Abstract
Background
Exercise has positive neuroplastic effects on the aging brain. It has also been shown that ingestion of beet root juice (BRJ) increases blood flow to the brain and enhances exercise performance. Here, we examined whether there are synergistic effects of BRJ and exercise on neuroplasticity in the aging brain.
Methods
Peak metabolic equivalent (MET) capacity and resting-state magnetic resonance imaging functional brain network organization are reported on 26 older (mean age = 65.4 years) participants randomly assigned to 6 weeks of exercise + BRJ or exercise + placebo.
Results
Somatomotor community structure consistency was significantly enhanced in the exercise + BRJ group following the intervention (MBRJ = −2.27, SE = 0.145, MPlacebo = −2.89, SE = 0.156, p = .007). Differences in second-order connections between the somatomotor cortex and insular cortex were also significant; the exercise + BRJ group (M = 3.28, SE = 0.167) had a significantly lower number of connections than exercise + placebo (M = 3.91, SE = 0.18, p = .017) following the intervention. Evaluation of peak MET capacity revealed a trend for the exercise + BRJ group to have higher MET capacity following the intervention.
Conclusions
Older adults who exercised and consumed BRJ demonstrated greater consistency within the motor community and fewer secondary connections with the insular cortex compared with those who exercised without BRJ. The exercise + BRJ group had brain networks that more closely resembled those of younger adults, showing the potential enhanced neuroplasticity conferred by combining exercise and BRJ consumption.
Keywords: Beet root juice, Neuroimaging, Brain health, Physical functioning
Researchers have established a link between brain plasticity and exercise in the aging brain (1,2). Researchers have also observed improvements in exercise performance and increased blood flow to the brain with the ingestion of beet root juice (BRJ) due to increased availability of nitric oxide (NO) (3–6). We hypothesized that there may be synergistic effects of BRJ and exercise on neuroplasticity in the aging brain. To that end, we designed a clinical trial to test whether exercise combined with regular ingestion of BRJ would induce neuroplasticity as measured by functional brain network community structure and improve exercise tolerance as measured by peak MET capacity compared with elderly participants who were only exercising. Specifically, in the current study, functional brain networks and exercise tolerance were assessed before and after a 6-week aerobic exercise intervention that was combined with either a BRJ or placebo supplement in a double-blind study design.
Exercise has been demonstrated to promote several mechanisms that support brain plasticity, leading to both structural and functional brain changes. Among older adults, both gray and white matter volume (7,8) and hippocampal volume (9) have been shown to increase following aerobic exercise interventions. In another study, older adults with a higher VO2 max, an index of cardiovascular fitness, demonstrated significantly greater activation in attentional control regions and significantly less activity in the anterior cingulate cortex during a cognitive task (10). More recently, Voss and colleagues conducted a 1-year aerobic intervention in older adults and found that not only did aerobic training improve older adults’ resting functional efficiency in cognitive networks, but it also increased the functional connectivity between their default mode network and frontal executive network (1).
BRJ has been shown to be an encouraging nutritional complement to exercise. Inorganic nitrate found in BRJ is metabolized by oral bacteria and converted to nitrite in the body (11,12), and the nitrite is reduced to NO allowing it to act beneficially to lower blood pressure (BP) and increase blood flow, especially to hypoxic areas of the body (4). When combined with exercise, the nitrate from BRJ has been shown to improve exercise performance. For example, researchers have demonstrated improved performance in well trained rowers following 6 days of drinking a BRJ supplement (3). Others have reported improved performance in a 5K run and lower ratings of perceived exertion (RPE) following consumption of baked beet root (13). Improved exercise performance measured by increased peak power and elevated work rate after 15 days of BRJ has also been reported (14). BRJ has also been shown to improve exercise endurance (5,15,16), and perhaps one of the most well-established benefits of BRJ in relation to exercise is the ability to reduce O2 cost during exercise, even at low doses (17–19). In addition, BRJ has been demonstrated to acutely improve regional cerebral blood flow (CBF) in frontal lobe white matter of elderly people (4) and has also been shown to modulate CBF of the prefrontal cortex during cognitive tasks with some improvement in task performance (20).
