Abstract
During normal aging process, the smell function declines significantly, starting from the sixth decade of age. While it has been shown that activity in the central olfactory system of seniors responding to odor stimulation is significantly less than that of young people, no information of the aging effect on the functions of this system during normal adulthood and early aging has been gathered. In this study, we used functional magnetic resonance imaging to investigate the olfaction-related brain activity in the central olfactory structures of 43 healthy adult volunteers aged from 22 to 64 years. The participants’ smell identification function was negatively correlated with age (r = −.32, p = .037). Significant negative correlation was observed between age and the olfaction-related activities in the bilateral dorsolateral prefrontal cortex, left insular cortex, and left orbitofrontal cortex (p < .001, corrected with cluster size ≥28 voxels). There was no significant correlation observed between age and the activity in the primary olfactory cortex detected in this age group. These results suggest that age-related functional decline in the human brain is more prominent in the secondary and higher-order central olfactory structures than the primary olfactory cortex in the early aging process.
Keywords: Olfaction, Age, Aging, Primary olfactory cortex, fMRI
The sense of smell is one of the basic functions that we have to perceive the environment around us. During the aging process, the overall smell function, including odor detection, discrimination, identification, and memory, goes down significantly starting from the sixth decade of age (1–10). Multiple factors have been documented that may contribute to this age-related functional decrease, for example, at the peripheral level—the loss of olfactory epithelium (11,12) and the functional decline of olfactory neurons (13), and at the central level—the atrophy and functional decline of the central olfactory system (14–23). Understanding the age-related functional decline of the central olfactory system is important, since it is the most dynamic system in the brain and has been shown to be involved in the two most common neurodegenerative diseases, that is, Alzheimer’s disease and Parkinson’s disease (24–30), in which olfactory deficits are prevalent and age is a significant risk factor.
We expected the level of brain activity in the central olfactory system to be age dependent. Previous studies have shown that the activity in the central olfactory system of the elderly (older than 65) is significantly weaker compared to that of the young (younger than 30) (14,16–18,21). However, no information of aging effect on the functions of this system during normal adulthood and early aging stage has been presented. To fill the gap of the aging curve in the central olfactory system, we evaluated a group of normal healthy adults younger than 65 years for the olfactory-related brain activation with functional magnetic resonance imaging (fMRI). Different to the previously used nonspecific olfactory stimulation methods (16–18,21), in this study, we assessed the central olfactory activities related to the sniffing of an odor (odor-sniffing) and the sniffing of odorless air (odorless-sniffing) separately, trying to identify the specific central structures with specific olfactory functions that are age dependent.
Methods
Human Participants
Forty-three healthy human volunteers (aged 22–64 years, average 40.9±15.0 years, 17 males, 3 left-handed) with no history of otorhinolaryngological, neurological, memory loss, or psychiatric conditions were recruited from the local community by advertisement. There was no significant age distribution difference between the male and female participants (two-sample t test, p = .43). All the participants provided written, informed consent prior to participation, in accord with the requirements of the Institutional Review Board of the Pennsylvania State University College of Medicine.
Psychophysical Test of Olfactory Function
The olfactory function of each participant was evaluated with the 40-component University of Pennsylvania Smell Identification Test (UPSIT, Sensonics, Haddon Heights, NJ) prior to the fMRI. The UPSIT is a self-administered, forced-choice test for the smell identification function. The participant UPSIT scores were analyzed for the aging effect on the smell identification function.
Olfactory Stimulation Paradigm
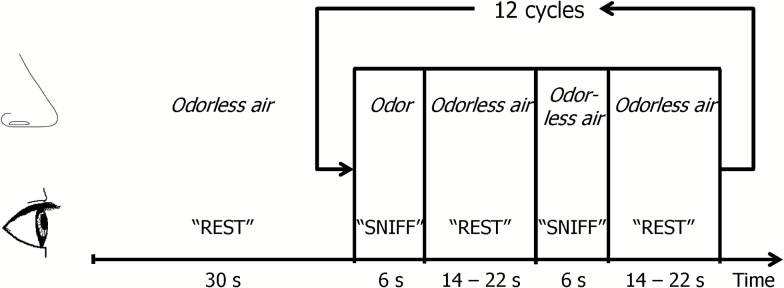
The olfactory stimulation paradigm contained visually prompted sniffing with either odorized air (lavender smell, lasting for 6 seconds) or odorless air delivered to the nose (Figure 1). Each condition was repeated 12 times and interleaved with 14–22 seconds odorless air at a constant air flow of 6L/min (3L/min through the odorant chamber when the odorant was delivered). The air flow was delivered to the both nostrils simultaneously through Teflon tubing (inner diameter 6.35mm) with a 50% relative humidity at room temperature (22°C). The intervals between the odor deliveries were pseudo-randomized to reduce any potential anticipatory effect on the olfactory system. The stimulation paradigm was executed with a programmable olfactometer (Emerging Tech Trans, LLC, Hershey, PA), which can deliver odorants to participant’s nostrils accurately without any optical, acoustic, thermal, or tactile cues to the participant. The olfactometer was located next to the MRI console out of the examination room, while the odorant containers were positioned next to the MRI scanner. Lavender oil (Givaudan Flavors Corporation, East Hanover, NJ) diluted in 1,2-propanediol (Sigma, St. Louis, MO) at 0.10% (volume/volume) was used as the olfactory stimulant. Lavender is one of the most effective olfactory stimulants with minimal to no propensity to stimulate the trigeminal system (31,32). The odorant was stored in six 300mL glass jars, each holding 50mL of the odorant. To keep the odor concentration stable during each odor presentation, the source of the odorant was switched sequentially among the six containers, with each container opened for 6 seconds. During the imaging session, the air in the magnet was constantly removed out of the examination room through a venting pipe to keep the room air odorless. Prior to the fMRI scan, the participants were trained to follow the visual prompt for sniffing. They were instructed to breathe normally and take a brief sniff when they see “SNIFF” on a LCD screen reflected in a mirror mounted on the MRI coil. The visual cues presented on the LCD screen next to the magnet were controlled by the ESys fMRI system (Invivo, Gainesville, FL) using an E-prime program (Psychology Software Tools, Sharpsburg, PA). The olfactory stimulation paradigm and MR image acquisition were synchronized using optical triggers from the MRI scanner. During the execution of the fMRI paradigm, the participants’ respiration trace was monitored via a pneumatic respiration sensor and recorded at a frequency of 10 Hz together with the odor delivery onsets and timing of image acquisition by the olfactometer. After fMRI scanning, the participants were asked to confirm if they sensed odors during the fMRI scans.
