Abstract
As with many other tissues and organs, the immune system is also affected by age. Immunosenescence is characterized by a decreased ability of immune cells to mount a productive response upon exposure to new antigens. Several studies have reported that members of families with exceptional longevity show improved immune function, which might contribute to the increased life- and health-span observed in those families. Autophagy is a catabolic process that delivers cytoplasmic material to the lysosomes for degradation. Defective autophagy is known to be associated with age in several cell types and tissues and its dysregulation is related to age-associated diseases. In T-cells, autophagy has an essential role in modulating protein and organelle homeostasis and in the regulation of activation-induced responses. In this study, using two different cohorts of individuals belonging to families with exceptional longevity, we show that CD4+ T-cells isolated from the offspring of parents with exceptional longevity show improved activation-induced autophagic activity compared with age-matched controls. Interestingly, increased autophagy is positively correlated with increased interferon-γ production. In conclusion, autophagy appears to be better maintained in members of families with extended longevity and positively correlates with improved T-cell function.
Keywords: Centenarians, Immunosenescence, CD4+ T-cell, Proteostasis, Macroautophagy
As individuals age a decline in many essential functions of the adaptive immune system occurs, which results in diminished ability to fight new pathogen infections and decreased efficacy of the response to vaccination (1). Defects in peripheral T-cell populations contribute to the overall loss of efficacy of immune responses with age (2). Indeed, inefficient cognate helper function of CD4+ T-cells has been identified as a major component of T-cell immunosenescence (3).
Lysosomal degradation of intracellular proteins via autophagy plays a central role in maintaining proper cell homeostasis by reducing the accumulation of damaged proteins and recycling amino acids for new protein synthesis, and can also provide alternative sources of energy or modify protein levels in response to extracellular signals (4). Interestingly, a decline in the function of mechanisms responsible for protein homeostasis, including autophagy, has been proposed to underlie some of the functional defects associated with aging in different cells and tissues (5–7). Decreased basal levels of autophagy have been reported in T-cells isolated from old subjects, suggesting that a decline in autophagy may underlie some of the functional defects that are characteristic of T-cells in old organisms (8).
Different explanations have been proposed to account for the exceptional longevity of certain populations. Evidence suggests that genetic traits in those populations account for some of the physiological properties of different tissues and organs that may contribute to explain increased longevity (9–12). Members of extended longevity families also show improved immune responses (13–15). For instance, a Dutch cohort of individuals enriched for familial longevity were found to exhibit less signs of immunosenescence compared to controls (16).
We have analyzed CD4+ T-cells obtained from two different populations of individuals belonging to families with exceptional longevity and we present evidence that supports that activation-induced autophagy in the CD4+ T-cell compartment is significantly better maintained in members of exceptional longevity families than in control subjects of the same age. Furthermore, levels of autophagic activity correlate with improved T-cell functional parameters, supporting that maintenance with age of this proteostatic and regulatory process might confer T-cells increased fitness.
Material and Methods
Study Design and Subjects
Eight Jewish Ashkenazi centenarians, 10 of their offspring (offspring of parents with exceptional longevity [OPEL]) and 8 age-matched controls (offspring of parents with usual survival [OPUS]) were randomly selected from the Einstein Longevity Aging Study that has recruited a previously described cohort of Ashkenazi Jews with exceptional longevity (17). A second set of samples was obtained from a randomly selected cohort of cytomegalovirus negative individuals from the Leiden Longevity Study and comprised six members of families with exceptional longevity and eight aged-matched partners. Selection of offspring was based on family history score in order to select for the offspring from families with greatest survival (average score: −2.8 ± 1.1) (18). The characteristics of this cohort have been described elsewhere (19). All analyses were performed in accordance with the policy of the Committee on Clinical Investigation of the Albert Einstein College of Medicine.
