Abstract
Background
Evidence highlights the importance of muscular strength as a protective factor for health and function across aging populations. The purpose of this study was to examine the extent to which low normalized grip strength (NGS) serves as a biomarker for both cardiometabolic disease and physical disability in U.S. and Chinese adults.
Methods
Middle aged and older adults from the U.S. National Health and Nutrition Examination Survey 2011–2012 and 2013–2014 combined surveys (n = 4,544), and the 2011 wave of the China Health and Retirement Longitudinal Study (n = 6,030) were included. Strength was assessed using a handgrip dynamometer, and was normalized to body mass. Weighted logistic regression models were used to assess the association between NGS and diabetes, hyperglycemia, hypertriglyceridemia, low HDL-cholesterol, hypertension, and physical disability status, while controlling for age, sex, and sociodemographic characteristics.
Results
Every 0.05 lower NGS was independently associated with a 1.49 (95% confidence interval [CI]: 1.42–1.56) and 1.17 (95% CI: 1.11–1.23) odds for diabetes; a 1.46 (95% CI: 1.39–1.53) and 1.11 (95% CI: 1.07–1.15) odds of hyperglycemia; a 1.15 (95% CI: 1.07–1.25) and 1.11 (95% CI: 1.08–1.14) odds of hypertriglyceridemia; a 1.22 (95% CI: 1.17–1.27) and 1.15 (95% CI: 1.12–1.18) odds of low HDL-cholesterol; a 1.19 (95% CI: 1.14–1.24) and 1.10 (95% CI: 1.07–1.14) odds of hypertension; and a 1.36 (95% CI: 1.29–1.42) and 1.10 (95% CI: 1.05–1.15) odds for physical disability status in U.S. and Chinese adults, respectively.
Conclusions
NGS was robustly associated with both cardiometabolic disease risk and physical disabilities in U.S. and Chinese aging adults.
Keywords: Disability, Diabetes, Muscle weakness, Aging, Epidemiology
Diabetes-related healthcare expenditures are highest in the United States and China, where in 2015, costs exceeded a combined 320 billion International Dollars (1). There is also a high prevalence of pre-diabetes, undiagnosed diabetes, and poor cardiovascular health in both countries and the burden tends to be elevated among mid-life (50–64 years) and older (≥65 years) adults (2,3). The expansion of the aging population combined with decreasing mortality has led to a diversification of cardiometabolic disease morbidity, including increased prevalence of aging-related mobility impairments and a substantial reduction in the number of nondisabled years in both countries (4–6). Despite fundamental differences in some cardiometabolic risk factors in both countries, such as higher smoking prevalence in China (7) and higher obesity prevalence in the United States (8), many shared lifestyle factors contribute to an elevated risk in the older adult populations. However, recent evidence has suggested a compression of morbidity and disability among older adults with diabetes in the United States, which may be a result of improved clinical management and public health advocacy regarding healthy lifestyles (9). Thus, early screening and continued health promotion efforts for healthy aging are vital to reduce the escalating burden associated with the multimorbidity of diabetes and disability.
Of relevance to both, there is a growing body of evidence linking muscular weakness, as determined by low grip strength, and a host of negative health outcomes including diabetes (10), disability (11), and early mortality (12–18). Given these links, grip strength has been labeled a “biomarker of ageing” (19); and yet, this limited interpretation precludes any potential role that weakness plays in a non-age-dependent context. Moreover, and despite the substantial covariance between body mass and strength capacity, few studies have considered low grip strength as a metric in proportion to body mass. We and others have recently shown an independent, inverse association between normalized grip strength (NGS) and cardiometabolic health among U.S. adults of all ages (20,21). What remains to be determined is if low NGS can serve as a biomarker for both cardiometabolic diseases and physical disabilities in an internationally-diverse population of aging adults. Given the rapid expansion of the aging and diabetic populations in both United States and China, the purpose of this study was to harmonize population-representative data and examine the association between NGS and diabetes, physical disabilities, and other cardiometabolic disease risk factors in nationally-representative samples of adults from both countries.
Research Design and Methods
Study Populations
The National Health and Nutrition Examination Surveys (NHANES) (https://www.cdc.gov/nchs/nhanes/index.htm) represent population-representative samples and program of studies designed to assess the health and nutritional status of adults and children in the United States. The NHANES 2011–2012 and 2013–2014 surveys were chosen based on information pertaining to health and measures of muscle strength capacity. The survey is unique in that it combines interviews and physical examinations; however, it does not include all the participants in the physical examination. Of the 5,267 participants who were 50 years and older, 4,544 had complete (1) demographic and anthropometric data (2); valid strength data from a handgrip dynamometer (3); the necessary blood samples obtained for non-fasting glycated hemoglobin determination; and (5) valid questionnaire data pertaining to impairments of functional limitation related to mobility. A subsample of 2,225 adults also had fasting measures for glucose, insulin and triglycerides. Ethical approval was obtained through the National Center for Health Statistics (NCHS) Research Ethics Review Board, and subsequent approval for secondary data analyses was not required.
