Abstract
Background
Musculoskeletal pain is highly prevalent and limits mobility in older adults. A potential mechanism by which pain affects mobility could be through its negative impact on the brain. We examined whether structural integrity of cerebral gray and white matter (WM) mediated the relationship between pain and mobility in community-dwelling older adults.
Methods
Musculoskeletal pain, gait speed, and neuroimaging data were obtained concurrently from the Health ABC study (mean age = 83/56% female, n = 212). Microstructural gray matter integrity was measured by mean diffusivity (MD), WM microstructure and macrostructure were measured by fractional anisotropy (FA) and WM hyperintensities (WMH), respectively. Regression models were adjusted for gray matter atrophy, age, gender, medication use, and obesity. Bootstrapped mediation methods were used (1,000 bootstrapped samples, 95% confidence intervals).
Results
The associations of musculoskeletal pain with WMH (β = .19, p < .05) and FA (β = −.18, p < .05) were robust to adjustment for gender, medication use, age, body mass index (BMI), and brain atrophy. Participants who experienced both knee and back pain had a significantly slower gait speed (~0.11 m/s) than those without knee or back pain (p < .05) independent of gender, medication, age, and BMI. WMH and FA significantly mediated the pain–gait speed relationship. Associations between pain and MD were not significant, and MD did not modify the association between pain and gait speed.
Conclusions
Cerebral WM integrity may contribute to the detrimental effects of musculoskeletal pain on mobility, although pre-existing WM integrity may also simultaneously amplify pain and decrease mobility. Future studies are needed to further understand whether successful pain management may significantly improve both brain health and mobility.
Keywords: Brain, Gait speed, Mobility, Musculoskeletal pain
Chronic pain is highly prevalent in older adults, with estimates as high as 70% in community settings (1,2). Chronic musculoskeletal pain significantly limits physical function and activities, negatively impacting quality of life (1,3,4). However, the neurobiological mechanisms underlying physical functional limitations in the presence of chronic musculoskeletal pain are not well characterized. As a consequence, targeted therapeutic strategies decreasing pain’s impact on physical function and disability over time are scarce (5).
Recent evidence suggests that chronic pain conditions are associated with neuroplastic changes including brain reorganization [(6,7) see (8) for a recent review). However, these associations have been scarcely investigated in relation to physical function. In 2008, Buckalew and colleagues first reported significantly lower white and gray matter (GM) volume in older adults with chronic low back pain compared to older adults without chronic low back pain (9). These findings were later supported by two other small follow-up studies in older adults (10,11). Indeed, a few studies have shown that cognitive function mediates the relationship between pain and physical performance, but the cerebral mechanisms have not been directly explored (12–14).
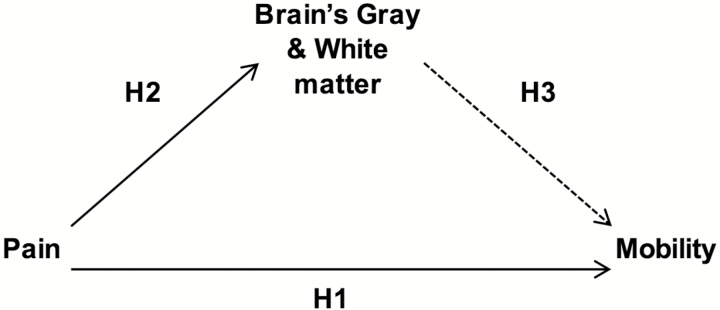
The present study was based on the hypothesis that chronic pain negatively impacts the central nervous system (CNS) and that this in turn, contributes to mobility limitations. We test the association between the presence and severity of musculoskeletal pain and gait speed with neuroimaging markers of brain structure. We examine both white and GM macrostructure and microstructure because of their known associations with mobility in older adults (15,16). We chose to focus the present study on gait speed given its sensitivity as a physical function outcome in predicting adverse outcomes in older adults (17,18). We hypothesized that there would be significant associations between: (a) pain presence and severity during the past 12 months and gait speed (H1 in Figure 1) and (b) pain presence and severity during the past 12 months and CNS measures of neuronal integrity (i.e., gray matter total mean diffusivity [MD]) and white matter (WM) abnormalities (i.e., total WM hyperintensities (WMH) and fractional anisotropy (FA) of normal-appearing WM; H2 in Figure 1). Last, we also hypothesized that (c) the CNS measures above would significantly mediate the relationship between pain and gait speed (H3 in Figure 1).
Figure 1.
Hypothesized model of the associations between presence and severity of pain and brain and gait speed variables.
