Abstract
Background
We lack a comprehensive assessment of the risks and benefits of calorie restriction in older adults at high risk for cardiometabolic disease. Calorie restriction may reduce visceral adipose tissue (VAT) but also have negative effects on lean mass and quality of life.
Methods
We conducted a 52-week, randomized controlled trial involving 164 older adults with obesity taking at least one medication for hyperlipidemia, hypertension, or diabetes. Interventions included an exercise intervention alone (Exercise), or with diet modification and body weight maintenance (Maintenance), or with diet modification and energy restriction (Weight Loss). The primary outcome was change in VAT at 12 months. Secondary outcomes included cardiometabolic risk factors, functional status, and quality of life.
Results
A total of 148 participants had measured weight at 12 months. Despite loss of −1.6% ± 0.3% body fat and 4.1% ± 0.7% initial body weight, Weight Loss did not have statistically greater loss of VAT (−192.6 ± 185.2 cm3) or lean mass (−0.4 ± 0.3 kg) compared with Exercise (VAT = −21.9 ± 173.7 cm3; lean mass = 0.3 ± 0.3 kg). Quality of life improved in all groups with no differences between groups. No significant changes in physical function were observed. Weight Loss had significantly greater improvements in blood glucose (−8.3 ± 3.6 mg/dL, p < .05) and HDL-cholesterol (5.3 ± 1.9, p < .01) compared with Exercise. There were no group differences in the frequency of adverse events.
Conclusions
While moderate calorie restriction did not significantly decrease VAT in older adults at high risk for cardiometabolic disease, it did reduce total body fat and cardiometabolic risk factors without significantly more adverse events and lean mass loss.
Keywords: Weight reduction, cardiometabolic risk, visceral adipose tissue, quality of life, physical function
In 2010, there were an estimated 40 million adults aged 65+ representing 13% of the U.S. population; and more than a third were obese (1,2). By 2030, older adults will comprise 20% of the population; and those who reach age 65 have a life expectancy that exceeds 15 years—most of which will be spent in relative good health (1). Despite increased longevity, many older adults experience hypertension, diabetes, arthritis, heart disease, and cancer and physical functional limitations that are associated with obesity (1). Weight loss or changes in diet quality/composition may reduce chronic disease in older adults, but few studies have addressed this hypothesis.
Part of the reason few studies have focused on older adults is the concern that weight loss may be harmful. In 2005, the American Society for Nutrition and The Obesity Society issued a joint Position Statement (3) concluding that appropriate treatment for obesity in older persons was “controversial” due to among other things, potential harmful effects on lean mass such as muscle and bone. By 2013, the latest Guidelines for the Management of Overweight and Obesity in Adults (4) similarly concluded that the safety of weight loss for those aged 65 and older remained “controversial” for many of the same reasons.
There is some evidence of the effect of lifestyle interventions, including dietary changes, exercise, and behavioral counseling, in older adults with obesity (5–10), but it is limited. Only nine randomized controlled trials comparing exercise alone and exercise with a calorie-restricted diet have been conducted in samples of adults with a mean age ≥ 65 years (11–18). Only three of these targeted older adults with cardiometabolic risk (defined as risk of developing atherosclerotic cardiovascular disease or diabetes as a consequence of underlying insulin resistance) (14,16,18); two of these had sample sizes less than 25 (16,18) and in the third (14) cardiometabolic outcomes (ie, plasma CRP and IL-6) were collected, but group differences were not reported. Furthermore, these studies did not isolate the effect of calorie restriction from changes in diet composition alone, a strategy that may improve the overall cardiometabolic risk profile while avoiding some of the potential risk associated with calorie restriction.
Given these gaps in knowledge, the goal of the Calorie Restriction in Overweight SeniorS: Response of Older Adults to a Dieting Study (CROSSROADS) was to conduct a prospective randomized controlled trial to compare the effects of changing diet composition with and without intentional weight loss (ie, calorie restriction) over 12 months on changes in body composition and adipose tissue distribution, cardiometabolic disease risk, and functional status and quality of life in adults aged 65 and older who were at risk for cardiometabolic disease due to obesity and associated risk factors. Our primary hypothesis was there would be a 10% difference in visceral adipose tissue (VAT) after 12 months of a weight-reducing diet compared with an exercise-only control intervention. Additionally, we hypothesized that there would be a similar difference in VAT after 12 months of an intervention that improved diet quality but was weight stable compared with an exercise-only control intervention.
Methods
The study was conducted between May 20, 2009 and October 1, 2014 at the University of Alabama at Birmingham (UAB). The research protocol was approved by the Institutional Review Board at the UAB and all participants gave written informed consent. The study had an external Data Safety Monitor. Additionally, CROSSROADS has been registered in ClinicalTrials.gov (Registration Number Identifier: NCT00955903). A comprehensive description of the methodology following recommendations of the CONSORT Statement for reporting of randomized controlled trials has been published previously (19).
Participants
Volunteers were recruited from the greater Birmingham-Hoover, Alabama metropolitan area, using various forms of advertisement and word-of-mouth recruitment techniques. Potential participants had to be at least 65 years old; be weight stable and obese (body mass index of 30–40 kg/m2); and prescribed at least one oral medication for control of lipids, blood pressure, and/or blood glucose, resulting in adequate control of the risk factor (eg, blood pressure < 160/100 mm Hg). Volunteers were excluded from participation during a series of one telephone and three in-person screening visits if they had significant medical, psychiatric, or physical limitations that would prevent adoption of the lifestyle recommendations or ongoing treatments that would independently affect body weight and composition (19).
