Abstract
Background
Resveratrol, a plant-derived polyphenol, has been reported to improve glucose metabolism and vascular function and to extend life span in animal models, but studies in humans have been inconclusive.
Methods
In a randomized, double-blind crossover study, we treated older glucose-intolerant adults (n = 30) with resveratrol (2−3 g/daily) or placebo, each for 6 weeks. A standard mixed-meal test was used to assess insulin sensitivity (Matsuda index) and secretion (C-peptide deconvolution) and vascular function by reactive hyperemia peripheral arterial tonometry. Skeletal muscle samples were obtained for gene expression using RNA-Seq analysis and to assess mitochondrial morphology.
Results
There were no changes in glucose tolerance, insulin sensitivity, weight, blood pressure, or lipid profile following resveratrol treatment. Fasting reactive hyperemia index improved with resveratrol (2.02 ± 0.2 vs 1.76 ± 0.02, p = .002). RNA-Seq analysis yielded 140 differentially expressed transcripts (corrected p-value ≤ .05), predominantly associated with mitochondrial genes and noncoding RNA. Ingenuity Pathway Analysis confirmed that mitochondrial dysfunction (p = 2.77 × 10−12) and oxidative phosphorylation (p = 1.41 × 10−11) were the most significantly perturbed pathways. Mitochondrial number, but not size, was increased.
Conclusions
Resveratrol treatment of older adults with impaired glucose regulation may have beneficial effects on vascular function, but not glucose metabolism or insulin sensitivity. Changes in gene expression suggest effects similar to those observed with caloric restriction, which has been shown to increase life and health span in animal models, although its significance for humans is uncertain. Future human studies should address the appropriate dose range and low bioavailability of resveratrol.
Keywords: Prediabetes, Gene expression, Polyphenols, Aging
Aging in humans is a well-established risk factor for many disabling and chronic conditions, among them diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. In fact, the risk of death from these causes is dramatically accelerated with increasing age, imposing a tremendous burden on health care systems across the world. For this reason, there is a need for effective interventions that have the potential to delay or attenuate age-related chronic diseases and to promote increased health span.
Resveratrol (trans-3, 5, 4′-trihydroxystilbene), a natural polyphenol found in fruits and medicinal plants, such as Japanese knotweed (Polygonum cuspidatum), has emerged as an attractive agent to counteract age-related diseases. Interest in this compound has accelerated in light of its association with the health benefits of red wine and its identification as a chemopreventive agent for cancer (1). Subsequent reports demonstrated resveratrol to be an activator of sirtuins, a family of NAD(+)-dependent deacetylase enzymes thought to mediate the beneficial effects of caloric restriction (2). In yeast, worms, and flies, resveratrol has been associated with sirtuin-dependent increase in life span (3). Further study in vitro and in animal models has shown resveratrol to have beneficial effects on glucose metabolism, vascular function, as well as anti-inflammatory and antioxidant properties (4). Resveratrol has also been shown to promote mitochondrial biogenesis and improve mitochondrial function (4,5). These potential benefits prompted the initiation of human studies to test resveratrol’s role in the prevention and treatment of chronic diseases such as diabetes and cardiovascular disease. However, formal studies to examine its metabolic effects are limited and inconclusive (6–10).
Our group previously conducted a pilot study to examine the effects of resveratrol on glucose metabolism and vascular function in older adults with impaired glucose tolerance (11). Promising effects were seen, including improvements in insulin sensitivity, postmeal plasma glucose, and vascular function. We therefore conducted this randomized double-blind placebo controlled crossover study to further characterize the effects of resveratrol treatment on metabolism, vascular function, and mitochondrial biogenesis in a similar cohort.
Methods
Participants
The study was approved by the Albert Einstein College of Medicine Institutional Review Board, and written informed consent was obtained from all participants. Adults aged 50–80 years without a prior diagnosis of diabetes were screened with a 75-g oral glucose tolerance test, and those with fasting plasma glucose of <126 mg/dL (or <140 if concurrent hemoglobin A1c [HbA1c] was <7%) and 2-hour glucose of >170 mg/dL were eligible. Exclusions included serious chronic or acute illness: active cancer (other than non-melanoma skin cancer) or history of estrogen-dependent neoplasm (eg, breast or endometrial cancer), symptomatic heart failure, chronic obstructive pulmonary disease, inflammatory conditions, significant liver disease or renal disease, recent (within 3 months) cardiovascular event (myocardial infarction, revascularization, or stroke), and prior bariatric or other gastric surgery or cigarette smoking. Also exclusionary was use of drugs known to influence glucose metabolism (eg, systemic glucocorticoids), antioxidant vitamins, warfarin, or antiplatelet drugs (other than aspirin). Because of potential resveratrol induced CYP450-related drug interactions, treatment with anti-epileptics, mexilitene, quinidine, cyclosporine, tacrolimus, HIV protease inhibitors, and high-dose statin therapy was also exclusionary. Thirty-eight participants were enrolled in the study; eight participants dropped out (three for study-related adverse events) before completion of the second treatment period and are not included in this analysis.