Figure 1.
Olfactory stimulation paradigm.
fMRI Study Protocol
The fMRI study was performed on a Siemens 3 T scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany) with an eight-channel head coil for signal reception. The participants were positioned in the supine position in a dark environment with their heads fit into a padded head restrainer to minimize motion and to provide correct positioning and comfort. The participants’ respiration and sniffing patterns were monitored to exclude any irregular respiration changes. A BOLD signal sensitive T2*-weighted echo-planar imaging sequence was used for fMRI image acquisition with repetition time/echo time/flip angle = 2,000 milliseconds/30 milliseconds/90°, field of view = 220×220mm, acquisition matrix = 80×80, 30 slices parallel to the anterior commissure–posterior commissure plane with a slice thickness = 4mm, parallel imaging acceleration factor = 2, number of repetitions = 239 with an acquisition time of 7 minutes 58 seconds. In addition, a T1-weighted anatomical image was acquired with a three-dimensional MPRAGE method with repetition time/echo time/flip angle = 2,300 milliseconds/2.98 milliseconds/9°, field of view = 256×256×160mm, acquisition matrix = 256×256×160, image resolution = 1×1 × 1mm, and parallel imaging acceleration factor = 2.
Data Processing and Analysis
The respiration trace, odor delivery timing, and image acquisition timing data were processed with the ONSET software (Olfactory Network Stimulation Editing Tool) for actual stimulation onset and duration vectors (http://www.pennstatehershey.org/web/nmrlab/resources/software) (33). In addition, the respiration volume was measured and compared between odor and odorless periods. The respiration volume was estimated with the area under the respiration trace for each inhalation and exhalation phase pair.
The first 10 fMRI images of the data series were discarded from data processing to remove any possible signal instability at the beginning of the echo-planar imaging sequence. The remaining fMRI data were processed with SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, London, UK) (34) following the standard procedure: (i) fMRI images were spatially realigned within the session to remove any minor head movements (translation < 1mm, rotation < 1°); (ii) co-registered with high-resolution anatomical image; (iii) normalized to the Montreal Neurological Institute (MNI) brain template (35) in a spatial resolution of 2×2 × 2mm; and (iv) smoothed with an 8×8 × 8mm (full width at half maximum) Gaussian smoothing kernel. A mask of central olfactory structures was generated based on a modified segmented brain template (Anatomical Automatic Labeling brain template, Neurofunctional Imaging Group, CYCERON, Caen, France) (36), which included the bilateral primary olfactory cortex (POC), amygdala, insula, hippocampus, parahippocampal cortex, frontal lobe and temporal lobe cortex, and cingulate cortex. Statistical parametric maps were generated at the individual level by fitting the stimulation paradigm to the functional data with a default 128-second high-pass filter, convolved with the canonical hemodynamic response function within the predefined mask of central olfactory structures with the six head movement parameters as nuisance covariates. Separate olfactory activation maps at the group level were generated for the odor-sniffing and odorless-sniffing (one-sample t tests, family-wise error corrected, p < .05, extent threshold = 6). The contrast between the odor-sniffing and odorless-sniffing conditions was generated at the group level (paired t test, p < .001, corrected using the AFNI AlphaSim program [http://afni.nimh.nih.gov] with a cluster size ≥45 voxels to achieve a corrected p <.05 [Monte Carlo simulations, p = .001, full width at half maximum = 8mm, cluster connection radius = 2mm, 1,000 iterations]). The aging effect on the olfactory activation in the central olfactory structures was studied with SPM and the masks of the group-average activation map for each contrast (multiple regression, p < .001, corrected using the AFNI AlphaSim program [http://afni.nimh.nih.gov] with cluster size ≥28 voxels to achieve a corrected p <.05 [Monte Carlo simulations, uncorrected p = .001, full width at half maximum = 8mm, cluster connection radius = 2mm, 1,000 iterations]). To further investigate the olfactory activation in the POC, the BOLD signals in the left and right POC responding to the odor-sniffing and odorless-sniffing were acquired from each individual participant with the peristimulus time histogram of the activation center in the predefined region of interest of the POC. BOLD signals responding to the odor-sniffing and odorless-sniffing were compared at the group level using a paired t test, and the correlations between peak BOLD signal in the POC with age and UPSIT score were evaluated with SPSS Version 21.0 (IBM Corp., Armonk, NY).