Methods
Participants
Participants were from the Healthy Brain Project ancillary to the Health, Aging, and Body Composition (Health ABC) study. Health ABC is a cohort of 3,075 well-functioning, white and black, men and women, aged 70–79 years from Pittsburgh, PA, and Memphis, TN, enrolled 1997–1998. In 2006–2007, 314 of the eligible 652 Health ABC participants at the Pittsburgh site were interested and eligible for MRI of the brain and were able to walk 20 meters. Medical histories were reviewed to rule out neurological and psychological illnesses. Participants in the Healthy Brain Project were similar to the Pittsburgh cohort of the Health ABC study (19). All subjects provided written informed consent, and the protocol was approved by the University of Pittsburgh institutional review board.
Self-Reported Presence and Severity of Pain
Presence of pain
Knee and back pain are the two most commonly reported pain types in older individuals (1). In the Health ABC Study, participants were asked concurrently with their MRI visit: “In the past 12 months, have you had any pain in your back?” and “In the past 12 months, have you had any pain in your right or left knee?” Responses were categorized by whether participants had experienced: (i) only knee, (ii) only back pain, (iii) both knee and back pains, or (iv) neither of these pains in the past 12 months.
Severity of knee pain
In addition, individuals who had experienced knee pain in the past 12 months were also asked about the severity of their knee pain while walking on a flat surface, going up or down stairs, at night while in bed, standing upright, getting in or out of a chair, and getting in or out of a car. Answer choices for each side (right and left) were: “None”, “Mild”, “Moderate”, “Severe”, or “Extreme”. The answers were assigned numerical values as follows: “Mild” = 1, “Moderate” = 2, “Severe” = 3, and “Extreme” = 4. Values were added for the left and right sides, encompassing a global measure of knee pain severity. Participants that had not experienced any knee pain in the past 12 months received a score of 0. Two individuals reported that they did not know how much pain they had experienced and were assigned 1 (mild pain).
Severity of back pain
Individuals who had experienced back pain in the past 12 months were also asked the following question: How severe was the back pain usually?” with answer choices: “Mild”, “Moderate”, “Severe”, or “Extreme”. The answers were assigned numerical values as follows: “Mild” = 1, “Moderate” = 2, “Severe” = 3, and “Extreme” = 4. Participants that had not experienced any back pain in the past 12 months received a score of 0. One person reported they did not know the severity of their back pain and we used a 1 (mild pain) in that instance.
Global pain index
Presence of pain alone is not enough to account for the negative impact chronic pain has on an individual’s life (20); therefore, we created a global pain index that accounted for the presence and severity of both back and/or knee pains. First, the knee and back pain severity variables (above) were both standardized to a mean of 0 and a standard deviation of 1. Second, we added the presence of knee and/or back pain (i.e., 0 = no knee/back pain, 1 = either knee or back pain only, 2 = both knee and back pain) to the standardized (z-score) severity of knee and back pains as calculated above. This derived composite continuous variable reflects equally the contribution of knee and back pain within an individual with higher numbers reflecting individuals experiencing both knee and back pain of greater severity.
Gait Speed
The GaitMatII (EQ Inc., Chalfonte, PA) is a 4-meter computerized gait instrument. We averaged participants’ gait speed taken over two traverses on the GaitMatII. Prior to data collection, participants performed two practice walks across the walkway.
Neuroimaging
Image acquisition
Details of the image acquisition protocol have been previously published (21). Images were obtained with a Siemens 12-channel head coil and 3T Siemens Tim Trio MR scanner at the Magnetic Resonance Research Center, University of Pittsburgh. T1-weighted, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted images were collected. Diffusion-weighted images were acquired using a single short spin-echo sequence (repetition time = 5300 ms, echo time = 88 ms, inversion time = 2500 ms, 90° flip angle, 256 × 256 mm field of view, two diffusion values of b = 0 and 1,000 s/mm, 12 diffusion directions, four repeats, 40 slices, 3 mm thick, 128 × 128 matrix size, 2 × 2 × 3 mm voxel size, and Generalized Autocalibrating Partial Parallel Acquisition = 2). A neuroradiologist examined each MRI for neurologic abnormalities.