Randomization
Following completion of baseline testing, participants were randomly assigned to one of three groups: Exercise Only (Exercise), Exercise + Diet Quality + Weight Maintenance (Maintenance), or Exercise + Diet Quality + Weight Loss (Weight Loss). The statistician generated blocked random assignments using a computer-based algorithm, stratified by age category (65–74, 75+), sex, and race. Allocations were concealed in sealed envelopes that were opened by a research assistant at the time of randomization. Because this was a behavioral intervention study, it was not possible for participants or all study personnel to be blinded to group assignment. However, study personnel involved in data collection were blinded to group assignments.
Description of the Intervention
The basis for the intervention was a behavioral lifestyle modification program that provided group-based counseling and healthy recommendations to improve physical activity in all groups, diet quality in the Maintenance group, and diet quality and body weight in the Weight Loss group. Because standard lifestyle interventions would generally include exercise prescriptions, the recommendations for exercise were consistent across all groups and included 90–150 min/wk of moderate to vigorous cardio-aerobic exercise such as walking based on monitoring their heart rate (20). Participants also received a written program to guide participation in two sessions/week of resistance training using resistance bands focused on major muscle groups of the extremities. The Exercise group served as a control, allowing for isolation of the effects of dietary changes and calorie restriction on body composition and physical function. Both the Maintenance group and the Weight Loss group received recommendations for improving diet composition by increasing consumption of low-energy dense fruits, vegetables, lean protein, and whole grains with a targeted macronutrient intake pattern of 25% of calories from protein, 47% from carbohydrates, and 28% from fat. In addition to recommended changes in diet composition, the Weight Loss group had a primary goal to reduce caloric intake by 500 kcal/d below estimated total energy needs based on measured resting energy expenditure, with a minimum intake of 1,000 kcal/d. Dietary intake was monitored with three 24-hour dietary recalls, including one weekend day, via the multiple pass approach at each time point. All groups received behavioral group counseling weekly for the first 24 weeks of the intervention, then every 2 weeks for the remainder of the 12-month intervention, to provide a high-frequency contact intervention consistent with obesity treatment guidelines (4). Each session that took place in our research facility lasted 60 minutes and included 30 minutes of group discussion related to a dietary, exercise, or behavioral topic, followed by 30 minutes of supervised exercise using resistance band exercises. All remaining exercise was unsupervised, and participants self-reported exercise using written diaries.
Outcome Measures
Blinded study personnel assessed outcome measures at baseline, 6 months, and 12 months, except for magnetic resonance imaging (MRI), which was completed at baseline and 12 months. Detailed methodology has been previously published (19); key measures are briefly described here. Body weight was measured in light clothing on calibrated electronic scales to the nearest 0.1 pound and converted to kilograms. The primary study outcome was change in VAT at 1 year measured by MRI on a 3-Tesla Philips Achieva system using previously described techniques (19). Other MRI body composition outcomes of interest included abdominal subcutaneous adipose tissue (SAT), thigh skeletal muscle, and thigh SAT volumes. Fat and lean mass (total, trunk, and appendicular) and bone mineral density at the lumbar spine were measured with dual-energy X-ray absorptiometry (DXA) using a Lunar DPX-L densitometer using the body composition Adult Software Version 1.33 (Lunar Corp).
We assessed biomarkers of cardiometabolic disease risk factors using fasting blood samples, including: glucose, insulin, lipids, and highly sensitive CRP (assessed by turbidometric methodology with reagents obtained from Pointe Scientific, Canton, MI) using a SIRRIS analyzer (Stanbio Laboratory, Boerne, TX); leptin and adiponectin (assessed by RIA reagents and radioimmunoassay, respectively, by Millipore, St. Charles, MO); and TNF-α and IL-6, both using electrochemiluminscence (Meso Scale Discovery, Rockville, MD). Blood pressure was measured by trained research staff using automated blood pressure devices (Omron HEM 907-XL) in duplicates measured 30 seconds apart then averaged. All prescription medications were recorded and categorized based on indication for diabetes, hypertension, or hyperlipidemia.
We assessed functional status using the UAB LifeSpace Assessment (21), 6-minute walk test (22), Short Physical Performance Battery (SPPB) (23), measurements of hand grip and knee extension strength, and chair sit and reach test (24). We assessed quality of life (QOL) generically and specifically using the SF-36v2 (25) and the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) (26,27), respectively.
Adverse Event Monitoring
We wanted to assess the full range of adverse events and symptoms reported by this group of older adults engaged in behavioral lifestyle interventions. Participants were interviewed at baseline for signs and symptoms and then screened every 3 months for any adverse medical events, signs, or symptoms of any nature. Additionally, participants self-reported any of these throughout the study.
Data Analyses
The prespecified comparisons of interests included Exercise versus Weight Loss, Exercise versus Maintenance, and Maintenance versus Weight Loss. We expected to be able to detect a 10% difference in VAT change between Weight Loss and Exercise and Weight Loss and Maintenance with 80% power to detect a change of 0.60 SD units with a retained sample size of 48 participants per group at follow-up.
