Abstract
Neurotrophins, including nerve-growth factor and brain-derived neurotrophic factor, have been implicated in Alzheimer’s disease (AD). Associations between AD and neurotrophin signaling genes have been inconsistent, with few studies examining sex differences in risk. We examined four single-nucleotide polymorphisms (SNPs) involved in neurotrophin signaling (rs6265, rs56164415, rs2289656, rs2072446) and risk for AD by sex in a population-based sample of older adults. Three thousand four hundred and ninety-nine individuals without dementia at baseline [mean (standard deviation) age = 74.64 (6.84), 58% female] underwent dementia screening and assessment over four triennial waves. Cox regression was used to examine time to AD or right censoring for each SNP. Female carriers of the minor T allele for rs2072446 and rs56164415 had a 60% (hazard ratio [HR] = 1.60, 95% confidence interval [CI] = 1.02–2.51) and 93% (HR = 1.93, 95% CI = 1.30–2.84) higher hazard for AD, respectively, than male noncarriers of the T allele. Furthermore, male carriers of the T allele of rs2072446 had a 61% lower hazard (HR = 0.39, 95% CI = 0.14–1.06) than male noncarriers at trend-level significance (p = .07). The association between certain neurotrophin gene polymorphisms and AD differs by sex and may explain inconsistent findings in the literature.
Keywords: Single-nucleotide polymorphisms, Brain-derived neurotrophic factor, Nerve growth factor
Alzheimer’s disease (AD) is a pervasive neurodegenerative disease that affects an estimated 5.3 million individuals in the United States, approximately two-thirds of whom are women (1). Although many studies have suggested that the sex discrepancy in rates of AD reflects the higher life expectancy of women (2), other research implicates sex differences in factors that contribute to the development of AD (3). Neurotrophins [e.g., nerve-growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5)] are trophic factors with well-established roles in neuronal differentiation, health, and survival in the central nervous system across the life span (4). Recent studies suggest an association between neurotrophins, neurotrophin-related genes, and the pathogenesis of AD (5). BDNF serum concentrations have been found to be significantly higher in individuals in the early stages of AD compared to those in more advanced stages (6). Additionally, in a study of persons with mild-to-moderate AD, mean baseline serum BDNF levels were significantly lower for individuals categorized with more rapid cognitive decline (>4 points per year) on the Mini-Mental State Examination compared to those with slower decline (7). In this sample, the rate of cognitive decline over a 1-year follow up was significantly associated with baseline serum BDNF levels. In the Framingham Heart Study, increased serum BDNF levels were associated with a 35% reduction in AD risk, but only among women (8). With respect to AD pathogenesis, disruptions in NGF and BDNF protein synthesis, transport, and signaling reportedly contribute to both a cholinergic deficit and the formation of plaques and neurofibrillary tangles (9,10). Exogenous administration of neurotrophic factors to neurons in vitro or in vivo (infusion into rodent hippocampus), appears to protect against, reduce, or reverse degenerative effects and restore function (11,12). Taken together, these studies suggest a possible role for neurotrophins in AD risk and rate of progression.
Several single-nucleotide polymorphisms (SNPs) have been shown to modulate the synthesis or function of BDNF, NGF, or their receptors including rs6265 (BDNF Val66Met), rs56164415 (BDNF C270T) rs2289656 (BDNF receptor trkB), and rs2072446 (NGF/BDNF receptor p75NTR). The Met allele of BDNF Val66Met is associated with lower BDNF secretion and poor episodic memory (13) and lower scores on tests of delayed recall, processing speed, and general intelligence (14). However, additional research suggests modifying factors such as age (15) in that carriers of the Met allele are susceptible to reduced brain volume and functioning in early adult life, whereas Val/Val homozygotes are susceptible to similar reductions in late life. Still other research has found no significant association between the Val66Met polymorphism and brain volume or cognitive function (16). The BDNF C270T (rs56164415) polymorphism occurs in the 5’ untranslated region of the BDNF gene. This noncoding region has a promotor function and thus C270T is hypothesized to affect BDNF translation (17). The minor T allele of rs56164415 was significantly associated with a nearly 4-fold increase in risk of late-onset AD (LOAD) in both Japanese (18) and Caucasian (19) populations, and with executive functions in a sample of Japanese individuals with AD (17). It has also been implicated in risk for schizophrenia (20). With respect to the p75NTR receptor, the minor T allele of rs2072446 was associated with 72% lower risk in familial AD (21). Despite the positive associations discussed above, other studies have found no association between neurotrophic genes and AD risk (21,22) or rate of cognitive decline after the onset of AD (23). Recent studies, however, raise the possibility that men and women are differentially susceptible to the effects of neurotrophin gene polymorphisms. A meta-analysis showed that carriers of the Met allele of Val66Met had increased AD risk in Caucasian women, with no significant associations in men (24). Sex differences have also been found with neuropsychiatric symptoms after AD onset with increased psychosis in male carriers of the Met allele, but not in female carriers (25).