Isolation and Culture of CD4+ T-Cells
Previously frozen buffy coats containing peripheral blood mononuclear cells from venous blood were used to isolate CD4+ T-cells using anti-human CD4-coupled-Dynabeads (Life sciences). Cells were expanded using human T-activator CD3/CD28 Dynabeads (Life sciences) for 6 days in RPMI medium 1640 (Life Sciences), supplemented with 10% fetal calf serum, 1% L-glutamine, 1% penicillin/streptomycin, and activated using human T-activator CD3/CD28 Dynabeads (Life sciences) for 2 days. Beads were removed and cells were cultured for 4 additional days before being used to assess autophagy activity in response to T-cell receptor (TCR) engagement.
Enzyme-Linked Immunosorbent Assay
CD4+ T-cells prepared from expanded cultures as described earlier were activated with human T-activator CD3/CD28 Dynabeads for 24 hours. Supernatants were then collected and cytokine production was determined with a sandwich enzyme-linked immunosorbent assay using antibody-pairs against human IL2 (5344.111 and biotinylated B33-2 from BD-Biosciences) or interferon-γ (IFNγ) (NIB42 and biotinylated 4S.B3 from e-Bioscience).
Analysis of T-Cell Phenotype by FACS
Freshly isolated T-cells from thawed peripheral blood mononuclear cell frozen stocks were incubated with fluorochrome-conjugated antibodies against CD4 (RPA-T4, BD Phar), CD8 (HIT-8a, eBioscience), CD45RO (UCHL1, eBioscience), CD62L (DREG-56, eBioscience), and/or CD28 (CD28-2, BD) for 30 minutes at 4°C in PBS. Cells were then washed with PBS and analyzed using an LSR II flow cytometer (Beckton Dickinson). Percentages of populations expressing a given marker and mean fluorescence intensity of specific markers were calculated using Flowjo software.
Measurement of Autophagy Flux
Turnover of LC3 was quantified using immunofluorescence by comparing the levels of LC3+ structures in untreated T-cells (resting or activated with plate bound anti-CD3 and antiCD28 antibodies for 24 hours) and in T-cells that were treated with 20 mM NH4Cl and 100 µM leupeptin to block lysosomal proteases for the last 3 hours of culture in each condition analyzed. Autophagy flux was determined as the ratio of the two deltas of the average number of puncta/nucleus in the four studied conditions: resting (R), resting with lysosomal inhibitors (RLI), activated (A), and activated with lysosomal inhibitors (ALI), with the following formula: . All statistical analyses were performed with SPSSStatistics 20 (IBM).
Immunofluorescence
T-cells were deposited on microscope slides using a cytospin centrifuge. Cells then fixed in 4% paraformaldehyde-phosphate buffered saline (PBS) and permeabilized in PBS −0.3% Triton. An anti-LC3 antibody (MBL, PM036) and an Alexa Fluor 488-coupled secondary antibody were used to visualize LC3+ vesicles. Images were acquired using a fluorescence microscope with apotome technology (Zeiss). From each slide, three fields were acquired, each containing 15–50 viable cells. Slides were blinded to the investigators and quantification of autophagosome puncta was performed using ImageJ (National Institutes of Health).
Results
To determine whether familial longevity could be associated with a reduced decline in activation-induced autophagy with age, we analyzed the induction of autophagy in CD4+ T-cells isolated from aged subjects and compared them with cells obtained from individuals belonging to families with exceptional longevity. Samples were obtained from two different ongoing studies: one at the Albert Einstein College of Medicine in the United States and a second one at Leiden University Medical Center in The Netherlands. The participants’ characteristics are described in Table 1.
Table 1.