This was an intention-to-treat study. All analyses, unless otherwise specified, include all randomized cases. The statistical analyses were conducted using SAS software (version 9.3, SAS Institute Inc., Cary, NC). Prior to testing the specific aims, descriptive statistics were used to characterize the sample. For each outcome variable, baseline means and standard deviations were calculated. Groups were compared using the chi-square tests and analysis of variance to determine whether baseline differences between groups existed post-randomization. We used a multiple imputation strategy using PROC MI and PROC MIANALYZE in SAS to create eight data sets to impute missing data at 6 and 12 months because it makes use of the broad array of data available to produce unbiased parameter estimates (28,29). To evaluate our hypotheses, differences between intervention groups were calculated using multiple linear regression and differences within groups were calculated using generalized linear models. Both sets of analyses adjusted for the baseline value of the outcome measure, age, race, and sex and for category of medication when the related risk factor was the outcome (eg, diabetes medications for blood glucose; antihypertensives for blood pressure; anti-lipid medications for lipids). Results using the multiple imputation strategy showed consistency with a completers analysis.
Results
Study Population
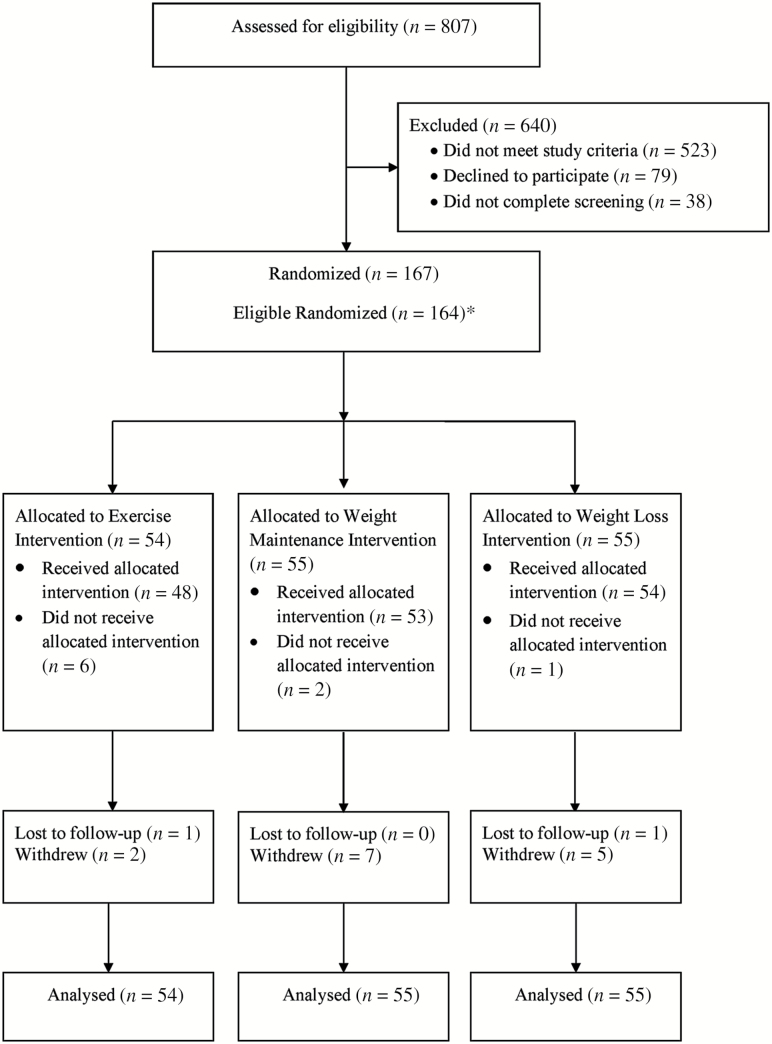
We randomized 164 eligible participants (see Figure 1); 148 participants (90.2%) had measured weight at 12 months (see Table 1 for baseline characteristics). At baseline, 156 were able to complete MRI scans for our primary body composition outcome; 133 of these individuals (81.1% of total randomized, 85.2% of those with baseline MRI scans) completed MRI scans at 12 months.
Figure 1.
Flow diagram of study participants throughout CROSSROADS trial. *Immediately following randomization and prior to receipt of intervention, three participants were diagnosed with cancer. Because cancer is an exclusion criterion and because we believe these participants very likely had undiagnosed cancer at the time of randomization, in the spirit of a controlled randomized trial and intention-to-treat, we do not include these three persons in any of our analyses. *Lost to follow-up and withdrew indicate those individuals for whom 12-month follow-up data were not collected.
Table 1.