The current study examined the relationship between several neurotrophin gene polymorphisms (SNPs) and risk for AD in the population sample of older adults from the Cache County Study on Memory in Aging (CCSMA). The CCSMA consists of a cohort of over 5,000 participants followed prospectively and who underwent dementia screening and evaluation in four triennial waves that spanned up to 13 years. Specifically, we investigated the association of BDNF SNPs rs6265 and rs56164415, BDNF receptor trkB rs2289656, and NGF/BDNF receptor p75NTR rs2072446 in the risk for AD, as well as possible sex differences in risk.
Method
Subjects
Data used in these analyses were from participants of the CCSMA who were dementia-free in Wave 1 (baseline) but identified with AD and those without cognitive impairment (noncases) from three subsequent triennial waves of a multistaged dementia screening and assessment protocol. The procedures for the CCSMA have been published previously (26,27). Briefly, 5,092 (90%) of the 5,657 older adult residents of Cache County, Utah, who were aged 65 years or older completed baseline cognitive screening with an adapted version (28) of the Modified Mini-Mental State Exam (3MS) (29). A risk factor interview (health, educational, occupational, family histories) was also completed at that visit. For those unable to complete the 3MS or who scored less than 60 points on the total or 15 or fewer points on the Orientation section, a proxy informant completed the Informant Questionnaire for Cognitive Decline (IQCODE) (30), and a risk factor interview on behalf of the participant. Participants who scored below a sensory-motor and education-adjusted cut point of 87 on the 3MS or above 3.27 on the IQCODE were recruited for the second stage of screening that involved a knowledgeable informant who completed a phone interview with the Dementia Questionnaire (DQ). As reviewed by study neuropsychologists and geropsychiatrists, participants whose DQ interview suggested significant cognitive impairment or suspected dementia were asked to complete a clinical assessment which consisted of a clinical and health interview from a knowledgeable informant and neurological, neuropsychological, and physical examination of the participant. Additionally, a random sample of CCSMA participants, matched to each AD case by age and apolipoprotein E (APOE) genotype (the designated subsample), was asked to complete all stages of dementia screening and assessment regardless of 3MS scores. Those aged 90 and older were automatically sent to the DQ stage (26) which was followed with a clinical assessment if rated positive at that stage.
Data from the clinical assessment were reviewed by a study geropsychiatrist and neuropsychologist who assigned preliminary diagnoses of dementia, its prodrome, or other cognitive syndromes if present. Persons who were diagnosed with suspected dementia or prodromal AD were asked to complete standard laboratory studies for dementia (26), and a brain MRI scan along with a physician visit. Participants were also invited to undergo a brain autopsy for neuropathological studies. After completion of the clinical assessment and labs, brain MRI, physician visits, and brain autopsy (as available), an expert panel of clinicians reviewed all available clinical information (with exception of APOE genotype) to assign final study diagnoses. A diagnosis of dementia was assigned according to criteria of the Diagnostic and Statistical Manual of Mental Disorders (3rd ed.) Revised (DSM-III-R) (31) and dementia type followed standard research criteria. For example, dementia of the Alzheimer’s type was assigned according to the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease (NINCDS) and Related Disorders Association (ADRDA) criteria (32). Age of dementia onset was assigned as the age at which individuals first met DSM-III-R criteria for dementia based on the chronology of symptom onset. Persons with a working diagnosis of dementia or prodromal AD were asked to complete a follow-up clinical assessment at 18 months to clarify diagnoses.
Subsequent waves (2 through 4) of dementia screening and assessment (and follow-up risk factor interviews) were conducted for surviving persons who were not identified with dementia in prior waves, using a similar protocol. Exceptions to the protocol included an adjustment to the 3MS cut point (including decline of 3 or more points) [to identify cases of mild cognitive impairment (MCI) and other mild forms of cognitive impairment in Wave 2 (27)] and the elimination of the DQ stage in Waves 3 and 4. All study procedures were approved by the Institutional Review Boards of Duke University, Johns Hopkins University, and Utah State University.
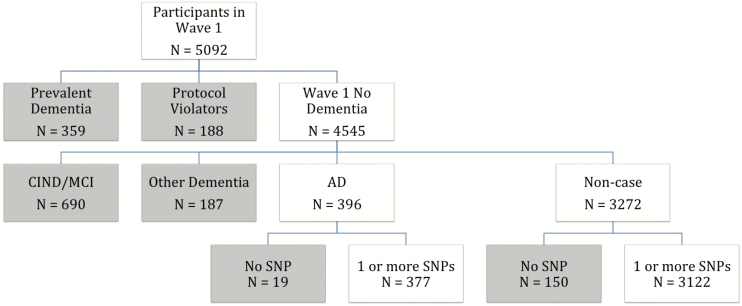
As a result of the four waves of screening and evaluation, a total of 942 cases of dementia were identified, 359 of which were prevalent at Wave 1 and thus excluded from the present analyses. Of the remaining 583 cases of dementia, 396 met criteria for possible, probable, or definite AD. We retrospectively identified persons without cognitive impairment for comparison via all available cognitive screening and assessment data who met the following criteria at the last study wave completed: (a) clinical assessment outcome of noncase or if lacking, (b) negative on all stages of screening. Persons with a final diagnosis of a non-AD dementia, MCI, or cognitive impairment no dementia were excluded from analyses as were those with incomplete screening and assessment (“protocol violators” who were screened positive but did not complete the next stage of screening or assessment). In this way, we identified 396 persons with incident AD and 3,272 noncases (without cognitive impairment); 168 individuals were missing genotype data from APOE or one of the neurotrophin SNPs (see the following) to yield a final sample of 3,499. Figure 1 displays the individuals included in the present analyses.