Finally, recent work by Hugenschmidt and colleagues demonstrated that the integrity of the brain is associated with mobility in older adults (21). Resting-state functional magnetic resonance imaging (fMRI) data were collected in older participants who were divided into three levels of physical functioning based on scores from the Short Physical Performance Battery (SPPB) (22). Significant differences in somatomotor cortex connectivity and secondary connections to the insula based on the SPPB classification were seen. Specifically, older adults with low levels of mobility showed reduced consistency in the motor community and a greater number of connections with the insular region as compared with a population of younger adults.
Here, we expanded on the work of Hugenschmidt and colleagues and tested whether there are synergistic effects of BRJ and exercise on neuroplasticity in the aging brain. Our primary hypothesis was that the combined effects of exercise + BRJ would lead to greater connectivity in the brain motor community than exercise + placebo. In addition, we hypothesized that the brain motor community of participants in the exercise + BRJ group would have fewer secondary connections with the insula than those randomized to exercise + placebo. Finally, given the ergogenic effect of BRJ on exercise performance, we hypothesized that participants in the exercise + BRJ group would have improved peak aerobic capacity during a treadmill test to exhaustion as compared with exercise + placebo.
Methods
Design and Participants
This was a randomized controlled double-blind placebo-controlled trial. Twenty-seven community-dwelling, hypertensive, older men and women were recruited for this study. One participant was removed from data analysis due to an MRI data error resulting in a final N = 26. Participants were 55 years or older, had a systolic BP between 130 and 160 mm Hg taking no more than two hypertensive medications, and were sedentary (defined as performing <60 minutes of moderate levels of exercise each week performed in bouts >10 minutes). Hypertensive participants were selected because of the known effects of BRJ on the cardiovascular system and the potential benefits that that BRJ might have for this population. Exclusion criteria included the use of tobacco products, a Modified Mini-Mental State Exam score less than 80, or a diagnosis of active neurological dysfunction. Furthermore, participants were ineligible if they were taking medications known to interfere with nitrate/nitrite metabolism.
BRJ Supplement
Each week participants were given 7 days of BEET IT Sport Shot (http://www.beet-it.com/) with 560 mg of nitrate or the Placebo BEET IT Sport Shot (http://www.beet-it.com/) containing very little (1.1 mg) nitrate, depending on the randomization. The participants were instructed to consume one 70-mL (2.4 oz.) beverage each day within 30 minutes of opening the bottle and to consume the beverage 1 hour before the exercise intervention.
Exercise Intervention
The aerobic exercise intervention consisted of a center-based, individualized, moderately intense walking program of 18 sessions: three 50-minute sessions per week for 6 weeks. All participants walked on motorized Life Fitness TR-9500HR treadmills at an RPE of 12–13 on the Borg Rating of Perceived Exertion Scale. The first 4 weeks were used to acclimatize participants to the training protocol and progressively increase the walking time toward the goal of 50 minutes per session. Participants were guided through a series of stretching exercises before and after walking.
Network Organization of Somatomotor Cortex
All participants completed two brain MRI scans: one preintervention, prior to randomization and one following the 6-week exercise intervention. The preintervention and postintervention scans were performed at the same time of day for each participant and were performed within 2 hours of ingestion of the drink (BRJ supplement or placebo). The two brain MRI scans were performed on nonintervention days: The first scan was within 2 days of the first exercise intervention, and the second scan was within 2 days after the final exercise intervention.
Detailed methodology on the acquisition of images and the calculation of brain networks for analysis from the MRI data can be found in Supplementary Material. The community structure of the brain network from each individual participant for the resting-state fMRI scans was determined using network modularity (23) and the Louvain algorithm (24). Communities within the brain are defined as groups of nodes that are more connected to each other than to nodes in other groups. Once the community structure was determined for each participant, the consistency of community structure across participants was assessed using scaled inclusivity (SI) (25). SI evaluates the overlap of modules across participants while penalizing for disjunction (26). It produces values ranging between 0 and 1, where 1 represents absolute consistency across all participants and values less than 1 indicate disjunction of modules. SI values were compared across groups.
Given that the overarching hypothesis of the current work was that exercise and BRJ would have synergistic effects on motor cortex organization, analyses focused on somatomotor communities. A region of interest (ROI) was generated based on the somatomotor community structure of a group of 19 older adults with high physical functioning from a prior study (Supplementary Figure 1) (21). SI was computed for the entire brain of each participant using the somatomotor ROI as the referent. The mean SI was then computed across the voxels within the somatomotor ROI.