Study Design
The study was a randomized double-blind placebo controlled crossover study. Resveratrol capsules were obtained from RevGenetics Corporation, and independent verification of the resveratrol content of the capsules used in this study was performed in the Proteomics Facility, Laboratory for Macromolecular Analysis and Proteomics at the Albert Einstein College of Medicine. A resveratrol dose of 1,500 mg twice daily was administered to the initial nine participants; however, because of gastrointenstinal side effects, subsequent participants received 1,000 mg twice daily. Randomization, blinding, and dispensing were conducted in the research pharmacy of Montefiore Medical Center. Participants were instructed to abstain from nutritional supplements containing resveratrol and to maintain their usual dietary and physical activity patterns. The compliance rate, defined as the proportion of tablets ingested relative to the intended number, was calculated based on remaining tablets returned at the end of the treatment period.
Study Visits and Interventions
Following a screening visit, the study consisted of two randomly assigned 6-week treatment periods (resveratrol and placebo). The duration of resveratrol treatment that may be required to demonstrate metabolic effects in humans has not been established. In an animal model, a single oral resveratrol dose acutely improved glucose tolerance (12), whereas other studies have reported improved glucose tolerance with chronic treatment (4) and our own preliminary studies suggest measurable effect after 4 weeks of treatment (11). We reasoned that some signal of efficacy should be apparent within 6 weeks, even if the full metabolic effects might take longer to emerge. Participants were instructed to begin study drug on the evening following the baseline measurements and continue through the evening prior to metabolic testing at the end of each treatment period. Following a 3-week washout period, the participants crossed over to the other intervention for the second 6-week treatment period.
Standard Mixed-Meal Test
Participants were studied following an overnight fast and after a standard breakfast consisting of 110-g carbohydrates, 20-g protein, and 20-g fat, at the beginning and end of each treatment period. Participants were requested to maintain consistent nutrient intake on the days prior to each standard meal test. Blood sampling for glucose and insulin levels was performed fasting (Time 0) and 30, 60, 90, 120, 150, and 180 minutes following the mixed-meal through an indwelling catheter. The assigned treatment (resveratrol or placebo) was administered with the meal at the test conducted at the end of the 6-week treatment period. Insulin sensitivity was estimated using homeostasis model assessment (HOMA-IR) (13) and also from insulin and glucose levels obtained following the standard meal challenge using the Matsuda index (14,15). Insulin secretion was estimated using the C-peptide deconvolution method as described by Van Cauter and colleagues (16). β-Cell function was assessed with the oral disposition index (DIo) calculated using the formula: (ΔI0–30/ΔG0–30) × (1/I0) (17). Insulin and glucose area under the curve (AUC) were calculated using the trapezoidal method.
Assays were performed in the core laboratories of the Einstein Institute for Clinical and Translational Research: glucose, HbA1c, lipoproteins, insulin (radioimmunoassay), C-peptide (radioimmunoassay), high-sensitivity C-reactive protein (hs-CRP; latex-enhanced turbidimetric assay), lipoprotein phospholipase A2 (Lp-PLA2; ELISA, R&D Systems), and adiponectin (radioimmunoassay; Linco). Blood chemistries, complete blood count, and urinalysis were performed in the clinical laboratories of Montefiore Medical Center.
Levels of resveratrol and metabolites were measured at 0, 30, 60, 120, and 180 minutes during the standard mixed-meal test (SMMT) in the resveratrol treatment period. As a control, resveratrol and metabolites were measured in samples obtained from three randomly selected participants during a placebo period SMMT to confirm the sensitivity of the assay to distinguish between samples obtained during the resveratrol and placebo treatment periods. The concentrations of resveratrol and its metabolites in plasma were determined by high-performance liquid chromatography–tandem mass spectrometry analysis, using a previously described protocol (18).
Endothelial Function Testing
Endothelial function testing was performed fasting and 90 minutes following the standard meal, using reactive hyperemia peripheral arterial tonometry (19), which measures arterial pulse wave amplitude in the finger before and after 5 minutes of blood flow occlusion using a standard blood pressure cuff (EndoPAT; Itamar Medical). The reactive hyperemia index (RHI) is the ratio of the average pulse amplitude in the posthyperemic phase divided by the average baseline amplitude, with normalization to the signal in the control arm to compensate for any systemic changes. Augmentation index (a measure of arterial stiffness) is also derived from the PAT signal.