A sample was also included from the China Health and Retirement Longitudinal Study (CHARLS), a nationally-representative survey of the middle-aged and elderly population (45 years old and above) in China. The CHARLS baseline national survey was fielded between June 2011 and March 2012. This is a multi-stage, stratified, random sample drawn at the county, neighborhood, and household levels (22). A total of 450 villages and urban communities from 28 provinces were selected so that the survey would represent a mix of urban and rural settings, and included a total of 17,708 participants. The CHARLS is based on the U.S. Health and Retirement Study (HRS) (http://hrsonline.isr.umich.edu/), and detailed information about the sampling procedure and data quality management can be found at http://charls.ccer.edu.cn/en. Of the 13,833 participants who were 50 years and older, 6,030 had complete data (demographic and anthropometric data; valid strength data from a handgrip dynamometer; fasting blood samples; and valid questionnaire data pertaining to self-reported diabetes, hypertension, and activities of daily living [ADLs]).
Demographic and Anthropometric Factors
NHANES
Sociodemographic characteristics were all assessed by self-report during the in-home interview. Race/ethnicity was categorized as: non-Hispanic white, non-Hispanic black, Mexican American or other Hispanic, or Other-including multi-racial. Education was categorized as: less than high school graduate, high school graduate/general educational development or equivalent, and/or some college or Associate’s degree, or college graduate or above. Marital status was dichotomized as married or not married. Weight (kg) was measured using a digital Toledo scale (Mettler-Toledo International, Inc., Columbus, OH). Height (m) was measured using a fixed stadiometer. Waist circumference was measured to the nearest 0.1 cm at the level of the iliac crest. Standard cut points for abdominal obesity in men (>102 cm) and women (>88 cm) were used.
CHARLS
Education was categorized as: illiterate, literate (had some informal education, able to read/write), primary education, middle school education, high school education and above. Marital status was dichotomized as married or not married. Per capita expenditures were used as measure of household resources. We used the log of per capita expenditures, since it was closer to a normal distribution, which was then discretized into tertiles using dummy variables to capture expected nonlinearities. Body weight and height were measured in light indoor clothes using a health meter (HN-286; Omron, Kyoto, Japan) and a stadiometer (Seca 213; Seca, Hamburg, Germany). Abdominal obesity was defined as waist circumference ≥90 cm in men and ≥80 cm in women.
For both NHANES and CHARLS, age was categorized as middle-age (50–64.9 years) and older (≥65 years), and used as a continuous variable in regression models. Body Mass Index was calculated as weight divided by height in meters squared (kg/m2). Individuals with Body Mass Index < 18.5 kg/m2 were excluded, due to the known association between underweight status and diabetes (23). Standard categories were applied to determine if each participant was normal weight (18.5–24.9), overweight (25–29.9), or obese (≥30) for U.S. adults and normal weight (18.5–23.9), overweight (24–27.9), or obese (≥28) for Chinese adults (24).
Cardiometabolic Parameters
NHANES
Resting systolic and diastolic blood pressures were measured three to four times with a mercury sphygmomanometer by trained staff. Self-reported data on diabetes was obtained by the question, “Have you ever been told by a doctor or other health professional that you had diabetes?” Non-fasting serum measures of glycated hemoglobin (HbA1c) were included as a diagnostic test for diabetes. Participants with diabetes that were being treated with only insulin alone were excluded, as they were considered likely to have type 1 diabetes. Self-reported data on hypertension was obtained by the question, “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” For a subset of individuals, morning fasting measures were also obtained for plasma glucose, insulin, and triglycerides.
CHARLS
Self-reported data on hypertension was obtained by the question, “Have you been diagnosed with hypertension by a doctor?” As part of the physical examination, blood pressure was measured three times (~45 seconds apart) in a single occasion, using an electronic monitor (Omron model HEM-7112). Self-reported information on diabetes was obtained by the question, “Have you been diagnosed with diabetes by a doctor?” Blood samples provided estimates of HbA1c which were obtained using a boronate affinity high-performance liquid chromatography assay. Bioassays were performed at the Youanmen Center for Clinical Laboratory of Capital Medical University, including total cholesterol, HDL, and triglycerides (25).
For both NHANES and CHARLS, The diagnostic criterion for diabetes was defined as self-reported diabetes or HbA1c values ≥6.5% (≥48 mmol/mol) (26). Moreover, hypertension was defined as self-reported history of hypertension, or a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg. Hypertriglyceridemia was determined as ≥150 mg/dL, elevated fasting glucose was determined as ≥126 mg/dL, and low high-density lipoprotein (HDL)-cholesterol was determined as <40 mg/dL and <50 mg/dL for men and women, respectively.
