Abstract
Disruption of the growth hormone (GH) signaling pathway promotes insulin sensitivity and is associated with both delayed aging and extended longevity. Two kinds of long-lived mice—Ames dwarfs (df/df) and GH receptor gene–disrupted knockouts (GHRKO) are characterized by a suppressed GH axis with a significant reduction of body size and decreased plasma insulin-like growth factor-1 (IGF-1) and insulin levels. Ames dwarf mice are deficient in GH, prolactin, and thyrotropin, whereas GHRKOs are GH resistant and are dwarf with decreased circulating IGF-1 and increased GH. Crossing Ames dwarfs and GHRKOs produced a new mouse line (df/KO), lacking both GH and GH receptor. These mice are characterized by improved glucose tolerance and increased adiponectin level, which could imply that these mice should be also characterized by additional life-span extension when comparing with GHRKOs and Ames dwarfs. Importantly, our longevity experiments showed that df/KO mice maintain extended longevity when comparing with N control mice; however, they do not live longer than GHRKO and Ames df/df mice. These important findings indicate that silencing GH signal is important to extend the life span; however, further decrease of body size in mice with already inhibited GH signal does not extend the life span regardless of improved some health-span markers.
Keywords: Ames dwarf mice, GHRKO mice, Dwarfism, Longevity, Insulin signaling
Ames dwarf (df/df) mice are characterized by a spontaneous recessive loss-of-function mutation in the Prophet of pituitary factor-1 (Prop-1) gene (1), which is upstream of pituitary factor-1 (Pit-1) (2) and responsible for differentiation of somatotrophs, lactotrophs, and thyrotrophs (1). This mutation promotes severe hormonal disturbances, including dramatic declines in circulating growth hormone (GH), prolactin (PRL), and thyrotropin (TSH). Moreover, these animals also display extremely low circulating insulin-like growth factor-1 (IGF-1) levels (3), enhanced insulin sensitivity (4,5), increased circulating adiponectin, and decreased proinflammatory mediators (tumor necrosis factor α [TNFα] and interleukin [IL]-6) (6), with reduced DNA and protein oxidation in the liver (7). As a consequence, df/df mice live up to ~70% longer than their normal counterparts (8).
Mice homozygous for targeted disruption of the GH receptor (GHR)/GH-binding protein gene (Ghr/bp gene), known as GHR/GH-binding protein knockout (GHR knockout [GHRKO]; Ghr/bp−/−) or “Laron dwarfs” (9,10), constitute a mouse model for human Laron syndrome. Similar to df/df, GHRKO mice live much longer (~55% longer) than normal littermates and are characterized by reduced body size with concurrent obesity, elevated serum GH levels, and greatly reduced circulating insulin and IGF-1 levels with improved insulin sensitivity. GHRKO mice also have reduced oxidative damage and enhanced oxidative stress resistance (9,11–19). GHRKO mice also display decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis, including peroxisome proliferator-activated receptor gamma [PPARγ] coactivator-1α (PGC-1α) (20), lower incidence and delayed onset of fatal neoplastic diseases (21), and reduced proinflammatory mediators with beneficial changes in circulating apolipoproteins (22).
It is well established that adipose tissue is as an important endocrine organ that secretes a myriad of adipokines, cytokines, and chemokines, thereby modulating systemic metabolic and inflammatory homeostasis (23). Moreover, the site of fat accumulation may be relevant for (patho)physiological consequences. Namely, visceral (intra-abdominal, “central”) obesity may promote insulin resistance and development of type 2 diabetes (24). Conversely, peripheral obesity (increased amount of subcutaneous fat) may increase insulin sensitivity and decrease risk of type 2 diabetes (25,26). Therefore, the mechanisms that regulate the depot-specific production and secretion of adipokines, cytokines, and chemokines are important to be understood in the context of longevity. Overall, high levels of inflammatory cytokines, including IL-1, IL-6, and TNFα, as well as macrophage inflammatory proteins (MIPs; eg, MIP-1α [CCL3] and MIP-1β [CCL4]) (27) can have detrimental effects on health span and promote aging. In turn, IL-10 is an anti-inflammatory cytokine and may inhibit the synthesis of proinflammatory cytokines such as interferon γ (IFNγ) or TNFα. Moreover, increased level of plasminogen activator inhibitor-1 (PAI-1) is a known risk factor for thrombosis and atherosclerosis (28). Another factor, vascular endothelial growth factor (VEGF), is a signal protein that stimulates angiogenesis. Also, the mitogen-activated protein kinases (MAPKs), including p38α/β, are involved in different physiological pathways and participate in the regulation of cell proliferation, differentiation, or survival. These diverse functions of different cytokines and delicate balance between proinflammatory and anti-inflammatory cytokines during aging process make it important to determine the levels of these cytokines in our studied model, to assess the level of inflammation in healthy, long-living animals when comparing with N control mice.
Our previous studies showed that both df/df and GHRKO mice are not only living longer but also characterized by improved health span with concomitant improvement of insulin sensitivity and low inflammatory status (5,6,20). However, regardless of similar prolongevity characteristics observed in df/df and GHRKO mice, we found some differences indicating that these two different mutant mice might have some overlapping characteristics, but the mechanism responsible for improved health span is not identical. One of the most striking differences was different response to calorie restriction. Assuming that these two unique animal models might carry different and more importantly independent mechanisms promoting extended longevity and health span, we have decided to evaluate the effects of crossing Ames dwarfs and GHRKO knockouts and to determine if the double mutant (df/KO) will display synergistic effects on adipose tissue, metabolic homeostasis, inflammatory parameters, and longevity. In the present study, we assessed df/KO life span and body weights, as well as levels of proinflammatory (IL-6, TNFα, IFNγ) and anti-inflammatory cytokines (IL-10, MIPs [MIP-1α and MIP-2]), PAI-1, and the angiogenic factor (VEGF) in a subcutaneous and epididymal visceral adipose tissue. We have also assessed the gene expression of factors involved in lipid metabolism (peroxisome proliferator-activated receptor α [PPARα] and PPARγ), key regulators of mitochondrial biogenesis (PGC-1α and sirtuin-1 [Sirt-1]), insulin signaling-related components (insulin receptor [IR], glucose transporter type 2 [GLUT-2], AKT-1), IGF-1, and MAPK signaling pathways (p38 MAPK) in the liver, pituitary, and hypothalamus. Furthermore, we assessed insulin sensitivity of these animals and the absolute and relative weights (percent of body weight) of several organs of df/KO mice. Interestingly, the relative weight of some organs, for example, brain, is intriguingly increased in dwarf mice when compared to normal animals (29).