Skeletal Muscle Samples
Skeletal muscle biopsies were performed in the fasting state prior to the SMMT in 16 participants. A muscle sample of ~50–100 mg was obtained with a spring-loaded biopsy needle (Bard Instruments) in the mid-thigh region (vastus lateralis) following local anesthesia extending into the muscle area. A ~1 mg muscle sample was preserved in glutaraldehyde for electron microscopy (EM). The remaining sample was immediately homogenized in Trizol, frozen in liquid nitrogen, and stored at −80°C for subsequent mRNA extraction and analysis of gene expression. The RNA-Seq analysis was performed at Einstein’s Sequencing Core Facility, using multiplexed 100 bp single-end sequencing on an Illumina HiSeq2500 (http://www.illumina.com/technology/mrna_seq.ilmn).
EM was performed on skeletal muscle samples postfixed with 1% osmium tetroxide followed by 1% uranyl acetate. Sections (80 nm) were prepared and stained with uranyl acetate followed by lead citrate. No fewer than 10 images per sample were analyzed at 8,000× magnification using a JEOL 1200EX transmission electron microscope at 80 kV. EM was performed on five randomly selected muscle sample pairs.
Statistical Analysis
Data are presented as mean (± SD) for baseline values. Placebo versus resveratrol variables (eg, peak and AUC glucose, insulin, Matsuda index, RHI, etc.) were compared using a paired t test. A nonparametric test (Wilcoxon’s test) was used if data were not normally distributed. The study was designed to have 90% power to detect a 20 mg/dL difference in the primary study outcome, glucose AUC, using data from our earlier pilot study (11), in which we observed glucose AUC of 469 ± 23 versus 428 ± 19 (p = .001), at baseline and after 4 weeks of resveratrol, respectively. The possibility of a carry-over effect between treatment periods was analyzed using a mixed effects model controlling for the baseline value at the beginning of each treatment period. No evidence of carry-over effect was observed. Data analysis was performed using SAS version 9.4.
The raw RNA-Seq reads were quality controlled and subsequently aligned to the hg19 build of the human genome using GSNAP (20). The mapped reads were then processed using the edgeR package (21) within the R/Bioconductor environment to identify differentially expressed transcripts between pre- and post-treatment cohorts with a false discovery rate of <0.05 (21,22). These gene lists were subsequently imported into Ingenuity Pathway Analysis (23) to identify enriched pathways, Gene Ontology terms, and disease-relevant associations.
Results
Baseline Characteristics
Thirty participants (19 men, 11 women) with a mean age of 67 ± 7 completed the study. The participants were overweight to obese, with a mean body mass index of 31.5 ± 5.3 and waist circumference of 103 ± 12 cm, moderately insulin resistant, with baseline HOMA-IR of 4.08 ± 2.7 and HbA1c was 6.1% ± 0.4%. Mean fasting glucose and 2-hour plasma glucose were 111 ± 14 and 173 ± 36 mg/dL, respectively. Hypertension (antihypertensive treatment or clinic blood pressure of >140/90) was present in 16/30, statin treatment in 7/30, and aspirin therapy in 8/30. Baseline characteristics of the participants (n = 8) who dropped out prior to completion of the study did not differ significantly from these who completed (Supplementary Table 1).
Metabolic Parameters
Cardiometabolic variables are shown in Table 1. There were no significant differences in metabolic parameters observed during the resveratrol or placebo treatment periods. Fasting plasma glucose (111 ± 13 vs 113 ± 13 mg/dL, p = .28), 3-hour glucose AUC (508 ± 88 vs 513 ± 80, p = .73), and 3-hour insulin AUC (330 ± 156 vs 366 ± 228, p = .47) were observed during resveratrol and placebo treatment periods, respectively. Insulin sensitivity assessed by the Matsuda index was unchanged (2.3 ± 1.7 vs 2.0 ± 0.99, p = .46). Insulin secretion assessed by C-peptide deconvolution was slightly reduced (8.9 ± 8 vs 8.1 ± 3 pmol/kg/min, p = .03) after resveratrol treatment, but β-cell function assessed by the disposition index (1.65 ± 0.8 vs 1.53 ± 0.7 mM−1, p = .45) showed no difference between resveratrol or placebo treatment. Weight, blood pressure, HbA1c, low-density lipid cholesterol, hs-CRP, Lp-PLA2, and adiponectin were similarly unchanged.
Table 1.