Figure 1.
Flow chart of final sample for analysis. Participants included in analyses (n = 3499) with data on one or more SNPs: AD or noncase as determined in Waves 2 through 4 of the CCSMA. Gray boxes represent subjects excluded from analyses at each level. AD = Alzheimer’s disease; CCSMA = Cache County Study on Memory in Aging; SNP = single-nucleotide polymorphism.
Genotyping
At the Wave 1 screening, participants gave a buccal skin sample from which DNA was extracted. At Waves 3 and 4, surviving participants were asked to donate a sample of blood, which was processed for DNA. DNA from buccal cells were processed using polymerase chain reaction (33) to identify genotype at APOE. Blood DNA (or if missing, buccal DNA) was used to process genotypes using standard TaqMan Assays (Life Technologies) for the following markers: rs6265, rs56164415, rs2289656, and rs2072446.
Data Analysis
Basic associations between each SNP and case–control status were examined using logistic regression. Cox proportional hazards regression models were used to examine the association of each SNP from the neurotrophin panel and time to dementia onset or right censoring. The observation time for those who developed dementia over the study period was calculated from the time (in years) from the age of dementia onset minus the age at Wave 1 screening. For those who never developed dementia during the study period, the observation time was calculated as the age of last full participation in designated study stages (i.e., screen negative or diagnosis of noncase after the clinical assessment) minus the age at Wave 1 screening. To examine differential effects of each neurotrophin SNP by sex, we coded each SNP by sex using a multilevel categorical variable according to the presence or absence of the minor allele to produce four dominant-model categories: Male GG; Male Ga/aa, Female GG, and Female Ga/aa. In statistical models for each SNP, the category of males with the homozygous major allele (Male GG) was selected as the reference. Where the sample size allowed, we also tested other models of inheritance, for example, log additive, recessive, and unrestricted models (34).
Covariates tested in Cox regression models included age at Wave 1 screening, education, and number of APOE E4 alleles. Because death is a competing risk for AD, we also examined whether each SNP was associated with the hazard of death in separate Cox regression models. Proportional hazards assumptions were examined by visual inspection of the log-minus-log plots. Statistical significance was set at the 95% confidence level in all analyses. All statistical models were run using SPSS software version 22.
Results
There were 3,499 individuals (57.8% female) who met inclusion criteria. Mean age of the sample was 74.64 years (standard deviation [SD] = 6.8) and mean years of education was 13.3 (SD = 2.88). Table 1 depicts additional descriptive statistics of the sample for those included in or excluded from analyses due to missing genotype data. Years of education differed between those with and without SNP data [t(187) = −3.45, p< .01]. No other significant differences in demographic factors were found.
Table 1.
Subject Characteristics With and Without Genotype Data
| 1 or more SNP | No SNP data | χ 2 or t-test | p value | |
|---|---|---|---|---|
| Sex, N (%) | Males = 1,476 (42.2%) | Males = 74 (43.8%) | 0.170 | .680 |
| Females = 2,023 (57.8%) | Females = 95 (56.2%) | |||
| Age, M (SD) | 74.64 (6.80) | 75.42 (6.67) | 1.48 | .145 |
| Education, M (SD) | 13.30 (2.88) | 12.56 (2.72) | −3.45* | .010* |
| APOE, N (%) | 0.184 | .668 | ||
| 1 or more E4 | 1,011 (29%) | 31 (31%) | ||
| No E4 | 2,472 (71%) | 69 (69%) | ||
| Cognitive group, N (%) | 0.037 | .848 | ||
| Any AD | 377 (10.8%) | 19 (11.2%) | ||
| No dementia | 3,122 (89.2%) | 150 (88.8%) |
Note: AD = Alzheimer’s disease; APOE = apolipoprotein E; M = mean; SD = standard deviation; SNP = single-nucleotide polymorphism.
*p value < 0.05.
Hardy–Weinberg equilibrium (HWE) was calculated using χ2 goodness-of-fit, using the criterion of p <0.001 as in other large scale association studies. In our sample, all SNPs were above the established threshold with the exception of rs56164415 (χ2 = 11.99, p = .0001). There was very low minor allele frequency of rs56164415 in the sample, with 381 heterozygotes and no homozygous individuals. Genotype frequencies did not vary by sex as depicted in Table 2.
Table 2.
