study.
Informed consent was obtained from all participants. SATSA was approved by the Regional Ethics Board at Karolinska Institutet, Stockholm, Sweden. Confidentiality and anonymity were guaranteed as part of the informed consent. Participants were informed that their involvement in the study was voluntary and that they were free to withdraw from the study at any point in time.
Data Collection
Information on demographics (ie, age, sex, and education) and lifestyle factors (ie, smoking, alcohol consumption, and physical exercise) were collected through the baseline survey. Blood pressure, weight, and height were measured by nurses at baseline and at each follow-up. Information on medical conditions and medical drug use was obtained based on self-report at each wave.
Education was dichotomized as low (elementary or vocational, ≤9 years) and high (high school or above, >9 years). Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters (kg/m2) and categorized into four groups: underweight (<20.0), normal weight (20–25), overweight (≥25–30), and obese (≥30). Smoking status was dichotomized as nonsmoking (participants who had never smoked) and smoking (including former and current smokers). Alcohol consumption was dichotomized as never-drinkers (those who never drink alcohol) and drinkers (including former and current drinkers). Information on physical exercise was based on the participant’s self-reported amount of exercise throughout the year and categorized as “no exercise/light” and “physical exercise” (including moderate to heavy exercise) (14). Cerebro- and cardio-vascular diseases (CVDs) included hypertension (defined as blood pressure ≥140/90 mmHg) (15), heart diseases (heart failure, coronary heart diseases, and heart attack), and stroke, and were assessed based on self-reported medical history.
Assessment of Diabetes and Prediabetes
Blood samples were taken from all participants at baseline with either fasting (n = 721, 91.0%) or non-fasting (n = 71, 9.0%) status and also at each follow-up at which information on hours fasting was recorded. Fasting blood glucose (FBG) was defined as blood glucose measured in blood samples taken after at least 8 hours of no caloric intake (16). Blood glucose concentration was tested using an enzymatic (glucose-oxidase) method (KODAK Ektachem).
Diabetes was ascertained at baseline and each follow-up based on self-reported medical history, use of hypoglycaemic medications (oral hypoglycemic agents or insulin), or FBG ≥7.0 mmol/L, or non-fasting blood glucose (noFBG) ≥11 mmol/L (4,16). Prediabetes was defined as FBG 5.6–7.0 mmol/L (or noFBG 7.8–11.0 mmol/L) in diabetes-free individuals (16).
Assessment of Cognitive Domains, Cognitive Impairment, and Dementia
The cognitive battery in SATSA included 11 tests assessing the four specific cognitive domains including verbal abilities (Information, Synonyms, and Analogies), spatial/fluid (Figure logic, Kohs Block Design, and Card rotations), memory (Digit span forwards and backward, Thurstone’s pictures memory, Name and faces immediate and delayed recall), and perceptual speed (Symbol digit, and Figure identification) (17). These domains were identified by principal-component analysis (PCA) as previously described by Finkel and colleagues (18). Briefly, factor analysis was used to construct latent factors from the individual cognitive tests within each domain. An invariant definition of factors at each wave was created by standardizing the cognitive measures relative to the respective means and variances at IPT1, and the loadings from the factor analyses conducted at IPT1 were used to construct the four factors, each of which represented a cognitive domain. Finally, for ease of interpretation, all cognitive factor scores were scaled into T-scores, using factor means and variances from IPT1 (19).
CIND was considered as having objective cognitive impairment, but not severe enough to meet the criteria for dementia diagnosis. Age- and education-specific norms were calculated in the dementia-free population at baseline in SATSA. A person was categorized as having CIND if their Mini-Mental State Examination (MMSE) score at study entry was at least 1 SD or 2 SDs below the age- and education-specific mean MMSE in people aged 50–75 years or ≥75 years, respectively (20). Dementia was diagnosed at baseline and follow-up examinations according to the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Third or Fourth editions (DSM-III or DSM-IV) (21,22).