Cardiometabolic Variables During Standard Meal Challenge Test; Placebo Versus Resveratrol (n = 30)
| Variable | Resveratrol | Placebo | p |
|---|---|---|---|
| Age, y | 67 (7) | — | |
| Male gender, n (%) | 19 (63) | — | |
| BMI (kg/m2) | 32 (5) | — | |
| Fasting plasma glucose (mg/dL) | 111 (12) | 113 (13) | .28 |
| Glucose AUC0-180 | 508 (88) | 513 (80) | .73 |
| Peak postmeal glucose (mg/dL) | 200 (34) | 202 (34) | .84 |
| HbA1c (%) | 6.4 (0.4) | 6.3 (0.4) | .15 |
| Fasting insulin (µU/mL) | 17.0 (6.6) | 17.5 (7.4) | .54 |
| Insulin AUC0-180 | 330 (156) | 366 (228) | .47 |
| HOMA-IR | 4.8 (1.9) | 5.1 (2.3) | .40 |
| Matsuda index | 2.3 (1.7) | 2.0 (1.0) | .46 |
| Insulin secretion (pmol/kg/min) | 8.9 (3.0) | 8.1 (2.5) | .03 |
| Disposition index (mM−1) | 1.65 (0.8) | 1.53 (0.7) | .45 |
| Weight (kg) | 90.3 (17.6) | 89.7 (17.2) | .13 |
| Percent body fat (BIA) | 29.2 (10.6) | 28.7 (11.2) | .87 |
| Systolic blood pressure (mmHg) | 132 (11) | 130 (13) | .38 |
| Diastolic blood pressure (mmHg) | 79 (8) | 78 (7) | .19 |
| HDL cholesterol (mg/dL) | 44 (11) | 43 (10) | .71 |
| LDL cholesterol (mg/dL) | 116 (40) | 109 (35) | .21 |
| Triglycerides (mg/dL) | 157 (118) | 125 (50) | .08 |
| hs-CRP (mg/L) | 2.4 (2.1) | 2.6 (2.3) | .60 |
| Adiponectin (µg/mL) | 10.1 (6.8) | 11.4 (10.3) | .20 |
| Lp-PLA2 (ng/mL) | 185.0 (65.2) | 177.5 (58.7) | .36 |
Note: AUC = area under the curve; BIA = bioimpedance analysis; BMI = body mass index; HDL = high-density lipid; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipid; Lp-PLA2 = lipoprotein phospholipase A2. Results are mean (SD).
Endothelial Function
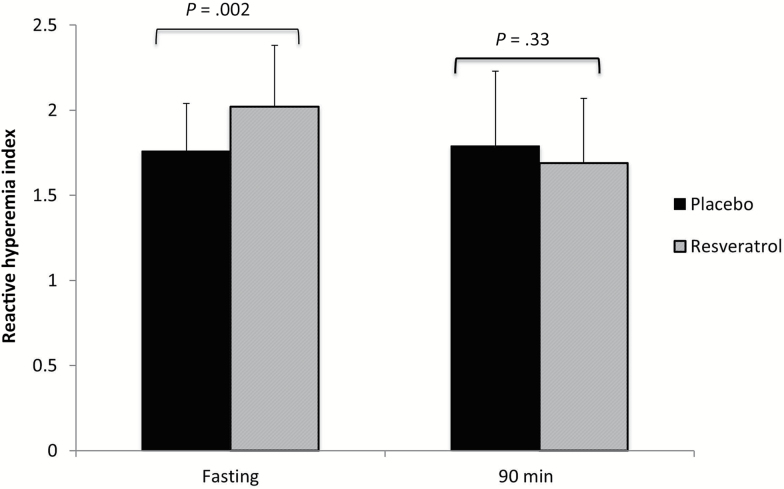
Fasting RHI was significantly better following resveratrol treatment (2.02 ± 0.2 vs 1.76 ± 0.02, for resveratrol and placebo, respectively, p = .002; Figure 1). No difference in postprandial RHI was seen following resveratrol treatment as compared to placebo (1.79 ± 0.4 vs 1.69 ± 0.38, p = .38). Augmentation index did not differ between resveratrol and placebo treatment periods, in either the fasting (16.1 ± 15.4 vs 17.3 ± 13.4, p = .9) or postmeal conditions (6.2 ± 9.5 vs 5.7 ± 8.9, p = .8).
Figure 1.
Reactive hyperemia index (RHI) in resveratrol versus placebo treatment periods. RHI is the ratio of posthyperemia pulse amplitude divided by baseline amplitude (see Methods for details).
