Abstract
Background
Despite the widespread belief that napping is common among older adults, little is known about the correlates of napping. We examined the prevalence and correlates of self-reported and objectively measured napping among very old women.
Methods
We studied 2,675 community-dwelling women (mean age 84.5 ± 3.7 years; range 79–96). Self-reported napping was defined as a report of regular napping for ≥1 hour per day. Individual objective naps were defined as ≥5 consecutive minutes of inactivity as measured by actigraphy and women were characterized as “objective nappers” if they had at least 60 minutes of naps per day.
Results
Seven percent of the women only had self-reported napping, 29% only had objective napping, and 14% met the criteria for both. Multinomial logistic regression showed that the independent correlates of “both subjective and objective napping” were age (per 5 year odds ratio [OR] = 1.59; 95% CI: 1.31–1.93), depressive symptoms (per SD of score, OR = 1.53; 1.32–1.77), obesity (OR =1.93; 1.42–2.61), current smoking (OR = 3.37; 1.56–7.30), heavier alcohol drinking (OR = 0.49; 0.34–0.71), history of stroke (OR = 1.56; 1.08–2.26), diabetes (OR = 2.40; 1.61–3.57), dementia (OR = 3.31; 1.27–8.62), and Parkinson’s disease (OR = 7.43; 1.87–29.50). Besides, having objective napping alone was associated with age and diabetes, whereas subjective napping was associated with stroke and myocardial infarction. These associations were independent of nighttime sleep duration and fragmentation.
Conclusions
Daytime napping is very common in women living in their ninth decade and both subjective and objective napping were significantly related to age and comorbidities. Future studies are needed to better understand napping and its health implications.
Keywords: Daytime napping, Siesta, Sleep, Actigraphy, Predictor
Sleep duration and disturbances tend to increase in later life, particularly as a result of chronic health problems or other aging-related lifestyle factors (1,2). One common sleep complaint in older adults is daytime napping and the report of regular napping is believed to be associated with a number of factors, such as chronic pain, depression, medication use, and increased daytime sleepiness (3). Although napping was traditionally viewed as a form of recovery sleep to improve performance and alertness (4,5), more recent studies have suggested that self-reported napping might be a risk factor, prodromal symptom, or result of adverse health outcomes, including mortality, cardiovascular diseases, diabetes, and Parkinson’s disease (PD) (6–9). Although it is unclear whether napping is beneficial or harmful, it is possible that napping might be a result of specific, ongoing chronic diseases processes. However, little is known about the correlates of napping in older adults.
Surprisingly, napping is not well defined in the elderly population and epidemiological studies have rarely examined how frequently napping occurs. Previous studies have mostly focused on self-reported napping (7), despite concerns over the meaning of self-reported napping (10,11). It is likely that self-reported napping reflects perceived sleepiness and intended naps but is less likely to capture unplanned naps such as dozing off in front of the television. Few studies have compared napping measured subjectively and objectively. Examining the correlates of both measures would help to explain the nature of this behavior and inform the choice of these measures in future studies.
We set out to examine the prevalence of objectively measured and self-reported napping in a large cohort of older women. In particular, we aimed to study the factors that are independently associated with napping in elderly women.
Methods
Participants
We studied women enrolled in the Study of Osteoporotic Fractures (SOF), an ongoing multicenter cohort study of aging. From 1986 to 1988, a total of 9,704 community-dwelling Caucasian women aged 65 years or older were enrolled from population-based listings in four U.S. areas: Baltimore, MD; Minneapolis, MN; Pittsburgh, PA; and Portland, OR. Women who reported bilateral hip replacement or required assistance with ambulation at the baseline examination were excluded from participation. Details of the study protocol have been described previously (12). The current analysis focused on participants from visit 8, which took place between January 2002 and April 2004 and included both objective and subjective assessment of daytime napping. Briefly, a total of 4,261 women (with a response rate of 87.8%) attended this visit, of whom 2,789 had a clinic visit and 501 had a limited visit performed at home. Wrist actigraphy data on napping were successfully collected on 2,688 participants who completed a clinic or home visit. The institutional review boards at each site approved the study and all participants provided written informed consent.
