Abstract
Background
Although the definition of multimorbidity as “the simultaneous presence of two or more chronic diseases” is well established, its operationalization is not yet agreed. This study aims to provide a clinically driven comprehensive list of chronic conditions to be included when measuring multimorbidity.
Methods
Based on a consensus definition of chronic disease, all four-digit level codes from the International Classification of Diseases, 10th revision (ICD-10) were classified as chronic or not by an international and multidisciplinary team. Chronic ICD-10 codes were subsequently grouped into broader categories according to clinical criteria. Last, we showed proof of concept by applying the classification to older adults from the Swedish National study of Aging and Care in Kungsholmen (SNAC-K) using also inpatient data from the Swedish National Patient Register.
Results
A disease or condition was considered to be chronic if it had a prolonged duration and either (a) left residual disability or worsening quality of life or (b) required a long period of care, treatment, or rehabilitation. After applying this definition in relation to populations of older adults, 918 chronic ICD-10 codes were identified and grouped into 60 chronic disease categories. In SNAC-K, 88.6% had ≥2 of these 60 disease categories, 73.2% had ≥3, and 55.8% had ≥4.
Conclusions
This operational measure of multimorbidity, which can be implemented using either or both clinical and administrative data, may facilitate its monitoring and international comparison. Once validated, it may enable the advancement and evolution of conceptual and theoretical aspects of multimorbidity that will eventually lead to better care.
Keywords: Chronic disease, Epidemiology, International Classification of Diseases
Multimorbidity, or the coexistence of multiple chronic conditions in one person, is one of the main challenges facing health systems worldwide. It has an adverse multiplicative effect on all health outcomes (1). Multimorbidity also represents a challenge for medical services that often provide highly specialized care focused on single diseases, which results in a fragmentation of the care process (2).
Although the study of multimorbidity has attracted increasing levels of attention during the past two decades, its epidemiological assessment and measurement is still identified as an area with important knowledge gaps, and filling these gaps remains a priority within the global research agenda (3). In particular, although the definition of multimorbidity as “the simultaneous presence of two or more chronic diseases” (4) is well established and accepted by the scientific community, the identification of which, how many, and in what manner diseases should be assessed in order to measure multimorbidity is still a matter of debate. As a result of the lack of explicit disease selection criteria, the number of conditions and specific diagnoses considered in the multimorbidity literature are very diverse. In a systematic review of multimorbidity indices, the number of different conditions ranged from 4 to 102 across the 39 indices identified (5). This leads to great heterogeneity not only in terms of prevalence estimates (ranging from 55% to 98% in older persons) (6) but also regarding the patterns of chronic disease co-occurrence (with up to 97 different patterns composed of two or more diseases) (7).
Two major aspects have impeded the evolution of a more precise and universally accepted operational measure of multimorbidity. First, a clear definition of chronic disease is lacking in most studies. In some cases, diseases tend to be selected according to predefined criteria such as their prevalence (8), the risk for adverse outcomes (9), or the treatability of the conditions (10). But it is also common that researchers determine their list based on pragmatic reasons, such as the availability of data (11), without further explanation. Second, establishing the level of disease categorization is a challenge that further increases the variation in the number of conditions across studies. Although all existing chronic conditions should ideally be considered in order to avoid bias toward the most prevalent—instead of relevant—conditions, the double counting of disease entities with a similar pathophysiology can be questionable (12). For example, cancer can be counted as a unique condition or as individual malignancies separately, and the term “heart diseases” is often used to group angina, atrial fibrillation, and heart failure.
Although various approaches to assess and measure multimorbidity have been suggested (13–18), a standardized instrument adapted to the older population has not prevailed so far. The development of a specific assessment tool is necessary to drive research that leads to improvements in prognosis, diagnosis, treatment, and care of older persons with multiple health conditions. Moreover, this will support researchers in increasing the comparability of their multimorbidity data, both across countries and over time. If we aim to capture the complete constellation of chronic health problems affecting the older population, the identification of such health problems should be based on the clinical assessment of a comprehensive classification of medical diagnoses following transparent criteria.
The aims of this study were to provide a clinically driven procedure for the assessment and measurement of multimorbidity in the older population and to show proof of concept by applying it to a population-based cohort of adults aged 60 years and older.
Method
The following procedure was agreed by the study team: (a) definition of chronic disease and formulation of the inclusion criteria; (b) identification of the codes from the International Classification of Diseases, 10th revision (ICD-10) (19) that fulfilled such criteria; and (c) grouping of the selected ICD-10 codes into broader disease categories according to clinical criteria.