Resveratrol Levels
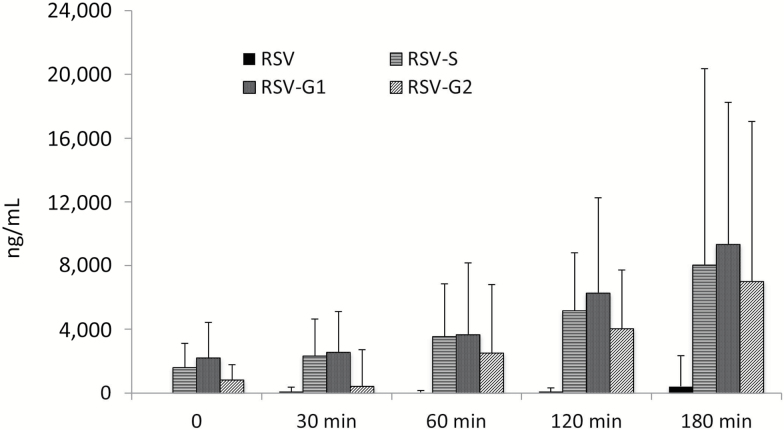
Levels of resveratrol and metabolites were measured at 0, 30, 60, 120, and 180 minutes during the SMMT in the resveratrol treatment period (Figure 2). No unmodified resveratrol was detectable at Time 0, but became detectable at low levels at subsequent time points. In contrast, levels of resveratrol metabolites (resveratrol-3-O-sulfate, resveratrol-3-O-glucuronide, and resveratrol-4′-O-glucuronide) were detectable at Time 0 and increased substantially over the 180 minutes following resveratrol administration. As a control, resveratrol and metabolites were measured in samples obtained from three randomly selected participants during a placebo period SMMT and none was detected.
Figure 2.
Levels of RSV and its metabolites at 0, 30, 60, 120, and 180 minutes during the SMMT in the resveratrol treatment period. RSV = resveratrol; RSV-G1 = resveratrol glucuronide 1; RSV-G2 = resveratrol glucuronide 2; RSV-S = resveratrol sulfate; SMMT = standard mixed-meal test.
Mitochondrial Function
RNA extracted from skeletal muscle samples underwent deep transcriptomic study using RNA-Seq and yielded 140 differentially expressed transcripts (corrected p-value ≤ .05), with the dominant ones being associated with mitochondrial genes and noncoding RNA (Supplementary Table 2). Ingenuity Pathway Analysis confirmed that mitochondrial dysfunction (p = 2.77 × 10−12) and oxidative phosphorylation (p = 1.41 × 10−11) were the most significantly perturbed (downregulated) pathways following resveratrol treatment (Table 2). The significantly differentially expressed genes included ENDOG, IGFBP7, and QDPR, all of which have been previously implicated in modulating mitochondrial function in aging/longevity contexts (24–26). Pathways involving immune function (regulation of IL-2 expression and IL-1 signaling) were also significantly affected by resveratrol. EM performed in five pairs (resveratrol and placebo) of muscle samples showed mitochondrial number was increased with resveratrol treatment in all cases, but mitochondrial area and morphology did not change (Supplementary Figure 1).
Table 2.
Predominant Canonical Pathways Identified by Ingenuity Pathways Analysis of Skeletal Muscle Gene Expression
| Canonical Pathway | p Value | Ratio | Molecules |
|---|---|---|---|
| Mitochondrial dysfunction | 2.5 × 10−12 | 12/71 | MT-CO1, MT-ATP6, MT-ND5, MT-ND6, NDUFS7, MT-CYB, MT- ND4, MT-CO3, MAPK9, SDHC, MT-Co2, MT-ND3 |
| Oxidative phosphorylation | 1.6 × 10−11 | 10/109 | MT-Co1, MT-ATP6, MT-ND5, NDUFS7, MT-CYB, MT-ND4, MT- CO3, SDHC, MT-CO2, MT-ND3 |
| Regulation of IL-2 expression in activated and anergic T lymphocytes | 3.7 × 10−3 | 3/79 | RAC1, MAPK9, TOB1 |
| IL-1 signaling | 5.5 × 10−3 | 3/91 | TAB2, MAPK9, GNG7 |
| SAPK/JNK signaling | 6.0 × 10−3 | 3/94 | RAC1, MAPK9, GNG7 |
Safety and Adherence
The first nine participants enrolled in the study were treated with 3 g of resveratrol daily. Three of these participants experienced severe gastrointestinal symptoms, one requiring hospitalization (Supplementary Table 3). Subsequently, the resveratrol dose was lowered to 2 g/d for remaining participants, and no further gastrointestinal symptoms were reported. There were no other serious adverse events or changes in laboratory safety parameters.
Adherence to study drug (assessed by pill count) was excellent, with 94% and 92% of expected drug consumed during the resveratrol and placebo periods, respectively.
Discussion
In this cohort of older adults with impaired glucose tolerance, we found no evidence of resveratrol effect on weight, carbohydrate tolerance, insulin sensitivity, or β-cell function. Beneficial effects were seen on endothelial function, although other cardiovascular risk factors (eg, lipids, blood pressure, hs-CRP) were largely unaffected. Importantly, we show evidence that resveratrol may increase mitochondrial number and modulate pathways involved in oxidative phosphorylation and inflammation in humans.