Materials and Methods
Animals
Normal wild-type (N), GHR knockout (GHRKO), and Ames dwarf (df/df) mice were produced in our breeding colony. Importantly, as a basic goal of our studies, we produced a new double-mutant mouse lacking both circulating GH and GHR (df/KO) by crossing Ames dwarfs and GHRKO. df/KO male and female animals were housed under controlled temperature and light conditions (22±2°C with a 12-hour light/12-hour dark cycle) and were provided food ad libitum with a nutritionally balanced diet (Rodent Laboratory Chow 5001: 23.4% protein, 4.5% fat, 5.8% crude fiber; LabDiet PMI Feeds, St Louis, MO). All animal procedures were approved by the Laboratory Animal Care and Use Committee at the Southern Illinois University School of Medicine (Springfield, IL). For cytokines, hormones, and chemokines analyses, the animals comprised four experimental groups including male mice only: N (n = 10), GHRKO (n = 12), df/df (n = 11), and df/KO (n = 10).
Life-Span Experiment
All animals (n = 23–43 per genotype per sex) were checked daily for health and survival and were handled for cage changes without any experimental manipulations. Following our previous protocol and approved IACUC criteria, natural death was considered for collecting longevity data unless animal appeared to be near death (listless, unable to walk, and cold to the touch) or had large bleeding tumors or neoplastic growth approaching 10% of body weight; then, the euthanasia was performed and the date of euthanasia was considered the date of death.
RNA Extraction and Complementary DNA Transcription
Before the collection of tissues, the mice were fasted overnight, anesthetized using ketamine/xylazine, bled by cardiac puncture, and euthanized by decapitation. Livers, pituitary glands, and hypothalami, as well as subcutaneous and epididymal adipose tissues were rapidly collected, snap frozen on dry ice, and stored at −80°C until processed. Whole blood was centrifuged in order to isolate the plasma supernatant, which was also stored at −80°C.
RNA was extracted from the liver, pituitary, and hypothalamus homogenates, using a miRNeasy Mini Kit (Qiagen) in accordance with the manufacturer’s instruction. RNA quantity and quality were analyzed using a NanoDrop 1000 Spectrophotometer (Thermo Scientific). The RNA concentration was measured spectrophotometrically at 260nm. One microgram of total RNA was subjected to electrophoresis on a 1.5% agarose gel to confirm RNA integrity. Potentially contaminating residual genomic DNA was eliminated using DNase I (Promega, Madison, WI). Reverse transcription was performed, and complementary DNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instruction.
Real-Time Polymerase Chain Reaction
The real-time polymerase chain reaction (RT-PCR) was carried out using the Smart Cycler instrument (Cepheid, Sunnyvale, CA) with iQ SYBR Green Supermix (Bio-Rad Laboratories). The three steps of the PCR included denaturation at 94°C for 2 minutes, annealing at 62°C for 30 seconds with fluorescence reading, and extension at 72°C for 30 seconds. In addition, a melting curve was done for each reaction to evaluate the potential of nonspecific products. β2-Microglobulin (B2M), which was previously validated in our laboratory as the most appropriate gene for normalizing the data (eg, 5, 20), was used as a housekeeping gene. Gene expression was assessed by measuring steady state levels of mRNA. Relative expression from RT-PCR was calculated using the equation 2A−B/2C−D (where A = cycle threshold [Ct] number for the gene of interest in the first control sample; B = Ct number for the gene of interest in the analyzed sample; C = Ct number for the housekeeping gene in the first control sample; D = Ct number for housekeeping gene in the analyzed sample). The first control was expressed as 1.00 by this equation, and all other samples were calculated in relation to this value. Then, the results in the control group (N) were averaged. All other outputs were divided by the mean value of the relative expression in the control group to yield the fold change of the expression of genes of interest compared to the control group. For RT-PCR, the primers used are listed in Supplementary Table S1.
Assessment of Blood Chemistry
Glucose was measured in blood collected via the tail vein using a glucometer ONE Touch Ultra (Life Scan, Milpitas, CA). Plasma was used for assessment of insulin and adiponectin. Plasma insulin levels were determined using Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem, Downers Grove, IL), and plasma adiponectin levels were determined using Mouse Adiponectin ELISA Kit (Linco Research, St Charles, MO) according to the manufacturers’ protocol.
Insulin Tolerance Test
Approximately 11-month-old male mice were injected intraperitoneally with 0.75 IU insulin/kg of body weight (n = 11–20 per group). Blood glucose levels were measured at the time points: 0, 15, 30, and 60 minutes, using a glucometer ONE Touch Ultra (Life Scan).
Glucose tolerance Test
Approximately 11-month-old male mice were injected with 2g of glucose per 1kg of body weight, and the glucose levels were measured at different time points (n = 10–15 per group): 0, 15, 30, 60, and 120 minutes, using a glucometer ONE Touch Ultra (Life Scan).