Definition of Chronic Disease
Based on a literature review of existing definitions of chronic disease across scientific papers and reports from international health organizations (eg, World Health Oroganization, U.S. Department of Health and Human Services, Australian Institute of Health and Welfare, etc.), the following key features were identified and discussed concerning their pertinence and suitability in older populations: duration (prolonged or lasting for a specified time), course (progressive, recurrent or steady), reversibility (of symptoms and of pathological alterations), treatment (pharmacological, nonpharmacological, and care setting), and consequences (disability, quality of life, etc.). An international and multidisciplinary team of physicians (R.J.F.M., F.M., A.M., L.F.) and epidemiologists (S.A., D.R., A.K.W.) with clinical and research expertise in chronic diseases and multimorbidity took part in the discussions. As a result, a definition of chronic disease was established, in order to identify chronic ICD-10 codes.
Identification of Chronic ICD-10 Codes
All four-digit level codes from the ICD-10 system were classified as being chronic or not, according to the definition as derived earlier. Two geriatricians (D.L.V., A.C.) and two general practitioners (L.A.G.F., C.C.S.) independently did the initial assessment, with the subsequent involvement of three other geriatricians (A.M., G.O., M.S.P.) in case of disagreement. The latter three geriatricians also confirmed their agreement to the entire final list of four-digit level chronic ICD-10 codes.
Grouping of Chronic Diseases Into Broader Categories
The same group of physicians clustered those chronic ICD-10 codes according to clinical criteria and relevance (pathophysiological pathway, treatment, prognosis, and prevalence) in order to create broader disease categories. Whenever possible, the original ICD-10 coding hierarchical structure was followed. However, there were some exceptions. First, some ICD-10 codes were segregated from others belonging to the same nosographic group given their high prevalence or their specificity in terms of clinical characteristics, management, and prognosis, leading to a new specific disease category. For instance, this was the case for diabetes, multiple sclerosis, or inflammatory bowel diseases, which were considered separately from other metabolic, autoimmune, or colitis-related diseases, respectively. Second, other codes were moved from one specific nosographic group to another given their higher clinical homogeneity with this second group. For example, chronic viral hepatitis was subtracted from the category “Chronic infectious diseases” and grouped together with “Chronic liver diseases.” Third, additional categories were created that included a heterogeneous range of infrequent codes with common body organ/system involvement, pathophysiology, or treatment approach (eg, “Ear, nose and mouth disorders,” “Other neurological conditions,” etc.). Fourth, certain codes were included in more than one category when their labels referred to more than one disease entity. Such was for instance the case of spondylosis with myelopathy (“Dorsopathies” + “Other neurological disorders”) or varicose veins of lower extremities with ulcer (“Venous and lymphatic disorders” + “Chronic ulcer of the skin”).
Proof of Concept
The feasibility of our procedure was verified using both clinical and administrative data for a population-based cohort of older adults: (a) baseline data collected in the Swedish National study of Aging and Care in Kungsholmen (SNAC-K) (20) and (b) inpatient data from the Swedish National Patient Register (21) for baseline SNAC-K participants. Both use ICD-10 as the medical diagnoses coding system.
The SNAC-K population consists of a geographically based sample of people aged 60 years and older living at home and in institutions in the Kungsholmen area of central Stockholm. Baseline examinations were carried out from March 2001 through August 2004 on a sample of 3,363 people (73.3% participation rate). Participants underwent extensive face-to-face interviews, clinical examinations, and testing in order to comprehensively assess their physical, neurological, psychiatric, and cognitive function. Physicians in SNAC-K made clinical diagnoses based on participant self-report, medical charts, anamnestic details, and information gathered from participants’ proxies, in order to increase the reliability of the diagnoses. All drugs used by SNAC-K participants were collected by the examining physician and coded according to the Anatomical Therapeutic Chemical (ATC) classification (22). Before the interview, participants were instructed to bring a list of currently used medications and for cognitively impaired persons, a proxy or caregiver was asked instead. If the person was living in a nursing home, information on medication use was collected from medical records. In SNAC-K, additional clinical, lab, and drug-related parameters complement physicians’ assessment for certain conditions (Supplementary Table 1).