Studies in animal models have demonstrated positive effects of resveratrol on glucose metabolism, including enhanced insulin sensitivity and improvements in insulin secretion and glucose tolerance (4,5). Human studies have mostly been small and of short duration, used widely varying resveratrol doses and have shown mixed results (27). The largest study (n = 214) of resveratrol in patients with established diabetes reported improvement in fasting and postchallenge glucose with doses up to 5 g/d, but no change in HbA1c or insulin levels, making the results difficult to interpret (28). Among the more rigorous studies, Timmers and colleagues demonstrated improved metabolic profile (lower HOMA, triglyceride, and leptin levels), reduced intrahepatic lipid content, and improved mitochondrial function in skeletal muscle in obese men using 150 mg/d of resveratrol (9). Subsequently, exhaustive studies in healthy obese men (1,500 mg/d) and healthy nonobese postmenopausal women (75 mg/d) failed to demonstrate any evidence of resveratrol effects on insulin sensitivity, body composition, or energy expenditure (8,10). It has been suggested that resveratrol’s effect may be limited to individuals with metabolic compromise (29), as is the case in rodent models (30). Our cohort, selected to have obesity, insulin resistance, and impaired glucose tolerance would appear ideal to test resveratrol’s metabolic effects, while avoiding the potential confounding of established diabetes and its treatment. Our failure, then, to demonstrate any detectable effect on glucose metabolism suggests other study design issues—for example, dose or duration of treatment, may be critical. Some evidence suggests that high-dose resveratrol (as used in our study) may act via a SIRT1-independent pathway and could actually impair insulin action via excess activation of PGC1α (31). Possibly, longer treatment with lower dose resveratrol may be required to modulate metabolism, as was the case in the rodent studies (30). Furthermore, although the Matsuda index appears to have better predictive value as a surrogate index of insulin sensitivity relative to HOMA-IR, both measures are recognized to have limitations as longitudinal measures of insulin sensitivity in response to therapeutic interventions (32). However, it may also be the case that resveratrol simply does not have important effects on glucose metabolism in humans.
The rapid metabolism of resveratrol, and thus very low resveratrol levels following oral dosing, has been a conundrum in the field. Unmodified resveratrol was present at very low levels following administration during the SMMT, but metabolites showed a robust increase. Furthermore, resveratrol metabolites were detectable at Time 0, prior to supervised dose administration, providing evidence of adherence to chronic dosing during the study. Others have demonstrated that specific resveratrol metabolites retain biological activity in some tissues (33) and that intracellular reservoirs of resveratrol metabolites may undergo deconjugation and result in significant intracellular levels of resveratrol (34). Although “therapeutic” resveratrol levels have not been defined, we feel confident that our participants had substantial resveratrol exposure during the 6-week treatment period, including during the SMMT. Levels of resveratrol and metabolites were not measured in every participant during placebo treatment. However, detectable levels resulting from dietary intake—estimated to rarely exceed 6–8 mg/d (35)—appear unlikely.
Resveratrol treatment has been reported to improve a variety of vascular biomarkers, including expression of adhesion molecules (VCAM, ICAM) and inflammatory cytokines (IL-6, TNF-α, PAI-1) (7,9,36–38), as well as reduction in oxidative stress (39). Endothelial function, assessed by flow-mediated vasodilation, has been reported to improve following acute (40) and chronic (41) resveratrol dosing of as little as 10 mg. We observed a modest, but significant improvement in RHI (a nitric oxide–dependent phenomenon) (42) in response to chronic resveratrol treatment. This is consistent with resveratrol’s reported ability to increase activity of endothelial nitric oxide synthase and inhibit the vasoconstrictor, endothelin (43). However, when administered with the standard meal, no additional effects of acute administration were apparent. No effect of resveratrol on augmentation index was demonstrable in our study. However, others have reported that resveratrol improves arterial pulse wave velocity (a measure of arterial stiffness) in nonhuman primates (44), and there is an ongoing study in humans to explore this (45).
The gene expression studies showed evidence for a pronounced perturbation of mitochondrial activity in the muscle samples taken with resveratrol versus placebo. The most notably perturbed pathways identified by Ingenuity Pathway Analysis are defined as “mitochondrial dysfunction” and “oxidative phosphorylation,” with the majority of mitochondrial transcripts involved in both pathways being downregulated. Furthermore, the relevant transcripts are all associated with the functioning of the four mt-DNA encoded mitochondrial complexes (I, III, IV, and V) involved in the electron transport chain. Interestingly, metformin—an antidiabetic drug known to target a number of aging-related mechanisms—is shown to inhibit mitochondrial complex I and to influence reactive oxygen species production and mitochondrial bioenergetics, suggesting some similarity to the action of resveratrol (46,47). Downregulation of genes associated with mitochondrial bioenergetics has been previously reported in caloric restriction studies in rhesus monkeys (48), where it was suggested that the caloric restriction group was effectively in a hypometabolic state associated with reduced activity of the mitochondrial electron transport system. Analysis of adenosine triphosphate production and mitochondrial energy metabolism in resveratrol-treated participants can provide further insights into the role of resveratrol on mitochondrial activity and its role in targeting aging. Pathways involved in immune regulation were also perturbed with resveratrol, although two circulating inflammatory markers, hs-CRP and Lp-PLA2, were unchanged.