Next we sought to identify how the somatomotor cortex connected to areas beyond its community boundaries using second-order connectivity analyses. We did not assess first-order connections (nodes directly connected to the somatomotor cortex) because first-order connections often remain within the community of interest. Therefore, second-order connections provide a better idea of the somatomotor cortex’s connectivity to other communities. Based on the findings of Hugenschmidt and colleagues (21), we determined the number of second-order connections from the somatomotor cortex to the insula. The insula was defined using the Automated Anatomical Labeling Atlas implemented in WFU Pickatlas software (27). The left and right insula were combined into a single ROI, and the average number of connections across all voxels within the ROI was computed (Supplementary Figure 1).
Assessment of Peak MET Capacity
To evaluate change in cardiovascular fitness, participants completed a graded exercise test to determine peak volume of oxygen consumption (VO2peak) on a motorized treadmill and metabolic cart (Medical Graphics Ultima). Metabolic equivalent (MET) capacity was calculated by dividing the VO2peak in mL/kg/min by a standard resting metabolic rate of 3.5 mL/kg/min. A physician-supervised, individualized ramp treadmill protocol was conducted whereby participants walked at a brisk pace with the speed based on their comfort and fitness. For lower fit individuals, the grade was increased at a rate of 1% per minute, and for higher fit individuals, the grade was increased at a rate of 2% per minute. Heart rate, rhythm, BP, and oxygen uptake were monitored during the test and throughout a 6-minute recovery period.
Statistical Analyses
Means and standard deviations as well as frequency distributions were used to describe participant characteristics, whereas group differences for the network metrics and peak MET capacity were evaluated via general linear models. In these analyses, the pretest for each outcome variable was used as a covariate enabling us to examine group differences by comparing least squared means at the time of follow-up testing. Sex was also used as a covariate in the peak MET capacity analysis because it is well known that men have higher MET capacities than women. A natural log transformation was employed to normalize the network metric data prior to conducting the general linear models because the raw data for these metrics were not normally distributed.
Results
Descriptive characteristics for the study sample can be found in Table 1. The two experimental groups of older adults were equivalent in age, distribution by sex, body mass index, years of education, and resting systolic BP.
Table 1.
Demographic Characteristics: Means ± SD or %
| Variable | BRJ (n = 14) | Placebo (n = 13) |
|---|---|---|
| Age (y) | 64.9 ± 4.1 | 66 ± 6.6 |
| Women n (%) | 8 (57.14%) | 6 (50%) |
| Body mass index (kg/m2) | 33.7 ± 5.9 | 35.3 ± 5.9 |
| Education (y) | 15.7 (3.4) | 14.4 (1.9) |
| Systolic BP (mm Hg) | 138.9 ± 11.7 | 137.8 ± 9.6 |
Note: BP = blood pressure; BRJ = beet root juice.
Intervention Adherence
Participants were asked to complete 18 exercise sessions over a period of 6 weeks; 85% of the participants (22 of 26) had 100% attendance. Two individuals in the BRJ group had adherence rates of 89% and 96%, whereas two individuals in the placebo group had adherence rates of 83% and 96%. The first 4 weeks were used to acclimatize participants to treadmill walking at an RPE of 12–13 for 50 minutes three times each week. From Weeks 5 to 18, the mean (SD) distance walked each session for the BRJ group was 2.46 miles (0.52), whereas it was 2.45 miles (0.44) for the placebo group, a nonsignificant difference (p = .68).
Supplement Adherence
Based on daily log data, 23 of 26 participants were 100% adherent to supplement intake. One person in the placebo group had an adherence rate of 86%, whereas two participants in the BRJ group had rates of 98%.
Nitrate/Nitrite Response to Supplement Consumption
Analysis of the blood samples revealed that the groups had similar levels of nitrate and nitrite at baseline before beverage consumption (26 ± 28 mM nitrate for Placebo and 84 ± 72 mM nitrate for BRJ, and 0.15 ± 0.07 mM nitrite for Placebo and 0.18 ± 0.10 mM nitrite for BRJ). Nitrate/nitrite levels after consumption of BRJ were much higher than for placebo: 21 ± 8 mM nitrate for Placebo and 422 ± 127 mM nitrate for BRJ, and 0.14 ± 0.07 mM nitrite for Placebo and 0.32 ± 0.18 mM nitrite BRJ; p < .001 for group differences for both nitrate and nitrite levels. The nitrite/nitrate data are the average levels before and after taking BRJ on each clinic visit.