Subcutaneous and Epididymal Fat Tissue Assay
Milliplex Luminex 200 system (Merck, KGaA, Darmstadt, Germany) was used to determine the protein levels of leptin, TNFα, PAI-1, IL-6, IFNγ, MIP-1α, MIP-2, VEGF, and IL-10 following the protocol provided by manufacturer.
Statistical Analysis
These data are expressed as mean ± SEM. To evaluate the effects of the genotype, we used two-way analysis of variance. For analyzing differences between group means, we used a Bonferroni post hoc test. A value of p < .05 was considered statistically significant. All statistical calculations were conducted using SPSS version 17.0 (SPSS, Chicago, IL) with α = 0.05. Survival analysis was performed on GraphPad Prism 5 using the log rank test, with female and males curves being compared separately. All graphs were created using Prism 4.02 (GraphPad Software, San Diego, CA).
Results
Life Span
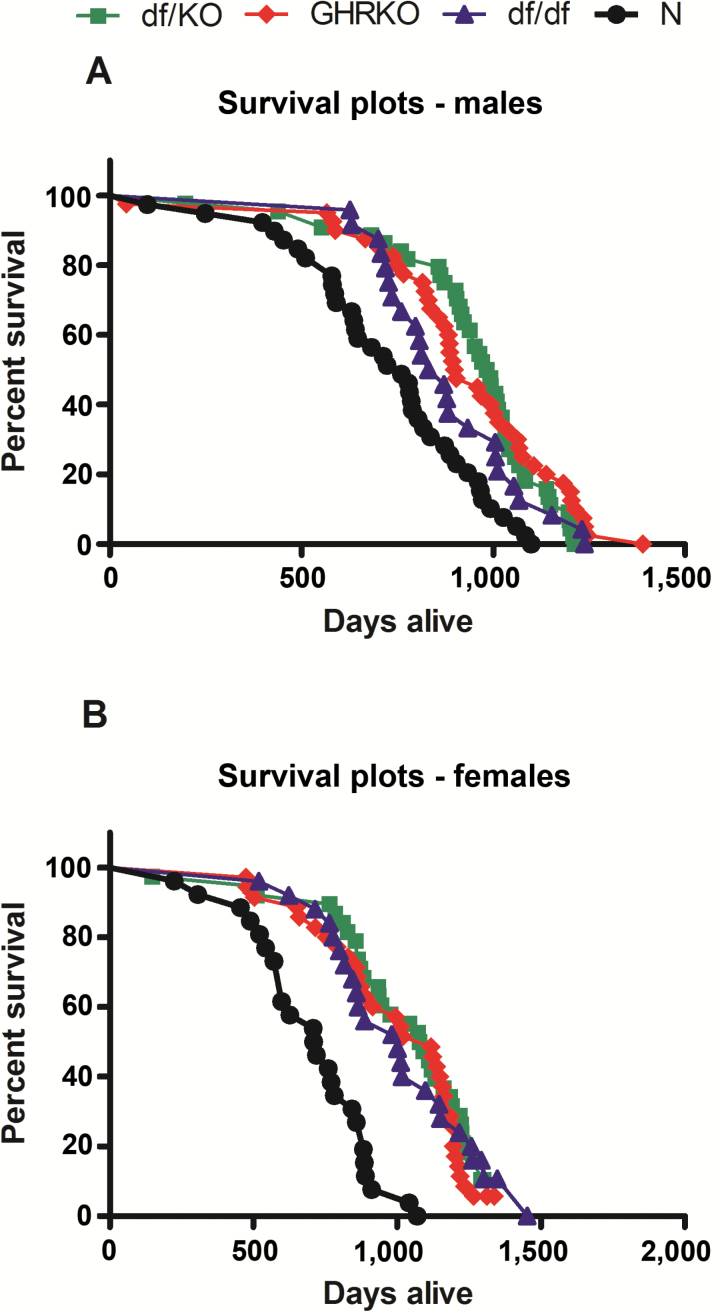
Both male and female double-mutant df/KO mice lived longer (mean life span) when compared to N mice (p < .001 each), although there were no significant differences between these mutants and GHRKO or df/df animals (Figure 1A and B). Also, male and female GHRKO and Ames df/df mice lived longer than N mice (males: p = .001, p = .044 vs N mice, respectively; females: p < 0.001 vs N mice both; Figure 1A and B). The mean, minimum, median, and maximum life span for female and male df/KO, GHRKO, Ames df/df, and N mice are shown in Supplementary Table S2.
Figure 1.
Survival plot of normal (N), growth hormone receptor knockout (GHRKO), Ames dwarf (df/df), and double-mutant (df/KO) mice for males (A) and females (B).