Self-Reported Napping Habits
Napping habits were reported through a self-administered questionnaire. Participants were asked “Do you take naps regularly?” and those who answered in the affirmative were asked the questions “How many days per week do you usually nap?” and “On average, how many hours do you nap each time?” with responding categories: <1 hour, 1–2 hours, and >2 hours. Self-reported napping was defined as reporting at least 1 hour of napping each time.
Actigraphic Measurements of Objective Napping
Napping was also measured objectively using wrist actigraphy, a previously validated noninvasive tool for estimating sleep–wake activity, including napping behavior (13,14). Sleepwatch-O (Ambulatory Monitoring, Ardsley, NY) was used and was worn on the nondominant wrist for a minimum of 3 consecutive 24-hour periods (mean 4.1 ± 0.7 days). For each nap parameter, we calculated a daily average across all days of recording. Movement was measured using a piezoelectric biomorph-ceramic cantilevered beam, which generated a voltage when the actigraph moved. These voltages were gathered continuously and stored in 1-minute epochs. Data were collected using the proportional integration mode (PIM) (14) and the University of California, San Diego (UCSD) scoring algorithm in the Action W-2 software was used to differentiate sleep from wake times (15,16). Participants also completed sleep diaries, which included information about times they got into and out of bed, when the actigraph was removed and times they napped. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep and to delete time from analyses when the actigraph was removed. The information on nap times was used to confirm that those times that were deleted because of a removal were not truly a nap. High interscorer reliability has been reported for this standardized scoring protocol (17).
By convention, a nap was defined as ≥5 consecutive minutes scored as sleep (inactivity) outside of the main sleep interval (18,19). For purposes of analyses, objective napping was defined as when the total duration of ≥5-minute naps added up to 60 minutes or more per day. Nighttime sleep duration and efficiency (SE; the average percentage of time asleep while in bed) were also recorded to represent nighttime sleep fragmentation.
Other Measurements
All participants completed questionnaires, which included information on demographics, smoking, physical activity, and medical history. Educational attainment was reported at baseline as the highest grade or year of school one completed and was divided into “less than college” and “college or higher.” Physical activity was assessed by asking participants if they walked for exercise. Heavier alcohol drinking was defined as being in the top quartile of the number of drinks consumed per week. Depressive symptoms were assessed using the Geriatric Depression Scale (GDS), a validated 15-item questionnaire commonly used for evaluation of depressive symptoms in older adults, with a higher score representing more depressive symptoms (20). The Mini-Mental State Examination (MMSE) was used to examine global cognitive function, with a higher score representing higher global cognitive function (21). Body mass index (weight in kilograms divided by height in meters squared) was measured at a physical examination. Obesity was defined as a body mass index ≥30 kg/m2. Reported medical conditions that were of particular interest to the current analysis were stroke, hypertension, diabetes, myocardial infarction, dementia or Alzheimer’s disease, and PD. In addition, participants were asked to bring in all medications used daily or almost daily during the prior 30 days and the medications were categorized according to a computerized dictionary (22). Medications examined in the current study included use of selective serotonin reuptake inhibitors, benzodiazepine, and hypnotic medications.
Statistical Analysis
The analysis cohort is comprised of 4,100 and 2,688 women with data on self-reported and actigraphic napping, respectively, with 2,675 having data for both measures. We first present the prevalence and distribution of self-reported and objective napping in this cohort.
Participants were categorized into four of the following groups: non-nappers, women with subjective napping only, women with objective napping only, and women with both subjective and objective napping. Characteristics of the participants were compared by these four categories using the one-way analysis of variance (ANOVA) for normally distributed continuous variables, Kruskal–Wallis tests for skewed variables, and chi-square tests for categorical variables. Potential correlates of interest were chosen a-priori based on existing literature and included age, obesity, smoking, alcohol drinking, physical activity, depressive symptoms, cognition, medical conditions, and current medication use (6,23–26).
Multinomial logistic regression was used to study how each characteristic independently correlates with subjective napping, objective napping, and both subjective and objective napping. Correlates that were associated with either napping outcome at p less than .10 were simultaneously included in the multivariable model and results are presented as odds ratios and 95% CI. In addition, several secondary analyses were performed, with (a) further adjustment for nighttime sleep duration or efficiency, to test if the associations were independent of short or fragmented nighttime sleep; (b) test of other subjective napping parameters (eg, reported regular napping or subjective number of days per week); and (c) test of objective napping defined as when the total duration of individual naps added up to 20 minutes or more per day. All statistical tests were two-sided and a p value less than .05 was considered statistically significant. Analyses were conducted using Stata, version 14.1 (Stata Corp LP, College Station, TX).