Peak MET Capacity
Table 2 provides the raw means for the peak MET data by treatment group and time of assessment along with the least squared means which represent adjusted posttest scores controlling for the peak MET value at the time of pretesting and participants’ sex. A general linear model showed a group effect that approached, but did not achieve, significance (p = .11) for the exercise + BRJ group to have higher MET capacity following exercise training than exercise + placebo with a partial eta2 of .11.
Table 2.
Means for Peak MET Capacity by Treatment Group
| Group | Pretest | Posttest | Adjusted Posttesta |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SE) | |
| Exercise + BRJ | 5.61 (1.46) | 6.12 (1.21) | 5.88 (0.18) |
| Exercise + Placebo | 4.85 (1.36) | 5.19 (1.14) | 5.44 (0.18) |
Note: BRJ = beet root juice; MET = metabolic equivalent.
aFor purposes of interpretation, we back calculated the log values in raw scaled units.
Connectivity of the Motor Cortex
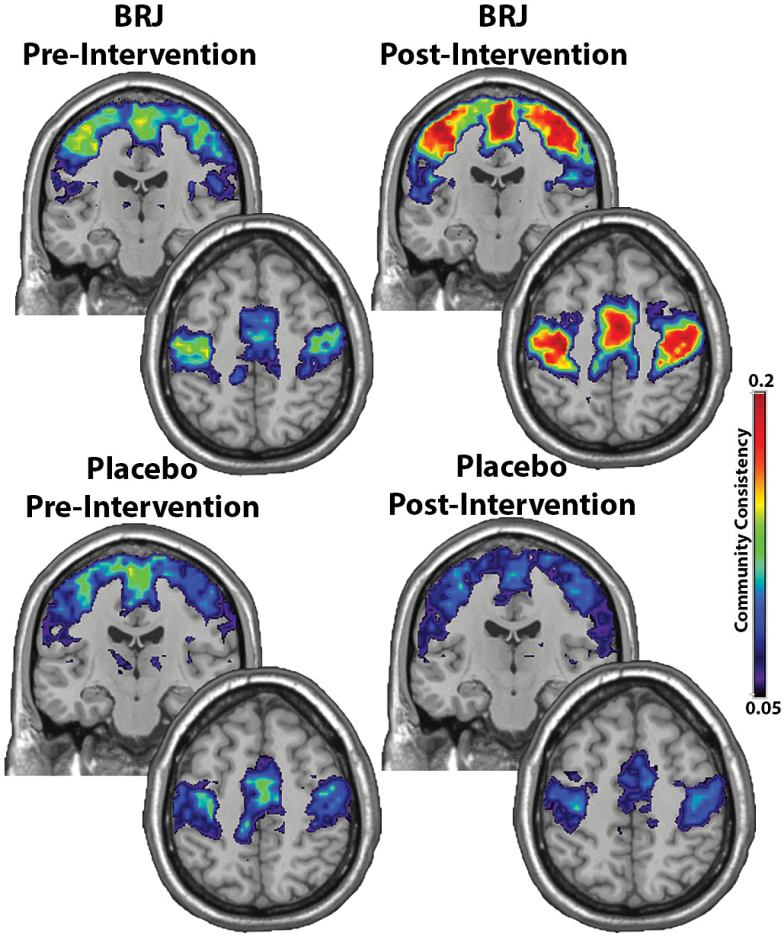
Figure 1 shows the consistency of the somatomotor network community prior to and following the exercise training for both the placebo and BRJ groups. For these images, the calibration bar represents the consistency (SI) of the motor community across participants. As depicted in Figure 1, the high values represent maximal consistency in the population while the low values represent lower consistency across participants.
Figure 1.
The consistency of the somatomotor community across participants. There is increased somatomotor community consistency following exercise in the BRJ group. This community included the primary motor cortex, somatosensory cortex, and midline supplementary motor regions. Both the upper coronal images and lower axial images show the somatomotor community.