Physical Disability Status
NHANES
The Physical Functioning Questionnaire (PFQ) was designed as a way of assessing level of disability, as self-reported difficulty performing specific, everyday tasks. The following PFQ items were incorporated to reflect specific factors pertaining to lower-extremity mobility, strength and dynamic balance: “By yourself and without using any special equipment, how much difficulty do you have: (PFQ061B) walking for a quarter of a mile (that is about two or three blocks)?; (PFQ061C) walking up ten steps without resting?; (PFQ061D) stooping, crouching or kneeling?; (PFQ061E) lifting or carrying something as heavy as ten pounds (like a sack of potatoes or rice)?; (PFQ061F) doing chores around the house (like vacuuming, sweeping, dusting, or straightening up)? (PFQ061H) walking from one room to another on the same level?; (PFQ061I) standing up from an armless straight chair? Response options for each task include: “no difficulty,” “some difficulty,” “much difficulty,” “unable to do,” “do not do this activity,” “refused” and “don’t know.” Subjects were classified on the basis of functional disability, defined as ≥1 tasks with any difficulty. We assigned missing values for any subject that answered “do not do this activity,” “refused” and “don’t know.”
CHARLS
Subjects were asked to identify whether they had difficulty in performing the following ADLs on their own: dressing, feeding, using the toilet, bathing, getting into or out of bed, controlling urination, and defecation. Response options for each task included: “no difficulty,” “have difficulty but can still do it,” “have difficulty and need help,” and “cannot do it.” Physical disability was defined as ≥1 ADL tasks with any difficulty.
Normalized Grip Strength
NHANES
Grip strength was assessed using a hydraulic handgrip dynamometer (Takei Digital Grip Strength Dynamometer, Model T.K.K.5401). Detailed descriptions of the protocol are provided in the Muscle Strength/Grip Test Procedure Manual (27). Each hand was tested three times, alternating hands between trials with a 60-second rest between measurements on the same hand. The grip test was performed in the standing position unless the participant was physically limited. Participants who had surgery on either hand or wrist in the last 3 months were not tested on that particular hand.
CHARLS
Grip strength was assessed using a hydraulic handgrip dynamometer (YuejianTM WL-1000 dynamometer). Each hand was tested two times, alternating hands between trials with a 30-second rest between measurements on the same hand. The grip test was performed in the standing position unless the participant was physically limited. Participants were excluded from this component if they were unable to hold the handgrip dynamometer and perform strength testing with both hands.
For NHANES and CHARLS, grip strength was normalized (NGS) as grip strength per body mass (ie, ) and examined as a continuous predictor with units set to 0.05.
Statistical Analysis
All statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and STATA version 13.
NHANES
Four-year sample weights were used to adjust for oversampling, survey nonresponse, and post-stratification. We took into account subsample weights since the analyses were conducted on persons with both non-fasting and fasting serum measures. To obtain correct variance estimation, information on strata and primary sampling unit (PSU) were utilized. Descriptive characteristics are provided as means, standard errors, and weighted percentages. Differences in these characteristics across age categories and between men and women were tested using linear regression (proc surveyreg) and logistic regression (proc surveylogistic) for continuous and categorical variables respectively, after creating categories and dummy coding for each variable.
CHARLS
All calculations were weighted to represent the overall Chinese adult population. The weights included corrections for household and respondent-level nonresponse based on propensity scores from a logistic regression of the household that was measured or the individual giving blood as recently described (28). Descriptive characteristics are provided as means, standard errors, or weighted percentages. We used t tests to determine the differences in these characteristics across age categories and between men and women.
To assess the odds of diabetes, physical disability, and cardiometabolic disease risk factors, separate weighted, a multivariate logistic regression modeling approach was used. Risk factors and covariates, including sex, age, race (NHANES only), education, marital status, per capita expenditures (CHARLS only) and NGS, were included in all of the adjusted models.
To compare descriptive data between U.S. and Chinese adults, within the equivalent age categories and sexes, analysis of variance (ANOVA) and chi-squared tests were used for continuous and dichotomous variables, respectively. Bonferroni adjustments were made to account for multiple comparisons among the subgroups. Lastly, by pooling the samples from both countries and statistically testing the interactions between grip strength and country dummy variable for each dependent variable, we were able to determine if the marginal effects are different between the United States and China.
Results
Descriptive data across national samples, sexes, and age categories are presented in Table 1. In United States and Chinese adults, and for both men and women, prevalence of physical disabilities were higher among older adults (≥65 years) as compared to middle-aged adults (50–64.9 years) (all p < .001). Physical disability status, obesity and abdominal obesity were more prevalent among U.S. versus Chinese adults for both men and women in both age categories (all p < .001). In the U.S. sample, diabetes prevalence was higher with increasing age for men only, and was 18.6% and 23.5% (p < .01) for ages 50–64.9 years and ≥65 years old, respectively. Among the Chinese, diabetes prevalence was higher for women than men in both age groups, but was not significantly different between middle-aged and older adults. Diabetes prevalence was higher in U.S. than Chinese men in both age categories; however, diabetes prevalence was higher in Chinese versus U.S. women in both age categories (all p < .001).