Results
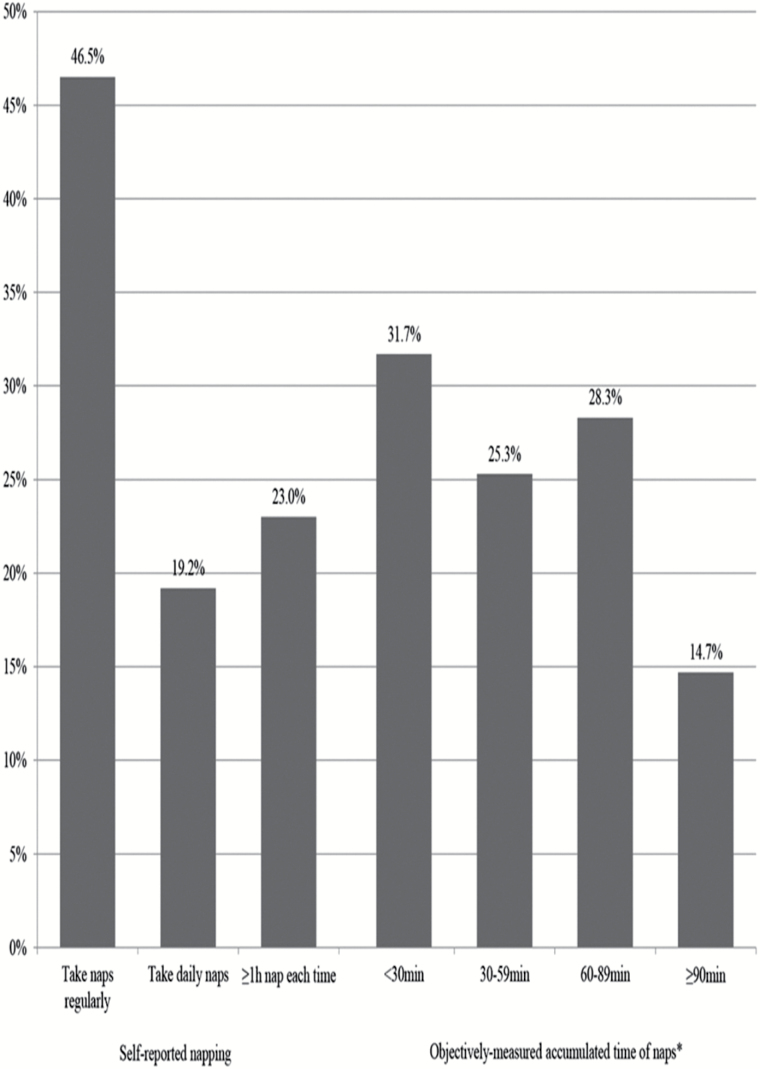
On average, the women were 84.5 ± 3.7 years old. As shown in Figure 1, 47% women reported taking naps regularly, 19% women reported taking daily naps, and 23% reported napping for ≥1 hour. Based on actigraphy data, the average accumulated daily duration of naps was 65 minutes and 43% of the women were classified as objective nappers. Thirty-two percent of the objective nappers also reported regular napping and 65% of the self-reported nappers were confirmed as objective nappers.
Figure 1.
Distribution of self-reported napping patterns (n = 4,100) and objectively measured napping (n = 2,688) among older women. *A nap was defined by actigraphy as ≥5 consecutive minutes scored as sleep (inactivity) outside of the main sleep interval.
The characteristics of the women according to subjective and objective napping are shown in Table 1. Overall, 7% of the women had subjective napping alone, 29% had objective napping alone, whereas 14% met the criteria for both subjective and objective napping. Compared to the non-nappers, women who had subjective and/or objective napping were older, more often obese, more likely to be current smokers, less likely to be heavier drinkers, had more depressive symptoms and lower global cognition, and were less likely to take walks for exercise. Moreover, they were more likely to have a history of stroke, hypertension, diabetes, myocardial infarction, dementia, and PD; more likely to be using selective serotonin reuptake inhibitor; and to have shorter nighttime sleep durations and lower sleep efficiency. Test of overall association across the four napping categories was statistically significant for all these factors.