Image Processing
Microstructural measures of the GM and WM [MD and FA, respectively] were obtained using previously published methods (21), briefly described below. Volumes for GM, WM, and cerebrospinal fluid were calculated by segmenting the skull-stripped T1-weighted image in native anatomical space. Volumes were estimated in cubic millimeters by summing tissue-specific voxels. Intracranial volume was contained within the inner skull. An atrophy index was calculated as 1-GM volume/intracranial volume. Diffusion tensor imaging estimates the microstructural integrity of brain tissues with MD estimating an average magnitude of water diffusion. Greater restriction of water diffusion is associated with a lower MD value. FA is an index of WM tract integrity with higher values indicating greater integrity (22). The diffusion-weighted images were preprocessed as previously reported (21). Mean FA and MD were calculated for normal-appearing WM and GM only. T2-weighted FLAIR images were acquired to obtain the WM hyperintensity volume (WMH). The WMH quantification was done using a semi-automated method for quantification and localization of WMH a fuzzy connected algorithm (23).
Brain Measures
For the present study, FA was used to capture microscopic details about tissue architecture providing information about fiber density, axonal diameter, and myelination in WM. WM hyperintensity volume (WMH) are brighter white patches on MRIs seen in normal aging and various neurological diseases. A greater WMH volume is thought to reflect damage to small blood vessels, hemorrhages, gliosis, and ischemia.
Potential Health- and Pain-Related Covariates
Variables known to be associated with brain health and pain were included as potential covariates. Age, gender, race, and current medications were self-reported. Body mass index (BMI) was calculated by the standard formula (weight in kilograms)/(height in meters)2. Diabetes was determined by self-report, use of hypoglycemia medication, a fasting glucose of ≥126 mg/dL, or a 2-h glucose tolerance test >200 mg/dL at baseline or during follow-up until time of MRI. Prevalent hypertension and stroke were defined by self-report or current medication use. Muscle strength was measured as the peak torque from isokinetic knee extension on a dynamometer (model 125AP, Kin-Com, Chattanooga, TN). The right leg was measured unless contraindicated due to prior surgery, injury, or pain. Depressive symptoms were assessed by the short form Center for Epidemiologic Studies–Depression (CES-D) scale and reported as number of symptoms endorsed (24).
Statistical Analysis
Statistical analyses were performed in IBM SPSS 23. Analyses of variance were used to compare baseline characteristics of the Health ABC participants between the pain groups. Hierarchical linear regressions were used to determine the association of pain with gait speed and whole brain WMH, MD, and FA after adjustment for covariates. Three models were tested: model 1 = presence and severity of pain alone, model 2 = model 1 plus covariates that were significantly different (p value ≤ .05) in bivariate associations, and model 3 = model 2 plus age, BMI, and atrophy index). Model assumptions were tested including tests of normality distribution and homogeneity of variances.
Mediation Analysis
Separate mediation analyses were conducted to assess both the presence of pain and the presence plus severity of pain, as described above. We tested the total indirect effect of pain on gait speed mediated through brain measures. The mediation analysis controlled for gender, nonsteroidal anti-inflammatory medication (NSAID) use, age, BMI, and brain atrophy.
Bootstrapping
We used bootstrapping procedures to obtain estimates and confidence intervals around the indirect effects to overcome potential problems caused by unmet assumptions in mediation analysis (25). We used the Hayes PROCESS macro (model 4) (25) that estimates the model coefficients in mediation while also providing modern inferential methods for inference about indirect effects including bootstrap confidence intervals.
Results
Of the 314 participants who took part in the imaging substudy, 212 participants had complete neuroimaging, pain, physical function, and all covariate data and are included in the present study. There were no significant differences between included and excluded individuals on demographics or health-related characteristics, including the presence of back and knee pain during the past 12 months (p > .05). The analytic sample had an average age of 82.8 years and was 56% female (Table 1).
Table 1.