Plasma Adiponectin and Insulin
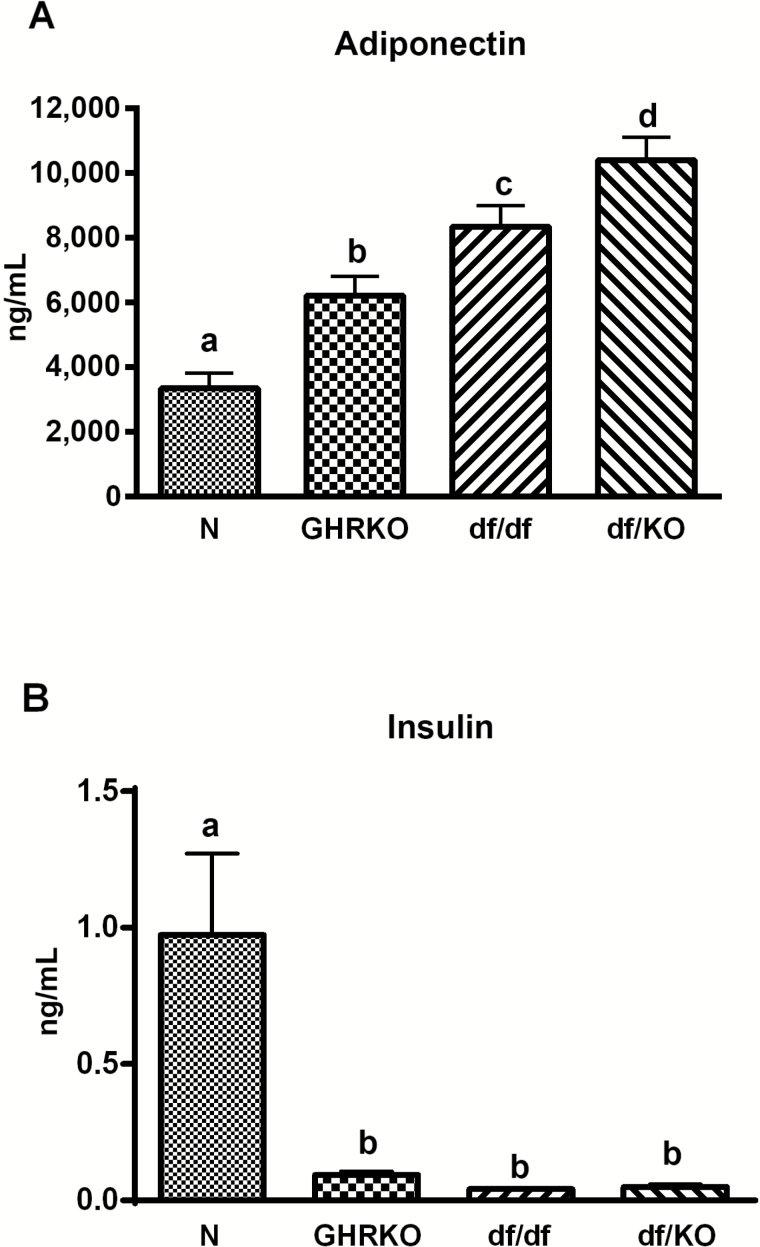
Plasma adiponectin was increased in df/KO mice when comparing with N, GHRKO, and df/df animals (p < .001, p < .024, and p < .0457, respectively). The level of this adipokine was also increased in df/df mutants and GHRKO animals in comparison to N mice (p < .001 and p = .012, respectively; Figure 2A). In contrast, plasma insulin levels were severely decreased in df/df mice (p < .001 vs N mice) as well as in df/KO and GHRKO animals (p = .001 vs N for both mutant animals; Figure 2B).
Figure 2.
Plasma level of adiponectin (A) and insulin (B) in normal (N), growth hormone receptor knockout (GHRKO), Ames dwarf (df/df), and double-mutant (df/KO) mice. Values are means ± SEM. a, b, c—values that do not share the same letter in the superscript are statistically different (p < .05).
Insulin Tolerance Test and Glucose Tolerance Test
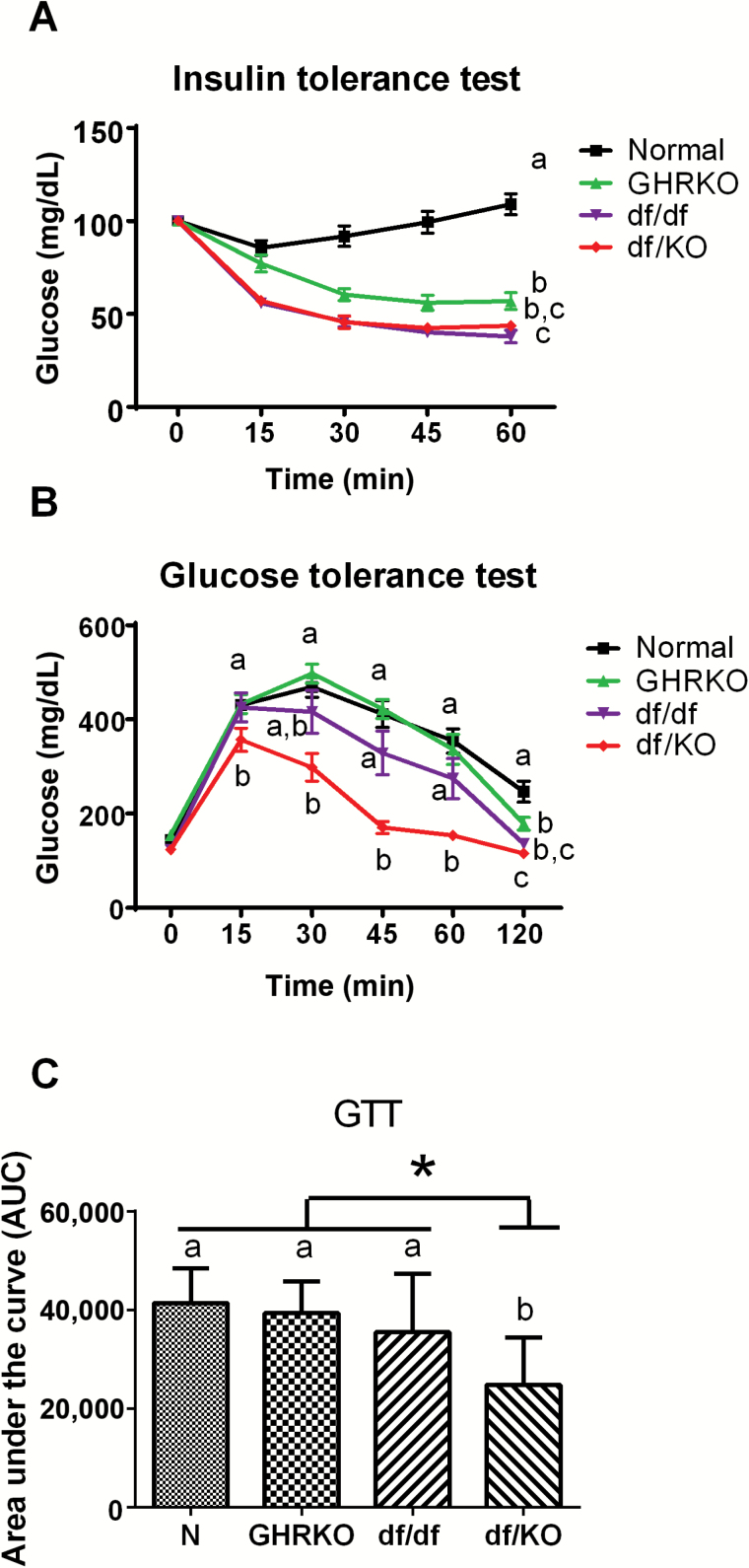
The insulin tolerance test (ITT) revealed that df/KO mice had greater insulin responsiveness than GHRKO and N mice (p = .029 and p < .001, respectively; Figure 3A), whereas no difference was observed between df/KO and df/df mice (Figure 3A). In alignment with these observations, the glucose tolerance test (GTT) revealed that df/KO mice had greater capacity for glucose disposal at 45 minutes (p < .001, p = .001, and p < .001, respectively) and 60 minutes (p < .001, p = .017, and p < .001, respectively) when compared to GHRKO, df/df, and N mice (Figure 3B). For example, there was already a difference between df/KO and GHRKO or N mice after 15 minutes (p < .001 and p = .002, respectively), which persisted after 120 minutes (p = .006 and p < .001, respectively; Figure 3B).
Figure 3.
Insulin tolerance test (ITT) (A), glucose tolerance test (GTT) (B), and area under the curve (AUC) (C) in normal (N), growth hormone receptor knockout (GHRKO), Ames dwarf (df/df), and double-mutant (df/KO) mice. Values are means ± SEM. a, b, c—values that do not share the same letter in the superscript are statistically different (*p < .05).
Additionally, the area under the curve of performed GTT also indicated improved glucose tolerance in df/KO mice when comparing with N, GHRKO, and df/df animals (p < .0001, p < .0004, and p < .0290, respectively; Figure 3C).
Subcutaneous Adipose Tissue