Genotype Frequencies by Sex
| Male [N, (%)] | Female [N, (%)] | χ 2 (df) | p value | ||
|---|---|---|---|---|---|
| rs6265 | G/G | 963 (66.1) | 1,283 (64.2) | 1.973 (2) | 0.373 |
| G/A | 433 (29.7) | 617 (30.9) | |||
| A/A | 60 (4.1) | 98 (4.9) | |||
| rs56164415 | C/C | 1,282 (89.2) | 1,733 (88.5) | 0.468 (1) | 0.494 |
| C/T | 155 (10.8) | 226 (11.5) | |||
| T/T | 0 (0) | 0 (0) | |||
| rs2072446 | C/C | 1,317 (91.2) | 1,813 (91.7) | 1.562 (2) | 0.458 |
| C/T | 123 (8.5) | 155 (7.8) | |||
| T/T | 4 (0.3) | 10 (0.5) | |||
| rs2289656 | C/C | 964 (66.9) | 1,293 (65.9) | 1.083 (2) | 0.582 |
| C/T | 416 (28.9) | 596 (30.4) | |||
| T/T | 60 (4.2) | 74 (3.8) |
BDNF SNP Associations With AD
Binary logistic regression showed a 71% increase in AD risk for female carriers of the minor T allele of rs56164415 [odds ratio (OR) = 1.71, 95% confidence interval (CI) (1.15–2.53), p = .008], and a trend of 61% reduction in risk among male carriers of the minor T allele of rs2072446 [OR = 0.38, 95% CI (0.14–1.05), p = .061] (table available in Supplementary Material). Analyses using Cox proportional hazards regression with the inclusion of covariates showed female minor T allele carriers of rs56164415 had a 93% higher hazard of AD [hazard ratio (HR) = 1.93, 95% CI (1.30–2.84), p = .001] than male noncarriers. Additionally, female minor T allele carriers of rs2072446 had a 60% higher hazard of AD [HR = 1.60, 95% CI (1.02–2.51), p = .043] compared to male noncarriers. For rs2072446, male carriers of the minor T allele showed reduced AD risk compared to male noncarriers, although at trend-level significance (p = .066). There were no significant associations with AD risk for rs6265 and rs2289656 in either males or females. In examining each SNP with all-cause mortality, no significant associations were identified (data not shown). Table 3 displays the Cox regression models for each SNP. Additionally, Figure 2 displays the adjusted survival curves for AD from the Cox models for rs2072446 and Figure 3 for rs56164415. In examining other models of inheritance, only the log additive model was significant for rs2072446 in females, indicating a 62% increase in the hazard of AD with each additional T allele [HR = 1.62, 95% CI (1.07–2.44)]. For rs2289656, the recessive model was the most significant, with a 64% lower risk of AD in females homozygous for the T allele [HR = 0.36, 95% CI (0.15–0.88)]. We were unable to assess other models of inheritance for rs56164415 as no individuals were homozygous for the minor allele.
Table 3.
Cox Regression Results by SNP
| SNP | Hazard ratio (HR) | 95% Confidence interval | p value |
|---|---|---|---|
| Rs56164415 | |||
| Male, no minor allele (reference) | — | — | — |
| Male, minor allele | 0.68 | 0.34–1.34 | .263 |
| Female, no minor allele | 0.90 | 0.70–1.15 | .401 |
| Female, minor allele | 1.93 | 1.30–2.84 | .001* |
| Rs2072446 | |||
| Male, no minor allele (reference) | — | — | — |
| Male, minor allele | 0.39 | 0.14–1.06 | .066 |
| Female, no minor allele | 0.98 | 0.77–1.25 | .898 |
| Female, minor allele | 1.60 | 1.02–2.51 | .043* |
| Rs6265 | |||
| Male, no minor allele (reference) | — | — | — |
| Male, minor allele | 0.99 | 0.67–1.47 | .959 |
| Female, no minor allele | 1.00 | 0.75–1.35 | .977 |
| Female, minor allele | 1.28 | 0.93–1.75 | .131 |
| Rs2289656 | |||
| Male, no minor allele (reference) | — | — | — |
| Male, minor allele | 1.14 | 0.77–1.69 | .521 |
| Female, no minor allele | 1.23 | 0.92–1.65 | .171 |
| Female, minor allele | 1.00 | 0.71–1.40 | .985 |
Note: For all SNPs, the reference category was male, no minor allele. All models included covariates of age and APOE genotype. APOE = apolipoprotein E; SNP = single-nucleotide polymorphism.
*p value < 0.05.
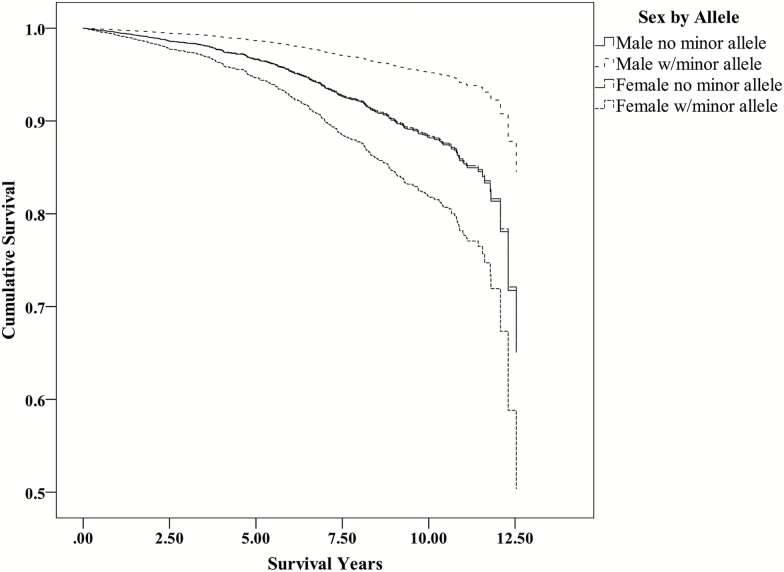
Figure 2.