Results
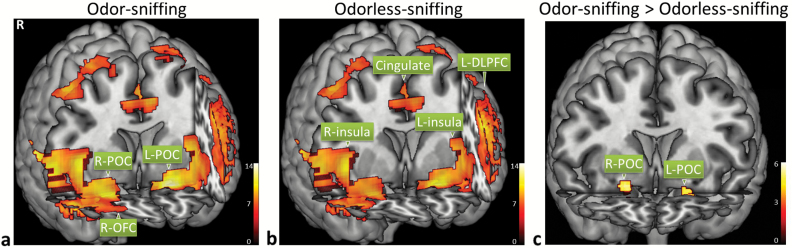
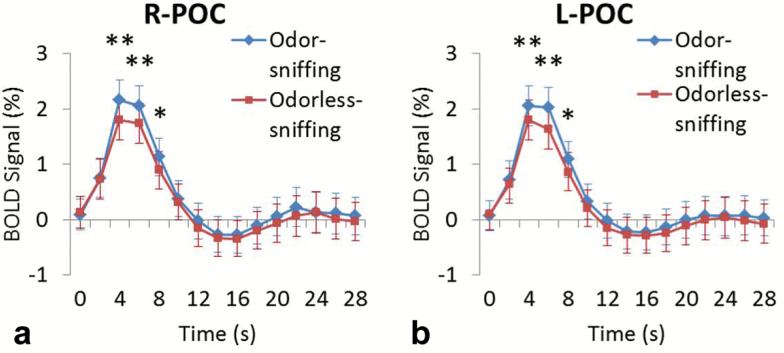
Both sniffing with or without concurrent odor presentation triggered significant activation in the bilateral POC and secondary olfactory structures, that is, insular cortex, orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), cingulate cortex, hippocampus, and parahippocampal cortex (Figure 2a and b; Tables 1 and 2, respectively). Voxel-based paired t test showed that odor-sniffing triggered a significantly stronger BOLD signal increase than odorless-sniffing in the bilateral POCs, namely, the right anterior piriform cortex, bilateral posterior piriform cortex, and bilateral periamygdaloid cortex (Figure 2c). Region of interest analysis of the BOLD signal time course showed that the peak BOLD signal in the bilateral POCs responding to odor-sniffing was significantly stronger than those responding to odorless-sniffing (paired t test, p < .01) (Figure 3a and b). The analysis of the respiration data showed that there was no significant difference in the sniff volume or respiratory rate between odor-sniffing and odorless-sniffing at either the individual level or group level (paired t test, p > .10).
Figure 2.
Both odor-sniffing (a) and odorless-sniffing (b) triggered significant activation in the bilateral primary olfactory cortex (POC), and secondary and higher-order olfactory structures, which includes the insular cortex, orbitofrontal cortex (OFC), cingulate cortex, dorsolateral prefrontal cortex (DLPFC), and temporal cortices (voxel-based one-sample t test, n = 43, family-wise-error [FWE] corrected, p < .001, cluster size ≥6 voxels). Odor-sniffing triggered significantly stronger POC activation than odorless sniffing (c) (voxel-based paired t test, n = 43, FWE corrected, p < .05, cluster size ≥6 voxels).
Table 1.
Olfactory Activations Triggered by Odor Sniffing
| Area | MNI Coordinates (mm) | Cluster Size (voxel) | Cluster Level | Voxel Level | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | p FWE-corr | p uncorr | p FWE-corr | p uncorr | t Value | ||
| L-POC/L-insula/L-OFC/L-DLPFC/L-hippocampus/L- parahippocampal/L-temporal_pole | −22 | 2 | −22 | 6,937 | .000 | .000 | .000 | .000 | 13.64 |
| R-insula/R-POC/R-OFC/R-hippocampus/R- parahippocampal/R-temporal_pole | 54 | 20 | −8 | 6,996 | .000 | .002 | .000 | .000 | 11.98 |
| R-DLPFC | 38 | 54 | 26 | 1,002 | .000 | .000 | .000 | .000 | 9.87 |
| L-cingulate/R-cingulate/L-sup_med_frontal/R-sup_ med_frontal | −8 | 4 | 44 | 3,386 | .000 | .000 | .000 | .000 | 9.65 |
| L-sup_frontal | −30 | −10 | 70 | 150 | .000 | .007 | .000 | .000 | 7.60 |
| L_mid_frontal | −46 | 6 | 54 | 10 | .000 | .438 | .000 | .000 | 6.65 |
| R-inf_frontal | 56 | 24 | 30 | 9 | .000 | .463 | .000 | .000 | 6.45 |
| L-mid_frontal | −34 | 4 | 64 | 6 | .001 | .556 | .001 | .000 | 6.18 |
Note: One-sample t test, FWE corrected, p < .001, cluster size ≥6 voxels. Coordinates are given in the left-posterior-inferior system in the Montreal Neurological Institute (MNI) brain template space. L = left; R = right. DLPFC = dorsolateral prefrontal cortex; FWE = family-wise error; OFC = orbitofrontal cortex; POC = primary olfactory cortex. inf = inferior; med = medial; mid = middle; sup = superior. corr = corrected; uncorr = uncorrected.
Table 2.
Olfactory Activations Triggered by Odorless Sniffing
| Area | MNI Coordinates (mm) | Cluster Size (voxel) | Cluster Level | Voxel Level | |||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | p FWE-corr | p uncorr | p FWE-corr | p uncorr | t Value | ||
| R-insula/R-POC/R-OFC/R-hippocampus/R- parahippocampal/R-temporal_pole | 50 | 18 | −10 | 7,163 | .000 | .000 | .000 | .000 | 12.93 |
| L-POC/L-insula/L-DLPFC/L-OFC | −22 | 2 | −20 | 5,972 | .000 | .000 | .000 | .000 | 12.38 |
| L-temporal_pole/L-hippocampus/L-parahippocampal | |||||||||
| R-cingulate/L-cingulate | 2 | 20 | 36 | 2,320 | .000 | .000 | .000 | .000 | 8.58 |
| R-sup_med_frontal | 6 | 26 | 64 | 31 | .000 | .163 | .000 | .000 | 7.39 |
| L-sup_frontal | −28 | −10 | 70 | 77 | .000 | .036 | .000 | .000 | 7.31 |
| L-mid_frontal | −46 | 6 | 54 | 9 | .000 | .451 | .000 | .000 | 7.05 |
| R-DLPFC | 24 | 26 | 62 | 8 | .000 | .479 | .000 | .000 | 6.44 |
| R-OFC | 30 | 34 | −22 | 7 | .001 | .510 | .000 | .000 | 6.44 |
| L-DLPFC | −36 | 28 | 52 | 12 | .000 | .381 | .000 | .000 | 6.27 |
Note: One-sample t test, FWE corrected, p < .001, cluster size ≥6 voxels. Coordinates are given in the left-posterior-inferior system in the Montreal Neurological Institute (MNI) brain template space. L = left; R = right. DLPFC = dorsolateral prefrontal cortex; FWE = family-wise error; OFC = orbitofrontal cortex; POC = primary olfactory cortex. med = medial; mid = middle; sup = superior. corr = corrected; uncorr = uncorrected.
