Abstract
Background
Age-related brain changes leading to altered socioemotional functioning may increase vulnerability to financial exploitation. If confirmed, this would suggest a novel mechanism leading to heightened financial exploitation risk in older adults. Development of predictive neural markers could facilitate increased vigilance and prevention. In this preliminary study, we sought to identify structural and functional brain differences associated with financial exploitation in older adults.
Methods
Financially exploited older adults (n = 13, 7 female) and a matched cohort of older adults who had been exposed to, but avoided, a potentially exploitative situation (n = 13, 7 female) were evaluated. Using magnetic resonance imaging, we examined cortical thickness and resting state functional connectivity. Behavioral data were collected using standardized cognitive assessments, self-report measures of mood and social functioning.
Results
The exploited group showed cortical thinning in anterior insula and posterior superior temporal cortices, regions associated with processing affective and social information, respectively. Functional connectivity encompassing these regions, within default and salience networks, was reduced, while between network connectivity was increased. Self-reported anger and hostility was higher for the exploited group.
Conclusions
We observed financial exploitation associated with brain differences in regions involved in socioemotional functioning. These exploratory and preliminary findings suggest that alterations in brain regions implicated in socioemotional functioning may be a marker of financial exploitation risk. Large-scale, prospective studies are necessary to validate this neural mechanism, and develop predictive markers for use in clinical practice.
Keywords: Cognitive aging, Neuroimaging, Geriatric assessment
Vulnerability to financial exploitation in older adulthood is an emerging public health crisis, warranting early detection and prevention. The estimated prevalence of financial exploitation in older adulthood is 4.7% with an annual incidence of 2.7%, providing sobering evidence of the potential scope of this problem (1,2). These data suggest approximately 1 in 20 adults can be expected to experience some form of financial exploitation past the age of 60, an incidence rate eclipsing many age-related diseases. These are almost certainly underestimates of the true prevalence as many older adults are unaware or unwilling to report exploitation (3,4). This latter point highlights the difficulty in conducting research in this area. Identifying older adults who have experienced exploitation is exceedingly challenging. Only a few studies have investigated behavioral correlates of financial exploitation in older adulthood, implicating demographic factors as well as individual differences in cognitive and social functioning as predictors of heightened exploitation risk in normal aging (4–6), mild cognitive impairment (7), and Alzheimer’s Disease (8,9) (and see (10,11) for a review of the determinants of financial exploitation risk in aging and brain disease).
Brain differences have been associated with reduced financial ability in mild cognitive impairment and Alzheimer’s disease (12,13). This suggests that altered neural functioning may also predict poor financial capacity and exploitation risk in normal aging. In this preliminary study, we examine both structural and functional brain differences in a cohort of older adults who have been victims of financial exploitation. Previous studies have sought to identify neural markers of financial capacity, decision-making, and exploitation risk in brain disease cohorts (13,14). However, no published studies have investigated neural markers of exploitation risk in normal aging (but see (15) for a discussion). While earlier neuroimaging investigations in brain disease populations have emphasized differences in brain regions associated with cognitive functioning, here we specifically target changes in regions implicated in socioemotional abilities, consistent with a recent model implicating altered social and emotional function in financial exploitation risk (11). This social cognitive neuroscience model includes two interacting pathways that can lead to increased exploitation risk in older adulthood. On the one hand, cognitive changes are associated with reduced financial skills, and increased risk of exploitation through financial mismanagement. On the other, changes in social cognition are associated with increased exploitation risk through undue social influence, coercion or deception at the hands of others. Both pathways are predicted to be associated with dissociable patterns of structural and functional brain change (11).
Exploitation is, by definition, a social transaction, and most exploitation is perpetrated within families or by close personal others (2,3). From this perspective, age-related brain changes associated with altered social and emotional capacity may be an important predictor of exploitation risk. Socioemotional selectivity theory (16) suggests that, with advancing age, time horizons narrow, increasing the importance of emotional and social experience. Increased attention to socioemotional cues, in turn, biases information processing toward more emotionally pleasant features of the environment and lived experience. This “positivity bias” (17) enhances the salience of positively valenced information, increasing attention, and memory for positive stimuli or events in later life. Conversely, negative information is given less attention [see (18), for a review]. While these changes are associated with well-being will advancing age (17), this bias may increase the risk of financial exploitation at the hands of others when potentially negative outcomes of decisions are dampened (19) and there is reduced capacity to discern social cues and the intentions of others (20).