Table 1.
Descriptive and Health Characteristics of the NHANES and CHARLS Populations for Middle-Aged (50–64.9 years) and Older (≥65 years) Adults
| NHANES: U.S. Population-Representative Sample (n = 5,267) | CHARLS: Chinese Population-Representative Sample (n = 6,566) | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| Age 50–64.9 | Age ≥ 65 | Age 50–64.9 | Age ≥ 65 | Age 50–64.9 | Age ≥ 65 | Age 50–64.9 | Age ≥ 65 | |
| n = 1,374 | n = 1,174 | n = 1,462 | n = 1,257 | n = 2,009 | n = 1,169 | n = 2,288 | n = 1,100 | |
| Age, years | 56.8 (0.17) | 72.7 (0.22)a,b | 56.7 (0.17) | 73.4 (0.17)a,b | 57.3 (0.11)c | 71.8 (0.25)a | 57.2 (0.12) | 72.3 (0.32)a |
| Body Mass Index (BMI), kg/m2 | 29.3 (0.25)b | 28.2 (0.26)b | 30.4 (0.34)a,b,c | 29.2 (0.25)b,c | 23.6 (0.09)a | 23.1 (0.23) | 24.8 (0.23)a,c | 24.1 (0.22)c |
| Obesity (BMI >30 or 28), % | 35.7b,c | 30.8b | 48.7b,c | 40.0b | 13.1 | 10.5 | 17.3c | 13.2c |
| Waist circumference, cm | 105.0 (0.58)b,c | 105.9 (0.74)b,c | 99.9 (0.73)b | 98.4 (0.69)b | 86.4 (0.27) | 86.7 (0.61) | 86.9 (0.24)c | 88.6 (0.44)a,c |
| Abdominal obesity, % | 47.3b | 53.0b,c | 75.7b | 76.9b | 5.6 | 5.7 | 45.7c | 49.8c |
| d,ePhysical disability, % | 25.7b | 31.6b | 48.6a,b,c | 64.6a,b,c | 10.9 | 20.3a | 13.8c | 25.7a,c |
| Grip strength, kg | 45.4 (0.43) | 38.4 (0.41)a | 28.8 (0.18)c | 23.6 (0.21)a,c | 41.1 (0.22)b | 34.0 (0.48)a,b | 27.5 (0.21)b,c | 22.6 (0.31)a,c |
| Normalized grip strength | 0.51 (0.01)b | 0.46 (0.01)a,b | 0.38 (0.01)b,c | 0.34 (0.01)a,b,c | 0.65 (0.00) | 0.57 (0.01)a | 0.48 (0.00)c | 0.42 (0.01)a,c |
| Glycated hemoglobin (HbA1c), % | 5.90 (0.04)b | 6.03 (0.03)a,b | 5.83 (0.03)b | 5.94 (0.03)a,b | 5.27 (0.02) | 5.25 (0.03) | 5.34 (0.02)c | 5.39 (0.04)c |
| fDiabetes, % | 18.6b | 23.5b,c | 16.1 | 17.6 | 16.7 | 15.7 | 19.5b,c | 20.5b,c |
| Glucose, mg/dL | 114.8 (1.60)b,c | 116.6 (2.44)b | 107.8 (2.06) | 112.0 (1.53)a | 111.0 (1.11) | 108.7 (0.98) | 110.5 (0.83)b | 112.7 (1.59)a,c |
| Insulin, μU/mL | 13.5 (1.09) | 13.9 (0.78) | 12.7 (0.56) | 12.4 (0.40) | n/a | n/a | n/a | n/a |
| Triglycerides, mg/dL | 131.1 (4.69) | 121.9 (4.55)b | 124.3 (1.40) | 125.3 (4.12) | 134.7 (3.41)a | 112.3 (3.55) | 139.7 (2.696)b,c | 145.2 (4.44)b,c |
| HDL-cholesterol, mg/dL | 48.4 (0.58)c | 49.8 (0.78)c | 60.3 (0.84) | 59.6 (0.63) | 49.5 (0.44)a | 50.1 (1.05) | 50.1 (0.43)b | 49.2 (1.26)b |
| LDL-cholesterol, md/dL | 114.9 (1.84)a | 99.9 (1.85) | 124.3 (0.63)a,c | 114.1 (1.43)c | 113.9 (1.04) | 110.5 (1.55)b | 123.1 (1.17)c | 122.2 (2.30)b,c |
| Systolic blood pressure, mmHg | 127.4 (0.83)c | 132.1 (0.81)a | 124.4 (0.63) | 135.8 (0.95)a,c | 129.7 (0.57) | 136.2 (0.90)a | 129.7 (1.17) | 139.6 (1.62)a,c |
| Diastolic blood pressure, mmHg | 75.8 (0.36)a,c | 69.6 (0.41) | 73.1 (0.43)a | 69.1 (0.45) | 77.6 (0.35)a,c | 73.8 (0.50)b | 76.0 (0.53)a,b | 74.4 (0.64)b |
Notes: BMI = body mass index; CHARLS = China Health and Retirement Longitudinal Study; HDL = high density lipoprotein; HOMA-Homeostasis Model of Assessment; LDL = low density lipoprotein; NHANES = National Health and Nutrition Examination Surveys; WC = waist circumference.