Baseline Demographic and Medication Characteristics, According to Study Groupa
| Variable | Entire Sample (N = 164) | Exercise Only (N = 54) | Weight Maintenance (N = 55) | Weight Loss (N = 55) |
|---|---|---|---|---|
| Age (year) | 70.3 ± 4.7 | 69.9 ± 4.5 | 70.5 ± 4.8 | 70.3 ± 4.8 |
| Male sex, no. (%) | 62 (37.8) | 17 (31.5) | 22 (40.0) | 23 (41.8) |
| Race/ethnicity, no. (%) | ||||
| African American | 39 (23.8) | 15 (27.8) | 12 (21.8) | 12 (21.8) |
| Asian American | 1 (0.6) | 0 (0) | 0 (0) | 1 (1.8) |
| European American | 124 (75.6) | 39 (72.2) | 43 (78.2) | 42 (76.4) |
| Education, no. (%) | ||||
| High school | 21 (12.8) | 9 (16.7) | 8 (14.6) | 4 (7.3) |
| Some college/technical | 40 (24.4) | 14 (25.9) | 10 (18.2) | 16 (29.1) |
| 2- or 4-year college | 57 (34.8) | 14 (26.0) | 24 (43.6) | 19 (34.5) |
| Graduate degree | 46 (28.0) | 17 (31.5) | 13 (23.7) | 16 (29.1) |
| Taking medications for select conditions (%) | ||||
| Hypertension | 88.6 | 90.7 | 90.9 | 87.3 |
| Lipids | 67.7 | 68.5 | 65.5 | 70.9 |
| Diabetes | 20.4 | 24.1 | 16.4 | 21.8 |
Plus-minus values are means ± SDs. All demographic information was based upon self-report. There were no statistically significant differences between the groups (p < .05).
Indications That the Intervention Targets Were Achieved
By 6 months, based upon self-report, the Weight Loss group had the greatest decrease in calories (−161.3 ± 62.4 kcal/d, p < .01) with corresponding increases in nonstarchy vegetables (1.0 ± 0.2 servings/d, p < .001) and fruit servings (0.4 ± 0.2 servings/d, p < .05) based upon results of three 24-hour dietary recalls. The Maintenance group had significant increases at 6 months in nonstarchy vegetables (0.6 ± 0.3 servings/d, p < .05) and fruit (0.8 ± 0.2 servings/d, p < .001) without any statistically significant increase in calories (Supplementary Table 1).
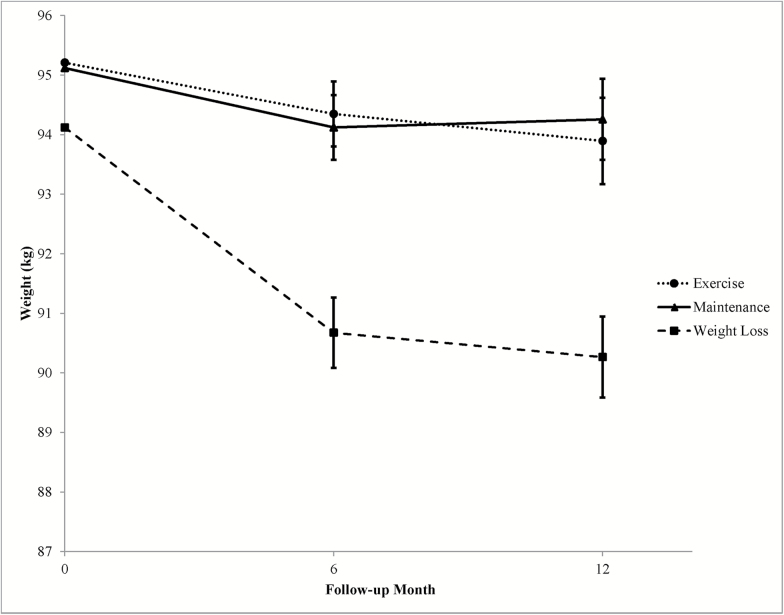
The Weight Loss group had a significant weight loss at 6 months and maintained a weight loss of 4.1% ± 0.7% of initial body weight through 12 months (see Figure 2). The Maintenance and Exercise groups did not have significant weight changes at 6 or 12 months. Compared with those in the Maintenance group, participants in the Weight Loss group had a 2.4 ± 0.7 kg (p < .001) and 3.0 ± 0.9 kg (p < .01) greater weight loss at 6 and 12 months, respectively. Compared with those in the Exercise group, participants in the Weight Loss group had a 2.6 ± 0.7 kg (p < .001) and 2.5 ± 0.9 kg (p < .01) greater weight loss at 6 and 12 months, respectively (Supplementary Table 1; Table 2). No statistically significant differences were observed between the Maintenance and Exercise groups.
Figure 2.
Effect of intervention on weight (lbs) at follow-up assessments. Data are adjusted for baseline values and covariates including age, sex, and race.
Table 2.