Figure 3.
The peak BOLD signal change in the right primary olfactory cortex (POC) (a) and left POC (b) responding to the odor-sniffing events was significantly stronger than that responding to the odorless-sniffing events at 5, 7, and 9s after the odor onset (paired t test, n = 43, *p < .05; **p < .01). All statistical parameter maps from SPM8 were overlaid on a standard anatomical template image (ch2better.nii) using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron).
The average UPSIT score of the group was 35.6±2.4. There was a significant aging effect on the smell function in this study group of 22–64 years of age. The UPSIT score was negatively correlated with age (r = −.32, p = .037). In addition, when the participants were separated by their sex, post hoc analysis showed that within each sex group, the UPSIT score was not significantly correlated with age for two-tailed tests of correlation coefficient (female: r = −.23, p = .25; male: r = −.41, p = .10). Based on the knowledge of a negative aging effect on olfactory functions, with a one-tailed test, the male group showed a borderline negative correlation between age and UPSIT score (p = .05). The female participants performed a little better than the male participants on the smell identification test (UPSIT score 35.9±2.4 vs 35.2±2.3); however, the difference was not significant (two-sample t test, p = .34).
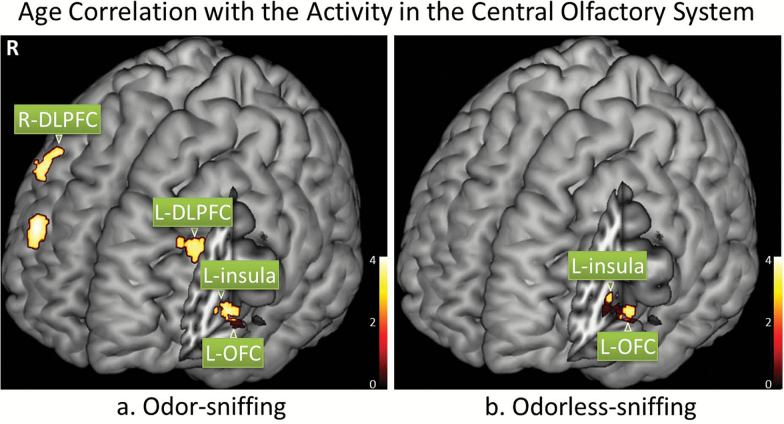
The fMRI data showed that there was a significant negative correlation between age and the BOLD signal in some secondary and higher-order olfactory structures. Specifically, the significant negative aging effect on the BOLD signal responding to odor-sniffing was located in the bilateral DLPFC, left OFC, and left insular cortex; when responding to odorless-sniffing the structures showing significant aging effect were the left OFC and left insular cortex (Figure 4 and Table 3). Neither voxel-based nor region of interest-based analysis showed any significant age correlation with the BOLD signal in the POC region. When the participants were separated into different age groups (ie, 22–30, 31–40, 41–50, 51–64 years) for comparison, there was no significant difference in the POC activation responding to either odor-sniffing or odorless-sniffing (two-sample t test, p > .17), even though the age group of 51–64 achieved significantly lower UPSIT scores (34.6±2.9) than the 22–30 age group (36.4±1.8) (two-sample t test, p = .03). Further post hoc analyses of the aging effect on the olfactory activation were conducted in the two separate sex groups. The only significant aging effect was observed in the left DLPFC, left insular cortex, and left OFC of male participants during odorless-sniffing (p < .005, corrected with cluster size ≥65 voxels to achieve a corrected p < .05) (Figure 5 and Table 4). No significant aging effect on the BOLD signal during odor-sniffing was observed for these male participants (corrected p = .43). For the female participants, there was no significant aging effect on the BOLD signal responding to either odor-sniffing or odorless-sniffing (corrected p ≥ .07).
Figure 4.
The BOLD signal responding to odor-sniffing and odorless-sniffing in some secondary central olfactory structures was significantly correlated with age (p < .001, corrected with cluster size ≥28 voxels). The statistical parameter maps from SPM8 were overlaid on a standard anatomical template image (ch2better.nii) using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron). DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex.
Table 3.
Age Correlation With the BOLD Signal in the Central Olfactory System
| Condition | Area | MNI Coordinates (mm) | Cluster Size (voxel) | Cluster Level | Voxel Level | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | p FWE-corr | p uncorr | p FWE-corr | p uncorr | t Value | |||
| Odor-sniff | R-DLPFC | 38 | 44 | 28 | 51 | .360 | .437 | .173 | .000 | 4.01 |
| R-DLPFC | 48 | 16 | 40 | 107 | .231 | .257 | .271 | .000 | 3.80 | |
| L-OFC/L-insula | −40 | 16 | −6 | 79 | .286 | .330 | .287 | .000 | 3.78 | |
| L-DLPFC | −36 | 32 | 32 | 32 | .427 | .545 | .458 | .001 | 3.53 | |
| Odorless-sniff | L-OFC/L-insula | −42 | 28 | 2 | 95 | .245 | .284 | .278 | .000 | 3.77 |
Note: The secondary olfactory structures showed significant negative aging effect on the olfactory activation signal (multiple regression, p < .001, corrected with cluster size ≥28 voxels). Coordinates are given in the left-posterior-inferior system in the Montreal Neurological Institute (MNI) brain template space. L = left; R = right. DLPFC = dorsolateral prefrontal cortex; FWE = family-wise error; OFC = orbitofrontal cortex. corr = corrected; uncorr = uncorrected.