The Swedish National Patient Register includes all inpatient care in Sweden since 1987 and, from 2001, it also includes specialized outpatient visits including day surgery and psychiatric care from both private and public care providers. The linkage with SNAC-K data is done using the personal identification number (23) once informed consent has been obtained from participants. The chronic ICD-10 codes registered 5 years previous to the SNAC-K baseline physician visit were included, both from the main and all nine possible secondary diagnoses.
Statistical Analysis
Prevalence rates and 95% confidence intervals of individual chronic conditions and of multimorbidity were calculated for the SNAC-K study population. The distribution of the number of chronic conditions across sex and age groups was graphically represented. To that end, the 11 original baseline age cohorts from SNAC-K were grouped into four broader categories: 60–66, 72–78, 81–87, and 90+. To account for the sampling design, sex and age-specific weights were applied. Finally, we calculated the number of people with two or more different ICD-10 codes within the same chronic disease category (eg, coexistence of renal calculosis and uterine prolapse in people with “Other genitourinary diseases”), in order to verify whether multimorbidity estimated with the disease categories would differ substantially from multimorbidity estimated with ungrouped individual ICD-10 codes.
Results
A disease or condition was considered to be chronic if it had a prolonged duration and either (a) left residual disability or worsening quality of life or (b) required a long period of care, treatment, or rehabilitation. By applying this definition in consideration of older populations, the group of physicians identified 918 one to four-digit level ICD-10 codes as chronic and grouped them into 60 chronic disease categories in a second phase (Supplementary Table 2).
When applying our operational measure of chronic disease to the SNAC-K cohort (mean age 74.6, SD 11.2; 46.9% 75 years or older; 64.9% women; 5.7% institutionalized) and when considering both available data sources, 13 out of the 60 disease categories showed a prevalence >10%, 35 were between 1% and 10%, and 12 were <1% (Table 1). Only 3.2% of participants were free from any of the 60 disease categories. One in every 10 participants was identified as having disease(s) in only one of the 60 disease categories, and 88.6%, 73.2%, and 55.8% were identified as having diseases in ≥2, ≥3, and ≥4 of the disease categories, respectively. The prevalence of almost all diseases slightly increased when we assessed their presence by complementing diagnoses from SNAC-K with hospital data (Supplementary Table 3), especially so for pancreas, biliary tract and gallbladder disorders (73.6% of all cases), chronic skin ulcers (66.7% of all cases), peripheral vascular disease (55.6% of all cases), and chronic infectious diseases (50%).
Table 1.
Prevalence per 100 Population (P/100) and 95% CI of Chronic Disease Categories Considering All Available Data Sourcesa (N = 3,363)
| Chronic Disease | P/100 | 95% CI | Chronic Disease | P/100 | 95% CI |
|---|---|---|---|---|---|
| Hypertension | 68.8 | 67.1–70.4 | Other cardiovascular diseasesb | 3.7 | 3.1–4.5 |
| Dyslipidemia | 45.8 | 44.1–47.6 | Neurotic, stress-related and somatoform diseases | 3.2 | 2.6–3.9 |
| Chronic kidney diseases | 37.5 | 35.9–39.1 | Other genitourinary diseasesb | 2.8 | 2.2–3.5 |