Effect of Exercise Intervention, Exercise + Diet Quality + Weight Maintenance Intervention, or Exercise + Diet Quality + Weight Loss Intervention on 1-Year Outcomes in Adults ≥ 65 Years (adjusted for baseline value and covariates)
| Exercise | Maintenance | Weight Loss | p Value | |||
|---|---|---|---|---|---|---|
| Exercise vs Maintenance | Exercise vs Weight Loss | Maintenance vs Weight Loss | ||||
| Body weight (kg): Baseline | 95.2 ± 1.7 | 95.1 ± 1.9 | 94.1 ± 2.1 | |||
| Change at 1 year | −1.3 ± 0.7 | −0.9 ± 0.7 | −3.9 ± 0.7a§ | 0. 62 | 0. 005 | 0. 001 |
| Visceral adipose tissue (cm3): Baseline | 3592.0 ± 239.4 | 3568.9 ± 242.5 | 3365.6 ± 276.1 | |||
| Change at 1 year | −21.9 ± 173.7 | −275.0 ± 189.2 | −192.6 ± 185.2 | 0. 291 | 0. 491 | 0. 745 |
| Abdominal SAT (cm3): Baseline | 5473.8 ± 290.3 | 5143.7 ± 245.8 | 4783.9 ± 224.1 | |||
| Change at 1 year | −237.2 ± 119.5b | −281.9 ± 120.6b | −280.2 ± 139.4b | 0. 777 | 0. 801 | 0. 992 |
| Total percent body fat: Baseline | 46.4 ± 0.8 | 46.4 ± 0.8 | 45.0 ± 0.8 | |||
| Change at 1 year | −0.7 ± 0.3b | −0.3 ± 0.3 | −1.6 ± 0.3a | 0. 38 | 0. 023 | 0. 002 |
| Total lean mass (kg): Baseline | 49.0 ± 1.3 | 49.3 ± 1.5 | 49.8 ± 1.5 | |||
| Change at 1 year | 0.3 ± 0.3 | 0.02 ± 0.3 | −0.4 ± 0.3 | 0. 548 | 0. 12 | 0. 328 |
| Glucose (mg/dL): Baseline | 110.2 ± 3.3 | 105.3 ± 2.6 | 107.5 ± 2.9 | |||
| Change at 1 year | 1.2 ± 2.5 | −1.2 ± 2.5 | −7.2 ± 2.8b | 0. 466 | 0. 023 | 0. 092 |
| HDL-cholesterol (mg/dL): Baseline | 54.4 ± 1.9 | 55.7 ± 2.2 | 52.3 ± 1.8 | |||
| Change at 1 year | −0.5 ± 1.3 | 2.5 ± 1.4 | 4.7 ± 1.5c | 0. 092 | 0. 007 | 0. 269 |
| Short Physical Performance Battery: Baseline | 9.6 ± 0.2 | 10.0 ± 0.2 | 10.3 ± 0.2 | |||
| Change at 1 year | −0.2 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.3 | 0. 293 | 0. 183 | 0. 814 |
| Impact of Weight on Quality of Life: Baseline | 72.5 ± 2.1 | 74.4 ± 2.0 | 76.4 ± 2.3 | |||
| Change at 1 year | 11.9 ± 1.5a | 9.7 ± 1.5a | 11.1 ± 1.5a | 0. 268 | 0. 697 | 0. 468 |
| Short Form-36 physical score: Baseline | 47.0 ± 1.1 | 44.8 ± 1.1 | 47.7 ± 0.9 | |||
| Change at 1 year | 1.5 ± 1.0 | 3.5 ± 1.0a | 1.9 ± 1.1 | 0. 144 | 0. 781 | 0. 26 |
| Short Form-36 mental score: Baseline | 48.4 ± 0.9 | 48.1 ± 1.3 | 49.6 ± 0.8 | |||
| Change at 1 year | −0.3 ± 1.2 | −2.1 ± 1.2 | 0.0 ± 1.5 | 0. 241 | 0. 899 | 0. 26 |
Note: SAT = subcutaneous adipose tissue. Bold values represent statistically significant p values.
p < .001 for within-group differences.
p < .05 for within-group differences.
p < .01 for within-group differences.
Symptoms and Adverse Events
The frequency of adverse events and reported symptoms was similar between all treatment groups (Supplementary Table 3). In the overall study sample, 70.7% of participants reported symptoms and adverse events for a total of 256 events. Thirty-one of those events (12.1%) were classified as serious adverse events. Ten events were definitely related to the study interventions and protocol (3.9% of all adverse events), being primarily musculoskeletal injuries that occurred during study prescribed exercise.