EM, performed in a subset of samples, demonstrated an increase in mitochondrial number, but not size. Resveratrol is known to increase the activity of PGC1α (4), an important regulator of mitochondrial mass and function. PGC1α’s stimulation of mitochondrial number appears to be mediated by ENDOG (which encodes the mitochondrial enzyme endonuclease G), the expression of which was increased in our participants following resveratrol treatment. Furthermore, because ENDOG expression has been reported to decline with aging (25) and is stimulated by resveratrol, this could provide a potential mechanism for some of resveratrol’s putative antiaging effects. The pathway analysis of our samples showing perturbation of pathways involved in mitochondrial dysfunction and oxidative phosphorylation provides indirect support for the significance of the change in mitochondrial number. Expression of several mitochondrial genes was downregulated with resveratrol (shown in Supplemental Table 2), but the specific functional consequences of these changes and the relationship to changes in mitochondrial number cannot be directly addressed from our data.
Formal safety testing of nutritional supplements such as resveratrol is generally not required by regulatory agencies, including the U.S. Food and Drug Administration, so safety data are limited. The gastrointestinal side effects of resveratrol observed in our study have been reported previously (49) and appear to be dose related. Although widely used and presumed safe, the potential adverse effects of long-term pharmacologic doses of resveratrol have not been studied.
Conclusions
Resveratrol treatment of older adults with impaired glucose regulation may have beneficial effects on vascular function, but not glucose metabolism or insulin sensitivity. Changes in gene expression suggest effects similar to those observed with caloric restriction, which has been shown to increase life and health span in many animal models; its significance for humans is uncertain. Future human studies with resveratrol will need to carefully address the appropriate dose range, low bioavailability, and side effect profile.
Supplementary Material
Supplementary data is available at The Journals of Gerontology Series A: Biological Sciences and Medical Sciences online.
Funding
This study was supported by the American Diabetes Association (1-11-CT-12), the Glenn Foundation for Medical Research, the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number UL1TR001073, the Einstein-Sinai Diabetes Research Center (NIH-5P60 DK20541, RO1 AG028730 to D.A.S.), and the Intramural Research Program at the National Institute on Aging (R.M.).
Supplementary Material
Acknowledgments
The authors thank the staff of the Einstein Clinical Research Center, Dr. Hillel Cohen for assistance with study design, K.S.S. Doussou for analysis of plasma resveratrol levels, and the study volunteers.
References
- 1. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi:10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 2. Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi:10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 3. Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi:10.1038/nrd3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi:10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi:10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 6. Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–541. doi:10.1016/j.nutres.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 7. Ghanim H, Sia CL, Abuaysheh S, et al. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi:10.1210/jc.2010-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi:10.2337/db12-0975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi:10.1016/j.cmet.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshino J, Conte C, Fontana L, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi:10.1016/j.cmet.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crandall JP, Oram V, Trandafirescu G, et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–1312. doi:10.1093/gerona/glr235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chi TC, Chen WP, Chi TL, et al. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007;80:1713–1720. doi:10.1016/j.lfs.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 13. Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi:10.1093/oxfordjournals.aje.a010187 [DOI] [PubMed] [Google Scholar]
- 14. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi:10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 15. Aloulou I, Brun JF, Mercier J. Evaluation of insulin sensitivity and glucose effectiveness during a standardized breakfast test: comparison with the minimal model analysis of an intravenous glucose tolerance test. Metabolism. 2006;55:676–690. doi:10.1016/j.metabol.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 16. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. doi:10.2337/diab.41.3.368 [DOI] [PubMed] [Google Scholar]
- 17. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi:10.2337/dc08-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiori JL, Shin YK, Kim W, et al. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi:10.2337/db13-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi:10.1016/j.jacc.2004.08.062 [DOI] [PubMed] [Google Scholar]