Characteristics of Subjects
| Characteristics | Einstein Longevity Study | Leiden Longevity Study | |||
|---|---|---|---|---|---|
| Centenarians (n = 8) | OPEL (n = 10) | OPUS (n = 8) | Offspring (n = 6) | Partners (n = 8) | |
| Demographic data | |||||
| Age (years) | 98.8 (4.5) | 75.1 (5.1) | 77.3 (6.5) | 64.8 (4.24) | 62.6 (8.7) |
| Female, % | 50 | 33.3 | 33.3 | 16.7 | 75 |
| T-cell phenotype | |||||
| % of CD62low CD45RO+ cells in the CD4+ T-cell population | 82.1 (12.5)a | 82.3 (12.8)c | 74.9 (17.3)c | 64.8 (17.2) | 66.0 (17.3) |
| % of CD62Llow CD45RO+ cells in the CD8+ T-cell population | 85.0 (13.0)b | 86.1 (7.8)b | 80.5 (18.1)a | 63.5 (10.5) | 68.0 (14.3) |
| % of CD28+ in the CD4+ T-cell population | 60.2b (12.3; MFI: 3,838) | 72.3a (9.9; MFI: 4,415) | 74.4a (9.6; MFI: 4,273) | ||
Note: MFI = mean fluorescence intensity. Means (SD) are provided, unless otherwise stated.
a n = 7. bn = 6. cn = 8.
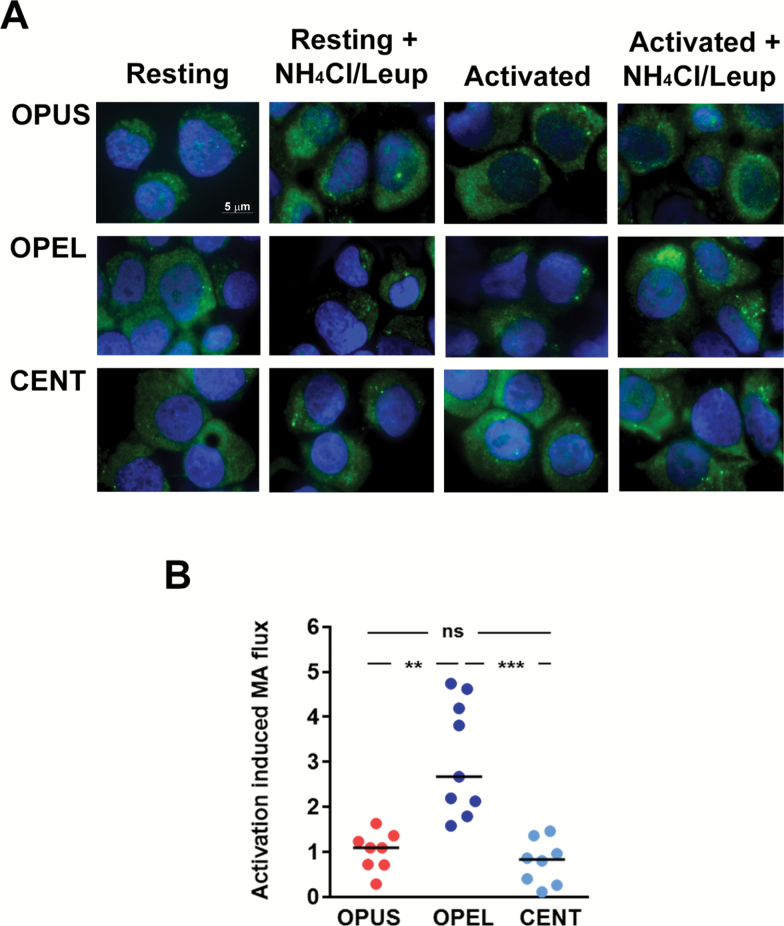
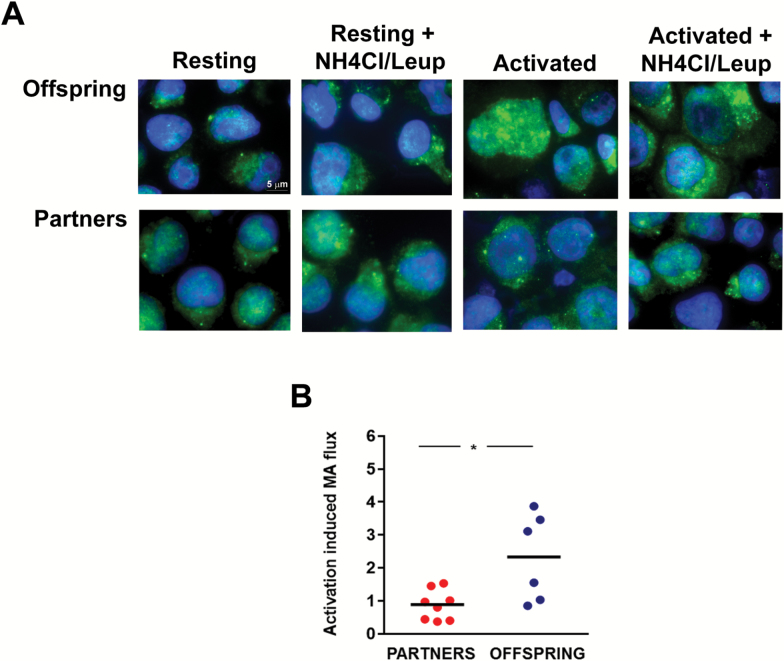
To quantify levels of activation-induced autophagy, we measured the rate of LC3 turnover, one of the most reliable parameters used to measured autophagy activity (20,21). Fold increase of LC3+ vesicles turnover in activated over resting T-cells was calculated for each sample. Analysis of samples from the Einstein Longevity Study revealed that induction of autophagy following activation was significantly compromised in CD4+ T-cells obtained from old control OPUS when compared with measurements performed in T-cells from the OPEL of similar age (Figure 1). Levels of autophagy in CD4+ T-cells obtained from OPUS were also markedly lower (average flux 1.1 ± 0.4 vs 3.8 ± 1.4) than those detected in CD4+ T-cells obtained from a limited number of young controls (average age 25.0 ± 3.6 years; n = 3), confirming previous studies that had shown decreased autophagy in T-cells with age (8). These results did not result from changes in levels of memory cells, which were not significantly different in both populations (Table 1). Similar results were obtained when samples from the Leiden Longevity Study were analyzed, comparing offspring from families with exceptional longevity with their partners, who did not belong to exceptional longevity families (Figure 2). As the data obtained with the samples of the Einstein Longevity study, increased in LC3 flux caused by T-cell activation, was significantly higher in T-cells obtained from offspring of families with exceptional longevity than in their partners. (Figures 1 and 2).