In the context of altered socioemotional abilities in older adults, differences in brain regions associated with emotional and social functioning may provide an early marker of exploitation risk. Age-related changes to activation of the anterior insula have been associated with a reduced ability to distinguish trustworthiness (19) and insensitivity to loss, but not gain, anticipation in a gambling task (20). Appraisal of emotionally valenced information is associated with an assembly of functionally connected brain regions, including anterior insula and dorsal anterior cingulate, known as the salience network (21). Inferring the thoughts or intentions of others is associated with medial prefrontal cortex (mPFC), a core node of the default network, which is broadly implicated in social cognition (22). In the first report to investigate these aspects of financial exploitation risk, here we examine whether brain differences in regions involved in socioemotional functioning are associated with financial exploitation in older adulthood. We investigated this hypothesis in older adults who have been victims of financial exploitation, and a matched sample who had avoided potential exploitation. We predicted reduced brain volume and altered functional connectivity in regions of the salience and default networks in the exploited cohort, suggestive of a potential neural marker of exploitation risk.
Methods
Thirteen older adults (age mean = 70.2 y; SD = 5.9; years of education = 18 y, SD = 2.6; 7 women) who had been financially exploited over the age of 60 years were enrolled. Financial exploitation was characterized by theft, misappropriation, or coercion resulting in financial loss, impersonation to obtain property or services, or hardship experienced due to loss of agreed upon financial contributions [see (2), for a similar approach to identify financially exploited older adults]. Participants needed to have experienced financial loss due to deception or based upon intentionally misleading or withheld information. Participants were excluded if the financial loss was not attributable to misleading information or deception (eg, physical theft or robbery, lost or stolen credit cards, etc.). Qualifying incidents of financial exploitation are listed in Supplementary e-Appendix 1.
Thirteen age, gender, education, global cognitive status, and site matched controls were used as a comparison group (mean age = 68.9 y, SD = 4.6; years of education = 16.9 y, SD = 2.1). Control older adults reported having been exposed to a potential instance of financial exploitation (telemarketing scheme) since the age of 60 years, but had successfully identified the scam and repelled the threat. Mini Mental State Exam scores were equal to or above 27. Participants gave written informed consent in accordance with the Institutional Review Boards of Cornell or York University.
Participants completed a neuropsychological and behavioral assessment. Behavioral testing included three primary categories: measures of cognition, personality and social interaction, and financial abilities. Participants also underwent structural and functional brain scanning with magnetic resonance imaging (see Supplementary e-Methods for full behavioral and neuroimaging protocols).
Results
Neuropsychological and Behavioral Assessment
All behavioral scores are reported in Supplementary e-Table 1. Financially exploited individuals self-reported higher levels of anger and hostility. All other behavioral measures did not differ significantly between groups.
Cortical Thickness
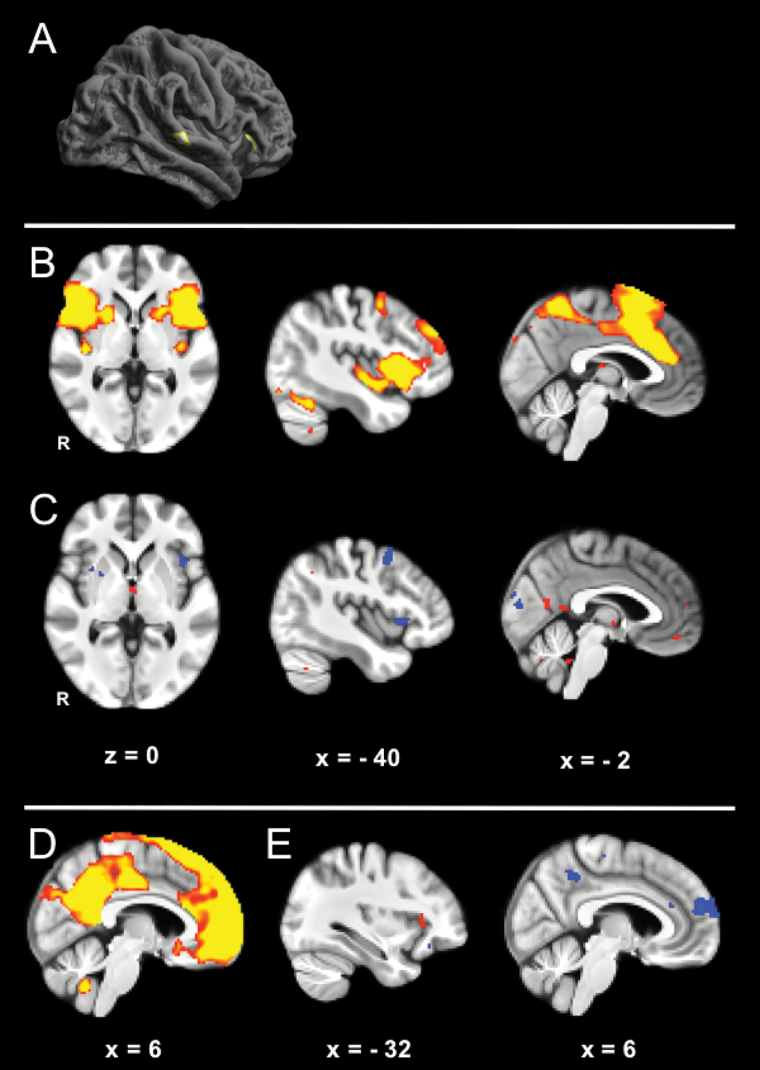