| Ischemic heart disease | 16.7 | 15.4–18.1 | Cardiac valve diseases | 2.7 | 2.1–3.3 |
| Anemia | 13.7 | 12.5–15.1 | Migraine and facial pain syndromes | 2.5 | 2.0–3.1 |
| Osteoarthritis and other degenerative joint diseases | 13.2 | 12.0–14.5 | Other psychiatric and behavioral diseasesb | 2.3 | 1.8–2.9 |
| Colitis and related diseases | 13.2 | 12.0–14.5 | Other neurological diseasesb | 1.9 | 1.5–2.5 |
| Deafness, hearing impairment | 12.3 | 11.2–13.5 | Sleep disorders | 1.9 | 1.5–2.4 |
| Heart failure | 11.7 | 10.6–12.9 | Bradycardias and conduction diseases | 1.9 | 1.4–2.4 |
| Obesity | 11.2 | 10.1–12.3 | Peripheral vascular disease | 1.8 | 1.3–2.4 |
| Thyroid diseases | 11.1 | 10.0–12.3 | Other metabolic diseasesb | 1.7 | 1.3–2.3 |
| Dementia | 10.5 | 9.5–11.6 | Peripheral neuropathy | 1.6 | 1.2–2.2 |
| Atrial fibrillation | 10.3 | 9.2–11.4 | Chronic pancreas, biliary tract and gallbladder diseases | 1.5 | 1.1–2.0 |
| Depression and mood diseases | 9.4 | 8.4–10.5 | Allergy | 1.4 | 1.1–1.9 |
| Solid neoplasms | 9.0 | 8.0–10.1 | Parkinson and parkinsonism | 1.3 | 0.9–1.8 |
| Diabetes | 8.9 | 7.9–10.0 | Other respiratory diseasesb | 1.1 | 0.8–1.6 |
| Cerebrovascular disease | 8.5 | 7.5–9.6 | Chronic ulcer of the skin | 1.0 | 0.7–1.5 |
| Osteoporosis | 7.6 | 6.7–8.7 | Epilepsy | 1.0 | 0.7–1.4 |
| Other musculoskeletal and joint diseasesb | 7.1 | 6.2–8.2 | Ear, nose, throat diseases | 0.9 | 0.6–1.3 |
| Dorsopathies | 6.6 | 5.8–7.6 | Inflammatory bowel diseases | 0.9 | 0.6–1.3 |
| Glaucoma | 6.5 | 5.6–7.5 | Hematological neoplasms | 0.8 | 0.5–1.2 |
| Cataract and other lens diseases | 6.3 | 5.4–7.2 | Venous and lymphatic diseases | 0.8 | 0.5–1.2 |
| Asthma | 6.0 | 5.2–6.9 | Schizophrenia and delusional diseases | 0.7 | 0.5–1.1 |
| Other eye diseasesb | 5.4 | 4.6–6.3 | Blood and blood forming organ diseases | 0.5 | 0.3–0.9 |
| COPD, emphysema, chronic bronchitis | 5.2 | 4.4–6.0 | Other digestive diseasesb | 0.5 | 0.3–0.8 |
| Autoimmune diseases | 4.8 | 4.1–5.7 | Chronic infectious diseases | 0.3 | 0.2–0.6 |
| Blindness, visual impairment | 4.5 | 3.8–5.3 | Chronic liver diseases | 0.2 | 0.1–0.4 |
| Esophagus, stomach, and duodenum diseases | 4.5 | 3.8–5.3 | Multiple sclerosis | 0.1 | 0.0–0.2 |
| Prostate diseases | 4.3 | 3.6–5.1 | Other skin diseasesb | 0.1 | 0.0–0.2 |
| Inflammatory arthropathies | 4.1 | 3.5–4.9 | Chromosomal abnormalities | 0.0 | — |
| 2+ chronic diseases | 88.6 | 87.6–89.5 | |||
| 3+ chronic diseases | 73.2 | 71.8–74.5 | |||
| 4+ chronic diseases | 55.8 | 54.2–57.4 |
Note: CI = confidence intervals; COPD = chronic obstructive pulmonary disease; SNAC-K = Swedish National study of Aging and Care in Kungsholmen. All prevalence rates are adjusted by sex and age-specific weights to account for the sampling design.
SNAC-K data + inpatient data from the National Patient Register.
These categories group all chronic diseases not included in the other system-specific categories. The list of diseases included in each category can be viewed in Supplementary Table 2.
We verified in the SNAC-K cohort that the percentage of people with two or more different ICD-10 codes within a given disease category was less than 1% of all persons presenting with that category (data not shown). Therefore, there should not be a substantial underestimation of multimorbidity when using our list of 60 disease categories, rather than ungrouped individual ICD-10 disease codes in the calculation of multimorbidity.