The community in the somatomotor cortex included the primary motor cortex, somatosensory cortex, and midline supplementary motor regions. As seen in Figure 1 and the log transformed means, the motor community was more consistent for participants following exercise training in the BRJ group (MBRJ = −2.27, SE = 0.145, MPlacebo = −2.89, SE = 0.156). Indeed this comparison was statistically significant, F(1,23) = 8.65, p = .007, and represents a large effect size (eta2 = 0.27); see descriptive data in Table 3.
Table 3.
Means for Scaled Inclusivity by Treatment Group
| Group | Pretest | Posttest | Adjusted Posttesta |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SE) | |
| Exercise + BRJ | 0.08 (0.05) | 0.12 (0.06) | 0.12 (0.01) |
| Exercise + Placebo | 0.08 (0.04) | 0.07 (0.05) | 0.06 (0.01) |
Note: BRJ = beet root juice.
aFor purposes of interpretation, we back calculated the log values in raw scaled units.
To show how the motor functional community interacted with other brain regions, second-order connectivity analyses between the somatomotor cortex and the insula were performed. The adjusted treatment means (SE) in Table 4 shows that the number of second-order connections between these regions was significantly lower following treatment in the BRJ condition (M = 29.82, SE = 9.5) compared with placebo (M = 68.42, SE = 10.3), F(1,23) = 6.67, p = .017. Once again the effect size for this comparison was large, eta2 = 0.225.
Table 4.
Means for Secondary Connections by Treatment Group
| Group | Pretest | Posttest | Adjusted Posttesta |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SE) | |
| Exercise + BRJ | 61.49 (46.37) | 29.46 (12.59) | 29.82 (9.5) |
| Exercise + Placebo | 63.09 (61.21) | 68.83 (62.62) | 68.42 (10.3) |
Note: BRJ = beet root juice.
aFor purposes of interpretation, we back calculated the log values in raw scaled units.
Discussion
We examined changes in functional brain networks associated with exercise + BRJ supplementation in older adults with a focus on the somatomotor cortex. In support of our primary hypothesis, we found that the motor community structure was more consistent in older adults in the exercise + BRJ supplementation group as compared with exercise + placebo. In addition, the number of connections between the somatomotor cortex and insula was much lower in the BRJ than placebo group following the intervention. Although the group difference for peak MET capacity was not statistically significant, the data did trend in the expected direction, with those in the exercise + BRJ group having a nonsignificantly higher peak MET capacity than exercise + placebo. To our knowledge, this is the first experiment to test the combined effects of exercise and BRJ on functional brain networks in the motor cortex and secondary connections of the motor cortex with the insula.
Brain plasticity refers to the phenomenon of altered neural structure, function, and connections in response to environmental or bodily demands (28). While neuroplasticity was once thought to be a phenomenon of younger brains, scientists have recognized that this process also occurs within the aging human brain (1). Specifically, cognitive interventions, aerobic exercise, and other interventions have recently been shown to affect brain structure and function of older adults (1,9,29–31). We hypothesized that BRJ might enhance neuroplasticity based on the fact that BRJ enhances exercise tolerance and performance (5,14–16) and modulates CBF, at least in an acute setting (4,20). Here, we show that BRJ, when used in conjunction with exercise, facilitates brain plasticity of somatomotor brain regions as compared with exercise + placebo alone. The fact that we observed trends, as opposed to statistically significant group differences in peak MET capacity, is likely due to the small sample size and the relatively brief period of training—6 weeks.
The discovery of greater consistency within the motor community in older adults who had exercised regularly while ingesting BRJ is consistent with Hugenschmidt and colleagues’ findings (21). In their study, functional brain networks were compared between three groups of older adults with varying levels of mobility as determined by the SPPB. They found decreased consistency in the somatomotor cortex among the lowest mobility group of older adults as compared with young adults. Of additional interest are our findings of a greater number of connections between the somatomotor cortex and insula in the placebo group. The insula has many functions, such as the integration of visual, somatosensory, vestibular, and cognitive input, which are all essential for optimal mobility (21). Hugenschmidt and colleagues observed an association between decreased mobility function and enhanced connectivity between the somatomotor cortex and a region including the posterior insula (21). They suggest that low levels of mobility may result in increasing such compensatory connections between the motor cortex and insula. In our study, participants in the exercise + placebo group showed an average number of secondary connections similar to the prior reported population with mid-level SPPB scores (21). Our participants who had exercised and consumed BRJ for 6 weeks had an average number of secondary connections comparable with the younger population (mean age = 26.4 years) in that same study. Although we cannot suggest that the exercise + BRJ resulted in brain health or mobility similar to the younger adults, the data do suggest that the combination of BRJ with exercise resulted in neuroplasticity that substantially exceeded exercise alone.