Financially exploited older adults had significantly reduced cortical thickness in right anterior insula cortex (Montreal Neurological Institute (MNI) peak coordinate x = 33, y = 29, z = 0) and right posterior superior temporal cortices, extending from auditory cortex into the sulcus (MNI peak coordinate x = 67, y = −32, z = 4; Figure 1A).
Figure 1.
Brain differences associated with financial exploitation. (A) Cortical thickness in financially exploited older adults is lower in the anterior insula and superior temporal sulcus or gyrus. Right lateral hemisphere. (B) Salience network in older and younger adults. (C) Differences in salience network connectivity in financially exploited and nonexploited older adults. Financially exploited older adults have reduced connectivity (cool colors) in the anterior insula, and greater connectivity (warm colors), in regions of the default network in posterior cingulate and medial prefrontal cortex. R = right hemisphere. (D) Default network in older and younger adults. (E) Differences in default network connectivity in financially exploited and nonexploited older adults. Financially exploited older adults have reduced connectivity (cool colors) in posterior cingulate cortex and mPFC, and greater connectivity (warm colors), in regions of the salience network in anterior insula. Please see online edition for color figure.
Functional Connectivity
We identified the salience and default networks with independent components analysis. The salience network included anterior insula bilaterally, anterior cingulate cortex, and other regions (Figure 1B) (21). The default network included mPFC, posterior cingulate cortex, inferior parietal lobule, lateral and medial temporal lobes (Figure 1D) (22). We compared the functional connectivity of these networks in financially exploited and nonexploited older adults. Financially exploited older adults had reduced salience network integrity, with significantly lower functional connectivity between left anterior insula and other regions (Figure 1C). These participants also showed reduced functional connectivity of core default network regions including mPFC and posterior cingulate (Figure 1E). Increased cross-network coupling was observed between these networks, specifically anterior insula, posterior cingulate, and mPFC regions (Figure 1C and E). Taken together, these differences were in line with predictions. Structural and functional differences in regions implicated in socioemotional functioning differentiated exploited from nonexploited older adults.
Discussion
Here, we present the first preliminary investigation of structural and functional brain differences associated with financial exploitation in normal aging. We conducted a targeted analysis, based on a recent model implicating altered social and emotional functioning in older adult financial exploitation (11). Older adults who had experienced financial exploitation showed cortical thinning in anterior insular cortex and posterior superior temporal cortices, regions implicated in affectively based decision-making (19) and social cognition (22). Exploited older adults also had lower functional connectivity within the salience network, and increased salience to default network connectivity. Similarly, functional connectivity within the default network was reduced in the exploited group and network connectivity was increased with salience regions, particularly the anterior insula, consistent with accounts of age-related functional connectivity differences associated with cognitive decline (23,24). Together, the findings provide early evidence that financial exploitation risk may be related to altered socioemotional circuitry in older adulthood.