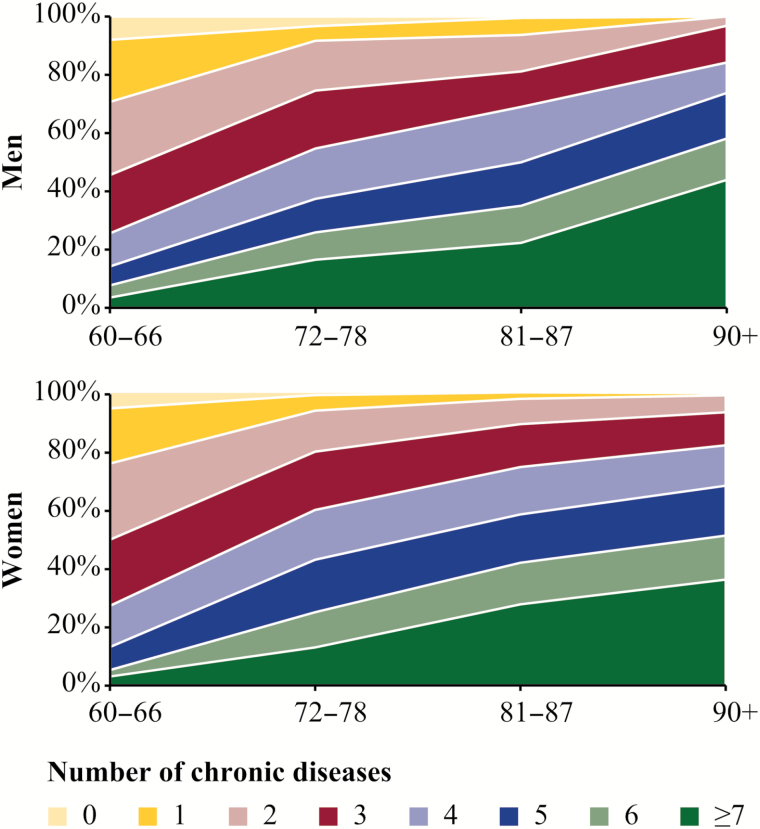
The number of chronic disease categories increased with age, consistently among men and women (Figure 1). The range and heterogeneity of the number of chronic disease categories was wide across all age groups. More than one third of the adults aged 90 years and older experienced chronic disease in seven or more disease categories.
Figure 1.
Percent distribution of number of chronic disease categories by sex and age group considering all available data sources (N = 3,363). Swedish National study of Aging and Care in Kungsholmen (SNAC-K) data + inpatient data from the National Patient Register. Within-age group prevalence rates are adjusted by age-specific weights to account for the sampling design.
Discussion
Multimorbidity, which primarily affects the older population, is still insufficiently understood from an epidemiological perspective (3). This is mainly due to the fact that no standard approach for its measurement exists so far. The selection of diseases across the multimorbidity literature is generally based on discretional criteria and dependent on the data available, which hinders comparability and generalizability of results.
In this study, we aimed to reach a consensus on which diseases fulfill the definition of chronicity, and how to categorize them into a manageable number of categories, based on the clinical judgment and agreement among an international and multidisciplinary team of geriatricians, general practitioners, and epidemiologists. When applied to the SNAC-K population, using both clinical data collected directly in SNAC-K and administrative data derived from the Swedish National Patient Register for that same population, the overall prevalence of multimorbidity was higher than that generally reported in primary care or even population-based studies (24), whether we measure it as the coexistence of two, three, or even four chronic conditions. This is largely due to the higher number of diseases considered in our comprehensive inclusion list and the integration of several sources of data (ie, lab, drug, and hospital data). The low discriminative power of any of the employed cut-off points to capture the heterogeneity in older adults’ morbidity is in line with recent claims suggesting to measure multimorbidity as a continuous grading scale of medical health problems rather than as a dichotomous yes/no phenomenon (25).
Strengths and Weaknesses
The transparency of the process used to develop this operational measure of chronic multimorbidity in older adults is one of the added values of the present methodology. Our definition of chronic disease is in accordance with most other approved definitions that also incorporate time, treatment, care, quality of life, and function-related aspects. Other dimensions such as disease progression (26), reversibility (27), sequelae (28), and prognosis (29) have also been considered occasionally, but we believe these would not add considerable accuracy to the criteria already used in this study. In fact, duration is the most common, and sometimes, the only criterion used to define chronic conditions (30).
The classification of ICD-10 codes according to their chronicity was done by an international team of geriatricians and general practitioners; two of the medical specialties that are best trained and suited to holistically assess and manage older patients with multiple coexisting chronic problems in the inpatient and outpatient settings (31).
Ours is among the most comprehensive lists of chronic conditions for measuring multimorbidity provided thus far. The need for more simple and succinct tools for multimorbidity detection has been proclaimed by some authors, mainly for practical reasons related to data access and processing (5,32), prevalence being the main driver for disease prioritization (33). However, rare diseases such as Parkinson’s disease (with a prevalence less than 2% in adults aged 60 years and older) (34) undeniably represent a heavy burden for affected older individuals. This is the case for many conditions that are less likely to lead to hospitalization but which may still significantly impact quality of life and other important outcomes.
The present classification may be thought of as a reference framework for chronic disease identification in different data sources, and as such aims to offer the most extensive list possible on the basis of a validated international system of disease classification. Yet, the list could be subject to the selection or exclusion of specific conditions, depending on the aims of the study. In this regard, the miscellaneous categories related to other diseases of a given organ/system may add little value to those studies focused on the pathophysiology underlying disease associations, but enable weighting for rare but relevant chronic health problems such as hydrocephalus, myasthenia gravis, or pneumoconiosis.