Double-mutant df/KO had increased levels of TNFα (p = .012 vs GHRKO mice) and IL-6 (p = .04 vs df/df mice) in subcutaneous fat (Supplementary Figures S1B and S1D, respectively). IL-10 showed only a tendency for elevated level in df/KOs compared to GHRKO, df/df, and N mice, but there were no significant differences between genotypes (p = .365; Supplementary Figure S2D). Furthermore, increased value of VEGF was observed in GHRKO dwarfs (p = .002 vs N mice; Supplementary Figure S2C). On the contrary, decreased values of leptin were found in df/df mice (p = .006 vs GHRKO and p = .008 vs N animals) and MIP-1α in GHRKOs and df/df animals (p = .002 vs N mice and p = .007 vs N animals, respectively; Supplementary Figures S1A and S2A, respectively).
Epididymal Adipose Tissue
In epididymal fat, decreased values for IL-6 (p = .016 vs N mice) and IFNγ (p = .02 vs N mice) were observed in df/KO double mutants (Supplementary Figures S3D and S3E, respectively). Moreover, a very low IFNγ level was observed in df/df mice (p = .036 vs N mice; Supplementary Figure S3E). Similar to subcutaneous fat tissue, decreased value of leptin was observed in df/df mice (p < .001 vs N animals; Supplementary Figure S3A). Also, a low leptin level was detected in df/KO animals (p = .003 vs N mice and p = .001 vs GHRKOs; Supplementary Figure S3A). Moreover, decreased value of MIP-1α was observed in df/df mice (p = .015 vs N mice; Supplementary Figure S3F).
Gene Expression
Importantly, highly suppressed mRNA levels for IGF-1 were detected in the liver of df/KO, GHRKO, and Ames dwarf mutants (all p < .001 vs N mice; Supplementary Figure S4A). Moreover, df/KO mice had increased hepatic gene expression levels for PPARα (p = .007 vs N mice), PPARγ (p < .001 vs N mice), IR (p < .001 vs N mice and p = .003 vs GHRKO mice), p38 (p = .046 vs N mice), and GLUT-2 (p = .015 vs N mice; Supplementary Figures S5A, S5B, S5C, S5E, and S5F, respectively). Sirtuin-1 only showed a slight tendency for higher mRNA levels in df/KO mice compared to KO, df/df, and N animals; however, there was no significant genotype effect (p = .336; Supplementary Figure S5D).
In the pituitary gland, df/KO mice had increased gene expression levels for PPARα (p = .004 vs N mice and p = .021 vs GHRKO mice), AKT-1 (p = .006 vs N mice and p = .052 vs GHRKO mice), PGC-1α (p = .02 vs N mice), and IGF-1 (p = .001 vs N mice, p = .0017 vs GHRKO mice; Supplementary Figures S6A, S6C, S6D, and S4B, respectively).
In the hypothalamus, only a tendency for increased mRNA level for IGF-1 was observed in df/KO mice, but there were no significant differences (p = .13; Supplementary Figure S4C). For PPARα, PPARγ, AKT-1, and PGC-1α, no significant genotype effects were observed (Supplementary Figures S7A–S7D).