Table 1.
Baseline Characteristics of Older Women by Subjective and Objective Napping
| Non-nappers (n = 1,327) | Only Subjective (n = 200) | Only Objective (n = 784) | Both Subjective and Objective (n = 364) | |
|---|---|---|---|---|
| Agea | 83.7 (3.3)* | 83.8 (3.1) | 84.2 (3.5) | 84.7 (3.6) |
| Depression scorea,b | 2.0 (2.3)*** | 2.6 (2.6) | 2.7 (2.7) | 3.5 (3.1) |
| MMSEa | 28.0 (1.8)* | 27.9 (2.1) | 27.8 (1.9) | 27.6 (2.3) |
| Obesity | 237 (18.1)* | 55 (27.8) | 184 (24.2) | 103 (29.7) |
| Educationc | 520 (39.3) | 80 (40.0) | 319 (40.7) | 166 (45.9) |
| Current smoking | 21 (1.6)* | 4 (2.0) | 21 (2.7) | 15 (4.1) |
| Heavier alcohol drinking | 360 (27.1)*** | 34 (17.0) | 117 (14.9) | 49 (13.5) |
| Exercise | 534 (40.9)** | 70 (35.4) | 271 (34.7) | 109 (30.5) |
| History of: | ||||
| Stroke | 133 (10.0)*** | 39 (19.5) | 104 (13.3) | 73 (20.1) |
| Hypertension | 736 (55.5)* | 114 (57.0) | 482 (61.5) | 229 (62.9) |
| Diabetes | 85 (6.4)*** | 19 (9.5) | 88 (11.2) | 61 (16.8) |
| Myocardial infarction | 141 (10.6)* | 38 (19.0) | 86 (11.0) | 59 (16.2) |
| Dementiad | 15 (1.1)*** | 3 (1.5) | 19 (2.4) | 17 (4.7) |
| Parkinson’s diseased | 4 (0.3)*** | 3 (1.5) | 8 (1.0) | 10 (2.7) |
| Current medication use | ||||
| SSRI | 97 (7.3)*** | 24 (12.1) | 63 (8.0) | 52 (14.3) |
| Sleep medication | 203 (15.3) | 24 (12.1) | 111 (14.2) | 54 (14.8) |
| Nighttime sleep | ||||
| Duration (hours)a | 6.9 (1.1)*** | 6.7 (1.4) | 6.7 (1.3) | 6.6 (1.5) |
| Efficiency (%)a | 78.3 (10.8)*** | 75.2 (13.9) | 78.9 (11.1) | 75.6 (13.9) |
Note: MMSE = Mini-Mental State Examination; SSRI = selective serotonin reuptake inhibitor.
Presented as mean (SD), the rest rows were all presented as number (%).
Geriatric Depression Scale.
College or higher.
Tested with Fisher’s exact test.
p < .05.
p < .01.
p < .00; p value was test of overall effects, tested by one-way analysis of variance (ANOVA), Kruskal–Wallis tests, or chi-square tests.
Multinomial logistic regression (Table 2) suggested that having subjective or objective napping alone shared a few common correlates, including depression score, obesity, and alcohol drinking. We also noted some discrepancy in the specific correlates of subjective and objective napping: those with a history of stroke or myocardial infarction were 90% or 63% more likely to have subjective napping, whereas for every 5-year increase in age or a history of diabetes, the odds of objective napping were 21% or 56% higher. We found a number of independent correlates for those with both subjective and objective napping including age, depression, obesity, current smoking, heavier alcohol drinking, and a history of stroke, diabetes, dementia, or PD. Those who were obese were 93% more likely to take both subjective and objective naps and the odds more than tripled for current smokers. Women who were in the top quartile of weekly alcohol consumption were 51% less likely to have both subjective and objective napping. Besides, the odds for women with a history of diabetes also doubled and for those with a history of dementia or PD, the odds of napping both subjectively and objectively were more than three times as high as those without these comorbidities. Secondary analysis shows that further adjustment for nighttime sleep duration or sleep efficiency did not attenuate the associations. Examination of the sample with both napping outcomes and test of other napping parameters (eg, subjective number of days per week or different cutoff for objective napping) showed similar associations and are not shown.