Baseline Characteristics in Health ABC Participants With and Without Back and/or Knee pain (n = 212)
| Characteristics (measured concurrent with MRI, unless otherwise noted) Mean, (SD), n (%) | No back/knee pain | Knee pain | Back pain | Back/knee pain |
|---|---|---|---|---|
| n = 63 | n = 40 | n = 46 | N = 63 | |
| Age | 82.8 ± 2.6 | 82.5 ± 2.6 | 82.8 ± 2.5 | 83.0 ± 2.9 |
| Female* | 29 (46.0) | 17 (42.5) | 26 (56.5) | 47 (74.6) |
| White | 51 (63.7) | 25 (51.0) | 44 (69.8) | 51 (53.7) |
| Education < HS | 10 (12.7) | 9 (18.4) | 9 (14.3) | 12 (12.6) |
| Center for Epidemiological Studies–Depression | 6.1 ± 5.8 | 5.1 ± 4.7 | 7.5 ± 5.9 | 7.9 ± 7.2 |
| Diabetes (at time of MRI) | 17 (21.3) | 12 (24.5) | 19 (30.2) | 28 (29.5) |
| Hypertension (at time of MRI) | 43 (68.3) | 25 (62.5) | 33 (71.8) | 48 (87.4) |
| Stroke (at time of MRI) | 6 (7.5) | 3 (6.1) | 2 (3.2) | 10 (10.5) |
| BMI (kg/m2) | 26.6 ± 4.4 | 28.3 ± 4.0 | 27.1 ± 4.5 | 28.2 ± 4.1 |
| Muscle strength | 88.5 ± 34.3 | 86.4 ± 29.8 | 83.6 ± 30.5 | 74.8 ± 24.6 |
| Total # of Medications* | 3.6 ± 2.3 | 4.0 ± 2.5 | 4.9 ± 2.9 | 5.2 ± 3.0 |
| Salicylates | 7 | 1 | 4 | 4 |
| NSAIDs* | 1 | 3 | 5 | 10 |
| Steroids | 1 | 0 | 1 | 0 |
| Other anti-inflammatories | 9 | 3 | 9 | 14 |
| Atrophy index | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 |
Note: BMI = body mass index; HS = higher secondary; MRI = magnetic resonance imaging; NSAID = nonsteroidal anti-inflammatory medications.
*ANOVA with p value ≤0.05.
Self-Reported Presence and Severity of Pain
Presence of pain
Approximately 29% of the sample reported having pain in both the knee and the back in the prior 12 months (n = 63), while 19% reported only knee pain (n = 40), 21% reported only back pain (n = 46), and 29% reported neither (n = 63). Table 1 summarizes the demographic and health-related characteristics by pain group. In general, participants with pain tended to be female and reported taking a significantly greater number of medications, specifically NSAIDs (p < .05). These and other demographic variables were subsequently included as covariates in separate regression models in the following paragraphs.
Pain severity
Among the participants who experienced knee pain, 51% reported that they did not experience pain when performing activities of daily living. Of those that reported knee pain during these activities, most individuals reported mild-to-moderate knee pain severity. Detailed information about the percentage and number of participants that found each of the activities painful is summarized in Supplementary Table 1. Approximately 25% of the participants who experienced back pain reported moderate pain severity (see Supplementary Table 2). One person reported they did not know the severity of their back pain, and we used a 1 (mild pain) for the subsequent statistical analysis in that instance.
Global pain index
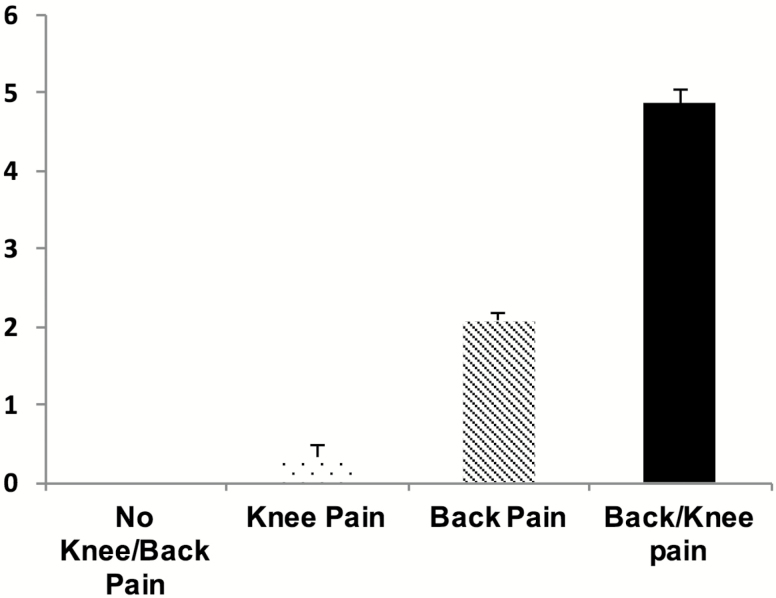
There were statistically significant differences on global pain severity between the participants that did not report knee and/or back pain compared to those that did report either or both pains with the latter experiencing the greatest global pain severity (all Bonferroni corrected p’s < .05; see Figure 2).
Figure 2.
Differences between the participants that did not report knee and/or back pain compared to those that did report either or both pains on the global pain index variable (Bonferroni corrected p’s < 0.05).