Survival function for rs2072446 from Cox regression models for males and females. Note that the plots for males and females who are not carriers of the minor (T) allele show nearly identical survival curves. Female carriers of the minor T allele have a significantly higher hazard and shortened survival duration for AD. AD = Alzheimer’s disease.
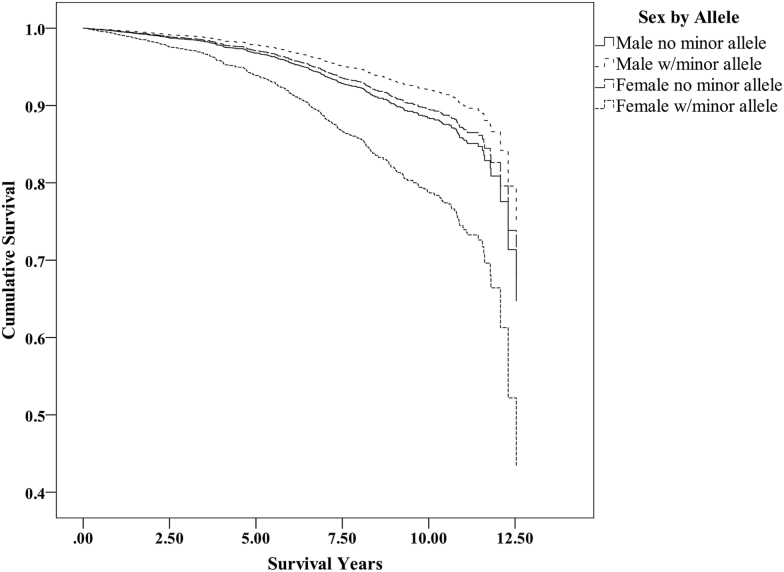
Figure 3.
Survival function for rs56164415 from Cox regression models for males and females. Female carriers of the minor T allele have a significantly higher hazard and shortened survival duration for AD. AD = Alzheimer’s disease.
Discussion
In a large population-based sample of 3,499 individuals, we found significant sex differences in associations between neurotrophin gene polymorphisms and AD under a dominant model of inheritance. Female carriers of the minor T allele of rs56164415 had a 93% higher risk of AD compared to male noncarriers, whereas female carriers of the minor T allele of rs2072446 showed a 60% higher risk. Rs2072446, which codes for NGF/BDNF receptor p75NTR, has not been studied extensively in humans for LOAD; however, the minor allele was associated with a reduction in risk of familial AD in an Italian sample (21). Familial AD in this study was defined as individuals who had at least two first-degree relatives in two generations with AD. Sex differences were not investigated in that sample. Other differences between that study and the current study included the nature of the samples, with the former study’s AD cases being nearly 50% familial AD, and the substantially younger age of participants [mean age of cases and controls approximately 65 years (cf. Cache County mean age of approximately 75 years)]. AD of later onset as in the Cache County Study often occurs in the context of comorbid conditions including other forms of dementia or comorbidities such as cerebrovascular disease (35), which also may have contributed to divergent results.
Few studies have reported on C270T (rs56164415), and their results are mixed. One study found that the presence of the minor T allele of C270T was associated with a 3.8-fold increase in risk for LOAD in a Japanese population (18). Similar results were found in a Caucasian sample (19), with presence of the minor T allele associated with a 3.8-fold increase in LOAD risk. However, other research has found no significant association between C270T and risk for AD (36). Our results suggest a sex-dependent effect of SNP C270T in risk for AD, which to the best of our knowledge has not been examined in other studies.
Val66Met (rs6265) has also been studied in several populations with conflicting results. No association between AD and Val66Met was found in the Italian sample described previously (21). A similar lack of association was also found in a Korean sample (22). However, a meta-analysis showed that female carriers of the minor Met allele of Val66Met had increased LOAD risk in Caucasians and that these associations were not present in men (24). In the present Cache County sample, we found no association between Val66Met and risk for AD in either men or women. Although not significant, the direction of effect regarding increased AD risk for women carrying the minor A allele was consistent with previous literature (24).