Table 2.
Multinomial Logistic Regression of Subjective and Objective Napping in Older Women (n = 2,675)
| Correlates | Subjective | Objective | Both Subjective and Objective |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age (per 5 y increase) | 1.08 (0.84, 1.39) | 1.21 (1.05, 1.41)* | 1.59 (1.31, 1.93)*** |
| Depression score (per SD increase) | 1.24 (1.02, 1.50)* | 1.34 (1.19, 1.51)*** | 1.53 (1.32, 1.77)*** |
| MMSE (per SD increase) | 0.99 (0.83, 1.19) | 0.99 (0.88, 1.10) | 0.99 (0.85, 1.14) |
| Obesity | 1.60 (1.10, 2.32)* | 1.36 (1.07, 1.72)* | 1.93 (1.42, 2.61)*** |
| Current smoking | 1.59 (0.52, 4.85) | 1.84 (0.93, 3.65) | 3.37 (1.56, 7.30)** |
| Heavier alcohol drinking | 0.63 (0.42, 0.95)* | 0.55 (0.43, 0.70)*** | 0.49 (0.34, 0.71)*** |
| Take walks for exercise | 0.92 (0.66, 1.27) | 0.90 (0.74, 1.10) | 0.85 (0.64, 1.13) |
| Stroke | 1.90 (1.23, 2.92)** | 1.14 (0.84, 1.55) | 1.56 (1.08, 2.26)* |
| Hypertension | 0.89 (0.65, 1.23) | 1.15 (0.94, 1.39) | 1.15 (0.88, 1.51) |
| Diabetes | 1.19 (0.67, 2.10) | 1.56 (1.11, 2.20)* | 2.40 (1.61, 3.57)*** |
| Myocardial infarction | 1.63 (1.06, 2.49)* | 0.85 (0.62, 1.16) | 1.03 (0.70, 1.52) |
| Dementia | 0.88 (0.18, 4.41) | 1.86 (0.77, 4.54) | 3.31 (1.27, 8.62)* |
| Parkinson’s disease | 3.39 (0.55, 20.95) | 3.01 (0.74, 12.33) | 7.43 (1.87, 29.50)** |
| SSRI use | 1.49 (0.89, 2.49) | 0.84 (0.58, 1.23) | 1.39 (0.90, 2.14) |
Note: MMSE = Mini-Mental State Examination; OR = odds ratio; SSRI = selective serotonin reuptake inhibitor. Multivariable model included all the above covariates (associated with either napping outcome in univariate analysis with p < .1).
p < .05.
p < .01.
p < .001.
Discussion
In this study of older women, 23% reported napping for at least 1 hour each time and 43% were objectively classified as nappers as assessed by actigraphy. Seven percent only had self-reported napping, 29% only had objective napping, and 14% met the criteria for both. Having self-reported or objective napping alone had a few different correlates, whereas those with both subjective and objective napping were independently correlated with most chronic health conditions and lifestyle factors. Women who were older, current smokers, who drank less, who were more often obese or had more depressive symptoms, and those with a history of stroke, diabetes, dementia, or PD were more likely to take naps, both objectively and subjectively. Sensitivity analysis using other cutoffs of napping parameters showed similar findings. Napping is common in older women and is particularly associated with comorbidities.
Daytime napping is known to be common in older adults but few studies have examined the prevalence, using both self-reported and objective measures. The prevalence of napping is high in this cohort of older women, even by using relatively stringent definition for napping, that is, requiring at least 5 consecutive minutes of no scored activity to be counted as a nap, and total duration of napping episodes adding up to at least 60 minutes per day. Based on these definitions, nearly half of older women were nappers. This is similar to findings from an earlier actigraphy study, which showed that 45% of individuals older than 65 years of age slept outside of their main nocturnal sleep period (27). Meanwhile, we found subjective napping to be less common, as was also shown in a previous study in older adults (19). It is possible that self-reported napping might only capture intended naps, whereas the unplanned “dozing-off” periods are more likely to be missed by self-report but are captured by actigraphy. This is a particular issue for older adults, who may tend to doze off frequently during the day but might have problems recalling these brief napping episodes. Moreover, we found some discrepancies in the occurrence of napping defined by subjective and objective measures—68% of the “objective nappers” did not match our criteria for “subjective nappers,” and 35% of the “subjective nappers” were not confirmed as “objective nappers.” This could partly reflect the limitation of using questionnaires alone in capturing napping behaviors. Besides, although we attempted to use relatively comparable criteria for subjective and objective napping, this is not a validation study by nature, thus the observed discrepancies should be interpreted with caution. Future studies should consider jointly examining subjective and objective napping given their potential differences, and longitudinal outcome studies are required to help understand the health implications of these measures.