Figure 1.
Autophagy flux induced by TCR and CD28 engagement in CD4+ T-cells from the Einstein Longevity Study. (A) Representative immunofluorescence fields showing LC3 staining (green) in CD4+ T-cells obtained from centenarians (CENT), the offspring of parents with exceptional longevity (OPEL), and the offspring of parents with usual survival (OPUS). Cells were either left resting or stimulated with antiCD3+anti-CD28-coated beads for 24 hours. During the last 3 hours, NH4Cl and leupeptine were added to the cell cultures to inhibit lysosomal proteases and assess LC3+ vesicle turnover (autophagy flux). Nuclei are stained with 4’,6-diamidino-2-phenylindole (DAPI) in blue. (B) Quantification of the autophagy flux induced by stimulation with anti-CD3 and anti-CD28 antibodies in CD4+ T-cells from control and exceptional longevity families. Individual data points and median of the fold increase in autophagy flux caused by T-cell activation are shown. Data were analyzed with a one-way analysis of variance and a Bonferroni post hoc test (see Table 1 for the size of the populations; **p < .01; ***p < .001).
Figure 2.
Autophagy flux induced by TCR and CD28 engagement in CD4+ T-cells from the Leiden Longevity Study. (A) Representative immunofluorescence fields showing LC3 staining (green) in CD4+ T-cells obtained from the offspring of extended longevity parents or their control partners that were either left resting or stimulated and anti-CD3+anti-CD28-coated beads for 24 hours. During the last 3 hours, NH4Cl and leupeptine were added to the cell cultures to inhibit lysosomal proteases and assess LC3+ vesicle turnover (autophagy flux). Nuclei are stained with DAPI in blue. (B) Quantification of autophagy flux induced by stimulation with antiCD3 and antiCD28 antibodies in CD4+ T-cells. Individual data points and median of the fold increase in autophagy flux caused by T-cell activation are shown. Data were analyzed with two-tail distribution t test (see Table 1 for the size of the populations; *p < .05).