The ICD used in this article is the standard for defining and reporting diseases, symptoms, and risk factors across WHO member states. Its most recent version (ICD-10) includes health problems that have not been classified as diseases traditionally, but which are long-standing and have an impact on individuals’ quality of life, such as developmental disorders, limb dysfunction, visual impairment, urinary incontinence, or behavioral problems (35). This contributes to making our methodology more usable for both patients and clinicians (36). The 11th version (ICD-11), to be finalized by 2018, will be comparable and consistent with ICD-10, so our list may be easily updated to guarantee its long-term use.
Complementing self- or clinician-rated disease assessments with drug, primary care (unavailable in this study), or hospital registry data has proved to provide more reliable estimates than those based on only a single source of information (24). This is especially relevant when studying multimorbidity rather than single morbidity, because the likelihood of incompleteness may be higher with increasing numbers of problems affecting an individual.
Still, some important limitations need to be made explicit and taken into consideration. The high degree of diagnostic specificity implicit under the ICD system may be relevant from the specialist care point of view, but is poorly adapted to the reality of primary care where episodes of care—instead of episodes of disease—are assessed (37). O’Halloran’s code-set of chronic conditions built around the International Classification of Primary Care (ICPC) (30) may adequately complement our list, although no disease aggregation was proposed by these authors, whose list comprises up to 129 conditions. Furthermore, for studies relying on patient self-report, disease designations may need to be adapted to make them understandable, clear, and distinguishable to lay participants.
We assigned no weights to individual conditions, although the differing impact of multimorbidity depending on the severity of disorders has been previously acknowledged (38). Weighting is particularly useful if the purpose of the measurement is to predict future outcomes such as mortality, but lacks interest beyond the specific outcome it is developed for or when no specific outcome is prioritized. Although osteoarthritis has a major impact on quality of life but not on mortality, the opposite is true for a small myocardial infarction.
The presence, number, or even combination of chronic health conditions alone may be of little informative value as a measure of an older person’s general health status (39). Other dimensions such as physical and cognitive function or coping strategies need to be considered if the well-being of older adults is to be assessed holistically (40). Moreover, our list is applicable only to older populations and therefore excludes relevant conditions such as endometriosis or the polycystic ovarian syndrome. Although multimorbidity is also common in younger populations (41), the physiological and clinical specificity of this phenomenon among older people deserves separate attention.
Comparison with Previous Research
Our list includes all health problems recommended by Diederichs and colleagues (5) to be included as core chronic diseases, and it vastly surpasses the minimum number of 12 conditions suggested by Fortin and colleagues to study prevalence estimates (24). Each of our 60 chronic disease categories identified has been incorporated in at least one of the most cited chronic disease inclusion lists for multimorbidity definitions (13–18). The broadness of our scope resulted in the inclusion of conditions, such as blood diseases, cataract and other lens diseases, chromosomal abnormalities, chronic infectious diseases, or chronic ulcers of the skin, which have so far only been considered by Salisbury and colleagues (17). The list published by these authors comprises 114 chronic conditions and is as yet the most exhaustive one, but it is part of a proprietary risk adjustment system whose algorithms are not available to the public. Other conditions have exceptionally been included by other authors: allergy (15), autoimmune and connective tissue diseases (16), glaucoma (16), multiple sclerosis (16), peripheral neuropathy (14), and venous and lymphatic disorders (15). Still, there are a few diseases considered in other lists that we decided to exclude given their symptomatic nature (eg, dizziness) (15) or a lack of clear concordance with the definition of chronic disease (eg, pulmonary embolism, as an acute event rather than a chronic disease) (17).
Other classification schemes for identifying chronic conditions have been developed in the United States (42–45). Some were developed for measuring chronic multimorbidity in the general population, but others for purposes such as the prediction of medical expenditures (42) or the evaluation of public health interventions (43). Compared with these, our operational measure of multimorbidity has the particularity of being specifically defined for older persons (ie, youth disorders such as autism (44) or attention-deficit/hyperactivity disorders (45) were excluded). Furthermore, our classification was based on an ad hoc clinical assessment of medical diagnoses by physicians and epidemiologists specialized in geriatrics and family medicine.