In addition to differences in their prevalence, a few specific correlates were also identified for self-reported or objective napping. For example, a history of stroke or myocardial infarction was associated with subjective napping only, whereas age or diabetes was associated with objective napping only. It is unclear why this difference exists. One explanation could be that stroke survivors might experience more sleepiness and fatigue (28) and were therefore more likely to over-report napping behaviors. Meanwhile, older adults who are generally healthy might have frequent unnoticed “doze-off” periods that are recorded by actigraphy but under-reported by the individuals themselves. Notably, one meta-analysis suggested that the development of sleep disturbances in later life is a result of aging-related pathological process rather than chronological age per se (2). We observed higher odds of napping as recorded by actigraphy in older adults, independent of other lifestyle factors or comorbidities. This again demonstrates the importance of using objective assessment of napping in the elderly participants. Future prospective studies are needed to examine whether napping increases longitudinally as individuals get older.
A number of independent correlates were identified for “both subjective and objective napping.” These correlates agreed with those reported by most previous studies and included age, smoking, obesity, depression, and medical history (23,29). The evidence is particularly robust for metabolic factors, with high body mass index frequently associated with napping and diabetes being the most significant predictor among all major medical conditions (24,30). The Health, Aging and Body Composition (Health ABC) study showed that among 235 older adults that the odds of day napping, as recorded by actigraphy, were five times higher in diabetics compared to nondiabetics (31). Moreover, there is growing attention recently on the relationship between daytime napping and risk of type 2 diabetes (8,32,33). We found both obesity and diabetes to be independent correlates of napping. One explanation might be through fatigue, a common symptom in diabetics, usually triggered by hypo- or hyperglycemia, glucose variability, diabetes-related complications, and emotional distress (34). Untreated type 2 diabetes has also been suggested as a cause of daytime somnolence (35). Another potential factor is sleep-disordered breathing, which has been associated with both obesity (36) and daytime napping (37). Since sleep-disordered breathing was only examined in a subsample of this cohort, we adjusted for sleep duration and efficiency in secondary analysis to account for the effects of nighttime sleep fragmentation and our results were nearly identical.
Interestingly, older women who had more alcohol consumption were less likely to take naps. It should be noted that alcohol consumption is generally low in this very old female population. Thus, it is likely that many of the participants had quitted drinking because of their health conditions and these less healthy individuals might also be more likely to nap due to fatigue or bodily pain. However, given the robust independent association found between alcohol drinking and both measures of napping, we cannot exclude the possibility that there exists a causal relationship between alcohol intake and napping. Future studies are needed to confirm our findings. Finally, a history of dementia or PD was associated with napping only when naps were measured by both self-report and actigraphy. It is possible that there might be more variations in the report of napping behaviors among dementia patients and the association between napping and dementia could have been masked if the naps were only recorded by self-reports. It is also worth noting recent findings on the association between self-reported short and extended time in bed and increased dementia incidence in a Swedish population (38). Although it remains controversial whether and how napping is associated with dementia or PD, studies with well-validated measures and a prospective design might help to better disentangle the association.
There are several strengths of our study. We studied a large sample of community-dwelling older adults, which provides an ideal setting for studying napping habits. Napping was measured both objectively by actigraphy and subjectively with several aspects of napping defined. This has enabled us, for the first time, to examine self-reported and objective measures of napping and their predictors in a large population-based sample. The consideration of a range of potential correlates helps to gain a more comprehensive understanding of napping behaviors in the elderly participants.