Body Composition Outcomes
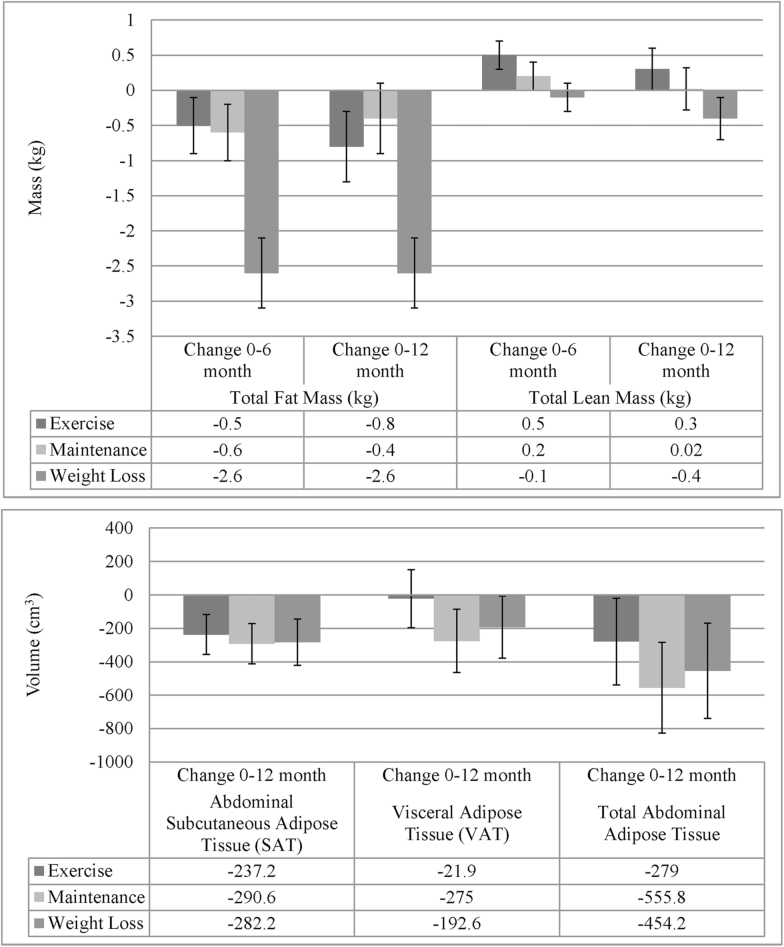
The Weight Loss group was the only treatment group to have significant within-group changes in fat mass measured by DXA when adjusted for baseline values and covariates at 6 (−2.6 ± 0.5 kg, p < .001; Supplementary Table 1) and 12 months (−2.66 ± 0.5 kg, p < .001; Table 2). The Weight Loss group’s changes in fat mass were significantly greater than the changes seen in the Exercise (−1.8 ± 0.7 kg, p < .05) and Maintenance (−2.1 ± 0.7 kg, p < .01) groups at 12 months (Figure 3). At 12 months, this corresponded to a 0.9% ± 0.4% (p < .05) and 1.3% ± 0.4% (p < .01) difference in body fat percentage for those in Weight Loss compared with Exercise and Maintenance, respectively. Truncal body fat percentages were significantly different at 12 months for Weight Loss compared with Exercise (−1.2% ± 0.5%, p < .05) and Maintenance (−1.8% ± 0.5%, p < .01). In contrast to the differences in fat mass, there were no statistically significant changes in total lean mass or lumbar bone density for any of the groups when adjusting for baseline and covariates, and there were no statistically significant differences between treatment groups.
Figure 3.
Change in body composition by intervention group. Data are adjusted for baseline values and covariates.
The Weight Loss group had significant changes in abdominal SAT by MRI but changes in VAT were nonsignificant. Similarly, the Maintenance group had notable decreases in the volume of abdominal SAT and VAT, being the only group to have significant changes in total abdominal fat volume (SAT + VAT) at 12 months (−555.8 ± 272.3 cm3, p < .05; Figure 3). The Exercise group also had decreases in abdominal SAT, but the magnitude of change in the VAT volume was 8%–11% of the volume change in the other treatment groups. However, contrasts between the groups for each depot volume were nonsignificant (Table 2). Fully adjusted models of change in MRI thigh skeletal muscle volume showed a decrease in the Weight Loss group and slight increases in the Maintenance and Exercise groups; however, the within-group changes and differences between groups were not statistically significant.
Cardiometabolic Risk Factors
At 6 months, the Weight Loss group had a significant decrease in triglycerides, and this decrease was statistically significant when compared with Exercise (−22.7 ± 9.4 mg/dL, p < .05; Supplementary Table 1). At 6 months, at least one marker of inflammation, hsCRP, was showing signs of increasing in the Exercise group while decreasing in the Maintenance group.
At 12 months, compared with Exercise, the Weight Loss group had significant changes in fasting blood glucose (−8.3 ± 3.6 mg/dL, p < .05) and HDL-cholesterol (5.3 ± 1.9, p < .01; Table 2). Triglycerides remained lower than baseline, but changes at 12 months were no longer statistically significant for the Weight Loss group. For the Maintenance group, total and LDL-cholesterol decreased significantly (Supplementary Table 2).
The Weight Loss group had an increase in adiponectin that lost statistical significance after full adjustment; however, the change at 12 months in adiponectin was statistically greater than Exercise (Supplementary Table 2). At 12 months, there were small, statistically nonsignificant decreases in the inflammatory markers TNF-α, IL-6, and hsCRP in the Weight Loss and Maintenance conditions; the difference in TNF-α change was statistically different between Exercise and Weight Loss.
Physical Function
There were no statistically significant within-group changes in the SPPB total score, our primary measure of physical function, for any of the groups (Table 2). There were also no statistically significant differences between groups for the SPPB total score. At 12 months, isometric knee extension strength, hand grip, 6-minute walk, and chair sit and reach showed no differences overall by treatment assignment (Supplementary Table 2).
Quality of Life
Quality of life measured by the SF-36v2 demonstrated significant improvements only in the physical function domains within the Maintenance group (Table 2). All domains of the IWQOL demonstrated significant within-group improvements for all groups, with the self-esteem domain showing statistically greater improvement in the Exercise and Weight Loss groups compared with Maintenance (Supplementary Table 2).
Discussion
The CROSSROADS trial was designed to isolate the effect of caloric restriction for intentional weight loss from changes in diet quality and/or exercise among obese older adults with at least one cardiometabolic risk factor. At the end of the 12-month intervention, the Weight Loss group had significant decreases in body weight, primarily due to decreases in body fat. Conversely, lean mass changes in all groups were minimal. Improvements in blood glucose and HDL-cholesterol were significantly greater in the Weight Loss group. Furthermore, calorie restriction had minimal impact on physical function but improved quality of life similar to the other groups. In summary, these data suggest that moderate calorie restriction in this group of high-risk older adults did not selectively decrease VAT more than the other interventions but did lead to favorable improvements in total body composition, cardiometabolic risk, and quality of life without diminishing physical function or increasing the risk of adverse events.