aSignificant difference between ages 50–64.9 years and ≥65 years within the same sex, and in the same country: Denoted as group with higher risk.
bSignificant difference between United States and China, within the equivalent age category and the same sex: denoted as group with higher risk.
cSignificant difference between men and women in the equivalent age category, and in the same country: denoted as group with higher risk.
dNHANES (United States): ≥1 Self-Reported Limitations from Physical Function Questionnaire (PFQ).
eCHARLS (China): Self-Reported Difficulty with any Activity of Daily Living (ADL).
fSelf-reported diabetes or HbA1c ≥ 6.5%.
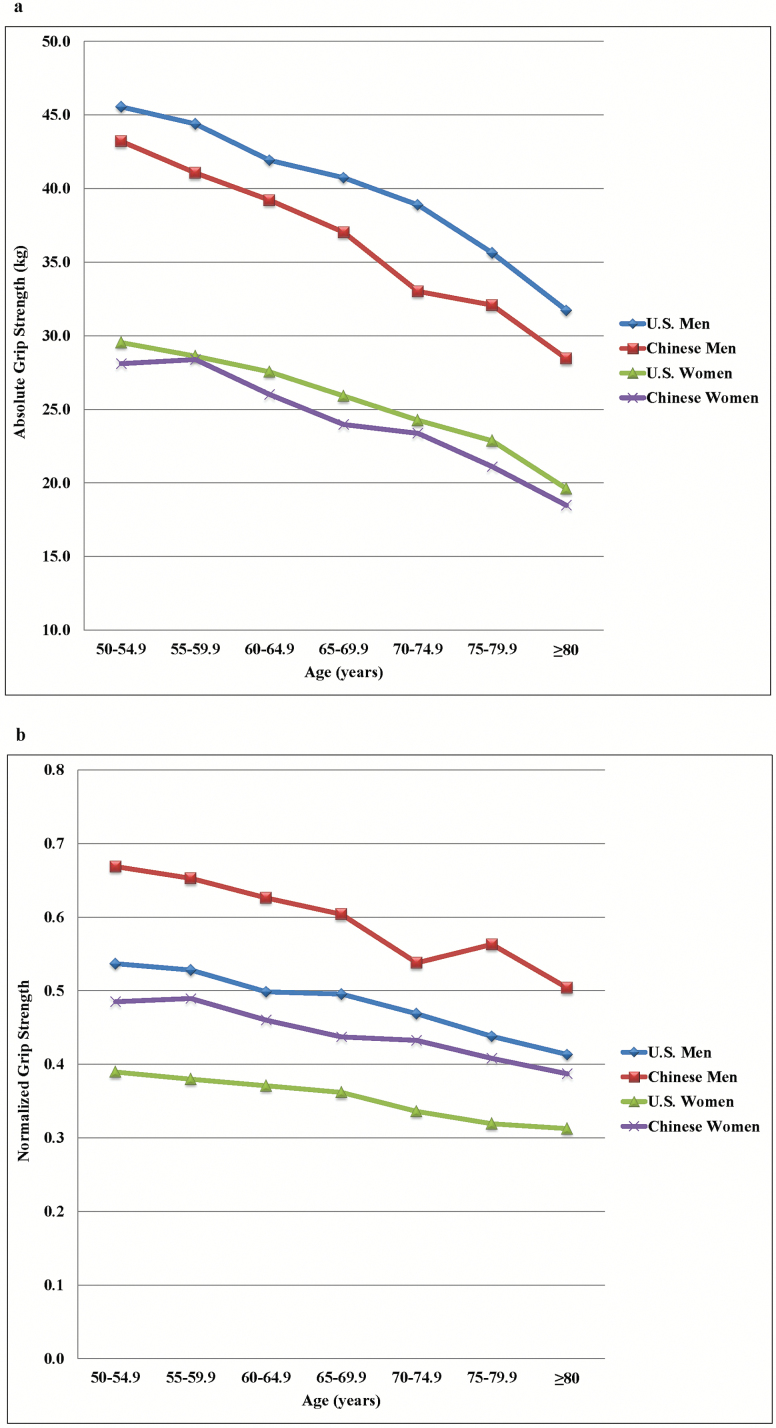
Chinese men and women had higher NGS than U.S. men and women for both age categories (all p < .001) (Figure 1). The marginal effects of NGS for diabetes (β = −0.13; p = .005), high glucose (β = −0.20; p = .002), and physical disability status (β = −0.62; p < .001) were significantly greater for U.S. adults than those for Chinese adults, but there were no significant difference in the marginal effects for hypertriglyceridemia, low HDL-cholesterol, or hypertension between countries.