Limitations of the study included limited generalizability as the sample involves only older Caucasian women. Temporal relationships could not be established due to the cross-sectional design of the study. The current study set out to identify potential correlates of daytime napping and thereby provide a basis for subsequent in-depth analysis on relevant outcomes. Longitudinal studies are required in the future to examine whether napping is a marker or risk factor of adverse health outcomes. Actigraphy was used to obtain objective data on napping and it is possible that some of the daytime sleep captured by actigraphy only reflects periods of inactive wakefulness. In order to address this limitation, we only considered periods of 5 or more consecutive minutes scored as inactivity, as was done in most previous studies (18,19) and defined objective napping by accumulated daytime naps of at least 60 minutes across the day. Furthermore, we found similar results by using different criteria for objective napping (accumulated naps of at least 20 minutes per day), indicating that the association was unlikely to be solely explained by measurement error. Although our approach does not eliminate the problem of misclassification, it is impossible to completely differentiate napping from inactive wakefulness using actigraphy alone. The use of actigraphy is a well-acknowledged method to assess 24-hour sleep–wake activities and has been validated against polysomnography to examine napping in young adults (13) but evidence is lacking for the very old population. Further studies are desired to identify the best measure for daytime napping in the very old. Meanwhile, it might be important to consider both actigraphy and subjective information to uncover the nature of napping in this population. Finally, although a number of factors were considered in the analysis, some correlates might not have been measured. The physiological effects of sleep-disordered breathing could not be fully controlled, as sleep fragmentation was used as a proxy. Future studies should incorporate measures of sleep-disordered breathing to help understand the independent correlates of napping.
In conclusion, napping is common in older women. There are some discrepancies in the prevalence and correlates of self-reported and objective napping. Having both subjective and objective napping was significantly correlated with age, depression, current smoking, alcohol drinking, obesity and a history of stroke, diabetes, dementia, or PD. It is critical to consider both subjective and objective measures in future studies of napping in the older population. Longitudinal studies are required to examine how subjective and objective measures of napping might predict future disease risks and help gain understanding of this common and complex behavior in the elderly participants.
Funding
This work was entirely funded by the National Institutes of Health. The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: NIH K24AG031155, R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. The sponsor played no role in design, methods, subject recruitment, data collection, analysis, or preparation of the manuscript.
Conflict of interest
S.A.-I. has been a consultant to Merck, Lacrima, Eisai, and Pfizer. The other authors declared no conflict of interest.
References
- 1. Smagula SF, Koh WP, Wang R, Yuan JM. Chronic disease and lifestyle factors associated with change in sleep duration among older adults in the Singapore Chinese Health Study. J Sleep Res. 2016;25:57–61. doi:10.1111/jsr.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. doi:10.1016/j.smrv.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–350. doi:10.1097/01.JGP.0000249385.50101.67 [DOI] [PubMed] [Google Scholar]
- 4. Milner CE, Cote KA. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18:272–281. doi:10.1111/j.1365-2869.2008.00718.x [DOI] [PubMed] [Google Scholar]
- 5. Faraut B, Boudjeltia KZ, Dyzma M, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi:10.1016/j.bbi.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 6. Gao J, Huang X, Park Y, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol. 2011;173:1032–1038. doi:10.1093/aje/kwq478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamada T, Hara K, Shojima N, Yamauchi T, Kadowaki T. Daytime napping and the risk of cardiovascular disease and all-cause mortality: a prospective study and dose-response meta-analysis. Sleep. 2015;38(12):1945–53. doi:10.5665/sleep.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi:10.2337/dc09-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leng Y, Ahmadi-Abhari S, Wainwright NW, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open. 2014;4:e006071. doi:10.1136/bmjopen-2014-006071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jean-Louis G, Kripke DF, Assmus JD, Langer RD. Sleep-wake patterns among postmenopausal women: a 24-hour unattended polysomnographic study. J Gerontol A Biol Sci Med Sci. 2000;55:M120–M123. [DOI] [PubMed] [Google Scholar]