This study provides a unique perspective on the question: “Is intentional weight loss beneficial and safe for older adults with obesity and associated comorbidities?” If intentional weight loss is potentially harmful for older adults, the alternative lifestyle modification options would include increasing exercise and/or improving diet quality. The CROSSROADS study design allowed us to understand some of the differential responses to calorie restriction in the setting of an otherwise comprehensive lifestyle intervention. Each treatment group experienced some benefits, such as improvements in self-reported quality of life, as a result of participating in the behavioral interventions in a group setting. However, there were clear benefits for the addition of calorie restriction to the prescription of increased exercise and changes in diet composition. These findings are consistent with other recent reports of weight loss interventions in older adults where significant improvements in risk factors are only observed when exercise is combined with diet-induced weight loss (17,30).
Changes in body composition, particularly fat mass, are likely markers for downstream changes in cardiometabolic risk factors (31). One study of weight loss plus exercise compared with exercise alone in older adults showed that reductions in fat mass and/or total body weight were associated with significant improvements in diastolic blood pressure, glucose, insulin, HDL-cholesterol, and triglycerides (32). In CROSSROADS, all treatment groups had some reduction in abdominal adipose tissue volume assessed by MRI. However, only the Maintenance group had significant within-group decreases in total abdominal adipose tissue volume. The cumulative changes in abdominal subcutaneous and VAT depots for the Maintenance group that did not have energy restriction requires additional study, as we might hypothesize this as a potential mechanism for how isocaloric changes in diet composition lead to improvements in cardiometabolic risk factors. Previous feeding studies like the DASH trial or the Omni-Heart trial have demonstrated the impact of altering diet pattern in populations with younger samples (33,34). However, to see a broader array of improvements in risk factors and changes in adipokines or markers of inflammation, our data suggest that the addition of calorie restriction is advantageous.
There have been nine randomized controlled studies conducted that have used a lifestyle intervention similar to that used in our study that targeted older adults (6). It is difficult to make comparisons with our study primarily because the populations targeted were different (eg, frail and sedentary, osteoarthritis, cardiometabolic disease), the primary outcomes were different, and the interventions varied according to precise components and duration. All studies demonstrated weight loss of between 5% and 10%, which was more than experienced in our study, and may be due to our less intensive intervention. This lower weight loss compared with other studies may also help to explain nonsignificant findings of our primary outcome of VAT.
To provide a broad assessment of the net benefit of intentional weight loss, we were also interested in understanding the impact of calorie restriction on safety and adverse outcomes. Older adults, who may be prone to falls or other traumatic injuries, have traditionally been considered to be at high risk of adverse outcomes when engaging in intentional weight loss. For many clinicians there has been a hesitancy to recommend intentional weight loss to older patients because of these risks and concerns that additional loss of lean mass would worsen physical function, thereby diminishing any potential benefit gained by losing weight. The data from this trial suggest that moderate calorie restriction does not increase the risk of adverse events above that associated with a recommendation for healthy exercise. In addition, the loss of lean mass was generally limited and not associated with any declines in physical function; conversely, quality of life in the weight loss group improved similarly to those who engaged in exercise only or weight maintenance.
The limitations of the CROSSROADS trial should be considered when placing the results into the context of clinical practice and future research efforts. While this study was based in a community setting, it was conducted by trained clinicians with specific expertise in behavioral interventions for lifestyle modification. As such, the outcomes achieved with this intervention in a different setting or with different providers may not be the same. However, because we did not provide participants with food or meal replacements or provide a large number of supervised exercise sessions, the amount of weight loss achieved is not so large that it is outside of the scope of most nonspecialist providers or community-based programs to achieve in a similar patient population. Perhaps as a result of this approach to the intervention, the participants lost approximately 4% of their body weight, and this may not have been enough to lead to larger changes in outcomes like VAT or physical function. It is also the case that based upon self-reported caloric intake that participants may not have achieved the 500 kcal/d that was desired and necessary to cause weight loss. Another limitation of this study is that the population of participants had high levels of physical function at baseline. As such, the true impact of weight reduction or the other interventions on physical function may be limited due to a ceiling effect. Other studies that focused on lower functioning older adults have shown larger effects on physical function compared with those we observed in CROSSROADS, albeit with more intensive exercise intervention strategies (12,17). Lastly, we accounted for medication usage, but we may not have been able to completely measure the potential effect modification on body composition or other outcomes of interest.
In conclusion, our data suggest that modest weight loss in older adults at high risk for cardiometabolic disease is beneficial for several key outcomes, and these benefits appear to outweigh the potential risks of losing weight intentionally in older adulthood. While participating in an intervention to improve exercise levels or dietary intake patterns had measureable benefits, the addition of a calorie-restricted diet was necessary to significantly decrease total fat mass and improve some key risk factors. The addition of the modest calorie restriction improved the overall benefit achieved without any evidence of increased risk due to loss of lean mass, increased adverse events, or negative effects on quality of life. When combined with prior evidence demonstrating positive benefits of weight reduction in older adults (8), we believe this study provides a compelling evidence base to support recommendations for intentional weight loss in older adults with obesity and associated risk factors. Future studies are warranted that evaluate the long-term impact of these interventions and more intensive levels of calorie restriction and exercise that might produce greater volumes of weight loss with potentially different outcomes than those observed in CROSSROADS. Additionally, evaluation of other diets that demonstrate selective loss of visceral fat and preservation of lean mass underweight maintenance conditions is warranted (35).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (research grant R01AG033094).