Body and Organ Weights
Double-mutant df/KO had decreased body weight as compared to N and GHRKO mice (p < .001 and p = .003, respectively; Supplementary Figure S8A). df/KO mice also had decreased weight of the brain, heart, liver, kidney, spleen, pancreas, epididymal fat (p < .001 vs N mice all; Supplementary Figures S8B, S8D, S8F, S8H, S9A, S9C, and S10A, respectively), perinephric fat (p = .004 vs N mice; Supplementary Figure S10C), and subcutaneous fat (p = .003 vs N mice; Supplementary Figure S10E). Moreover, when compared to N mice, GHRKO and Ames dwarf mice had lower weights of brain (p = .001 both; Supplementary Figure S8B), heart (p < .001 both; Supplementary Figure S8D), liver (p < .001 both; Supplementary Figure S8F), kidney (p < .001 both; Supplementary Figure S8H), spleen (p = .001 and p = .005, respectively; Supplementary Figure S9A), pancreas (p < .001 both; Supplementary Figure S9C), epididymal fat (p < .001 both; Supplementary Figure S10A), and subcutaneous fat (p = .039 and p = .019, respectively; Supplementary Figure S10E).
On the contrary, df/KO animals had the highest relative brain weight in comparison to N mice (p < .001) but also when compared to GHRKO and Ames dwarf mice (p < .001 both; Supplementary Figure S8C). For the liver, the relative weights were trended to be increased in df/KOs and Ames df/df mice in comparison to GHRKOs; however, the results were not significant (Supplementary Figure S8G); for the heart relative weight, there was a difference between df/KO and GHRKO mice only (p = .006; Supplementary Figure S8E). Surprisingly, the relative weights of the kidney, spleen, and epididymal fat were decreased in df/KO mice (p = .002, p = .033, and p < .001 vs N mice, respectively; Supplementary Figures S8I, S9B, and S10B, respectively). For perinephric fat, the relative weight was also trended to be decreased in df/KO mice but without any significance (Supplementary Figure S10D).
Discussion
As reported above, both male and female double-mutant df/KO mice live much longer in comparison with N mice; however, there were no differences in life span between these double mutants and GHRKO and df/df animals, each of which exhibited an extension in longevity relative to N mice confirming previous observations (8,30). These findings provide new important insight for the correlation between body size and life span. It is well accepted in aging literature that within the same species the body size strongly correlates with expected longevity, indicating that smaller animals outlive the large one. Most of the studies seem to prove this observation. Our study with early-life GH treatment in df/df mice showed that treating df/df mice for 6 weeks only early in life caused increased body size and normalized longevity of these long-living mutants (31). However, in the same study, we have shown that the same intervention with T4 also caused increase of body size without any effect on life span (31). This could suggest that just altering the body size is not enough to alter the life span, but direct action of GH/IGF-1 axis is crucial to affect the life span, whereas observed body size changes provide only visible phenotypic side effects. Based on this observation, we can assume that GH/IGF-1 axis similarly suppressed in either df/df, GHRKO, or df/KO mice and additional decrease of body size will not provide any further life-span extension. Therefore, the crossing between df/df and GHRKO mice did not lead to further increase the life span in df/KO animals, likely due to overlapping mechanisms of life-span extension.
Importantly, plasma adiponectin level was increased and insulin level was decreased in df/KO mice, compared to N mice. This could have been anticipated, given that the similar changes in levels of these hormones were previously observed in long-lived GHRKO (32–34) and df/df mice (4–6,35,36). Therefore, it may confirm the hypothesis that these changes that seem to be involved in the regulation of life span are also contributing to the extended longevity of df/KO mice. However, the adiponectin data suggest that maintaining high levels of this anti-inflammatory adipokines in long-living df/KO, df/df, and GHRKO mice might be important for extended health span and life span, and it also indicated that additional increases observed in df/KO mice when comparing with df/df and GHRKO mice did not support additional life-span extension.
In the same way, the ITT and GTT showed improved insulin sensitivity and glucose handling in df/KO double-mutant mice when compared to normal and GHRKO or df/df mice. Importantly, as it has been demonstrated earlier, that the two types of dwarf mice (GHRKO and Ames) are more insulin sensitive than normal animals and it seems to be a key element of life extension (37). However, ITT and GTT measure general glucose handling or sensitivity to injected insulin, yet the detailed tissue specific regulation was not determined using this method. Moreover, based on our published clamp study performed on df/df mice, we can expect that either df/KO and GHRKO are characterized by hepatic insulin sensitivity as well as are characterized by upregulated glucose uptake in skeletal muscle and adipose tissue similarly to df/df mice (38).
In the present study, decreased levels of two proinflammatory cytokines, that is, IL-6 and IFNγ, as well as leptin, were observed in the epididymal fat in df/KO double-mutant mice. Consistently, IL-6 level was decreased in the same fat tissue in GHRKO mice, and a tendency for lower leptin level in GHRKO mice compared to N mice was previously found (34), which was not clear in the present study. Similarly, in df/df mice, reduced IL-6 level in epididymal fat tissue was detected (36), although not significant in the current study. We previously showed a decreased level of another proinflammatory cytokine, that is, TNFα in epididymal fat of df/df animals (36); however, in the current study, no genotype effect was observed. Therefore, these findings indicate an even more reduced level of proinflammatory cytokines in long-lived df/KO double mutants, which should be considered beneficial for the improved insulin sensitivity observed. Thus, we hypothesize that this is one of many important mechanisms responsible for extended longevity in these mice.