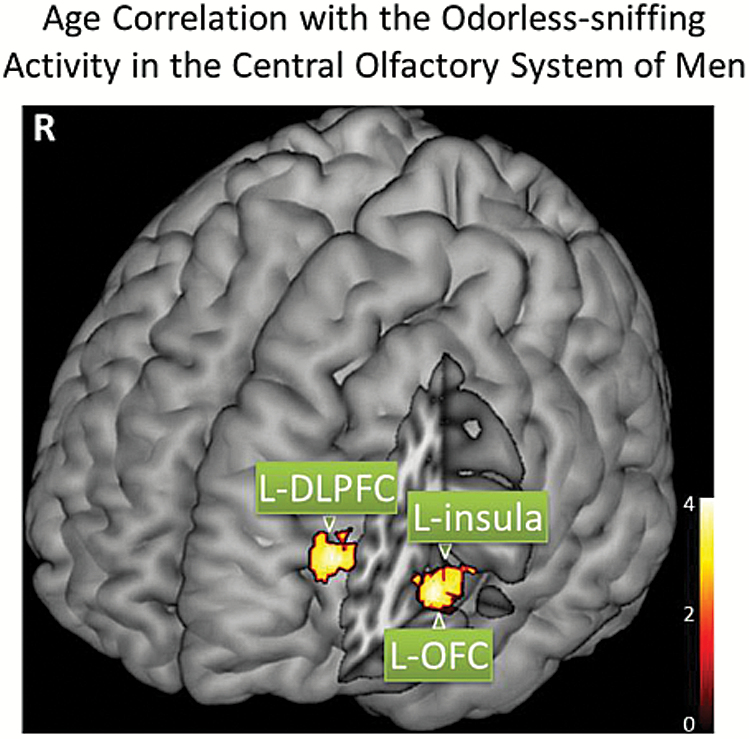
Figure 5.
The BOLD signal responding to odor-sniffing in some secondary central olfactory structures of men was significantly correlated with age (p < .005, corrected with cluster size ≥65 voxels). DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex.
Table 4.
Age Correlation With the BOLD Signal in the Central Olfactory System of Men
| Condition | Area | MNI Coordinates (mm) | Cluster Size (voxel) | Cluster Level | Voxel Level | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | p FWE-corr | p uncorr | p FWE-corr | p uncorr | t Value | |||
| Odorless-sniff | L-DLPFC | −30 | 42 | 16 | 67 | .838 | .481 | .590 | .001 | 3.92 |
| L-insula/L-OFC | −40 | 14 | −6 | 115 | .734 | .350 | .644 | .001 | 3.82 | |
Note: Within the male group, significant negative aging effect on the olfactory activation signal responding to odorless-sniffing was observed in some secondary olfactory structures (multiple regression, p < .005, corrected with cluster size ≥65 voxels). Coordinates are given in the left-posterior-inferior system in the Montreal Neurological Institute (MNI) brain template space. L = left. DLPFC = dorsolateral prefrontal cortex; FWE = family-wise error; OFC = orbitofrontal cortex. corr = corrected; uncorr = uncorrected.
Discussion
We demonstrated a significant age-related olfactory functional decline in the central olfactory system during normal adulthood and early aging. Consistent with previous observations (1), the smell identification test results showed a significant smell function decline in normal healthy adults before the age of 65 years. Our fMRI data revealed that the functional activity related to olfaction in the bilateral DLPFC, left insula, and left OFC was negatively correlated with age. The OFC and insular cortex are secondary olfactory structures, which receive inputs of olfactory information from the POC. The OFC is a key structure in odor perception (37–44), and the insular cortex plays an important role in the multisensory integration (42,45–49). Previous group comparisons between young and old healthy volunteers have shown the olfactory activation in the insular cortex to be age dependent (16). On the other hand, the DLPFCs are higher-order structures in the central olfactory system, which receive olfactory inputs from the OFC, amygdala, insular cortex, medial prefrontal cortex, and anterior cingulate cortex. They are highly involved in odor memory and cognitive functions that related to the olfactory tasks (50–53). A previous study of 15 healthy young and middle-aged women has also shown some significant aging effect on the olfactory-related activation in the DLPFC (51). These findings suggest that the central olfactory system plays a significant role in the age-related olfactory functional decline.
Interestingly, the brain structures that showed significant functional age dependency during normal adulthood and early aging stages were secondary and higher-order central olfactory structures. In contrast, we did not observe a significant aging effect on the POC activity in this age range. Our previous data from an older volunteer group showed that the BOLD signal in their bilateral POC was significantly weaker than that in the young volunteers (16). Taken together, it suggests that aging effect on the functions of the POC is nonlinear: the functions are relatively stable during normal adulthood and early aging stages and then experience a significant decline in the later aging period. In contrast, the age-related decrease of olfactory activity in the DLPFC, OFC, and insula happens at an earlier stage. Besides the fMRI findings in this study, one piece of evidence that supports this notion is the selective atrophy of secondary olfactory structures, for example, the OFC, but not the POC during normal aging (54). Another piece of evidence comes from a neuroelectrophysiological study of a 140 healthy volunteers aged 16–79 years (55), which electrophysiological data showed significant decrease in olfactory-related brain activity (mainly coming from secondary and higher-order cortices), but psychophysical assessments of those participants’ smell functions did not detect significant age-related changes in the odor detection threshold (a smell function controlled by the POC).
Women usually have better smell functions than men of the same age, and comparing to men, they usually experience age-related olfactory functional decline at a later age (1,9,56,57). Thus, sex might be a confounding factor in the relationship between age and the functions of central olfactory structures. When the participants were separated by their sex, significant BOLD signal decrease along with age was only observed in the secondary olfactory structures of men and during odorless-sniffing. This finding is consistent with the borderline negative correlation between these participants’ smell identification function and their age. This age-related functional decline in men during the early aging stage likely indicates men’s vulnerability to age-related olfactory deficits at a later age. For women participants, no significant age correlation was observed with either the activity of their central olfactory systems or their smell identification function. These results suggest that age-related functional changes in the central olfactory system of men and women may follow a different time course. Therefore, sex should be considered in the study of aging effect on human olfaction.