Among the neurotrophins, BDNF is particularly important for learning and memory, through its functions in neuronal plasticity and synapse formation (37–39). NGF is essential for the survival and normal function of the basal forebrain cholinergic neurons projecting to the hippocampus and cortex, a population of neurons affected by pathological processes early in AD (10,40). Besides their importance for neuronal physiology, neurotrophins have known roles in neuroinflammatory processes (41,42) and neovascularization (43–45) which are of great significance for both neurodegenerative diseases and stroke. Our results highlight sex differences in the association of neurotrophin gene polymorphisms on risk of AD, with carriers of the minor alleles of SNPs rs56164415 and rs2072446 showing an increased risk in women only. These results may highlight potential interactions between neurotrophin signaling and sex-dependent factors. Gonadal hormones, such as estrogens, progesterone, and androgens have been shown to modulate BDNF production and signaling through distinct pathways (46); these hormones may differentially influence BDNF production and signaling between sexes.
Based on the literature, future disease-modifying strategies for neurodegenerative disorders, including AD, may be oriented toward modulating NGF signaling (47) and BDNF-based synaptic repair (48). Already new research has identified benefits in preventing cognitive impairment in rodent models of AD for a small molecule inhibitor of p75NTR (LM11A-31) (49), as well as for a trkB agonist (7,8-dihydroxyflavone) (50–52). Further studies are needed to assess the efficacy of these molecules in AD, particularly in view of the identified sex differences in the pathological processes. Lifestyle factors may also present potential avenues for intervention. Diets low in fats and refined sugars (53) and aerobic physical activity (54) promote BDNF levels. Additionally, certain medications [e.g., antidepressants (55)] can increase BDNF. Future examination of gene–lifestyle/environment interactions for AD risk may reveal significant associations.
The current study’s limitations include the relative homogeneity of the study population with 99% being Caucasian and 90% members of the Church of Jesus Christ of Latter Day Saints. The Cache County population, however, is not a religious isolate unlike the Hutterites, Amish, or Mennonites. The state’s population originated from 20,000 founders, predominantly from the New England and Midwestern states, augmented by several waves of immigration from Europe (56). The genetic structure of the Utah population has been studied extensively and is characterized by low inbreeding coefficients, reflecting the largely unrelated members of the founding pioneers (57–59). Furthermore, the Cache County Study subpopulation is similar in ethnic diversity to Caucasians in the AD Neuroimaging Initiative samples from centers across the United States, and less homogeneous than the HapMap CEU population (60). Although rs56164415 violated HWE, disequilibrium in other SNPs is relatively unusual in the Cache County population (61). Finally, identification of cases and controls was based on a multistaged dementia screening and assessment protocol rather than a clinical evaluation of all participants. Although this design involved clinical evaluation of all cases of AD, with imperfect screening sensitivity, individuals with AD and other dementias may have erroneously been categorized as controls. Nonetheless, an examination of the screening methods reveals approximately 85% sensitivity for prevalent (62) and incident (63) dementia. Augmenting these dementia cases were those identified via clinical assessment of the designated subsample, which included screen negative individuals. Additionally, the repeated, longitudinal screenings and evaluations over 12 years of follow-up and the exclusion of indeterminate cases (protocol violators) and those with mild cognitive syndromes further reduced the possibility of classification error.
Strengths of the study included the high participation rate (90% at enrollment) and three waves of follow-up. The study design and data on dementia onset allowed for greater precision in statistical methods (e.g., using survival analyses as compared to logistic regression). Furthermore, with the extensive information on mortality data (with ages of death), we were able to examine any SNP associations with competing risks from death, of which there were none.
Conclusion
Our results indicated several sex differences in the associations between neurotrophin SNPs and AD risk, suggesting the possibility that sex-dependent effects on neurotrophin signaling modify the risk for AD and highlight the importance of investigating sex-dependent effects in sexually dimorphic disease, such as AD. Future studies of interest include the role of potential modifying factors such as diet, exercise, antidepressant medication and hormone-replacement use, as well as gene–gene interactions to elucidate the role of neurotrophins and risk for AD.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging grant R01AG11380 and a Research Catalyst grant from the Office of Research and Graduate Studies, Utah State University.
Conflict of Interest
The authors declared no conflict of interest.
Supplementary Material
Acknowledgments
The authors are indebted to the original Cache County Study investigators and the study participants who continue to inspire our work. A preliminary version of this work was presented at the annual scientific meeting of the Gerontological Society of America, November 2016, New Orleans, LA.