BRJ is clearly an encouraging nutritional supplement that may improve functional health in older adults, and the proposed primary mechanism of benefit of BRJ is the rise in plasma nitrite caused by the high levels of dietary nitrate in BRJ (32). Consumed nitrate, once absorbed from the intestine, is taken up from the plasma by salivary glands and concentrated in saliva; nitrate is subsequently reduced to nitrite by oral bacteria and ultimately absorbed into the circulatory system (32,33). Nitrite appears to be reduced to NO during hypoxia. NO is an antioxidant and a potent vasodilator (34,35), is a critical relaxation factor synthesized in endothelial cells (36,37), and is key to vascular compliance. For this study, we hypothesized that reductions in brain blood flow associated with hypertension and aging-associated leukoaraiosis result in low-grade hypoxia (38) and that these reductions might be offset by the NO-mediated vasodilation in hypoxic regions due to the increased amount of circulating nitrite from the BRJ ingestion. Indeed, results from our lab have shown that 24 hours of a high nitrate diet supplemented with a single dose of BRJ leads to increased regional CBF in older adults (39). Coupled with exercise (a hypoxia-inducing activity), we propose that the biological mechanism underlying the neural plasticity shown in Figure 1 resulted from increased NO bioavailability after drinking BRJ.
Of note, some studies have suggested that there may be a risk, although unlikely, of cancer from having a diet that is very high in nitrates. A recent review panel sponsored by the NIH recently published a review of dietary nitrate and cardiovascular disease and concluded that the very low cancer risk seems to be with meat and very high levels from water sources of nitrate, but not vegetable sources, like beetroot juice (40).
This study has limitations. For example, as noted earlier, the treatment was relatively brief (6 weeks), and the sample size was small. In addition, for financial reasons, we were not able to design a full factorial study crossing BRJ supplementation (yes/no) with exercise training (yes/no). Finally, the population validity of these findings is restricted to older adults with controlled hypertension.
In summary, despite limitations, we believe our findings are compelling and warrant a large-scale clinical trial given the aging of the population, the concerns in geriatric medicine related to both physical functioning and brain health, and the growing interest in nutritional supplements. We know that exercise promotes both structural and functional neuroplasticity and that BRJ improves exercise performance (1,3,7,15,19). In the current study, we observed that exercise + BRJ improved functional connections within the motor community and resulted in fewer compensatory connections between the motor cortex and insula than exercise + placebo. Although the data on peak MET capacity did not reach conventional levels of statistical significance, the fact the exercise + BRJ resulted in an improvement over exercise + placebo by 0.44 in peak MET capacity is notable and clearly has potential clinical relevance. In future studies, we would also recommend that investigators include novel motor-based tasks that involve mobility with cognitive decision making and/or agility.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the Translational Science Center of Wake Forest University. Funding was also provided from the National Institutes of Health (HL058091).
Conflict of Interest
None of the authors declare any conflict of interest related to this manuscript.