As noted previously, dramatic suppression in hepatic mRNA level for IGF-1 was detected in df/KO mice. The reduction in IGF-1 level is assumed to be one of the most crucial features leading to extended longevity (39,40). On the other hand, deletion of liver IGF-1 gene at 1 year of age reduced serum IGF-1 but distinctly impaired health span of the iLID (inducible liver IGF-1-deficient) mice (41). In the present study, there were no differences in IGF-1 mRNA levels between double-mutant df/KOs and both GHRKO and df/df mice. Thus, the lack of differences in extended life span observed between df/KOs and GHRKOs, as well as between df/KO animals and df/df mice, may be a consequence of the three mouse lines having similar suppressed levels of liver IGF-1 production compared to normal mice. Importantly, our findings are consistent with results published previously by our group, also demonstrating greatly suppressed levels of IGF-1 in the liver and skeletal muscles of GHRKO mice (42), as well as in the hearts of these mutants (43). Moreover, severely reduced plasma IGF-1 concentrations in GHRKO mice (12) and df/df mice (35) were demonstrated previously. Previously, we have reported (44) strongly decreased IGF-1 gene expression level in hepatic tissue of df/df mice. Intriguingly, our findings indicate an increased pituitary IGF-1 mRNA level in df/KO double mutants, with a tendency for higher IGF-1 gene expression levels in GHRKO and df/df mice compared to normal animals. These results are not unexpected, given that it is known that IGF-1 is locally produced in different extrahepatic tissues, including the pituitary gland (eg, ref. (45)). Therefore, increased IGF-1 gene expression in the pituitary may be a compensatory effect against severely decreased production of this growth factor by the liver and other tissues.
In our study, the df/KO mice were characterized by increased hepatic gene expression for two transcription factors, that is, PPARα and PPARγ. This is consistent with previous results, showing elevated levels of PPARα and PPARγ mRNAs and proteins in the liver of GHRKO mice (46,47). However, although PPARγ mRNA and protein levels were also increased in the liver of df/df compared to N mice, the same was not observed for PPARα (44). Intriguingly, PPARα and PPARγ protein levels, but not gene expression, were decreased in the skeletal muscles of GHRKO mice (48). Similarly, PPARα and PPARγ mRNAs were not altered in the hearts of GHRKO mice (43). As reported in the present study, PPARα and PPARγ mRNA levels were also elevated in the pituitary glands of df/KO mutants. Moreover, pituitary mRNA level of PGC-1α—the master regulator of mitochondrial biogenesis—is increased in df/KO mutants compared to normal animals. Consistently, PGC-1α gene expression increased in the skeletal muscles and kidneys of GHRKO dwarfs (20,49). We also showed increased IR and p38 gene expression in the liver in df/KO double mutants. High IR protein level was demonstrated previously in hepatic and muscle tissue in GHRKO mice (33). Consistently, IR gene expression increased in the liver (42) and did not change in the heart (43) of GHRKO mice compared to N animals. IR protein level was also elevated in hepatic tissue in df/df mice (4). In turn, increased hepatic phosphorylated p38 (p-p38) protein level in GHRKO mice was previously reported (32), which is the opposite of df/df mice, in which p-p38 protein level decreased in the liver in comparison with N mice (5). In conclusion, the above changes in genes expression observed in df/KO mice may be considered beneficial in the context of extended life span of these animals, because these results were previously independently observed in both GHRKO and df/df long-lived mice.
As reported above, df/KO double mutants had distinctly decreased body weight compared not only to normal mice but also to GHRKO dwarfs, with a tendency for decrease in comparison with df/df mice. Consistently, both GHRKO (29,33,50) and df/df mice (4,44) had lower body weight as compared to normal animals. Intriguingly, df/KO double mutants had the highest (among all presently examined mouse strains) relative brain weight (expressed as percent of body weight), while simultaneously having—as in the cases of all other examined organs—decreased absolute weight of this organ compared to normal rodents. However, such data could be expected, given that GHRKO mice also have higher relative brain weight compared to N mice in the current and in a previous study (29), and an improved long-term memory in these mutants as they age has been reported (51). Similarly, df/df mice also have improved cognitive function in advanced age (52) and had a similar relative brain weight comparing to GHRKO in the current study.
In summary, taking into account all of the above-mentioned biochemical, physiological, and anatomical features of df/KO double-mutant mice, and remembering the potential beneficial role of numerous parameters, as examined in our study regarding the regulation of the insulin action, inflammation, immunity, and/or life span, one could conclude that the newly created mouse model, characterized by a lack of circulating GH and GHR, may be considered as a new and unique experimental animal model for aging studies. Elimination of both GH and GHR may produce a more severe growth phenotype, associated with improvements in insulin signaling, glucose metabolism, and higher adiponectin levels. All these characteristics could provide strong suggestion that df/KO mice will live longer than either df/df and GHRKO mice. However, lack of further life-span extension might suggest that once GH/IGF-1 signaling is silenced, then any further suppression of body size will not provide additional benefits on longevity. These new findings suggest that alteration of GH/IGF-1 signaling pathway represents the most robust mechanistic action for life-span extension, whereas concomitant body size reduction might represent just phenotypic side effect. Based on this, we will need to continue more in-depth study of detailed mechanism to be able to determine if we could reach the same 40%–60% of life-span extension by targeting some genes related to GH/IGF-1 signaling pathway without producing dwarfism effects.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging (NIA; grant numbers AG031736, AG032290, AG19899) and Polish National Science Centre (DEC-2012/04/M/NZ4/00198; grant number 507/1-107-05/507-10-050 of the Medical University of Lodz, Poland to A.G.). J.J.K. is supported by the State of Ohio’s Eminent Scholar Program that includes a grant from Milton and Lawrence Goll.
Conflict of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supplementary Material
Acknowledgments
The authors thank Dr. Chris Wright and Adam Spong for their work on the feasibility of producing these double mutants.