- 20. Wu JD, Xu XH, Zhu J, et al. Effect of exenatide on inflammatory and oxidative stress markers in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2011;13:143–148. doi:10.1089/dia.2010.0048 [DOI] [PubMed] [Google Scholar]
- 21. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi:10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber W, Carey VJ, Gentleman R, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–121. doi:10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Data Were Analyzed Through the Use of QIAGEN’s Ingenuity Pathway Analysis Redwood City, CA: IPA, QIAGEN; http://www.qiagen.com/ingenuity Accessed March 3, 2017. [Google Scholar]
- 24. Alic N, Hoddinott MP, Vinti G, Partridge L. Lifespan extension by increased expression of the Drosophila homologue of the IGFBP7 tumour suppressor. Aging Cell. 2011;10:137–147. doi:10.1111/j.1474-9726.2010.00653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerber RA, O’Brien E, Cawthon RM. Gene expression profiles associated with aging and mortality in humans. Aging Cell. 2009;8:239–250. doi:10.1111/j.1474-9726.2009.00467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su J, Ekman C, Oskolkov N, et al. A novel atlas of gene expression in human skeletal muscle reveals molecular changes associated with aging. Skelet Muscle. 2015;5:35. doi:10.1186/s13395-015-0059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pollack RM, Crandall JP. Resveratrol: therapeutic potential for improving cardiometabolic health. Am J Hypertens. 2013;26:1260–1268. doi:10.1093/ajh/hpt165 [DOI] [PubMed] [Google Scholar]
- 28. Elliot PJ, Walpote SM, Morelli L, et al. Resveratrol/SRT501. Drugs Fut. 2009;34:291–295. doi:10.1358/dof.2009.034.04.1360696 [Google Scholar]
- 29. Crandall JP, Barzilai N. Exploring the promise of resveratrol: where do we go from here? Diabetes. 2013;62:1022–1023. doi:10.2337/db12-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turrens JF, Lariccia J, Nair MG. Resveratrol has no effect on lipoprotein profile and does not prevent peroxidation of serum lipids in normal rats. Free Radic Res. 1997;27:557–562. doi:10.3109/10715769709097859 [DOI] [PubMed] [Google Scholar]
- 31. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi:10.1016/j.cmet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiang AH, Watanabe RM, Buchanan TA. HOMA and Matsuda indices of insulin sensitivity: poor correlation with minimal model-based estimates of insulin sensitivity in longitudinal settings. Diabetologia. 2014;57:334–338. doi:10.1007/s00125-013-3121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruotolo R, Calani L, Fietta E, et al. Anti-estrogenic activity of a human resveratrol metabolite. Nutr Metab Cardiovasc Dis. 2013;23:1086–1092. doi:10.1016/j.numecd.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 34. Andreadi C, Britton RG, Patel KR, Brown K. Resveratrol-sulfates provide an intracellular reservoir for generation of parent resveratrol, which induces autophagy in cancer cells. Autophagy. 2014;10:524–525. doi:10.4161/auto.27593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, Martin JH. Resveratrol – pills to replace a healthy diet? Br J Clin Pharmacol. 2011;72:27–38. doi:10.1111/j.1365-2125.2011.03966.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97:198–207. doi:10.1210/jc.2011-1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tome-Carneiro J, Gonzalvez M, Larrosa M, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol Nutr Food Res. 2012;56:810–821. doi:10.1002/mnfr.201100673 [DOI] [PubMed] [Google Scholar]
- 38. Tomé-Carneiro J, Larrosa M, Yáñez-Gascón MJ, et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol Res. 2013;72:69–82. doi:10.1016/j.phrs.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 39. De Groote D, Van Belleghem K, Devière J, Van Brussel W, Mukaneza A, Amininejad L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab. 2012;61:15–24. doi:10.1159/000338634 [DOI] [PubMed] [Google Scholar]
- 40. Wong RH, Howe PR, Buckley JD, Coates AM, Kunz I, Berry NM. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–856. doi:10.1016/j.numecd.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 41. Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–1827. doi:10.1097/HJH.0b013e328362b9d6 [DOI] [PubMed] [Google Scholar]
- 42. Dakak N, Husain S, Mulcahy D, et al. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension. 1998;32:9–15. doi:10.1161/01.HYP.32.1.9 [DOI] [PubMed] [Google Scholar]
- 43. Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi:10.1016/j.bcp.2008.11.027 [DOI] [PubMed] [Google Scholar]
- 44. Mattison JA, Wang M, Bernier M, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi:10.1016/j.cmet.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. National Institute of Aging (NIA). Resveratrol and Cardiovascular Health in the Elderly Bethesda, MD: National Library of Medicine; https:// clinicaltrials.gov/ct2/show/NCT01842399 Cited May 11, 2016. Accessed March 3, 2017. [Google Scholar]
- 46. Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–487. doi:10.1042/BJ2014062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Algire C, Moiseeva O, Deschênes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). 2012;5:536–543. doi:10.1158/1940-6207.CAPR-11-0536 [DOI] [PubMed] [Google Scholar]
- 48. Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci USA. 2001;98:5093–5098. doi:10.1073/pnas.081061898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi:10.1158/0008-5472.CAN-10-2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.