Statistical Analysis
Differences in baseline characteristics of the participants by diabetes status were assessed using chi-square (χ2) or two-tailed one-way ANOVA followed by pairwise mean comparisons using the Bonferroni correction method. Multivariable linear regressions were used to compare the mean differences in cognitive performances at baseline among different groups (diabetes-free, prediabetes, and diabetes). Linear mixed-effects models were used to estimate the association of diabetes or prediabetes with intercept and annual change in cognitive performance using age as the time scale. Each cognitive domain was used as a separate outcome. As previous studies on cognitive domains in SATSA indicated small practice effect (23,24), we included a time-varying retest covariate (“First cognitive assessment” vs “Follow-up assessment”) in the part of the model where the fixed effect is modeled. The final model included diabetes status (diabetes-free, prediabetes, diabetes), linear term for age at time of assessment, and their interaction terms (diabetes status × age) to estimate the differences in annual rate of change in cognitive performance associated with diabetes status. All models also included a random intercept and a random slope for age allowing individual differences at intercept and over time. The random effect accounted for both the repeated measures for each person and the presence of twin-pairs by using a person-specific identifier and a common twin-pair identifier, in the part where the covariance is modeled. An unstructured variance-covariance matrix was employed in all models. As we intended to observe the cognitive trajectories associated with diabetes status before dementia occurrence, the cognitive data were censored after dementia diagnosis. We also tested whether the cognitive changes over time differed between domains using multivariate mixed-effects models. These models included the scores of the cognitive domains as the dependent variable, and as independent variables: (a) an indicator of the type of cognitive domain (CD type); (b) the diabetes status at study entry; (c) the age at time of the assessment; and (d) a three-way interaction between CD type, diabetes status, and age (CD type × diabetes status × age). The reference domain was memory. Age was centered at 65 years (spatial/fluid, memory, and perceptual speed) or 70 years (verbal) in the analyses based on previous SATSA findings (25). Demographics, birth cohort (19), baseline lifestyle factors, BMI, and CVDs were considered as potential confounders and entered in the mixed-effects models not in interaction with age. We also conducted the following supplementary analyses: (a) modeled diabetes status as a time-varying variable; (b) assessed cognitive trajectories related to incident diabetes and diabetes duration; (c) included participants with CIND and dementia; and (d) sensitivity analysis with multiple imputation. Details on mixed-effects models testing, retest covariate, age centering, and supplementary analyses are provided in the Supplementary Methods.
All statistical analyses were performed with Stata SE 14.0 (StataCorp LP., College Station, TX).
Results
Characteristics of the Study Population at Baseline
Forty-five participants (5.7%) had diabetes and 68 (8.6%) had prediabetes at baseline. Over the follow-up period, 284 (35.8%) participated in all the waves, 120 (15.1%) participated in at least two follow-ups, 28 (3.5%) participated only at baseline assessment, and 361 participants (45.5%) died. Overall, 98 participants (12.4%) had incident diabetes, and 54 (6.8%) developed dementia during the follow-up. At baseline, those participants who developed dementia were significantly older (mean = 68.4, SD = 7.1) than those who remained dementia-free (mean = 62.9, SD = 8.7, p < .001). Compared to diabetes-free participants, those with diabetes or prediabetes were more likely to be older, have a lower MMSE score, and have lower performance in memory and perceptual speed tasks, but were more likely to have hypertension, or a higher BMI (Table 1). In the linear regression models, diabetes and prediabetes were associated with lower performance in memory (β −2.61; 95% CI −5.11, −0.11; p = .040) and speed (β −3.95; 95% CI −7.08, −0.81; p = .014). However, these associations were no longer significant after adjustment for age, sex, education, and birth cohort.
Table 1.