References
- 1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dementia. 2015;11:332–384. doi:10.1016/j.jalz.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. 2001;153:132–136. doi:10.1093/aje/153.2.132 [DOI] [PubMed] [Google Scholar]
- 3. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi:10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi:10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathol Appl Neurobiol. 2003;29:211–230. doi:10.1046/j.1365-2990.2003.00487.x [DOI] [PubMed] [Google Scholar]
- 6. Laske C, Stransky E, Leyhe T et al. . Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm (Vienna). 2006;113:1217–1224. doi:10.1007/s00702-005-0397-y [DOI] [PubMed] [Google Scholar]
- 7. Laske C, Stellos K, Hoffmann N et al. . Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. doi:10.1017/S1461145710001008 [DOI] [PubMed] [Google Scholar]
- 8. Weinstein G, Beiser AS, Choi SH et al. . Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi:10.1001/jamaneurol.2013.4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen SJ, Watson JJ, Dawbarn D. The neurotrophins and their role in Alzheimer’s disease. Curr Neuropharmacol. 2011;9:559–573. doi:10.2174/157015911798376190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iulita MF, Do Carmo S, Ower AK et al. . Nerve growth factor metabolic dysfunction in Down’s syndrome brains. Brain. 2014;137:860–872. doi:10.1093/brain/awt372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cattaneo A, Calissano P. Nerve growth factor and Alzheimer’s disease: new facts for an old hypothesis. Mol Neurobiol. 2011;46:588–604. doi:10.1007/s12035-012-8310-9 [DOI] [PubMed] [Google Scholar]
- 12. Diniz BS, Teixeira AL. Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. Neuromolecular Med. 2011;13:217–222. doi:10.1007/s12017-011-8154-x [DOI] [PubMed] [Google Scholar]
- 13. Egan MF, Kojima M, Callicott JH et al. . The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi:10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- 14. Miyajima F, Ollier W, Mayes A et al. . Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–417. doi:10.1111/j.1601-183X.2007.00363.x [DOI] [PubMed] [Google Scholar]
- 15. Voineskos AN, Lerch JP, Felsky D et al. . The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Arch Gen Psychiatry. 2011;68:198–206. doi:10.1001/archgenpsychiatry.2010.194 [DOI] [PubMed] [Google Scholar]
- 16. Kim A, Fagan AM, Goate AM et al. . Lack of an association of BDNF val66met polymorphism and plasma BDNF with hippocampal volume and memory. Cogn Affect Behav Neurosci. 2015;15:625–643. doi:10.3758/s13415-015-0343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagata T, Shinagawa S, Nukariya K et al. . Association between brain-derived neurotrophic factor (BDNF) gene polymorphisms and executive function in Japanese patients with Alzheimer’s disease. Psychogeriatrics. 2011;11:141–149. doi:10.1111/j.1479-8301.2011.00364.x [DOI] [PubMed] [Google Scholar]
- 18. Kunugi H, Ueki A, Otsuka M et al. . A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer’s disease. Mol Psychiatry. 2001;6:83–86. doi:10.1038/sj.mp.4000792 [DOI] [PubMed] [Google Scholar]
- 19. Olin D, MacMurray J, Comings DE. Risk of late-onset Alzheimer’s disease associated with BDNF C270T polymorphism. Neurosci Lett. 2005;381:275–278. doi:10.1016/j.neulet.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe Y, Nunokawa A, Someya T. Association of the BDNF C270T polymorphism with schizophrenia: updated meta-analysis. Psychiatry Clin Neurosci. 2013;67:123–125. doi:10.1111/pcn.12018 [DOI] [PubMed] [Google Scholar]
- 21. Cozza A, Melissari E, Iacopetti P et al. . SNPs in neurotrophin system genes and Alzheimer’s disease in an Italian population. J Alzheimers Dis. 2008;15:61–70. doi:10.3233/jad-2008-15105 [DOI] [PubMed] [Google Scholar]
- 22. Kim Y, Kong M, Lee C. Lack of common genetic factors for susceptibility to vascular dementia and Alzheimer’s disease. Gene. 2012;497:298–300. doi:10.1016/j.gene.2012.01.087 [DOI] [PubMed] [Google Scholar]
- 23. Chuu JY, Taylor JL, Noda A, Yesavage J, Murphy GM Jr. The brain-derived neurotrophic factor val66met polymorphism and rate of decline in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2006;9:43–49. doi:10.3233/jad-2006-9104 [DOI] [PubMed] [Google Scholar]
- 24. Lin Y, Cheng S, Xie Z, Zhang D. Association of rs6265 and rs2030324 polymorphisms in brain-derived neurotrophic factor gene with Alzheimer’s disease: a meta-analysis. PLoS One. 2014; 9:e94961. doi:10.1371/journal.pone.0094961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pivac N, Nikolac M, Nedic G et al. . Brain derived neurotrophic factor Val66Met polymorphism and psychotic symptoms in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:356–362. doi:10.1016/j.pnpbp.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 26. Breitner JC, Wyse BW, Anthony JC et al. . APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi:10.1212/wnl.53.2.321 [DOI] [PubMed] [Google Scholar]
- 27. Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: The Cache County study. Neurology. 2002;58:209–218. doi:10.1212/WNL.58.2.209 [DOI] [PubMed] [Google Scholar]
- 28. Tschanz JT, Welsh-Bohmer KA, Plassman BL, Norton MC, Wyse BW, Breitner JC; Cache County Study Group An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:28–38. [PubMed] [Google Scholar]
- 29. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 30. Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi:10.1017/s0033291700005742 [DOI] [PubMed] [Google Scholar]
- 31. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM), 3rd ed. (revised). Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 32. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi:10.1212/wnl.34.7.939 [DOI] [PubMed] [Google Scholar]