Pain, Gait Speed, and Brain Measures
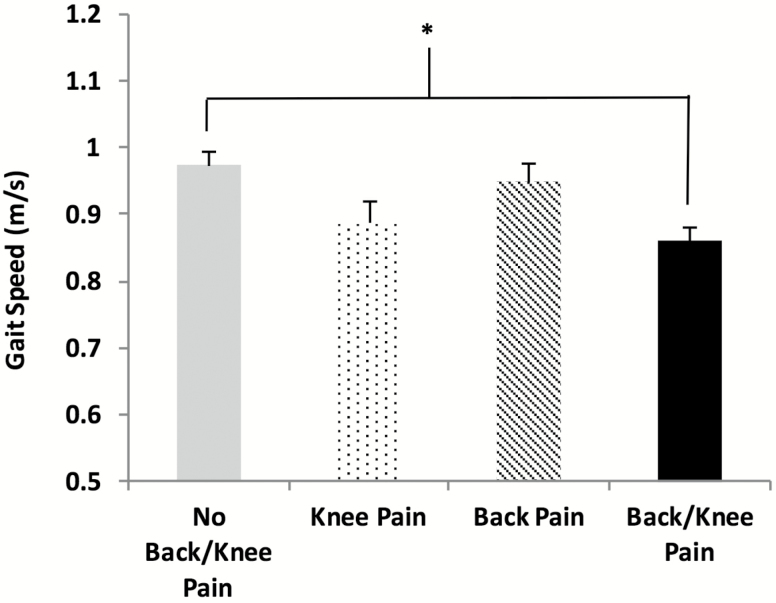
Compared to those without knee and/or back pain, those reporting both knee and back pain had significantly slower gait speed during usual walk, independent of gender and NSAID medication use (Figure 3). Adjustment for gender, NSAID medication use, age, and BMI did not attenuate the association between greater pain and slower gait.
Figure 3.
Gait speed differences between the participants that did not report knee and/or back pain compared to those that did report either or both pains (Bonferroni corrected *p < 0.05 adjusted for covariates).
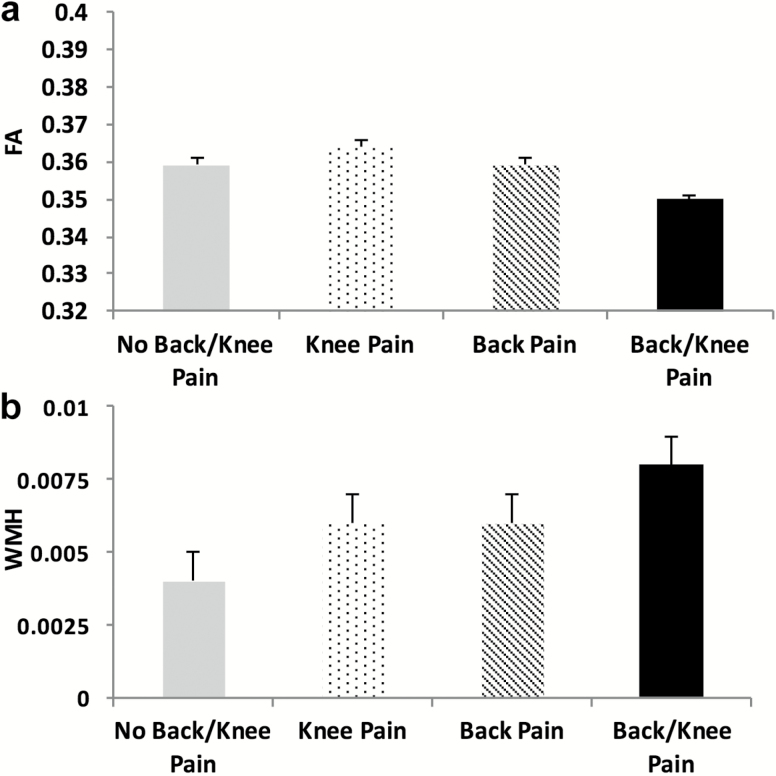
Compared to those without knee and/or back pain, those reporting knee pain had significantly lower FA independent of all other covariates (Bonferroni corrected p = .001; Figure 4a). Similarly, a significantly higher total WMH were present in those reporting both knee and back pain compared to those without knee and/or back pain (Bonferroni corrected p = .037; Figure 4b). There were no significant associations between clinical pain and MD, p > .05, Table 2.
Figure 4.
White matter differences between the participants that did not report knee and/or back pain compared to those that did report either or both pains. (a) fractional anisotropy (FA) and (b) white matter hyperintensities (WMH) (Bonferroni corrected p < 0.05 adjusted for covariates).
Table 2.