The anterior insula is a core node of the salience network, associated with salience-detection, affect-based decision-making and reward anticipation (19,21). Differences in the insula may impair threat detection in older adulthood, or disrupt the integration of threat-related information into decision-making processes during social interactions. Reduced threat detection may leave older adults at greater risk for exploitation, particularly in complex, or emotionally volatile contexts as would be the case within families, the most common context for elder exploitation (3).
Brain structure and connectivity differences in the default network were also observed in exploited versus nonexploited older adults. The default network is associated with social reasoning (22), necessary for fluid social interactions where complex, multimodal social cues must be integrated to accurately infer the intentions of others. Age-related alterations in default network brain regions may disrupt processing of social cues, leading to impairments in social reasoning, leaving older adults susceptible to deception or undue social influence, and vulnerable to exploitation. This is consistent with reports suggesting that older adults are at greater risk of fraud or deceit during in-person encounters (25). Further, greater functional interactions between salience and default networks suggests that exploited older adults may place greater reliance on low fidelity, and possibly misleading, social information to guide affectively based decision-making.
While we identified both structural and functional brain changes that differentiated our exploited and nonexploited cohorts, we observed few differences in behavioral performance on a battery of neurocognitive and self-report measures. Exploited older adults reported higher anger and hostility, as might be predicted. There were no differences identified between our groups on any other cognitive, socioemotional, or financial capacity assessments. Our sample of older adults was normal-to-high functioning on standardized assessments of intellectual functioning and closely matched on overall cognitive status. As such, we did not predict significant cognitive differences to emerge given the small group sizes. In line with our guiding hypothesis of financial exploitation risk as a deficit in socioemotional processing, we did expect differences between our groups on measures of social cognition and financial capacity. However, poor sensitivity of these behavioral measures in a cognitively normal older adult population, as well as the small sample size in this initial study, likely precluded detection of significant cohort differences. As we continue to investigate socioemotional determinants of financial exploitation risk, we recognize the necessity and importance of developing sensitive and specific behavioral assays of these functional domains.
As the first investigation of structural and functional brain differences in exploited older adults, we recognize the limitations inherent in the small sample sizes reported here. Financial exploitation is under-reported in community-dwelling older adults as victims are reluctant to acknowledge such experiences out of concerns for potential public embarrassment, infringement on privacy or, in some cases, personal safety (4). In this context, identifying participants is exceedingly difficult, as suggested by the relative paucity of studies in this area. Further, we were unable to independently confirm exploitation incidents, relying instead on self-report as is common in many demographic studies (eg, 2)—a limitation of the current work and challenge for the field. As reducing exploitation of older adults is increasingly recognized as an urgent public health priority (1,26) efforts to identify early markers of exploitation risk are necessary. Our findings provide preliminary yet reliable evidence implicating specific patterns of structural and functional brain differences in financial exploitation risk—group differences that are not reflected in standardized neuropsychological assessments. Given the preliminary and exploratory nature of this study, statistical tests were not corrected for multiple comparisons, raising the risk of false positives. However, the support provided here for our original hypotheses, associating structural and functional brain differences in regions implicated in socioemotional processing with exploitation risk, offers promising avenues for future research. From an immediate clinical perspective, these data argue that heightened vigilance for exploitation risk may be necessary when changes or damage to these brain regions have been diagnosed. The preliminary results also provide the basis for future large-scale, prospective studies to identify increasingly precise and clinically relevant neural markers of financial exploitation risk in older adulthood.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This study was funded by grants from the Alzheimer’s Association (NIRG-14-320049) and the Elder Justice Foundation to R.N.S. This project was also supported in part by NIH grant 1S10RR025145.
Conflicts of Interest
All authors report no disclosures.
Supplementary Material
Acknowledgments
We thank Mark Lachs, Karl Pillemer, Jason Karlawish, and M.T. Connolly for guidance and support for this project, our participants and their families, as well as the people on the front line helping older adults in our communities.