- 11. Vitiello MV. We have much more to learn about the relationships between napping and health in older adults. J Am Geriatr Soc. 2008;56:1753–1755. doi:10.1111/j.1532-5415.2008.01837.x [DOI] [PubMed] [Google Scholar]
- 12. Cummings SR, Black DM, Nevitt MC, et al. ; The Study of Osteoporotic Fractures Research Group. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75. [DOI] [PubMed] [Google Scholar]
- 13. Kanady JC, Drummond SP, Mednick SC. Actigraphic assessment of a polysomnographic-recorded nap: a validation study. J Sleep Res. 2011;20(1 Pt 2):214–222. doi:10.1111/j.1365-2869.2010.00858.x [DOI] [PubMed] [Google Scholar]
- 14. Blackwell T, Redline S, Ancoli-Israel S, et al. ; Study of Osteoporotic Fractures Research Group. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. [DOI] [PubMed] [Google Scholar]
- 16. Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–191. [DOI] [PubMed] [Google Scholar]
- 17. Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. [DOI] [PubMed] [Google Scholar]
- 18. Patel SR, Hayes AL, Blackwell T, et al. ; Osteoporotic Fractures in Men (MrOS); Study of Osteoporotic Fractures (SOF) Research Groups. The association between sleep patterns and obesity in older adults. Int J Obes (Lond). 2014;38:1159–1164. doi:10.1038/ijo.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dautovich ND, McCrae CS, Rowe M. Subjective and objective napping and sleep in older adults: are evening naps “bad” for nighttime sleep?J Am Geriatr Soc. 2008;56:1681–1686. doi:10.1111/j. 1532-5415.2008.01822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brink TL. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: Howarth Press; 1986. [Google Scholar]
- 21. Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 22. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. [DOI] [PubMed] [Google Scholar]
- 23. Cross N, Terpening Z, Rogers NL, et al. Napping in older people ‘at risk’ of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24:494–502. doi:10.1111/jsr.12313 [DOI] [PubMed] [Google Scholar]
- 24. Owens JF, Buysse DJ, Hall M, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. J Clin Sleep Med. 2010;6:330–335. [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon IY, Kripke DF, Youngstedt SD, Elliott JA. Actigraphy suggests age-related differences in napping and nocturnal sleep. J Sleep Res. 2003;12:87–93. [DOI] [PubMed] [Google Scholar]
- 26. Stang A, Dragano N, Moebus S, et al. ; Heinz Nixdorf Recall Investigative Group. Midday naps and the risk of coronary artery disease: results of the Heinz Nixdorf Recall Study. Sleep. 2012;35:1705–1712. doi:10.5665/sleep.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ancoli-Israel S, Kripke DF, Mason W, Kaplan OJ. Sleep apnea and periodic movements in an aging sample. J Gerontol. 1985;40:419–425. [DOI] [PubMed] [Google Scholar]
- 28. Ding Q, Whittemore R, Redeker N. Excessive daytime sleepiness in stroke survivors: an integrative review. Biol Res Nurs. 2016;18:420–431. doi:10.1177/1099800415625285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–4515. doi:10.1210/jc.2005-0035 [DOI] [PubMed] [Google Scholar]
- 30. Picarsic JL, Glynn NW, Taylor CA, et al. Self-reported napping and duration and quality of sleep in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2008;56:1674–1680. doi:10.1111/j.1532-5415.2008.01838.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldman SE, Hall M, Boudreau R, et al. Association between nighttime sleep and napping in older adults. Sleep. 2008;31:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hublin C, Lehtovirta M, Partinen M, Koskenvuo M, Kaprio J. Napping and the risk of type 2 diabetes: a population-based prospective study. Sleep Med. 2016;17:144–148. doi:10.1016/j.sleep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 33. Leng Y, Cappuccio FP, Surtees PG, Luben R, Brayne C, Khaw KT. Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutr Metab Cardiovasc Dis. 2016;26:996–1003. doi:10.1016/j.numecd.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fritschi C, Quinn L. Fatigue in patients with diabetes: a review. J Psychosom Res. 2010;69:33–41. doi:10.1016/j.jpsychores.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feinberg I. Untreated type 2 diabetes as a cause of daytime somnolence. Sleep. 1993;16:82. [PubMed] [Google Scholar]
- 36. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 37. Masa JF, Rubio M, Pérez P, Mota M, de Cos JS, Montserrat JM. Association between habitual naps and sleep apnea. Sleep. 2006;29:1463–1468. [DOI] [PubMed] [Google Scholar]
- 38. Bokenberger K, Ström P, Dahl Aslan AK, et al. Association between sleep characteristics and incident dementia accounting for baseline cognitive status: a prospective population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:134–139. doi:10.1093/gerona/glw127 [DOI] [PMC free article] [PubMed] [Google Scholar]