Regression Analyses of the Global Pain Index Predicting Physical Function and Brain Measures Among our Sample (n = 212)
| Dependent variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Standardized Beta | p value | Standardized Beta | p value | Standardized Beta | p value | |
| Mobility measures | ||||||
| Gait Mat walking speed (m/s) | −.24 | .000 | −.21 | .001 | −.14 | .021 |
| Whole brain measures | ||||||
| White matter macrostructure (WMH)* | .19 | .002 | .19 | .003 | .19 | .002 |
| White matter microstructure (FA)* | −.18 | .005 | −.13 | .036 | −.11 | .079 |
| Gray matter microstructure (MD)* | −.03 | .623 | −.03 | .675 | .00 | .999 |
Note: Model 1 = global pain index. Model 2 = model 1 + gender and NSAID medications (variables with p value ≤0.05 from Table 1). Model 3 = model 2 + age and BMI (*Also included atrophy index). FA = fractional anisotropy; MD = mean diffusivity; NSAID = nonsteroidal anti-inflammatory medications; WMH = white matter hyperintensities. The bold value signifies p < .05.
Pain and Physical Function Adjusted for Brain Measures
To test the total indirect effect of pain presence and severity on gait speed through the whole brain measures, bootstrapped mediation analyses (n = 1,000) were performed while controlling for gender, NSAID medication use, age, BMI, and brain atrophy. WMH and FA significantly mediated the relationship between pain and gait speed, whereas MD did not significantly mediate any of the relationships. Bias-corrected bootstrapped estimates and 95% confidence intervals for the indirect effect of pain on gait speed adjusting for covariates are presented in Table 3.
Table 3.
Bias Corrected Bootstrapped* Estimates and Confidence Intervals** for the Indirect Effect of Pain on Gait Speed Adjusting for Covariates.***
| Presence of pain | Global pain index | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Error | Lower bound | Upper bound | Effect | Error | Lower bound | Upper bound | |
| White matter macrostructure (WMH) | −.0064 | .0029 | −.0132 | −.0019 | −.0034 | .0014 | −.0070 | −.0011 |
| White matter microstructure (FA) | .0031 | .0022 | .0002 | .0092 | .0012 | .0010 | .0000 | .0044 |
| Gray matter microstructure (MD) | .0000 | .0017 | −.0041 | .0032 | .0002 | .0008 | −.0011 | .0024 |
Note: BMI = body mass index; FA = fractional anisotropy; MD = mean diffusivity; NSAID = nonsteroidal anti-inflammatory medications; WMH = white matter hyperintensities. The bold value signifies p < .05.
*n = 1,000 bootstrap samples.
**95% Bootstrapped confidence intervals.
***Adjusted for age, gender, BMI, NSAID medication use, and brain atrophy.
Sensitivity analyses determined that adding cognitive function to the models did not significantly change the findings (not shown).
Discussion
The present study provides neuroimaging evidence that lower integrity of the brain’s WM significantly mediates the relationship between pain and mobility in a sample of community-dwelling older adults. Specifically, two key findings emerged from our analyses: (i) individuals who experienced both knee and back pain reported greater pain severity, had a slower gait, and had worse WM indices compared to those who did not experience pain and (ii) measures of WM integrity, but not of GM integrity, significantly mediated the pain–gait speed association.
In the present study, individuals reported mild-to-moderate levels of pain severity, consistent with a well-functioning population of older adults. However, individuals who experienced both knee and back pain reported greater levels of pain severity that were associated with a “clinically meaningful” slower gait speed (i.e., ~0.11 m/s slower) (26). Slower gait has been associated with greater severity and impact of pain regardless of pain location (27). However, an investigation on the HABC parent cohort (n = 2,766) found that older adults who had back pain along with hip or knee pain had worse lower extremity performance compared with those with back pain alone (28). Indeed, an increased number of pain sites and greater pain severity were associated with poorer physical performance after adjusting for age, sex, height, and weight on a separate community-dwelling cohort of older adults (4). More recently, musculoskeletal pain at multiple sites predicted new onset of mobility difficulty 18-months later (5). Given the greater functional impact of multisite pain, it is not surprising that these individuals also had the lowest integrity of the brain’s WM, even after accounting for other factors. We found that individuals who experienced both knee and back pain compared to the other groups had lower FA, a microstructural measure of WM integrity, as well as greater number of WMH, a macrostructural measure of WM integrity. This is consistent with findings by Buckalew and colleagues (10) where greater WMH burden was associated with lower gait speeds within the older chronic pain participants (10). In older adults, lower WM FA is associated with worse balance performance (29) and slower fast-paced gait speed (30). Based on a separate analysis of the present study cohort not focused on pain, Rosario and colleagues (31) found that the inverse association between gait speed and WMH was only significant in those individuals with a low FA (31). A decreased FA in WM tracts is thought to reflect microstructural abnormalities, including demyelination and axonal loss while pathological findings in WMH regions include myelin pallor, tissue rarefaction, and mild gliosis (32). It is possible that the degeneration at the microscopic level (i.e., measured by FA) occurs before any overt sign at a macrostructural level (i.e., measured by WMH), but the differences or associations between WMH and FA are still not well understood. Nonetheless, a growing body of literature already supports decreased FA in persons that experience chronic pain (33,34) with lower FA predicting pain’s chronification (35). Although surprising that GM microstructure was not significantly associated with pain presence or severity in the present sample, it is possible that controlling for age-related changes to the GM macrostructure (i.e., atrophy) obscured detecting GM differences at a microstructural level.