- 33. Saunders AM, Strittmatter WJ, Schmechel D et al. . Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi:10.1212/wnl.43.8.1467 [DOI] [PubMed] [Google Scholar]
- 34. Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat Protoc. 2011;6:121–133. doi:10.1038/nprot.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi:10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saarela MS, Lehtimaki T, Rinne JO et al. . No association between the brain-derived neurotrophic factor 196 G>A or 270 C>T polymorphisms and Alzheimer’s or Parkinson’s disease. Folia Neuropathol. 2006;44:12–16. [PubMed] [Google Scholar]
- 37. Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76 Pt C:677–683. doi:10.1016/j.neuropharm.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 38. Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014;76 Pt C:628–638. doi:10.1016/j.neuropharm.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 39. Deinhardt K, Chao MV. Shaping neurons: Long and short range effects of mature and proBDNF signalling upon neuronal structure. Neuropharmacology. 2014;76 Pt C:603–609. doi:10.1016/j.neuropharm.2013.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cuello AC, Bruno MA, Allard S, Leon W, Iulita MF. Cholinergic involvement in Alzheimer’s disease. A link with NGF maturation and degradation. J Mol Neurosci. 2010;40:230–235. doi:10.1007/s12031-009-9238-z [DOI] [PubMed] [Google Scholar]
- 41. Chen JJ, Wang T, An CD, Jiang CY, Zhao J, Li S. Brain-derived neurotrophic factor: a mediator of inflammation-associated neurogenesis in Alzheimer’s disease. Rev Neurosci. 2016;27:793–811. doi:10.1515/revneuro-2016-0017 [DOI] [PubMed] [Google Scholar]
- 42. Skaper SD. Nerve growth factor: a neuroimmune crosstalk mediator for all seasons. Immunology. 2017;151:1–15. doi:10.1111/imm.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blais M, Lévesque P, Bellenfant S, Berthod F. Nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3 and glial-derived neurotrophic factor enhance angiogenesis in a tissue-engineered in vitro model. Tissue Eng Part A. 2013;19:1655–1664. doi:10.1089/ten.tea.2012.0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen J, Zhang C, Jiang H et al. . Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi:10.1038/sj.jcbfm.9600034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One. 2013;8:e55039. doi:10.1371/journal.pone.0055039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi:10.1016/j.neuroscience.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 47. Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8:1703–1718. doi:10.1586/14737175.8.11.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi:10.1038/nrn3505 [DOI] [PubMed] [Google Scholar]
- 49. Knowles JK, Simmons DA, Nguyen TV et al. . Small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol Aging. 2013;34:2052–2063. doi:10.1016/j.neurobiolaging.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434–444. doi:10.1038/npp.2011.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Z, Liu X, Schroeder JP et al. . 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:638–650. doi:10.1038/npp.2013.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao L, Tian M, Zhao HY et al. . TrkB activation by 7, 8-dihydroxyflavone increases synapse AMPA subunits and ameliorates spatial memory deficits in a mouse model of Alzheimer’s disease. J Neurochem. 2016;136:620–636. doi:10.1111/jnc.13432 [DOI] [PubMed] [Google Scholar]
- 53. Beilharz JE, Maniam J, Morris MJ. Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions. Nutrients. 2015;7:6719–6738. doi:10.3390/nu7085307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Heijnen S, Hommel B, Kibele A, Colzato LS. Neuromodulation of aerobic exercise-a review. Front Psychol. 2015;6:1890. doi:10.3389/fpsyg.2015.01890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee BH, Kim YK. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7:231–235. doi:10.4306/pi.2010.7.4.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Skolnick M. The Utah Geneological database: a resource for genetic epidemiology, in Banbury Report No. 4: Cancer incidence in defined populations. New York, NY: Cold Spring Harbor Laboratory Press; 1980:285–297. [Google Scholar]
- 57. Jorde LB. The genetic structure of the Utah Mormons: migration analysis. Hum Biol. 1982;54:583–597. [PubMed] [Google Scholar]
- 58. Jorde LB, Morgan K. Genetic structure of the Utah Mormons: isonymy analysis. Am J Phys Anthropol. 1987;72:403–412. doi:10.1002/ajpa.1330720313 [DOI] [PubMed] [Google Scholar]
- 59. O’Brien E, Rogers AR, Beesley J, Jorde LB. Genetic structure of the Utah Mormons: comparison of results based on RFLPs, blood groups, migration matrices, isonymy, and pedigrees. Hum Biol. 1994;66:743–759. [PubMed] [Google Scholar]
- 60. Sharp AR, Ridge PG, Bailey MH et al. . Population substructure in Cache county Utah: The Cache county study. BMC Bioinformatics 2014;15:S8. doi:10.1186/1471-2105-15-S7-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ebbert MT, Ridge PG, Wilson AR et al. . Population-based analysis of Alzheimer’s disease risk alleles implicates genetic interactions. Biol Psychiatry. 2014;75:732–737. doi:10.1016/j.biopsych.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two-stage dementia screen in a population sample. J Clin Epidemiol. 2000;53:531–540. doi:10.1016/S0895-4356(99)00196-1 [DOI] [PubMed] [Google Scholar]
- 63. Hayden KM, Khachaturian AS, Tschanz JT, Corcoran C, Norton M, Breitner JCS. Characteristics of a two-stage screen for incident dementia. J of Clin Epidemiol. 2003;56:1038–1045. doi:10.1016/S0895-4356(03)00247-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.