Future studies should analyze whether our proposed measure of multimorbidity outperforms these lists of chronic conditions regarding their predictive value for adverse health outcomes and their usefulness for clinical management and treatment decision support.
Applicability of Results
The demographic of older adults continues to grow, and thus, persons in this age range will continue to dominate health research in the coming decades. Therefore, a standardized comprehensive list of chronic conditions that can be pragmatically implemented using clinical and administrative data sources is necessary, in order to offer accurate, reproducible, and understandable information on time trends of the incidence and prevalence of multimorbidity. This will be crucial for the programing and forecasting of available health care resources in the future (46).
Moreover, if the greatest burdens of disability and death during old age arise from the co-occurrence of chronic diseases (6), studying the determinants of chronic disease development and accumulation will become a priority. This will also be vital to ensure that the extra years accruing from longevity are lived in good health. An open-access, reference list of chronic diseases is a prerequisite for the design and execution of this type of observational research. Epidemiological studies, where chronic multimorbidity is likely to be a confounder or effect modifier, may also benefit from our methodology (47).
Finally, one important step that is necessary in order to generate an evidence base for clinical practice in patients with multimorbidity is the focus on the associations of chronic diseases into so-called patterns of multimorbidity (41). Knowledge about such patterns provides essential information for developing guidelines that offer clinical management and treatment decision support for patients with multiple chronic diseases. However, unless a homogeneous list of chronic conditions is available, no contrastable information will be obtained from the myriad of disease associations identified across studies (7).
Conclusions
We provide a clinically driven comprehensive list of chronic conditions that can be implemented using either or both clinical and administrative data. The list is openly available to the entire research community as a basis for discussion and to be validated in other populations. It may enable the advancement and evolution of conceptual and theoretical aspects of multimorbidity that will eventually lead to better care for the vast number of older people suffering from the coexistence of multiple chronic conditions.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by the funders of the Swedish National study on Aging and Care (SNAC): the Ministry of Health and Social Affairs, Sweden, the participating County Councils and Municipalities, and the Swedish Research Council. Specific grants were obtained from the Swedish Research Council for Health, Working Life and Welfare (Forte, 2006-1612), the Swedish Research Council (Vetenskapsrådet, 521-2013-8676), the Loo and Hans Osterman Foundation (2016oste46287), Lindhés Advokatbyrå AB (LA2016-0239), and Stiftelsen Gamla Tjänarinnor (2015-00243).
Conflict of Interest
All authors declare no conflict of interest.
Supplementary Material
References
- 1. Arokiasamy P, Uttamacharya U, Jain K, et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015;13:178. doi:10.1186/s12916-015-0402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307:2493–2494. doi:10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Palmer K, Navickas R, et al. ; Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle (JA-CHRODIS) Time to face the challenge of multimorbidity. A European perspective from the joint action on chronic diseases and promoting healthy ageing across the life cycle (JA-CHRODIS). Eur J Intern Med. 2015;26:157–159. doi:10.1016/j.ejim.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 4. van den Akker M, Buntinx F, Knottnerus J. Comorbidity or multimorbidity: what’s in a name ? A review of literature. Eur J Gen Pract. 1996;2:65–70. doi:10.3109/13814789609162146 [Google Scholar]
- 5. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi:10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 6. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi:10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 7. Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67:254–266. doi:10.1016/j.jclinepi.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 8. Schellevis FG, van der Velden J, van de Lisdonk E, van Eijk JT, van Weel C. Comorbidity of chronic diseases in general practice. J Clin Epidemiol. 1993;46:469–473. [DOI] [PubMed] [Google Scholar]
- 9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi:10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 11. De Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi:10.1016/j.jclinepi.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Stewart M, Fortin M, Britt HC, Harrison CM, Maddocks HL. Comparisons of multi-morbidity in family practice—issues and biases. Fam Pract. 2013;30:473–480. doi:10.1093/fampra/cmt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Britt HC, Harrison CM, Miller GC, Knox SA. Prevalence and patterns of multimorbidity in Australia. Med J Aust. 2008;189:72–77. [DOI] [PubMed] [Google Scholar]