Supplementary Material
References
- 1. Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2. doi:10.3389/fnagi.2010.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burdette JH, Laurienti PJ, Espeland MA, et al. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci. 2010;2:23. doi:10.3389/fnagi.2010.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bond H, Morton L, Braakhuis AJ. Dietary nitrate supplementation improves rowing performance in well-trained rowers. Int J Sport Nutr Exerc Metab. 2012;22:251–256. [DOI] [PubMed] [Google Scholar]
- 4. Presley TD, Morgan AR, Bechtold E, et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi:10.1016/j.niox.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1441–R1450. doi:10.1152/ajpregu.00295.2013 [DOI] [PubMed] [Google Scholar]
- 6. Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide. 2014;38:45–57. doi:10.1016/j.niox.2014.03.162 [DOI] [PubMed] [Google Scholar]
- 7. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. [DOI] [PubMed] [Google Scholar]
- 8. Ruscheweyh R, Willemer C, Krüger K, et al. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32:1304–1319. doi:10.1016/j.neurobiolaging.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. doi:10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi:10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clements WT, Lee SR, Bloomer RJ. Nitrate ingestion: a review of the health and physical performance effects. Nutrients. 2014;6:5224–5264. doi:10.3390/nu6115224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoon MW, Johnson NA, Chapman PG, Burke LM. The effect of nitrate supplementation on exercise performance in healthy individuals: a systematic review and meta-analysis. Int J Sport Nutr Exerc Metab. 2013;23:522–532. [DOI] [PubMed] [Google Scholar]
- 13. Murphy M, Eliot K, Heuertz RM, Weiss E. Whole beetroot consumption acutely improves running performance. J Acad Nutr Diet. 2012;112:548–552. doi:10.1016/j.jand.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 14. Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–R1131. doi:10.1152/ajpregu.00206.2010 [DOI] [PubMed] [Google Scholar]
- 15. Bailey SJ, Fulford J, Vanhatalo A, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi:10.1152/japplphysiol.00046.2010 [DOI] [PubMed] [Google Scholar]
- 16. Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi:10.1152/japplphysiol.00722.2009 [DOI] [PubMed] [Google Scholar]
- 17. Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf). 2007;191:59–66. doi:10.1111/j.1748-1716.2007.01713.x [DOI] [PubMed] [Google Scholar]
- 18. Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22:64–71. [DOI] [PubMed] [Google Scholar]
- 19. Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc. 2014;46:143–150. doi:10.1249/MSS.0b013e3182a1dc51 [DOI] [PubMed] [Google Scholar]
- 20. Wightman EL, Haskell-Ramsay CF, Thompson KG, et al. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–158. doi:10.1016/j.physbeh.2015.05.035 [DOI] [PubMed] [Google Scholar]
- 21. Hugenschmidt CE, Burdette JH, Morgan AR, Williamson JD, Kritchevsky SB, Laurienti PJ. Graph theory analysis of functional brain networks and mobility disability in older adults. J Gerontol A Biol Sci Med Sci. 2014;69:1399–1406. doi:10.1093/gerona/glu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Puthoff ML. Outcome measures in cardiopulmonary physical therapy: short physical performance battery. Cardiopulm Phys Ther J. 2008;19:17–22. [PMC free article] [PubMed] [Google Scholar]
- 23. Newman ME. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–8582. doi:10.1073/pnas.0601602103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blondel V, Guillaume J, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008:10008. [Google Scholar]
- 25. Steen M, Hayasaka S, Joyce K, Laurienti P. Assessing the consistency of community structure in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;84:016111. doi:10.1103/PhysRevE.84.016111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moussa MN, Steen MR, Laurienti PJ, Hayasaka S. Consistency of network modules in resting-state FMRI connectome data. PLoS One. 2012;7:e44428. doi:10.1371/journal.pone.0044428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 28. Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi:10.1093/brain/awr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Engvig A, Fjell AM, Westlye LT, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi:10.1016/j.neuroimage.2010.05.041 [DOI] [PubMed] [Google Scholar]
- 30. Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C. The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Front Hum Neurosci. 2012;6:34. doi:10.3389/fnhum.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proc Natl Acad Sci USA. 2007;104:11483–11488. doi:10.1073/pnas.0606552104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. [DOI] [PubMed] [Google Scholar]
- 33. Lundberg JO, Gladwin MT, Ahluwalia A, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt PM, Schramm M, Schroder H, Wunder F, Stasch J-P. Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J Biol Chem. 2004;279:3025–3032. [DOI] [PubMed] [Google Scholar]
- 35. Patel RP, McAndrew J, Sellak H, et al. Biological aspects of reactive nitrogen species. Biochim Biophys Acta. 1999;1411:385–400. [DOI] [PubMed] [Google Scholar]
- 36. Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 37. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. [DOI] [PubMed] [Google Scholar]
- 38. Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. J Neurol Sci. 2009;283:28–31. doi:10.1016/j.jns.2009.02.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Presley TD, Morgan AR, Bechtold E, et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahluwalia A, Gladwin M, Coleman GD, et al. Dietary nitrate and the epidemiology of cardiovascular disease: report from a National Heart, Lung, and Blood Institute Workshop. J Am Heart Assoc. 2016;5. doi:10.1161/JAHA.116.003402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.