Baseline Characteristics of Cognitively Intact Participants at Baseline by Diabetes Status (n = 793)
| Characteristics | Diabetes-free | Prediabetes | Diabetes | p Value |
|---|---|---|---|---|
| n = 680 | n = 68 | n = 45 | ||
| Age (y) | 63.0 ± 8.2 | 63.4 ± 8.5 | 67.4 ± 8.2a | .004 |
| Female sex | 410 (60.3) | 36 (52.9) | 24 (53.3) | .353 |
| Education | ||||
| Low (≤ 9 y) | 387 (59.1) | 40 (61.5) | 28 (62.2) | .862 |
| High (> 9 y) | 268 (40.9) | 25 (38.5) | 17 (37.8) | |
| Hypertension (yes) | 310 (45.6) | 38 (55.9) | 33 (73.3) | .001 |
| Heart disease (yes) | 79 (11.8) | 10 (15.2) | 9 (20.5) | .200 |
| Stroke (yes) | 7 (1.1) | 0 (0.0) | 0 (0.0) | .559 |
| Any APOE ε4 | 171 (30.5) | 12 (22.2) | 12 (38.7) | .256 |
| BMI (kg/m2) | 25.4 ± 3.8 | 27.7 ± 4.6a | 26.5 ± 3.7 | <.001 |
| Underweight (<20.0) | 34 (5.0) | 1 (1.5) | 4 (8.9) | <.001 |
| Normal (20–25) | 315 (46.3) | 25 (36.8) | 6 (13.3) | |
| Overweight (25–30) | 253 (37.2) | 25 (36.8) | 30 (66.7) | |
| Obese (≥30) | 78 (11.5) | 17 (25.0) | 5 (11.1) | |
| Smoking | 313 (48.2) | 29 (42.7) | 18 (40.0) | .419 |
| Alcohol drinkers | 571 (84.0) | 56 (82.4) | 37 (82.2) | .905 |
| Physical exercise | 580 (88.8) | 55 (82.1) | 39 (86.7) | .256 |
| MMSE | 28.3 ± 1.4 | 28.2 ± 1.7 | 27.8 ± 1.6a | .022 |
| Cognitive performance | ||||
| Verbal abilities | 51.5 ± 9.8 | 52.2 ± 9.6 | 50.8 ± 11.1 | .772 |
| Spatial/fluid abilities | 52.0 ± 10.4 | 51.8 ± 10.9 | 49.4 ± 11.8 | .307 |
| Memory | 51.5 ± 9.9 | 48.9 ± 10.5 | 49.1 ± 8.4 | .048 |
| Perceptual speed | 51.9 ± 10.1 | 51.8 ± 9.1 | 48.0 ± 12.0a | .048 |
Notes: BMI = body mass index; MMSE = Mini-Mental State Examination. Data are presented as n (%) or mean ± SD. Data missing: 28 for education, 27 for MMSE, 15 for heart disease, 29 for stroke, 148 for APOE ε4, 30 for smoking, 28 for physical exercise, 50 for MMSE, 51 for verbal abilities, 62 for spatial/fluid abilities, 43 for memory, and 48 for perceptual speed.
aPairwise mean comparisons (diabetes-free vs prediabetes; diabetes-free vs diabetes; and prediabetes vs diabetes) adjusted with the Bonferroni correction: p value < .05 (the reference group was baseline diabetes-free participants).