We then proceeded to analyze autophagic activity in T-cells obtained from centenarians in the Einstein Longevity Study (average age 100.8 ± 2.5), to determine if the sustained activity of autophagy detected in OPEL could be maintained even in centenarians. Our data showed that activation-induced autophagy in this population showed a similar level of decline as the one detected in T-cells from OPUS, suggesting that the age-associated decline of autophagy in the T-cell compartment is delayed but not completely prevented in exceptional longevity families (Figure 1).
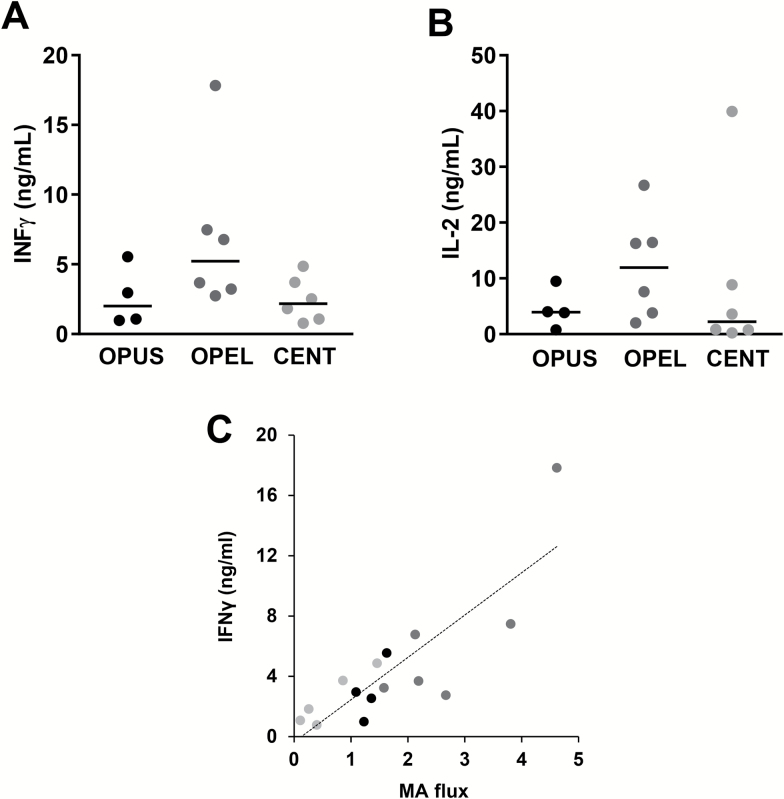
To determine whether better maintenance with age of autophagy activity in T-cells could support improved function in exceptional longevity families, we analyzed the expression of IL2 and IFNγ in T-cells. Confirming our hypothesis, CD4+ T-cells from OPEL produced markedly higher levels of IFNγ than T-cells from OPUS or centenarians (Figure 3A). T-cells from OPEL also produced higher amounts of IL2 than cells from OPUS (Figure 3B). Interestingly, we found a significant positive correlation between levels of activation-induced autophagy activity and IFNγ secretion in those cells (Figure 3C). For IL-2, however, T-cells from centenarians appear show a wider range of production and consequently, a correlation between IL-2 production and autophagy activity could not be established.
Figure 3.
Changes in autophagy flux with age correlate with levels of activation-induced interferon-γ (IFNγ) expression in CD4+ T-cells. IFNγ (A) and IL-2 (B) expression in activated CD4+ T-cells isolated from centenarians (CENT), the offspring of parents with exceptional longevity (OPEL), and the offspring of parents with usual survival (OPUS). Individual data points and median of the fold increase in autophagy flux caused by T-cell activation are shown. (C) Correlation plot between autophagy flux and IFNγ production (Pearson r = .835; p < .0001).