Although previous research has demonstrated the peripheral mechanisms contributing to the pain–gait association, to our knowledge, this is the first study to focus on potential cerebral mechanisms. Specifically, only measures of WM integrity (i.e., WMH, FA), but not GM integrity (i.e., MD), significantly mediated the pain–gait speed association. Based on these findings, it can be suggested that the central nervous system mechanisms associated with age-related mobility impairments may be accelerated by the presence of pain. Older adults with more severe pain or more pain interference have poorer cognitive function compared to those with no pain or less pain (36) supporting our findings of lower brain integrity. Thus, clinical pain assessment and treatment with the goal of decreasing the burden of chronic pain in older adults can have a multitude of beneficial effects. However, these relationships need to be further examined in a longitudinal study design, especially since recent neuroimaging evidence (35) suggests that pre-existing WM structure and function predispose individuals to chronic pain development. Nonetheless, it seems plausible to use interventions that take advantage of the brain’s neuroplastic capacity, both for decreasing pain’s impact on the brain as well as increasing physical functional outcomes including mobility.
Our results are limited by several factors. First, data were collected at a single time point and only cross-sectional associations were examined and not causal relationships. Future longitudinal studies examining these relationships are urgently needed. Second, the present study only examined total brain measures. It is possible that specific tracts and areas are differentially affected by pain and that total brain changes dilute changes in these specific areas. Future research designed to comprehensively assess pain’s impact on the brain along with mobility is urgently needed. Third, the present sample was a high functioning cohort of community-dwelling older adults experiencing mild-to-moderate levels of pain. Thus, our findings cannot be generalized to institutionalized older adults who often experience more severe levels of pain. Finally, our study only captured the two most common pain types in older adults. Future work should assess all pains experienced by an individual including its characteristics and analgesic medications taken.
Despite these limitations, the findings from this research provide the preliminary evidence needed to further investigate the effects of pain on the brain of older adults and its relation to age-related changes in mobility. Longitudinal studies should be designed to elucidate the directionality of the associations between cerebral WM, pain, and mobility, which are possibly different in subsets of individuals. Our findings also suggest that mild-to-moderate pain at multiple body sites can have a negative impact on the mobility of older individuals. Future studies are needed to understand whether pain management may improve both brain health and mobility.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
Health ABC was supported by National Institute on Aging (NIA) Contracts (N01AG6-2101, N01AG6-2103, N01-AG-6-2106, NIA grant R01AG028050, and NINR grant R01NR012459). This research was supported in part by the Intramural Research Program of the NIA (K23AG028966, R01AG029232) and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30AG024827-07. This work was also supported by NIA grant K01AG048259 to YC-A.
Conflict of Interest
None to declare.