Diabetes Status at Baseline and Cognitive Trajectories
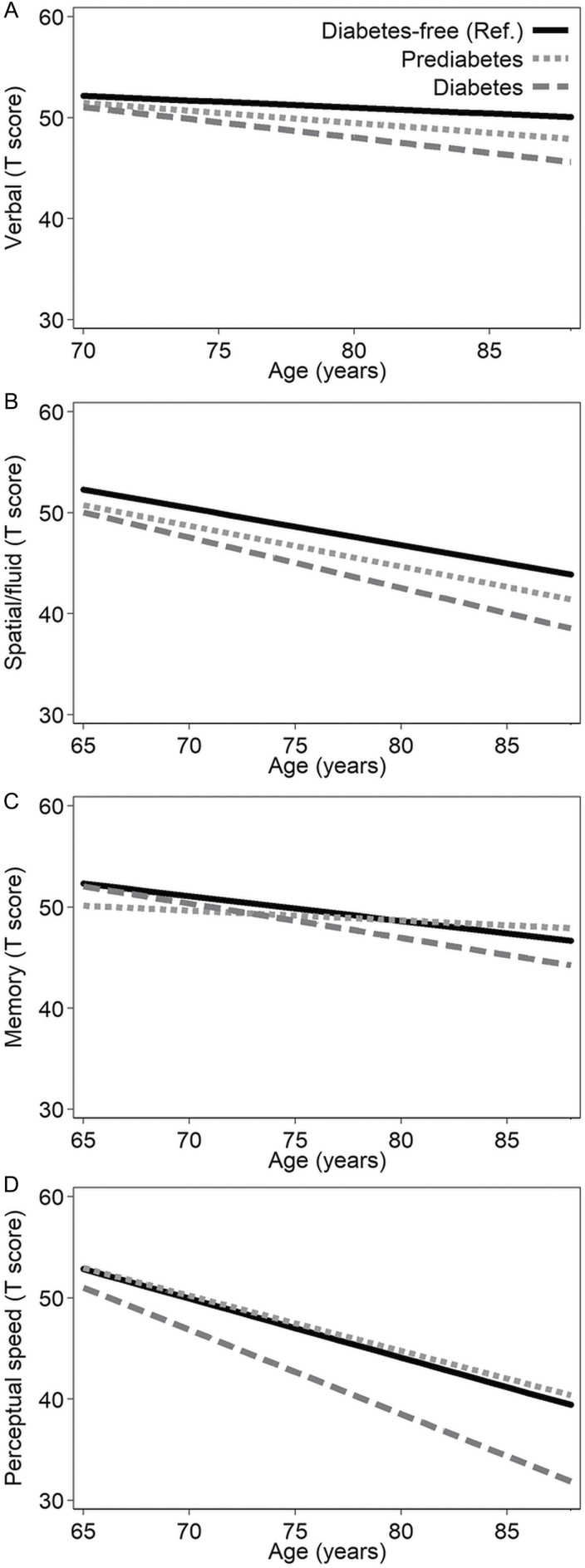
Basic-adjusted (sex, education, birth cohort, and practice effect) linear mixed-effects models showed that older adults with prediabetes had worse memory mean performance than diabetes-free individuals at age 65 (Table 2), but prediabetes was not associated with lower cognitive performance in verbal or spatial abilities, or perceptual speed. Over the follow-up period, compared to diabetes-free, prediabetes was associated with a less steep decline in memory, while diabetes was related to a faster decline in verbal abilities and perceptual speed (Table 2 and Figure 1). These results were similar after further adjustment for smoking, alcohol consumption, physical exercise, BMI, and CVDs (Supplementary Table 1). Figure 1 describes the cognitive trajectories in the four domains among participants with prediabetes and diabetes, compared to diabetes-free participants.
Table 2.