Discussion
Autophagy is an essential cellular process that controls a wide array of functions, ranging from the response to different types of stress to the modulation of cell differentiation and tissue development (4). Our data support that CD4+ T-cells isolated from old (>65 years) individuals that belong to families with exceptional longevity preserve the ability to increase autophagy flux in respond to TCR engagement, which is markedly reduced in control age-matched individuals. Interestingly, the higher capacity to induce autophagy activity in response to stimulation in T-cells from OPEL versus T-cells from OPUS, correlates with improved function as measured by increased IFNγ production.
Several studies have reported that decreases in autophagy occur with age in many cell types (7, 22), which results in a decreased ability of old cells to cope with different stresses and leads to dysregulated cell homeostasis and altered function. In T-cells, a similar reduction in the levels of autophagy with age has been reported in mice and humans (8, 23). Supporting that the age-associated altered regulation of autophagy may underlie some of the age-associated functional defects in T-cells, recent studies have shown that administration of spermidine to old mice leads to increased autophagy in T-cells, resulting in enhanced generation of CD8+ T-cell memory in response to viral infection (23). The correlation we observe in this study between levels of autophagy activity in T-cells and the magnitude of cytokine production in response to activation, suggests that, also in human CD4+ T-cells, an age-associated decrease in autophagy might be responsible for some of the deficiencies in the quality and/or magnitude of T-cell responses that characterize T-cell immunosenescence (2,24).
Autophagy has been proposed to play critical roles in the regulation of T-cell homeostasis and function (25). For instance, turnover of mitochondria through mitophagy controls homeostasis of this organelle in naive T-cells (26). Consequently, T-cells deficient in autophagy show altered mitochondrial function with increased production of reactive oxygen species (26,27). Furthermore, autophagy also contributes to the regulation of activation-induced responses in T-cells and cells deficient in essential Atg genes show reduced levels of proliferation and cytokine secretion in response to TCR engagement (28,29). Autophagy-mediated regulation of T-cell metabolism has also been proposed to ensure efficient generation of CD8+ T-cell memory and to maintain regulatory T-cell function (30–33). Maintenance of higher levels of autophagy in T-cells of OPELs, might, therefore, also lead to enhanced T-cell responses, increased generation of effective memory, and improved regulatory T-cell function. A general increased fitness of the T compartment would then ensure better responses to infection and reduced autoimmunity, which could contribute to the increased health-span observed in exceptional longevity families (15). Indeed, studies in centenarian offspring of Sicilian origin have reported that their T-cells show less signs of immunosenescence than those from age-matched controls, including increased presence of naive T-cells and reduced numbers of T-cells with markers of senescence (13). Those studies have also established correlations between the numbers and characteristics of T-cells present in cohorts of centenarians and their 5-year survival rate (13,34).
The mechanisms that can account for the presence of a T-cell compartment with less signs of immunosenescence in members of exceptional longevity families remain yet to be identified (14). Our data suggest that preserving activation-induced autophagy activity in CD4+ T-cells may be key to the maintenance of more effective T-cell responses with age. Interestingly, a recent study has reported that the ability to activate autophagy in response to TCR engagement is impaired in CD8+CD28- T-cells, functionally impaired CD8+ T-cells that accumulate with age (35). However, we did not detect any significant differences in the expression of CD28 in CD4+ T-cells obtained from OPEL or OPUS (Table 1), suggesting that other mechanisms might be at play to downregulate autophagy in the CD4+ T-cell compartment. We have previously shown that signaling through common gamma chain cytokine receptors, including the IL2 receptor, regulates the induction of autophagy in activated T-cells (36). Given that reduced IL2 production and diminished signaling through the IL2 receptor are well-defined signs of aging in T-cells (2), it is possible that the loss of activation-induced autophagy activity may respond to the decreased ability of aged T-cells to produce this cytokine and/or signal through its receptor. As not only common-gamma chain cytokines but also other cytokines may influence the ability of T-cells to activate autophagy, we should also consider that differences in the cytokine milieu that defines inflamm-aging in distinct human populations might contribute to changes in the regulation of autophagy in T-cells (36,37).