References
- 1. Lachs MS, Han SD. Age-associated financial vulnerability: an emerging public health issue. Ann Intern Med. 2015;163:877–878. doi:10.7326/M15-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peterson JC, Burnes DP, Caccamise PL, et al. Financial exploitation of older adults: a population-based prevalence study. J Gen Intern Med. 2014;29:1615–1623. doi:10.1007/s11606-014-2946-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100:292–297. doi:10.2105/AJPH.2009.163089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schafer MH, Koltai J. Does embeddedness protect? Personal network density and vulnerability to mistreatment among older American adults. J Gerontol B Psychol Sci Soc Sci. 2015;70:597–606. doi:10.1093/geronb/gbu071 [DOI] [PubMed] [Google Scholar]
- 5. Boyle PA, Yu L, Wilson RS, Gamble K, Buchman AS, Bennett DA. Poor decision making is a consequence of cognitive decline among older persons without Alzheimer’s disease or mild cognitive impairment. PLoS One. 2012;7:e43647. doi:10.1371/journal.pone.0043647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. James BD, Boyle PA, Bennett DA. Correlates of susceptibility to scams in older adults without dementia. J Elder Abuse Negl. 2014;26:107–122. doi:10.1080/08946566.2013.821809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han SD, Boyle PA, James BD, Yu L, Bennett DA. Mild cognitive impairment and susceptibility to scams in old age. J Alzheimers Dis. 2016;49:845–851. doi:10.3233/JAD-150442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Griffith HR, Belue K, Sicola A, et al. Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology. 2003;60:449–457. doi:http://dx.doi.org/10.1212/WNL.60.3.449 [DOI] [PubMed] [Google Scholar]
- 9. Marson DC, Sawrie SM, Snyder S, et al. Assessing financial capacity in patients with Alzheimer disease: a conceptual model and prototype instrument. Arch Neurol. 2000;57:877–884. doi:10.1001/archneur.57.6.877 [DOI] [PubMed] [Google Scholar]
- 10. Lichtenberg PA. Financial exploitation, financial capacity, and Alzheimer’s disease. Am Psychol. 2016;71:312–320. doi:10.1037/a0040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spreng RN, Karlawish J, Marson DC. Cognitive, social, and neural determinants of diminished decision-making and financial exploitation risk in aging and dementia: a review and new model. J Elder Abuse Negl. 2016;28:320–344. doi:10.1080/08946566.2016.1237918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffith HR, Okonkwo OC, den Hollander JA, et al. Brain metabolic correlates of decision making in amnestic mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010;17:492–504. doi:10.1080/13825581003646135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoeckel LE, Stewart CC, Griffith HR, et al. MRI volume of the medial frontal cortex predicts financial capacity in patients with mild Alzheimer’s disease. Brain Imaging Behav. 2013;7:282–292. doi:10.1007/s11682-013-9226-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith HR, Stewart CC, Stoeckel LE, et al. Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. J Am Geriatr Soc. 2010;58:265–274. doi:10.1111/j.1532-5415.2009.02679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samanez-Larkin GR. Financial decision making and the aging brain. APS Obs. 2013;26:30–33. doi:http://dx.doi.org/10.1037/0003-066X.54.3.165 [PMC free article] [PubMed] [Google Scholar]
- 16. Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am Psychol. 1999;54:165–181. [DOI] [PubMed] [Google Scholar]
- 17. Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9:496–502. doi:10.1016/j.tics.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 18. Charles ST, Carstensen LL. Social and emotional aging. Annu Rev Psychol. 2010;61:383–409. doi:10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samanez-Larkin GR, Knutson B. Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci. 2015;16:278–289. doi:10.1038/nrn3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castle E, Eisenberger NI, Seeman TE, et al. Neural and behavioral bases of age differences in perceptions of trust. Proc Natl Acad Sci USA. 2012;109:20848–20852. doi:10.1073/pnas.1218518109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi:10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- 22. Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. doi:10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 2014;111:E4997–E5006. doi:10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spreng RN, Stevens WD, Viviano JD, Schacter DL. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging. 2016;45:149–160. doi:10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanley JT, Blanchard-Fields F. Challenges older adults face in detecting deceit: the role of emotion recognition. Psychol Aging. 2008;23:24–32. doi:10.1037/0882-7974.23.1.24 [DOI] [PubMed] [Google Scholar]
- 26. Pillemer K, Connolly MT, Breckman R, Spreng N, Lachs MS. Elder mistreatment: priorities for consideration by the white house conference on aging. Gerontologist. 2015;55:320–327. doi:https://doi.org/10.1093/geront/gnu180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.