Mixed-Effect Models’ β Coefficients and 95% Confidence Intervals (95% CI) for the Annual Changes in Mean Cognitive Performance in Different Domains Related to Baseline Diabetes Status
| Mixed Models | Exposure Status | Verbal Abilities | Spatial/Fluid Abilities | Memory | Perceptual Speed | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI)a | p | β (95% CI)a | p | β (95% CI)a | p | β (95% CI)a | p | ||
| Interceptb | Diabetes-free (Ref.) | 49.23 (47.92; 50.54) | 51.43 (50.01; 53.84) | 48.28 (47.0; 49.60) | 48.93 (47.61; 50.26) | ||||
| Prediabetes | −0.70 (−2.57; 1.16) | .458 | −1.53 (−3.53; 0.47) | .133 | −2.19 (−4.16; 0.10) | .035 | 0.12 (−1.71; 1.94) | .900 | |
| Diabetes | −1.12 (−3.39; 1.16) | .337 | −2.23 (−4.89; 0.42) | .099 | −0.28 (−2.87; 2.64) | .839 | −1.83 (−4.21; 0.55) | .132 | |
| Annual decline (age, years)b | Diabetes-free | −0.12 (−0.15; −0.08) | .000 | −0.36 (−0.41; −0.32) | .000 | −0.25 (−0.30; −0.20) | .000 | −0.58 (−0.63; −0.53) | .000 |
| Prediabetes | −0.08 (−0.19; 0.03) | .159 | 0.04 (−0.18; 0.10) | .583 | 0.15 (0.00; 0.30) | .050 | 0.04 (−0.11; 0.18) | .628 | |
| Diabetes | −0.19 (−0.33; −0.04) | .014 | −0.14 (−0.32; 0.05) | .161 | −0.10 (−0.30; 0.11) | .374 | −0.25 (−0.44; −0.05) | .012 | |
Notes: aMixed-effect models adjusted for sex, education, birth cohort, and practice effect. Cognitive data were censored after diagnosis of dementia. The reference group was diabetes-free participants at baseline.
bAge was centered at age 65 (memory, spatial/fluid, and perceptual speed) or age 70 (verbal) years.
Figure 1.
Age-related cognitive trajectories in different domains by baseline diabetes status. The figure shows the age-related cognitive trajectories in verbal (A), spatial/fluid (B), memory (C), and perceptual speed (D) domains. Mixed-effect models are adjusted for sex, education, birth cohort, and practice effect. Cognitive data were censored after diagnosis of dementia. The presented cognitive trajectories were plotted based on the mean value of the covariates including sex, education, birth cohort, and practice effect. Age was centered at age 65 (spatial/fluid, memory, and perceptual speed) or age 70 (verbal). The reference group was baseline diabetes-free participants.
We also tested whether the annual cognitive changes differed between domains among individuals with diabetes (n = 45) compared to diabetes-free (n = 680) at baseline. Multivariate mixed models adjusted for education, birth cohort, and retest effect showed that the annual decline in verbal abilities (β −0.24; 95% CI −0.44, −0.06; p = .011) and spatial/fluid abilities (β −0.26; 95% CI −0.46, −0.06; p = .009) was greater than the annual decline in memory (reference domain). Similar but not statistically significant results were observed for perceptual speed (β −0.12; 95% CI −0.31, 0.07; p = .213).
Discussion
In this prospective population-based study of cognitively intact older people, we found that (a) baseline diabetes was associated with a faster decline in perceptual speed and verbal abilities over time; and (b) baseline prediabetes was associated with lower performance and less steep decline in memory over the follow-up.
Although prediabetes has been related to dementia risk (4), the association between prediabetes and specific cognitive abilities remains unclear. To our knowledge, only five studies have previously examined the relationship between prediabetes and cognitive decline in different domains, all reporting a lack of association (5,7,26–28). In our study, we observed that prediabetes at study-entry was related to poor memory performance, yet prediabetes is not a chronic condition, thus transitions may occur to diabetes or to diabetes-free over time. When we considered this non-chronicity in the data analyses, prediabetes became unassociated with poor memory function at any time point. Previous studies examining the association between diabetes and declines in different cognitive domains have produced mixed results (5,29–32). Diabetes was found to be associated with a faster decline in perceptual speed and executive function tasks in middle-aged adults, but not with episodic memory (32). Conversely, another large population-based study including middle-aged (mean age 54.4 years) dementia-free older adults found a faster memory decline over 10 years associated with diabetes, but not in the measure of executive function (5). Similar results were also found in other population-based studies with shorter follow-up times or including older participants (11,30,31). Finally, Köhler and colleagues (29) followed dementia-free participants aged 75 years and older for 4.5 years and found no effect of diabetes on any cognitive domain. Variability in these results may be explained by different methodological discrepancies, such as the different age ranges of the study populations, inclusion of people with cognitive impairment, shorter follow-up periods, assessments of cognitive function through screening tools or single cognitive tests rather than composite measures of the domains, and differences in the modeling of cognitive function over time. In our study, we also observed a faster cognitive decline in perceptual speed and verbal abilities among older adults with diabetes. This pattern differs from the cognitive progression observed in Alzheimer’s disease dementia, characterized by disturbances of episodic memory at early stages of the disease (33,34). Indeed, in our study we found no association between diabetes and memory decline, even when individuals with cognitive impairment and dementia were included in the analyses. These results seem to support the existence of vasculopathic process, rather than neurodegenerative, underpinning the diabetes-related cognitive decline. However, our results should be interpreted with caution until future replications are performed in other cohorts.