We have recently found that a specific form of selective autophagy, chaperone-mediated autophagy, plays an essential role in the regulation of T helper responses and is also decreased with age in mice and humans (38). Interestingly, restoring the age-associated decrease in the activation of chaperone-mediated autophagy leads to enhance proliferation and cytokine production in mouse T-cells (38). These data may indicate that not only the dysregulation of macroautophagy but also the decline in the activity of other autophagic pathways might contribute to the reduced magnitude of the T-cell response that occurs with age (5).
Given the sample sizes used in this study, results must be interpreted with caution. However, even using those sample sizes, we were able to detect marked significant differences in activation-induced autophagy flux in CD4+ T-cells associated with familial longevity, which were corroborated in two different cohorts. These results would support that further studies to determine the global impact of reduced autophagy flux in T-cells for morbidity and mortality in the elderly adults should be continued in larger prospective studies. In any case, the correlation between higher autophagic activity in T-cells from OPEL and the increase in cytokine production when compared with T-cells isolated from OPUS would support that interventions aimed at improving autophagy activity in the elderly might result in enhanced T-cell function and attenuate the effects of aging in the T helper cell compartment.
Funding
This work was funded by the National Institute on Aging of the National Institutes of Health (AG031782, to F.M.), the Irma T Hirschl Trust (to F.M.), and the Paul Glenn Foundation for Medical Research (Center for the Biology of Human Aging to N.B., F.M., and G.A.). Use of research cores was supported by the Nathan Shock Center of Excellence for the Biology of Aging (National Institute on Aging AG038072).
References
- 1. Aspinall R, Goronzy JJ. Immune senescence. Curr Opin Immunol. 2010;22:497–499. doi:10.1016/j.coi.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 2. Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi:10.1016/j.arr.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 3. Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi:10.1084/jem.20041395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi:10.1007/978-3-642-00302-8_3 [DOI] [PubMed] [Google Scholar]
- 5. Cuervo AM, Macian F. Autophagy and the immune function in aging. Curr Opin Immunol. 2014;29:97–104. doi:10.1016/j.coi.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi:10.1016/j.cell.2011.07.030 [DOI] [PubMed] [Google Scholar]
- 7. Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi:10.1016/j.tig.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phadwal K, Alegre-Abarrategui J, Watson AS, et al. A novel method for autophagy detection in primary cells: impaired levels of macroautophagy in immunosenescent T cells. Autophagy. 2012;8:677–689. doi:10.4161/auto.18935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conneely KN, Capell BC, Erdos MR, et al. Human longevity and common variations in the LMNA gene: a meta-analysis. Aging Cell. 2012;11:475–481. doi:10.1111/j.1474-9726.2012.00808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barzilai N, Guarente L, Kirkwood TB, Partridge L, Rando TA, Slagboom PE. The place of genetics in ageing research. Nat Rev Genet. 2012;13:589–594. doi:10.1038/nrg3290 [DOI] [PubMed] [Google Scholar]
- 11. Barzilai N, Gabriely I, Atzmon G, Suh Y, Rothenberg D, Bergman A. Genetic studies reveal the role of the endocrine and metabolic systems in aging. J Clin Endocrinol Metab. 2010;95:4493–4500. doi:10.1210/jc.2010-0859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pawlikowska L, Hu D, Huntsman S, et al. ; Study of Osteoporotic Fractures. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi:10.1111/j.1474-9726.2009.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pellicanò M, Buffa S, Goldeck D, et al. Evidence for less marked potential signs of T-cell immunosenescence in centenarian offspring than in the general age-matched population. J Gerontol A Biol Sci Med Sci. 2014;69:495–504. doi:10.1093/gerona/glt120 [DOI] [PubMed] [Google Scholar]