Supplementary Material
References
- 1. Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154:2649–2657. doi:10.1016/j.pain.2013.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karttunen NM, Turunen JH, Ahonen RS, Hartikainen SA. Persistence of noncancer-related musculoskeletal chronic pain among community-dwelling older people: a population-based longitudinal study in Finland. Clin J Pain. 2015;31:79–85. doi:10.1097/AJP.0000000000000089 [DOI] [PubMed] [Google Scholar]
- 3. Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302:2214–2221. doi:10.1001/jama.2009.1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eggermont LH, Bean JF, Guralnik JM, Leveille SG. Comparing pain severity versus pain location in the MOBILIZE Boston study: chronic pain and lower extremity function. J Gerontol A Biol Sci Med Sci. 2009;64:763–770. doi:10.1093/gerona/glp016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eggermont LH, Leveille SG, Shi L, et al. Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc. 2014;62:1007–1016. doi:10.1111/jgs.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9. doi:10.1038/nn.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vachon-Presseau E, Tétreault P, Petre B, et al. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139:1958–70. doi:10.1093/brain/aww100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coppieters I, Meeus M, Kregel J, et al. Relations between brain alterations and clinical pain measures in chronic musculoskeletal pain: a systematic review. J Pain. 2016; 17:949–962. doi:10.1016/j.jpain.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 9. Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain Med. 2008;9:240–248. doi:10.1111/j.1526-4637.2008.00412.x [DOI] [PubMed] [Google Scholar]
- 10. Buckalew N, Haut MW, Aizenstein H, Morrow L, Perera S, Kuwabara H, Weiner DK. Differences in brain structure and function in older adults with self-reported disabling and nondisabling chronic low back pain. Pain Med. 2010;11:1183–1197. doi:10.1111/j.1526-4637.2010.00899.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckalew N, Haut MW, Aizenstein H., et al. White matter hyperintensity burden and disability in older adults: is chronic pain a contributor? PM R. 2013;5:471–480; quiz 480. doi:10.1016/j.pmrj.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi:10.1111/j.1526-4637.2006.00091.x [DOI] [PubMed] [Google Scholar]
- 13. Pulles WL, Oosterman JM. The role of neuropsychological performance in the relationship between chronic pain and functional physical impairment. Pain Med. 2011;12:1769–1776. doi:10.1111/j.1526-4637.2011.01266.x [DOI] [PubMed] [Google Scholar]
- 14. Morone NE, Abebe KZ, Morrow LA, Weiner DK. Pain and decreased cognitive function negatively impact physical functioning in older adults with knee osteoarthritis. Pain Med. 2014;15:1481–1487. doi:10.1111/pme.12483 [DOI] [PubMed] [Google Scholar]
- 15. Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68:1379–1386. doi:10.1093/gerona/glt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PubMed] [Google Scholar]
- 18. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. doi:10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Venkatraman VK, Aizenstein HJ, Newman AB, et al. Lower digit symbol substitution score in the oldest old is related to magnetization transfer and diffusion tensor imaging of the white matter. Front Aging Neurosci. 2011;3:11. doi:10.3389/fnagi.2011.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. [DOI] [PubMed] [Google Scholar]
- 21. Rosano C, Aizenstein HJ, Newman AB, et al. Health ABC Study. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62:307–13. doi:10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi:10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi:10.1016/j.pscychresns.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 25. Hayes AF. (2013). Introduction to mediation, moderation, and conditional process analysis. New York: The Guilford Press. [Google Scholar]
- 26. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc, 2006;54:743–749. doi:10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 27. Morone NE, Karp JF, Lynch CS, Bost JE, El Khoudary SR, Weiner DK. Impact of chronic musculoskeletal pathology on older adults: a study of differences between knee OA and low back pain. Pain Med. 2009;10:693–701. doi:10.1111/j.1526-4637.2009.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiner DK, Haggerty CL, Kritchevsky SB, Harris T, Simonsick EM, Nevitt M, Newman A; Health, Aging, and Body Composition Research Group. How does low back pain impact physical function in independent, well-functioning older adults? Evidence from the Health ABC Cohort and implications for the future. Pain Med. 2003;4:311–20. [DOI] [PubMed] [Google Scholar]
- 29. Van Impe A, Coxon JP, Goble DJ, Doumas M, Swinnen SP. White matter fractional anisotropy predicts balance performance in older adults. Neurobiol Aging. 2012;33:1900–1912. doi:10.1016/j.neurobiolaging. 2011.06.013 [DOI] [PubMed] [Google Scholar]
- 30. Tian Q, Ferrucci L, Resnick SM, et al. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging Behav. 2015;10:697–706. doi:10.1007/s11682-015-9449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosario BL, Rosso AL, Aizenstein HJ, et al. ; Health ABC Study Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. 2016;71:968–973. doi:10.1093/gerona/glv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. [DOI] [PubMed] [Google Scholar]
- 33. Lieberman G, Shpaner M, Watts R, et al. White matter involvement in chronic musculoskeletal pain. J Pain. 2014;15:1110–1119. doi:10.1016/j.jpain.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilcox SL, Gustin SM, Macey PM, Peck CC, Murray GM, Henderson LA. Anatomical changes within the medullary dorsal horn in chronic temporomandibular disorder pain. Neuroimage. 2015;117:258–66. doi:10.1016/j.neuroimage.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 35. Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154:2160–2168. doi:10.1016/j.pain.2013.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Leeuw G, Eggermont LH, Shi L, et al. Pain and cognitive function among older adults living in the community. J Gerontol A Biol Sci Med Sci. 2016;71:398–405. doi:10.1093/gerona/glv166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.