Chronic hyperglycemia has been linked to poor cognitive perceptual speed in middle-aged adults without diabetes (35). The brain is highly susceptible to variations in glucose blood levels, and many negative consequences may result from hyperglycemia, such as neuronal death, which is known to lead to cognitive decline over time (36). At the biochemical level, hyperglycemia may induce alterations in both neuronal and glial cell functioning, and promote the production of reactive oxygen species (ROS), resulting in oxidative stress, formation of advanced glycosylation end-products (AGEs), activation of AGE receptors, and finally atherosclerosis (37,38). At the structural level, diabetes has been associated with brain atrophy, especially in the regions around the ventricles (such as subcortical grey matter or white matter regions) and an increased burden of small vessel diseases, suggesting that diabetes-related cognitive decline can be due to brain vascular damages rather than Alzheimer’s-type pathology (39). Additionally, a recent autopsy study has shown that diabetes is associated with brain infarction, especially lacunes, but not Alzheimer’s disease neuropathology, further supporting a vascular etiology in diabetes-related cognitive decline (40). Our supplementary findings show that long-duration diabetes (reflecting a long-term exposure to hyperglycemia) was associated with a decline in spatial/fluid abilities, reinforcing the importance of hyperglycemia in the etiology of cognitive impairment in diabetes. However, more research combining cognitive, biochemical, neuroimaging, and neuropathological data is needed to pinpoint the underlying mechanisms.
The major strengths of our study are the population-based cohort, the long-term follow-up period, and the repeated measures of diabetes status and cognitive functioning. Additionally, cognitive domains were assessed as composite outcome measures that have several advantages, such as increasing reliability, and reducing the measurement error (ie, ceiling and floor effects) and the error variance associated with single cognitive tests. However, some limitations need to be pointed out. First, the small sample size in the stratified analyses might have led to a lack of power to detect some of the group differences and to an underestimation of the point estimates. Second, diabetes is associated with an increased risk of dementia and mortality. Consequently, assessing dementia-free participants who survived through follow-ups might have led to an underestimation of the association between diabetes and cognitive decline. However, the information from individuals who became demented contributed to the analyses prior to dementia diagnoses, and presumably contributed to information on decline. Furthermore, analyses using multiple imputation produced estimates with similar magnitude and direction for most cognitive domains. Finally, blood glucose has been used as a “gold standard” for the diagnosis of diabetes for decades before glycated hemoglobin (HbA1C) was introduced in 2010 (41), many years after SATSA started. Fasting information (ie, numbers of hours and status) were self-reported in SATSA, thus recall bias could not be ruled out. However, participants were relatively cognitively intact and those tested before lunch or who had already diabetes were specifically instructed to fast before the blood sample collection. Our results can be generalized to Western populations with characteristics similar to those in the SATSA, but caution is needed when generalizing our results to younger populations.