During normal adulthood and early aging, the olfactory-related activation in the bilateral DLPFC, left OFC, and left insular cortex was negatively correlated with age. The pathobiological mechanisms underlying such functional declines in these central olfactory structures are unknown. A previous longitudinal study of a large cohort found that carotid intima media thickness and plaque score were associated with the development of olfactory impairments among participants younger than 60 years. That finding suggests that early atherosclerosis might be a cause of the development of olfactory deficit during early aging (58). Other contributing factors of the early aging effect on the central olfactory activity might include local brain atrophy (54) and the neurotransmitter changes, for example, dopaminergic denervation (22,23). It is interesting that the aging effect on the olfactory functions of the OFC and insular cortex was observed only on the left, but not in both hemispheres. In general, aging-related pathological changes in the brain are usually symmetric, for example, the atherosclerosis in the cerebral circulatory system, and beta-amyloid deposition and neurofibrillary tangle formation in the brain tissue. Thus, the lateralization observed in this study might be related to the functional asymmetry between the two hemispheres in processing olfactory information (43,59).
Sniffing of an odorant triggered significantly stronger POC activation than the sniffing of odorless air. This is consistent with the results from a previous positron emission tomography study that compared POC activity during odor-sniffing and odorless-sniffing (60). The difference of the activation in the POC confirms that the POC is the very site for the neural activities associated with odor detection and perception apart from the sniffing response and the somatosensory stimulation by the air flow (61). The same structural involvement in the odor-sniffing and odorless-sniffing conditions demonstrates a top–down mechanism in the central olfactory system during a sniffing-involved olfactory activity. Thus, specific olfactory functions related to odorant stimulations can be untangled from sniffing-related activities in the central olfactory system.
There are a few limitations in this study. First, this is a cross-sectional study to look at the aging effect on the function of central olfactory system. The fMRI results demonstrated an aging effect on the activity of specific secondary olfactory structures but not the POC. Considering the interindividual variations, a longitudinal study should be more sensitive to detect age-related functional changes. Secondly, in this study, we did not observe a significant UPSIT score difference between the two sex groups, and there was no significant age correlation with the UPSIT score in either the male or female group. It is known that significant decline of olfactory functions usually happens after age 65, and before that, the decrease of smell functions is relatively small. This study focused on the early aging effect on the central olfactory functions before age 65. We believe our sample size is not large enough for detecting the cross-sex UPSIT score difference and the association between UPSIT score and age when sex is considered. Thus, to investigate the sex effect on aging-related functional changes in the central olfactory system, a larger subject group with a wider age range should be used. Thirdly, there was no systemic cognitive assessment of the participants in this study. It is known that there is a significant aging effect on human cognitive functions (62) and human cognitive functions are highly correlated with olfactory functions, especially odor identification function (27,63,64). In this study, the study group was relatively young with no participant older than 64 years and none of the participants had reported memory or cognitive issues during the screening procedure for this study. In future studies of the aging effect on olfaction function involving senior participants, cognitive functions should be included as a covariant. Finally, we observed a significant aging effect on the smell identification ability of this normal adulthood and early aging study group; however, the aging effect on other olfactory functions was not evaluated, for example, the odor detection threshold, odor discrimination, and odor memory. A combination of comprehensive assessments of olfactory functions, cognitive functions, and a complementary fMRI of brain olfactory activities should be able to help identify the different roles of each central olfactory structure in the aging-related olfactory functional decline.
Conclusion
This study provided normative data of age-related olfactory functional decline during normal adulthood and early aging stage. Olfactory fMRI demonstrated that the central olfactory system was significantly involved in the age-related olfactory functional decline. Consistent with the smell function decline measured by the smell identification test, the brain activities of some secondary and higher-order olfactory structures, for example, bilateral DLPFC, left insular cortex, and left OFC, were significantly decreased. In contrast, the activity in the POC was not significantly correlated with age in this age range. These results suggest that age-related functional decline in the human central olfactory system starts from the secondary and higher-order central olfactory structures.
Funding
This work was supported by the DANA Foundation, the Pennsylvania Department of Health, and the National Institutes of Health grant number R01 AG027771.