References
- 1. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi:10.1038/384327a0 [DOI] [PubMed] [Google Scholar]
- 2. Andersen B, Pearse RV, 2nd, Jenne K, et al. The Ames dwarf gene is required for Pit-1 gene activation. Dev Biol. 1995;172:495–503. doi:10.1006/dbio.1995.8040 [DOI] [PubMed] [Google Scholar]
- 3. Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi:10.1016/S0070-2153(04)63006-7 [DOI] [PubMed] [Google Scholar]
- 4. Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi:10.1677/joe.0.1730081 [DOI] [PubMed] [Google Scholar]
- 5. Gesing A, Al-Regaiey KA, Bartke A, Masternak MM. Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp Gerontol. 2014;58:219–229. doi:10.1016/j.exger.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61:323–331. [DOI] [PubMed] [Google Scholar]
- 7. Brown-Borg H, Johnson WT, Rakoczy S, Romanick M. Mitochondrial oxidant generation and oxidative damage in Ames dwarf and GH transgenic mice. J Am Aging Assoc. 2001;24:85–96. doi:10.1007/s11357-001-0012-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi:10.1038/384033a0 [DOI] [PubMed] [Google Scholar]
- 9. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94:13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32:356–386. doi:10.1210/er.2010-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kopchick JJ, Laron Z. Is the Laron mouse an accurate model of Laron syndrome? Mol Genet Metab. 1999;68:232–236. doi:10.1006/mgme.1999.2890 [DOI] [PubMed] [Google Scholar]
- 12. Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi:10.1210/endo.141.7.7586 [DOI] [PubMed] [Google Scholar]
- 13. Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi:10.1054/npep.2002.0889 [DOI] [PubMed] [Google Scholar]
- 14. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi:10.1210/en.2003-0374 [DOI] [PubMed] [Google Scholar]
- 15. Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–E413. doi:10.1152/ajpendo.00423.2003 [DOI] [PubMed] [Google Scholar]
- 16. Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–E29. doi:10.1152/ajpendo.00575.2004 [DOI] [PubMed] [Google Scholar]
- 17. Coschigano KT. Aging-related characteristics of growth hormone receptor/binding protein gene-disrupted mice. Age (Dordr). 2006;28:191–200. doi:10.1007/s11357-006-9004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: influence of genes and nutrition. Mech Ageing Dev. 2006;127:687–694. doi:10.1016/j.mad.2006.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun LY, Steinbaugh MJ, Masternak MM, Bartke A, Miller RA. Fibroblasts from long-lived mutant mice show diminished ERK1/2 phosphorylation but exaggerated induction of immediate early genes. Free Radic Biol Med. 2009;47:1753–1761. doi:10.1016/j.freeradbiomed.2009.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gesing A, Masternak MM, Lewinski A, Karbownik-Lewinska M, Kopchick JJ, Bartke A. Decreased levels of proapoptotic factors and increased key regulators of mitochondrial biogenesis constitute new potential beneficial features of long-lived growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2013;68:639–651. doi:10.1093/gerona/gls231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi:10.1093/gerona/glp017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding J, Berryman DE, Jara A, Kopchick JJ. Age- and sex-associated plasma proteomic changes in growth hormone receptor gene-disrupted mice. J Gerontol A Biol Sci Med Sci. 2012;67:830–840. doi:10.1093/gerona/glr212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200. doi:10.5114/aoms.2013.33181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. [DOI] [PubMed] [Google Scholar]
- 25. Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997;5:93–99. doi:10.1002/j.1550-8528.1997.tb00648.x [DOI] [PubMed] [Google Scholar]
- 26. Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi:10.1161/01.CIR.0000057974.74060.68 [DOI] [PubMed] [Google Scholar]
- 27. Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi:10.1016/j.biocel.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 28. Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi:10.1111/j.1538-7836.2005.01420.x [DOI] [PubMed] [Google Scholar]
- 29. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi:10.1016/j.ghir.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 30. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi:10.1073/pnas.0600161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi:10.1096/fj.10-163253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi:10.1210/en.2004-1120 [DOI] [PubMed] [Google Scholar]
- 33. Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4:e4567. doi:10.1371/journal.pone.0004567 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Masternak MM, Bartke A, Wang F, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11:73–81. doi:10.1111/j.1474-9726.2011.00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT. Does growth hormone prevent or accelerate aging? Exp Gerontol. 1998;33:675–687. [DOI] [PubMed] [Google Scholar]
- 36. Menon V, Zhi X, Hossain T, et al. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13:497–506. doi:10.1111/acel.12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64:516–521. doi:10.1093/gerona/glp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiesenborn DS, Ayala JE, King E, Masternak MM. Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr). 2014;36:9709. doi:10.1007/s11357-014-9709-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi:10.1126/science.1077991 [DOI] [PubMed] [Google Scholar]
- 40. Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93:571–598. doi:10.1152/physrev.00006.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gong Z, Kennedy O, Sun H, et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi:10.1111/acel.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–684. doi:10.1016/j.exger.2005.06.003 [DOI] [PubMed] [Google Scholar]
- 43. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417–429. doi:10.1016/j.exger.2006.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masternak MM, Al-Regaiey K, Bonkowski MS, et al. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59:784–788. doi:10.1093/gerona/59.8.B784 [DOI] [PubMed] [Google Scholar]
- 45. Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–3202. doi:10.1172/JCI39375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1394–1398. doi:10.1093/gerona/60.11.1394 [DOI] [PubMed] [Google Scholar]
- 47. Masternak MM, Bartke A. PPARs in calorie restricted and genetically long-lived mice. PPAR Res. 2007;2007:28436. doi:10.1155/2007/28436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi:10.1093/gerona/60.10.1238 [DOI] [PubMed] [Google Scholar]
- 49. Gesing A, Masternak MM, Wang F, et al. Expression of the key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66:1062–1076. doi:10.1093/gerona/glr080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40. doi:10.1093/gerona/glp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72:653–660. doi:10.1016/S0031-9384(01)00423-1 [DOI] [PubMed] [Google Scholar]
- 52. Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi:10.1006/hbeh.2001.1654 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.