In summary, diabetes is associated with a faster decline in perceptual speed and verbal abilities, while prediabetes is associated with lower memory performance in middle-age. The detrimental effect of hyperglycemia on cognitive function may start before diabetes onset, already during the prediabetes stage in older adults. However, memory seems to be less affected by the detrimental effect of hyperglycemia over time. Our findings provide further support for the detrimental effect of diabetes on cognitive decline in older adults, and added new knowledge on the potential effect of prediabetes on cognitive decline. Future studies are need to further address whether an effective glycemic control in middle-age and its maintenance over time might be a key in hindering diabetes-related cognitive decline.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The Swedish Adoption/Twin Study of Aging was supported by grants from the National Institutes of Health (AG04563, AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Working Life and Social Research (97:0147:1B, FAS 2009-0795), and the Swedish Research Council (825-2007-7460, 825-2009-6141). This work was further supported by the Swedish Research Council for Health, Working Life and Welfare (2013–2292), the Swedish Research Council (521-2013-8689). This study also received grants from the Nationella forskarskolan om åldrande och hälsa (SWEAH), Gun och Bertil Stohnes Stiftelsen, Sigurd och Elsa Goljes Minne Stiftelsen, and Gamla Tjänarinnor Stiftelsen for the purpose of travel to international conferences. This project is also part of CoSTREAM (www.costream.eu) and received funding from the European Union’s Horizon 2020 research and innovation programme under the grant agreement (No 667375). W.X. was also supported by Demensfonden and Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse.
Conflict of Interest
No potential conflicts of interest relevant to this article were reported.
Supplementary Material
Acknowledgments
The authors would like to express their gratitude to the participants and staff involved in the data collection and management in the SATSA. In addition, we are grateful to Emerald G. Heiland, Aging Research Center, Karolinska Institutet, for her suggestions on the English in the article.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas, 7th ed 2015. Brussels, Belgium: International Diabetes Federation; http://www.diabetesatlas.org Accessed December 02, 2016. [Google Scholar]
- 2. Alzheimer’s Disease International. World Alzheimer report 2015: the global impact of dementia 2015. https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf Accessed December 02, 2016.
- 3. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi:10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 4. Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi:10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 5. Tuligenga RH, Dugravot A, Tabák AG et al. . Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. Lancet Diabetes Endocrinol. 2014;2:228–235. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayeda ER, Haan MN, Neuhaus J et al. . Type 2 diabetes and cognitive decline over 14 years in middle-aged African Americans and whites: the ARIC Brain MRI Study. Neuroepidemiology. 2014;43:220–227. doi:10.1159/000366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samaras K, Lutgers HL, Kochan NA et al. . The impact of glucose disorders on cognition and brain volumes in the elderly: the Sydney Memory and Ageing Study. Age (Dordr). 2014;36:977–993. doi:10.1007/s11357-013-9613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spauwen PJ, Köhler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care. 2013;36:1554–1561. doi:10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knopman DS, Mosley TH, Catellier DJ, Coker LH; Atherosclerosis Risk in Communities Study Brain MRI Study Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi:10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 10. van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015–2023. doi:10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 11. Hassing LB, Johansson B, Pedersen NL, Nilsson SE, Berg S, McClearn G. Type 2 diabetes mellitus and cognitive performance in a population-based sample of the oldest old: impact of comorbid dementia. Aging Neuropsychology and Cognition. 2003;10:99–107. doi:10.1076/anec.10.2.99.14458 [Google Scholar]
- 12. Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi:10.1046/j.1365-2796.2002.01032.x [DOI] [PubMed] [Google Scholar]
- 13. Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish Adoption/Twin Study of Aging. Aging Neuropsychology and Cognition. 2004;11:325–345. doi:10.1080/13825580490511152 [Google Scholar]