References
- 1. Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–1443. doi:10.1126/science.6505700 [DOI] [PubMed] [Google Scholar]
- 2. Stevens JC, Cain WS, Schiet FT, Oatley MW. Olfactory adaptation and recovery in old age. Perception. 1989;18(2):265–276. doi:10.1068/p180265 [DOI] [PubMed] [Google Scholar]
- 3. Cowart BJ. Relationships between taste and smell across the adult life span. Ann NY Acad Sci. 1989;561:39–55. doi:10.1111/j.1749-6632.1989.tb20968.x [DOI] [PubMed] [Google Scholar]
- 4. Kaneda H, Maeshima K, Goto N, Kobayakawa T, Ayabe-Kanamura S, Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem Senses. 2000;25(3):331–337. doi:10.1093/chemse/25.3.331 [DOI] [PubMed] [Google Scholar]
- 5. Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264(3):237–243. doi:10.1007/s00405-006-0173-0 [DOI] [PubMed] [Google Scholar]
- 6. Ship JA, Weiffenbach JM. Age, gender, medical treatment, and medication effects on smell identification. J Gerontol. 1993;48(1):M26–M32. doi:10.1093/geronj/48.1.M26 [DOI] [PubMed] [Google Scholar]
- 7. Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: elderly versus young persons. Am J Psychol. 1991;104(2):161–192. [PubMed] [Google Scholar]
- 8. Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol B Psychol Sci Soc Sci. 2000;55(5):P304–P310. doi:10.1093/geronb/55.5.P304 [DOI] [PubMed] [Google Scholar]
- 9. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The Rate of Age-Related Olfactory Decline Among the General Population of Older U.S. Adults. J Gerontol A Biol Sci Med Sci. 2015;70(11): 1435–1441. doi:10.1093/gerona/glv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schubert CR, Cruickshanks KJ, Fischer ME, et al. Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012;37(4):325–334. doi:10.1093/chemse/bjr102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118(7):731–738. doi:10.1001/archotol.1992.01880070061012 [DOI] [PubMed] [Google Scholar]
- 12. Nakashima T, Kimmelman CP, Snow JB., Jr Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110(10):641–646. [DOI] [PubMed] [Google Scholar]
- 13. Rawson NE, Gomez G, Cowart BJ, Kriete A, Pribitkin E, Restrepo D. Age-associated loss of selectivity in human olfactory sensory neurons. Neurobiol Aging. 2012;33(9):1913–1919. doi:10.1016/j.neurobiolaging.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evans WJ, Cui L, Starr A. Olfactory event-related potentials in normal human subjects: effects of age and gender. Electroencephalogr Clin Neurophysiol. 1995;95(4):293–301. [DOI] [PubMed] [Google Scholar]
- 15. Murphy C, Wetter S, Morgan CD, Ellison DW, Geisler MW. Age effects on central nervous system activity reflected in the olfactory event-related potential. Evidence for decline in middle age. Ann NY Acad Sci. 1998;855:598–607. doi:10.1111/j.1749-6632.1998.tb10630.x [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60(4):510–514. doi:10.1093/gerona/60.4.510 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki Y, Critchley HD, Suckling J, et al. Functional magnetic resonance imaging of odor identification: the effect of aging. J Gerontol A Biol Sci Med Sci. 2001;56(12):M756–M760. doi:10.1093/gerona/56.12.M756 [DOI] [PubMed] [Google Scholar]
- 18. Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986(1–2):39–53. doi:10.1016/S0006-8993(03)03168-8 [DOI] [PubMed] [Google Scholar]
- 19. Wang J, You H, Liu JF, Ni DF, Zhang ZX, Guan J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am J Neuroradiol. 2011;32(4):677–681. doi:10.3174/ajnr.A2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buschhüter D, Smitka M, Puschmann S, et al. Correlation between olfactory bulb volume and olfactory function. Neuroimage. 2008;42(2): 498–502. doi:10.1016/j.neuroimage.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 21. Yousem DM, Maldjian JA, Hummel T, et al. The effect of age on odor-stimulated functional MR imaging. AJNR Am J Neuroradiol. 1999;20(4):600–608. [PMC free article] [PubMed] [Google Scholar]
- 22. Larsson M, Farde L, Hummel T, Witt M, Lindroth NE, Bäckman L. Age-related loss of olfactory sensitivity: association to dopamine transporter binding in putamen. Neuroscience. 2009;161(2):422–426. doi:10.1016/j.neuroscience.2009.03.074 [DOI] [PubMed] [Google Scholar]
- 23. Wong KK, Muller ML, Kuwabara H, Studenski SA, Bohnen NI. Olfactory loss and nigrostriatal dopaminergic denervation in the elderly. Neurosci Lett. 2010;484(3):163–167. doi:10.1016/j.neulet.2010.08.037 [DOI] [PubMed] [Google Scholar]
- 24. Serby M, Corwin J, Conrad P, Rotrosen J. Olfactory dysfunction in Alzheimer’s disease and Parkinson’s disease. Am J Psychiatry. 1985;142(6): 781–782. doi:10.1176/ajp.142.6.aj1426781 [DOI] [PubMed] [Google Scholar]
- 25. Doty RL. Influence of age and age-related diseases on olfactory function. Ann NY Acad Sci. 1989;561:76–86. doi:10.1111/j.1749-6632.1989.tb20971.x [DOI] [PubMed] [Google Scholar]
- 26. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. doi:10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 27. Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53(7):1480–1487. [DOI] [PubMed] [Google Scholar]
- 28. Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–808. doi:10.1001/archpsyc.64.7.802 [DOI] [PubMed] [Google Scholar]
- 29. Devanand DP, Michaels-Marston KS, Liu X, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157(9):1399–1405. doi:10.1176/appi.ajp.157.9.1399 [DOI] [PubMed] [Google Scholar]
- 30. Lojkowska W, Sawicka B, Gugala M, et al. Follow-up study of olfactory deficits, cognitive functions, and volume loss of medial temporal lobe structures in patients with mild cognitive impairment. Curr Alzheimer Res. 2011;8(6):689–698. doi:10.2174/156720511796717212 [DOI] [PubMed] [Google Scholar]
- 31. Allen W. Studies on the level of anesthesia for the olfactory and trigeminal respiratory reflexes in dogs and rabbits. Am J Physiol. 1936;115: 579–587. [Google Scholar]
- 32. Patton HD. Taste, olfaction and visceral sensation. In: Ruth TC, Fulton JF, eds. Medical Physiology and Biophysics. Philadelphia, PA: Saunders; 1960: 375. [Google Scholar]