- 14. Pinti M, Nasi M, Lugli E, et al. T cell homeostasis in centenarians: from the thymus to the periphery. Curr Pharm Des. 2010;16:597–603. doi:10.2174/138161210790883705 [DOI] [PubMed] [Google Scholar]
- 15. Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi:10.1016/j.exger.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 16. Derhovanessian E, Maier AB, Beck R, et al. Hallmark features of immunosenescence are absent in familial longevity. J Immunol. 2010;185:4618–4624. doi:10.4049/jimmunol.1001629 [DOI] [PubMed] [Google Scholar]
- 17. Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290:2030–2040. doi:10.1001/jama.290.15.2030 [DOI] [PubMed] [Google Scholar]
- 18. Houwing-Duistermaat JJ, Callegaro A, Beekman M, Westendorp RG, Slagboom PE, van Houwelingen JC. Weighted statistics for aggregation and linkage analysis of human longevity in selected families: the Leiden Longevity Study. Stat Med. 2009;28:140–151. doi:10.1002/sim.3421 [DOI] [PubMed] [Google Scholar]
- 19. Schoenmaker M, de Craen AJ, de Meijer PH, et al. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet. 2006;14:79–84. doi:10.1038/sj.ejhg.5201508 [DOI] [PubMed] [Google Scholar]
- 20. Botbol Y, Macian F. Assays for monitoring macroautophagy activity in T cells. Methods Mol Biol. 2015;1343:143–153. doi:10.1007/978-1-4939-2963-4_12 [DOI] [PubMed] [Google Scholar]
- 21. Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi:10.4161/auto.1.2.1697 [DOI] [PubMed] [Google Scholar]
- 22. Morimoto RI, Cuervo AM. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S33–S38. doi:10.1093/gerona/glu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Puleston DJ, Zhang H, Powell TJ, et al. Autophagy is a critical regulator of memory CD8(+) T cell formation. Elife 2014;3:e03706. doi:10.7554/eLife.03706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi:10.1038/ni.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Botbol Y, Guerrero-Ros I, Macian F. Key roles of autophagy in regulating T-cell function. Eur J Immunol. 2016;46:1326–1334. doi:10.1002/eji.201545955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi:10.4049/jimmunol.0801143 [DOI] [PubMed] [Google Scholar]
- 27. Watanabe R, Fujii H, Shirai T, Saito S, Ishii T, Harigae H. Autophagy plays a protective role as an anti-oxidant system in human T cells and represents a novel strategy for induction of T-cell apoptosis. Eur J Immunol. 2014;44:2508–2520. doi:10.1002/eji.201344248 [DOI] [PubMed] [Google Scholar]
- 28. Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi:10.1084/jem.20061303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185:7349–7357. doi:10.4049/jimmunol.1000576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kovacs JR, Li C, Yang Q, et al. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi:10.1038/cdd.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev. 2013;12:520–534. doi:10.1016/j.arr.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 32. Dinkins C, Pilli M, Kehrl JH. Roles of autophagy in HIV infection. Immunol Cell Biol. 2015;93:11–17. doi:10.1038/icb.2014.88 [DOI] [PubMed] [Google Scholar]
- 33. Wei J, Long L, Yang K, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17:277–285. doi:10.1038/ni.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bucci L, Ostan R, Giampieri E, et al. Immune parameters identify Italian centenarians with a longer five-year survival independent of their health and functional status. Exp Gerontol. 2014;54:14–20. doi:10.1016/j.exger.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 35. Arnold CR, Pritz T, Brunner S, et al. T cell receptor-mediated activation is a potent inducer of macroautophagy in human CD8(+)CD28(+) T cells but not in CD8(+)CD28(-) T cells. Exp Gerontol. 2014;54:75–83. doi:10.1016/j.exger.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 36. Botbol Y, Patel B, Macian F. Common gamma-chain cytokine signaling is required for macroautophagy induction during CD4 T cell activation. Autophagy 2015;11:1864–1877. doi:10.1080/15548627.2015.1089374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morrisette-Thomas V, Cohen AA, Fülöp T, et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev. 2014;139:49–57. doi:10.1016/j.mad.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valdor R, Mocholi E, Botbol Y, et al. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat Immunol. 2014;15:1046–1054. doi:10.1038/ni.3003 [DOI] [PMC free article] [PubMed] [Google Scholar]