- 14. Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. Int J Obes (Lond). 2013;37:296–302. doi:10.1038/ijo.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi:10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes care. 2013;36(suppl 1):S11–S66. doi:10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3:346–352. doi:10.1111/j.1467-9280.1992.tb00045.x [Google Scholar]
- 18. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. doi:10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- 19. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Cohort differences in trajectories of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2007;62:P286–P294. doi:10.1093/geronb/62.5.P286 [DOI] [PubMed] [Google Scholar]
- 20. Marseglia A, Fratiglioni L, Laukka EJ et al. . Early cognitive deficits in type 2 diabetes: a population-based study. J Alzheimers Dis. 2016;53:1069–1078. doi:10.3233/JAD-160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gatz M, Pedersen NL, Berg S et al. . Heritability for Alzheimer’s disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–M125. doi:10.1093/gerona/52A.2.M117 [DOI] [PubMed] [Google Scholar]
- 22. Bokenberger K, Pedersen NL, Gatz M, Dahl AK. The type A behavior pattern and cardiovascular disease as predictors of dementia. Health Psychol. 2014;33:1593–1601. doi:10.1037/hea0000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finkel D, Reynolds CA, Larsson M, Gatz M, Pedersen NL. Both odor identification and ApoE-ε4 contribute to normative cognitive aging. Psychol Aging. 2011;26:872–883. doi:10.1037/a0023371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies G, Harris SE, Reynolds CA et al. . A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol Psychiatry. 2014;19:76–87. doi:10.1038/mp.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39:535–550. doi:10.1037/0012-1649.39.3.535 [DOI] [PubMed] [Google Scholar]
- 26. Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi:10.1212/01.WNL.0000134666.64593.BA [DOI] [PubMed] [Google Scholar]
- 27. Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med. 2004;164:1327–1333. doi:10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 28. Fontbonne A, Berr C, Ducimetière P, Alpérovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–370. doi:10.2337/diacare.24.2.366 [DOI] [PubMed] [Google Scholar]
- 29. Köhler M, Kliegel M, Kaduszkiewicz H et al. ; Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) study group. Effect of cardiovascular and metabolic disease on cognitive test performance and cognitive change in older adults. J Am Geriatr Soc. 2012;60:1286–1291. doi:10.1111/j.1532-5415.2012.04032.x. [DOI] [PubMed] [Google Scholar]
- 30. Maggi S, Limongi F, Noale M et al. ; ILSA Study Group. Diabetes as a risk factor for cognitive decline in older patients. Dement Geriatr Cogn Disord. 2009;27:24–33. doi:10.1159/000183842. [DOI] [PubMed] [Google Scholar]
- 31. Okereke OI, Kang JH, Cook NR et al. . Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. J Am Geriatr Soc. 2008;56:1028–1036. doi:10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 32. Rawlings AM, Sharrett AR, Schneider AL et al. . Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med. 2014;161:785–793. doi:10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dubois B, Feldman HH, Jacova C et al. . Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi:10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 34. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment.5th ed. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 35. Sanz CM, Ruidavets JB, Bongard V et al. . Relationship between markers of insulin resistance, markers of adiposity, HbA1c, and cognitive functions in a middle-aged population-based sample: the MONA LISA study. Diabetes Care. 2013;36:1512–1521. doi:10.2337/dc12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145–171. doi:10.1007/s12035-012-8339-9. [DOI] [PubMed] [Google Scholar]
- 37. Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a “glycoxidation-centric” point of view. Nutr Metab Cardiovasc Dis. 2013;23:913–919. doi:10.1016/j.numecd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 38. González-Reyes RE, Aliev G, Ávila-Rodrigues M, Barreto GE. Alterations in glucose metabolism on cognition: a possible link between diabetes and dementia. Curr Pharm Des. 2016;22:812–818. doi:10.2174/1381612822666151209152013 [DOI] [PubMed] [Google Scholar]
- 39. Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI?Diabetes. 2014;63:2244–2252. doi:10.2337/db14-0348. [DOI] [PubMed] [Google Scholar]
- 40. Abner EL, Nelson PT, Kryscio RJ et al. . Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12:882–889. doi:10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi:10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.