- 14. Marengoni A, Winblad B, Karp A, Fratiglioni L. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98:1198–1200. doi:10.2105/AJPH.2007.121137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Bussche H, Koller D, Kolonko T, et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health. 2011;11:101. doi:10.1186/1471-2458-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi:10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 17. Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi:10.1186/1477-7525-3-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Geneva, Switzerland: WHO; http://apps.who.int/classifications/icd10/browse/2016/en. Accessed May 17, 2016. [Google Scholar]
- 20. Lagergren M, Fratiglioni L, Hallberg IR, et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res. 2004;16:158–168. doi:10.1007/BF03324546 [DOI] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi:10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO Collaborating Centre for Drug Statistics Methodology (WHOCC). Anatomical Therapeutic Chemical (ATC) Classification System. Oslo, Norway: WHOCC; http://www.whocc.no/atc_ddd_index/. Accessed May 17, 2016. [Google Scholar]
- 23. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi:10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–151. doi:10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lowe D, Taylor M, Hill S. Changing definitions altered multimorbidity prevalence, but not burden associations, in a musculoskeletal population. J Clin Epidemiol. 2016;78:116–126. doi:10.1016/j.jclinepi.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 26. World Health Organization (WHO). Noncommunicable Diseases. Geneva, Switzerland: WHO. http://www.who.int/topics/noncommunicable_diseases/en/. Accessed May 17, 2016. [Google Scholar]
- 27. US Department of Health and Human Services. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: US Department of Health and Human Services; 2011. [Google Scholar]
- 28. Newman Dorland W. Dorland’s Illustrated Medical Dictionary. 28th ed. Philadelphia, PA: W.B. Saunders Company; 1994. [Google Scholar]
- 29. Australian Institute of Health and Welfare. Chronic Diseases and Associated Risk Factors in Australia, 2001. Canberra, Australia: Australian Institute of Health and Welfare; 2002. [Google Scholar]
- 30. O’Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract. 2004;21:381–386. doi:10.1093/fampra/cmh407 [DOI] [PubMed] [Google Scholar]
- 31. Ickowicz E. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc. 2012;60:E1–E25. doi:10.1111/j.1532-5415.2012.04188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diederichs C, Bartels DB, Berger K. Methodological challenges concerning the selection of diseases for a standardized multimorbidity index. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2011;54:972–978. doi:10.1007/s00103-011-1323-0. [DOI] [PubMed] [Google Scholar]
- 33. Van Den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54:675–679. [DOI] [PubMed] [Google Scholar]
- 34. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi:10.1002/mds.25945 [DOI] [PubMed] [Google Scholar]
- 35. Anderson G. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. http://www.rwjf.org/en/library/research/2010/01/chronic-care.html. Accessed May 17, 2016. [Google Scholar]
- 36. Willadsen TG, Bebe A, Køster-Rasmussen R, et al. The role of diseases, risk factors and symptoms in the definition of multimorbidity—a systematic review. Scand J Prim Health Care. 2016;34:112–121. doi:10.3109/02813432.2016.1153242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lamberts H, Hofmans-Okkes I. Episode of care: a core concept in family practice. J Fam Pract. 1996;42:161–167. [PubMed] [Google Scholar]
- 38. Diederichs CP, Wellmann J, Bartels DB, Ellert U, Hoffmann W, Berger K. How to weight chronic diseases in multimorbidity indices? Development of a new method on the basis of individual data from five population-based studies. J Clin Epidemiol. 2012;65:679–685. doi:10.1016/j.jclinepi.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 39. Young Y, Frick KD, Phelan EA. Can successful aging and chronic illness coexist in the same individual? A multidimensional concept of successful aging. J Am Med Dir Assoc. 2009;10:87–92. doi:10.1016/j.jamda.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization (WHO). World Report on Ageing and Health. Geneva, Switzerland: WHO; 2015. http://www.who.int/ageing/publications/world-report-2015/en. Accessed May 17, 2016. [Google Scholar]
- 41. Prados-Torres A, Poblador-Plou B, Calderón-Larrañaga A, et al. Multimorbidity patterns in primary care: interactions among chronic diseases using factor analysis. PLoS One. 2012;7:e32190. doi:10.1371/journal.pone.0032190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention. Chronic Disease Indicators. http://www.cdc.gov/cdi/overview.html. Accessed October 10, 2016. [Google Scholar]
- 44. Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi:10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Medicare and Medicaid Services. Chronic Condition Data Warehouse. https://www.ccwdata.org/web/guest/condition-categories. Accessed October 10, 2016. [Google Scholar]
- 46. Atun R. Transitioning health systems for multimorbidity. Lancet. 2015;386:721–722. doi:10.1016/S0140-6736(14)62254-6 [DOI] [PubMed] [Google Scholar]
- 47. Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci. 2007;62:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.