Figure 1.
Absolute (a) and normalized (b) grip strength for U.S. and Chinese adults.
NHANES
Every 0.05 lower NGS was independently associated with each of the following among U.S. adults: a 1.49 increased odds for diabetes; a 1.46 increased odds of hyperglycemia; a 1.15 increased odds of hypertriglyceridemia; a 1.22 increased odds of low HDL-cholesterol; a 1.19 increased odds of hypertension; and a 1.36 increased odds for disability status (Supplementary Tables 1a–6a).
U.S. women were at lower odds of having: diabetes (odds ratio [OR]: 0.30; 95% confidence interval [CI]: 0.22–0.42), hyperglycemia (OR: 0.25; 95% CI: 0.17–0.37), hypertriglyceridemia (OR: 0.55; 95% CI: 0.41–0.76), low HDL-cholesterol (OR: 0.62; 95% CI: 0.50–0.77), and disability status (OR: 0.71; 95% CI: 0.59–0.85). Age was associated with greater odds of hypertriglyceridemia, hypertension, low HDL-cholesterol, and disability status (all p < .01).
CHARLS
Every 0.05 lower NGS was independently associated with the following among Chinese adults: a 1.17 increased odds for diabetes; a 1.11 increased odds of hyperglycemia; a 1.11 increased odds of hypertriglyceridemia; a 1.15 increased odds of low HDL-cholesterol; a 1.10 increased odds of hypertension; and a 1.10 increased odds for disability status (Supplementary Tables 1b–6b). Chinese women were at higher odds of having hypertriglyceridemia (OR: 1.25; 95% CI: 1.01–1.56) and low HDL-cholesterol (OR: 2.08; 95% CI: 1.71–2.53). Age was associated with greater odds of hypertension and disability (all p < .01).
Table 2 is a summary table for the association between NGS (per each 0.05 unit lower) and cardiometabolic factors and disability status in U.S. and Chinese adults.
Table 2.
Regression Summary Table for the Association Between Normalized Grips Strength (per each 0.05 unit lower) and Cardiometabolic Factors and Disability Status
| NHANES | CHARLS | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio | Lower Limit | Upper Limit | p-Value | Odds Ratio | Lower Limit | Upper Limit | p-Value |
| Diabetes | 1.49 | 1.42 | 1.56 | <.001 | 1.17 | 1.11 | 1.23 | <.001 |
| Hyperglycemia | 1.46 | 1.39 | 1.53 | <.001 | 1.11 | 1.07 | 1.15 | <.001 |
| Hypertriglyceridemia | 1.15 | 1.07 | 1.25 | <.001 | 1.11 | 1.08 | 1.14 | <.001 |
| Low HDL-cholesterol | 1.22 | 1.17 | 1.27 | <.001 | 1.15 | 1.12 | 1.18 | <.001 |
| Hypertension | 1.19 | 1.14 | 1.24 | <.001 | 1.10 | 1.07 | 1.14 | <.001 |
| Physical disability status | 1.36 | 1.29 | 1.36 | <.001 | 1.10 | 1.06 | 1.15 | <.001 |
Note: CHARLS = China Health and Retirement Longitudinal Study; HDL = high density lipoprotein; NHANES = National Health and Nutrition Examination Surveys.
Discussion
The principal finding of this study was that low NGS was robustly associated with both cardiometabolic diseases and physical disabilities in middle-age to older U.S. and Chinese men and women. For every 0.05 decrement in NGS, there was a 49% and 17% increased odds for diabetes; a 46% and 11% increased odds of hyperglycemia; a 15% and 11% increased odds of hypertriglyceridemia; a 22% and 15% increased odds of low HDL-cholesterol; a 19% and 10% increased odds of hypertension; and a 36% and 11% increased odds for disability status in U.S. and Chinese adults, respectively. The results of this study provide further support that individuals with weak grip strength are at increased risk of cardiometabolic abnormalities and physical dysfunction compared to stronger individuals (10,11,17).
We have also uncovered interesting sex disparities between U.S. and Chinese adults. U.S. men had a significantly higher prevalence of diabetes than women for both middle age and older adults, whereas in Chinese adults, women had a higher prevalence of diabetes among both middle-age and older adults. Further, after adjusting for important sociodemographic covariates, U.S. women had lower odds of having diabetes and hyperglycemia, hypertriglyceridemia, and low HDL-cholesterol compared to U.S. men. By contrast, in similarly adjusted models, Chinese women were at higher odds of having diabetes, hyperglycemia, hypertriglyceridemia and low HDL-cholesterol compared to Chinese men. Lastly, Chinese women had a higher prevalence of hypertension than Chinese men.