- 33. Wang J, Sun X, Yang QX. Methods for olfactory fMRI studies: implication of respiration. Hum Brain Mapp. 2014;35(8):3616–3624. doi:10.1002/hbm.22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friston KJ, Tononi G, Reeke GN, Jr, Sporns O, Edelman GM. Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience. 1994;59(2):229–243. doi:10.1016/0306-4522(94)90592-4 [DOI] [PubMed] [Google Scholar]
- 35. Collins DL, Zijdenbos AP, Kollokian V, et al. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–468. doi:10.1109/42.712135 [DOI] [PubMed] [Google Scholar]
- 36. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi:10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 37. de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46(4):671–679. doi:10.1016/j.neuron.2005.04.021 [DOI] [PubMed] [Google Scholar]
- 38. Howard JD, Gottfried JA. Configural and elemental coding of natural odor mixture components in the human brain. Neuron. 2014;84(4):857–869. doi:10.1016/j.neuron.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu KN, Tan BK, Howard JD, Conley DB, Gottfried JA. Olfactory input is critical for sustaining odor quality codes in human orbitofrontal cortex. Nat Neurosci. 2012;15(9):1313–1319. doi:10.1038/nn.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52(6):1097–1108. doi:10.1016/j.neuron.2006.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boyle JA, Djordjevic J, Olsson MJ, Lundström JN, Jones-Gotman M. The human brain distinguishes between single odorants and binary mixtures. Cereb Cortex. 2009;19(1):66–71. doi:10.1093/cercor/bhn058 [DOI] [PubMed] [Google Scholar]
- 42. Djordjevic J, Zatorre RJ, Petrides M, Boyle JA, Jones-Gotman M. Functional neuroimaging of odor imagery. Neuroimage. 2005;24(3): 791–801. doi:10.1016/j.neuroimage.2004.09.035 [DOI] [PubMed] [Google Scholar]
- 43. Royet JP, Hudry J, Zald DH, et al. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13(3):506–519. doi:10.1006/nimg.2000.0704 [DOI] [PubMed] [Google Scholar]
- 44. Kjelvik G, Evensmoen HR, Brezova V, Håberg AK. The human brain representation of odor identification. J Neurophysiol. 2012;108(2):645–657. doi:10.1152/jn.01036.2010 [DOI] [PubMed] [Google Scholar]
- 45. Plailly J, Radnovich AJ, Sabri M, Royet JP, Kareken DA. Involvement of the left anterior insula and frontopolar gyrus in odor discrimination. Hum Brain Mapp. 2007;28(5):363–372. doi:10.1002/hbm.20290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qureshy A, Kawashima R, Imran MB, et al. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84(3):1656–1666. [DOI] [PubMed] [Google Scholar]
- 47. Mak YE, Simmons KB, Gitelman DR, Small DM. Taste and olfactory intensity perception changes following left insular stroke. Behav Neurosci. 2005;119(6):1693–1700. doi:10.1037/0735-7044.119.6.1693 [DOI] [PubMed] [Google Scholar]
- 48. Heining M, Young AW, Ioannou G, et al. Disgusting smells activate human anterior insula and ventral striatum. Ann NY Acad Sci. 2003;1000: 380–384. doi:10.1196/annals.1280.035 [DOI] [PubMed] [Google Scholar]
- 49. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214 (5–6):519–534. doi:10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karunanayaka P, Eslinger PJ, Wang JL, et al. Networks involved in olfaction and their dynamics using independent component analysis and unified structural equation modeling. Hum Brain Mapp. 2014;35(5): 2055–2072. doi:10.1002/hbm.22312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reske M, Kellermann T, Shah NJ, Schneider F, Habel U. Impact of valence and age on olfactory induced brain activation in healthy women. Behav Neurosci. 2010;124(3):414–422. doi:10.1037/a0019289 [DOI] [PubMed] [Google Scholar]
- 52. Rolls ET, Grabenhorst F, Parris BA. Neural systems underlying decisions about affective odors. J Cogn Neurosci. 2010;22(5):1069–1082. doi:10.1162/jocn.2009.21231 [DOI] [PubMed] [Google Scholar]
- 53. Dade LA, Zatorre RJ, Evans AC, Jones-Gotman M. Working memory in another dimension: functional imaging of human olfactory working memory. Neuroimage. 2001;14(3):650–660. doi:10.1006/nimg.2001.0868 [DOI] [PubMed] [Google Scholar]
- 54. Shen J, Kassir MA, Wu J, et al. MR volumetric study of piriform-cortical amygdala and orbitofrontal cortices: the aging effect. PLoS One. 2013;8(9):e74526. doi:10.1371/journal.pone.0074526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murphy C, Morgan CD, Geisler MW, et al. Olfactory event-related potentials and aging: normative data. Int J Psychophysiol. 2000;36(2):133–145. doi:10.1016/S0167-8760(99)00107-5 [DOI] [PubMed] [Google Scholar]
- 56. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489–502. doi:10.1016/0031-9384(84)90269-5 [DOI] [PubMed] [Google Scholar]
- 57. Kern DW, Wroblewski KE, Schumm LP, Pinto JM, Chen RC, McClintock MK. Olfactory function in Wave 2 of the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69(suppl 2):S134–S143. doi:10.1093/geronb/gbu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schubert CR, Cruickshanks KJ, Fischer ME, Klein BE, Klein R, Pinto AA. Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing. 2015;44(5):878–882. doi:10.1093/ageing/afv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li W, Lopez L, Osher J, Howard JD, Parrish TB, Gottfried JA. Right orbitofrontal cortex mediates conscious olfactory perception. Psychol Sci. 2010;21(10):1454–1463. doi:10.1177/0956797610382121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kareken DA, Sabri M, Radnovich AJ, et al. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage. 2004;22(1):456–465. doi:10.1016/j.neuroimage.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 61. Sobel N, Prabhakaran V, Desmond JE, et al. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392(6673):282–286. doi:10.1038/32654 [DOI] [PubMed] [Google Scholar]
- 62. Craik FIM, Salthouse TA. The Handbook of Aging and Cognition. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2000:ix, 755. [Google Scholar]
- 63. Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology. 2002;21(2):58–67. doi:10.1159/000048618 [DOI] [PubMed] [Google Scholar]
- 64. Royall DR, Chiodo LK, Polk MS, Jaramillo CJ. Severe dysosmia is specifically associated with Alzheimer-like memory deficits in nondemented elderly retirees. Neuroepidemiology. 2002;21(2):68–73. doi:10.1159/000048619 [DOI] [PubMed] [Google Scholar]