Supplementary Material
Acknowledgments
J.D.A. and J.L.L. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. There are no disclosures.
References
- 1. Fakhouri TH, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief. 2012;(106):1–8. [PubMed] [Google Scholar]
- 2. Federal Interagency Forum on Aging-Related Statistics. Older Americans 2012: Key Indicators of Well-Being http://www.agingstats.gov/agingstatsdotnet/main_site/default.aspx Accessed November 17, 2015.
- 3. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13(11):1849–1863. doi:10.1038/oby.2005.228 [DOI] [PubMed] [Google Scholar]
- 4. Jensen MD, Ryan DH, Apovian CM et al. . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102–S138. doi:10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13(1):46–51. doi:10.1097/MCO.0b013e32833309cf [DOI] [PubMed] [Google Scholar]
- 6. Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: do benefits exceed potential risks? Exp Gerontol. 2016. doi:10.1016/j.exger.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathus-Vliegen EM; Obesity Management Task Force of the European Association for the Study of Obesity Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5(3):460–483. doi:10.1159/000341193 [DOI] [PubMed] [Google Scholar]
- 8. Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: do the benefits outweigh the risks? Curr Nutr Rep. 2015;4(2):143–155. doi:10.1007/s13668-015-0123-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15(4):240–250. doi:10.1016/j.jamda.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol. 2013;48(10):1054–1061. doi:10.1016/j.exger.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Messier SP, Loeser RF, Miller GD et al. . Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi:10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 12. Messier SP, Mihalko SL, Legault C et al. . Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi:10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101(5):991–999. doi:10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rejeski WJ, Brubaker PH, Goff DC Jr et al. . Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–886. doi:10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santanasto AJ, Glynn NW, Newman MA et al. . Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011:Article ID 516576, 10 pages. doi:10.1155/2011/516576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon TP, Sistrun SN, Krishnan RK et al. . Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol (1985). 2008;104(5):1313–1319. doi:10.1152/japplphysiol.00890.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villareal DT, Chode S, Parimi N et al. . Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi:10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults–a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64(1):90–95. doi:10.1093/gerona/gln032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haas MC, Bodner EV, Brown CJ et al. . Calorie restriction in overweight seniors: response of older adults to a dieting study: the CROSSROADS randomized controlled clinical trial. J Nutr Gerontol Geriatr. 2014;33(4):376–400. doi:10.1080/21551197.2014.965993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. United States. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans: Be Active, Healthy, and Happy! Washington, DC: U.S. Dept. of Health and Human Services; 2008. [Google Scholar]
- 21. Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51(11):1610–1614. [DOI] [PubMed] [Google Scholar]
- 22. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. [DOI] [PubMed] [Google Scholar]
- 24. Jones CJ, Rikli RE, Max J, Noffal G. The reliability and validity of a chair sit-and-reach test as a measure of hamstring flexibility in older adults. Res Q Exerc Sport. 1998;69(4):338–343. doi:10.1080/02701367.1998.10607708 [DOI] [PubMed] [Google Scholar]
- 25. Sf36.Org. SF-36 Health Survey. http://www.sf-36.org Accessed December 2, 2015.
- 26. Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11(2):157–171. doi:10.1023/A:1015081805439 [DOI] [PubMed] [Google Scholar]
- 27. Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9(2):102–111. doi:10.1038/oby.2001.13 [DOI] [PubMed] [Google Scholar]
- 28. Elobeid MA, Padilla MA, McVie T et al. . Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS One. 2009;4(8):e6624. doi:10.1371/journal.pone.0006624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4(3):175–184. [DOI] [PubMed] [Google Scholar]
- 30. Bouchonville M, Armamento-Villareal R, Shah K et al. . Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond). 2014;38(3):423–431. doi:10.1038/ijo.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher D, Heshka S, Kelley DE et al. ; MRI Ancillary Study Group of Look AHEAD Research Group Changes in adipose tissue depots and metabolic markers following a 1-year diet and exercise intervention in overweight and obese patients with type 2 diabetes. Diabetes Care. 2014;37(12):3325–3332. doi:10.2337/dc14-1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beavers KM, Beavers DP, Nesbit BA et al. . Effect of an 18-month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity (Silver Spring). 2014;22(2):325–331. doi:10.1002/oby.20607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Appel LJ, Sacks FM, Carey VJ et al. ; OmniHeart Collaborative Research Group Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455–2464. doi:10.1001/jama.294.19.2455 [DOI] [PubMed] [Google Scholar]
- 34. Sacks FM, Svetkey LP, Vollmer WM et al. ; DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi:10.1056/NEJM200101043440101 [DOI] [PubMed] [Google Scholar]
- 35. Gower BA, Goss AM. A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. J Nutr. 2015;145(1):177S–183S. doi:10.3945/jn.114.195065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.