These findings are consistent with others that have examined the association between sex and other physical health domains in China (29–31). In another CHARLS study, women fared worse than men across both self-report and objective measures of health (32). Women aged 60 years and older were 27% more likely to need help with basic ADLs as compared to 20% of men, and were also disproportionately affected by prevalent hypertension compared to men (32). Health disparities have also been observed with respect to health care access and utilization. In a study that included over 156,000 Chinese adults, men were more likely to seek medical care, have longer duration of hospitalization and spend more on hospitalization compared to women even across the same medical conditions (33). When taken together, these results provide evidence that Chinese women may be vulnerable to a “dual burden” of aging compared to Chinese men, and this could be driven by differential access to power, education and/or medical care (34).
Our results indicate that if hand grip strength is modeled relative to body mass, it can be used as a biomarker for both obesity-related cardiometabolic diseases and age-related functional declines in aging U.S. and Chinese adults. These findings make a compelling case for the adoption of grip strength assessment in the clinical setting, to identify individuals who may be at greatest risk for future negative health outcomes, and that might benefit from lifestyle interventions to reduce risk. The inclusion of grip strength in contemporary primary care settings may be particularly salient, since China is currently overhauling their national healthcare system by aiming to provide “95% of the population with modest but comprehensive health coverage” (see: pg. 1283) (35). There is also growing emphasis of providing preventive services in the United States (36), particularly for older adults (37).
Strengths/Limitations
We were unable to harmonize all variables used in this study across the CHARLS and NHANES datasets. While both studies examined physical disability status as a primary outcome, we assessed physical disability status in NHANES with a PFQ, while CHARLS asked participants about their difficulty with six ADLs. Nevertheless, recent work has shown that both the PFQ and ADLs are adequate measures of assessing overall physical disability status (38), so we expected any differences in measurement error to be non-differential. Second, in order to achieve the greatest concordance across both datasets, we limited our analysis to individuals aged 50 years and older. While this decision was in keeping with the primary focus of our study, we were unable to include younger individuals in this analysis since the CHARLS dataset only includes middle-aged and older Chinese adults. Since disability rates among middle-aged Americans are increasing (39), there is growing interest in the role that strength plays as an early-life predictor of future physical functioning. Lastly, the design of this study was limited by the repeated cross-sectional waves in NHANES, which posed challenges in causal inference, especially with respect to reverse causation. Thus, we are unable to deduce whether low NGS leads to higher odds of cardiometabolic abnormalities and physical dysfunction, or conversely, whether poor cardiometabolic profiles and/or physical limitations lead to declines in muscle strength. Future longitudinal studies are needed to better understand how declines in muscle strength contribute to unhealthy aging.
Despite these limitations, this is the first study to harmonize and examine the relationships between NGS, physical disability, and cardiometabolic health in large, nationally-representative sample of adults in the United States and China. These findings can be generalized to U.S. and Chinese adult men and women aged 50 years and older, which is particularly important since aging individuals represent the fastest growing segments of the population in both countries (40,41). Further, our study examined the role of grip strength, which is a cost-effective and reliable proxy for total body muscle strength, that can be easily administered in any clinical setting (42). Lastly, the results of this study extend our previous work that used grip strength screening as a noninvasive diagnostic tool in identifying those most at risk for future cardiometabolic impairment or changes in their physical disability status (10).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the Behavioral and Social Research division of the National Institute on Aging of the National Institute of Health (grants R01AG037031 and R03AG049144), the Natural Science Foundation of China (grants 71130002, 71450001, 71273237 and 71603013), China Medical Board and Peking University.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support from Global REACH at the University of Michigan Medical School.
References
- 1. Grp IDA. IDF Diabetes Atlas: 7th Edition. Brussels, Belgium: International Diabetes Federation; 2015:144. [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. [DOI] [PubMed] [Google Scholar]
- 3. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi:10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 4. Bardenheier B, Lin J, Zhuo X, et al. Disability-free life-years lost among adults aged ≥50 years, with and without diabetes. Diabetes Care. 2016;39:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:106–114. [DOI] [PubMed] [Google Scholar]
- 6. Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016;4:537–547. doi:10.1016/S2213-8587(16)30010-9. [DOI] [PubMed] [Google Scholar]
- 7. Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–679. [DOI] [PubMed] [Google Scholar]
- 8. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi:10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 9. Bardenheier B, Lin J, Zhuo X, et al. Compression of disability between two birth cohorts of US adults with diabetes, 1992–2012: a prospective longitudinal analysis. Lancet Diabetes Endocrinol. 2016; 4:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson MD, Zhang P, Choksi P, Markides KS, Al Snih S. Muscle weakness thresholds for prediction of diabetes in adults. Sports Med. 2016;46:619–628. doi:10.1007/s40279-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. [DOI] [PubMed] [Google Scholar]
- 12. Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ. 2014;348:g2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez-Jaramillo P, Cohen DD, Gomez-Arbelaez D, et al. Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. Int J Cardiol. 2014;174:458–461. [DOI] [PubMed] [Google Scholar]
- 16. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci. 2014;69:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 18. Strand BH, Cooper R, Bergland A, et al. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromso Study. J Epidemiol Community Health. 2016;70:1214–1221. doi:10.1136/jech-2015-206776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sayer AA, Kirkwood TB. Grip strength and mortality: a biomarker of ageing? Lancet. 2015;386:226–227. doi:10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 20. Lawman HG, Troiano RP, Perna FM, Wang CY, Fryar CD, Ogden CL. Associations of relative handgrip strength and cardiovascular disease biomarkers in U.S. adults, 2011-2012. Am J Prev Med. 2016;50:677–683. doi:10.1016/j.amepre.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson MD, Zhang P, Choksi P, Markides KS, Al Snih S. Muscle weakness thresholds for prediction of diabetes in adults. Sports Med. 2016;46:619–628. doi:10.1007/s40279-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43:61–68. doi:10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sairenchi T, Iso H, Irie F, Fukasawa N, Ota H, Muto T. Underweight as a predictor of diabetes in older adults: a large cohort study. Diabetes Care. 2008;31:583–584. doi: 10.2337/dc07-1390. [DOI] [PubMed] [Google Scholar]
- 24. Zhou BF, Work CM-aG. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr. 2002;11:S685–S693. [PubMed] [Google Scholar]
- 25. Zhao Y, Crimmons E, Hu P, et al. China Health and Retirement Longitudinal Study: 2011–2012 National Baseline Blood Data Users’ Guide. Beijing, China: China Center for Economic Research-Peking University; 2014. [Google Scholar]
- 26. International Expert C ; Nathan DM, Balkau B, Bonora E, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NHANES. Muscle Strength Procedures Manual. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2011. [Google Scholar]
- 28. Zhao Y, Crimmins E, Hu P, et al. Prevalence, diagnosis, and management of diabetes mellitus among older Chinese: results from the China Health and Retirement Longitudinal Study. Int J Public Health. 2016;61:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pu Y, Liu L, Wang Y, et al. Geographic and sex difference in the distribution of intracranial atherosclerosis in China. Stroke. 2013;44:2109–2114. [DOI] [PubMed] [Google Scholar]
- 30. Zhao W, An Z, Hong Y, Zhou G, Liu B, Guo J. Sex differences in long-term outcomes among acute ischemic stroke patients with diabetes in China. Biol Sex Differ. 2015;6:29. doi:10.1186/s13293-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Ning X, Yang L, et al. Sex differences in trends of incidence and mortality of first-ever stroke in rural Tianjin, China, from 1992 to 2012. Stroke. 2014;45:1626–1631. [DOI] [PubMed] [Google Scholar]
- 32. Smith JP, Strauss J, Zhao Y. Healthy aging in China. J Econ Ageing. 2014;4:37–43. doi:10.1016/j.jeoa.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song Y, Bian Y. Gender differences in the use of health care in China: cross-sectional analysis. Int J Equity Health. 2014;13:8. doi:10.1186/1475-9276-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization. Integrating Equity, Gender, Human Rights and Social Determinants Into the Work of WHO. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 35. Blumenthal D, Hsiao W. Lessons from the East--China’s rapidly evolving health care system. NEJM. 2015;372:1281–1285. [DOI] [PubMed] [Google Scholar]
- 36. Koh H, Sebelius K. Promoting prevention through the affordable care act. NEJM. 2010;363:1296–1299. [DOI] [PubMed] [Google Scholar]
- 37. Chung S, Lesser L, Lauderdale D, Johns N, Palaniappan L, Luft H. Increasing preventive care with expanded medicare coverage under the Affordable Care Act. J Patient-Cent Res Rev. 2015;2:120. [Google Scholar]
- 38. Louie GH, Ward MM. Association of measured physical performance and demographic and health characteristics with self-reported physical function: implications for the interpretation of self-reported limitations. Health Qual Life Outcomes. 2010;8:84. doi:10.1186/1477-7525-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martin L, Freedman V, Schoeni R, Andreski P. Trends in disability and related chronic conditions among people ages fifty to sixty-four. Health Affairs. 2010;29:725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luo H, Wong GH, Lum TY, Luo M, Gong CH, Kendig H. Health expectancies in adults aged 50 years or older in China. J Aging Health. 2016;28:758–774. doi:10.1177/0898264315611663. [DOI] [PubMed] [Google Scholar]
- 41. Ortman J, Velkoff V, Hogan H. An Aging Nation: The Older Population in the United States Current Population Reports. Washington, DC: United States Census Bureau; 2014. [Google Scholar]
- 42. Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31:3–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.