Abstract
Background
Changes in cerebral blood flow velocity (CBF) in response to a cognitive task (task-related ΔCBF) have been shown by Transcranial Doppler ultrasonography (TCD) to be reduced in slow walkers. However, it is unknown whether reduced task-related ΔCBF is associated with reduced neural activity in specific brain regions, as measured by blood-oxygen-level dependent (BOLD) functional magnetic resonance imaging (fMRI).
Methods
We assessed the regional changes in neural activity associated with reduced middle cerebral artery (MCA) task-related ΔCBF to an executive task and slow walking speed in 67 community-dwelling older adults from the MOBILIZE Boston Study. Participants underwent walking assessments and TCD ultrasonography measures of MCA ΔCBF during the n-back task of executive function. A subset of participants (n = 27) completed the same task during fMRI. Individual BOLD activation maps for the n-back task were correlated with TCD measures and network-level averages were associated with TCD and preferred walking speed.
Results
Participants with diminished task-related ΔCBF walked more slowly (β = .39, p = .001). fMRI revealed significant associations between task-related ΔCBF and regional BOLD activation in several brain regions/networks supplied by the MCA. Of these regions and networks, those within the executive network were most strongly associated with walking speed (β = .36, p = .01).
Conclusions
Task-related ΔCBF during an executive function task is related to activation in several neural networks and impairment in the ability to recruit the executive network in particular is associated with slow walking speed in older adults.
Keywords: Gait speed, Neural activation, Cerebral blood flow velocity, Executive function, Neurovascular coupling
Slow walking speed is associated with several adverse outcomes, including the development of dementia (1) and increased mortality (2). In healthy adults, functional neuroimaging studies reveal locomotion to be mainly controlled by supraspinal system regions like the primary motor cortex, supplementary motor area, and premotor cortex (3,4). However, neurodegenerative changes in these and other regions due to the aged brain and disease-related processes may slow walking speed (3).
Several studies have also shown a decline in mobility to be associated with a reduced capacity to redistribute cerebral blood flow (CBF) to compensatory frontal regions (5–9). The aged brain and disease have been separately associated with impaired hemodynamics (10,11), diminished recruitment of the executive and attention networks (12,13), and mobility deficits (14). For example, using Transcranial Doppler (TCD) ultrasonography, Sorond and colleagues (6) observed that reduced CBF velocity in response to an executive task within the middle cerebral artery (MCA) was associated with slow walking speed. This suggests that activation of executive control networks is important for normal gait performance. However, it is unknown whether reduced response is associated with reduced neural activity in specific brain networks. Thus, we used functional magnetic resonance imaging (fMRI) to cross-sectionally assess regional changes in neural activity associated with MCA CBF velocity and walking speed. We hypothesized that CBF velocity during an executive task is associated with neural recruitment of executive regions supplied by the MCA, and that reduced executive neural recruitment is associated with slow walking speed.
Methods
Participants
Sixty-seven participants were recruited from the MOBILIZE Boston Study (Maintenance of Balance, Independent Living, Intellect and Zest in the Elderly of Boston Study [MBS]), which is a longitudinal population-based study launched in 2005 to identify novel risk factors for falls in older adults. As a part of the original cohort, interested individuals had to be: 70+ years of age (or 65+ if living with an already-enrolled participant); able to understand and communicate in English; expect to live in the area for at least 2 years; and able to walk 20 feet without personal assistance. Individuals were excluded if they had a terminal disease; severe vision or hearing deficits; or significant cognitive impairment (ie, Mini-Mental State Exam [MMSE] score <18). A full description of the protocol is provided in Leveille and colleagues (15). The current study further excluded individuals with a diagnosis of Parkinson’s disease, limb deformity, severe arthritis, and/or an inadequate temporal bone window for TCD insonation.
Those with complete TCD data and no contraindications to MRI were also invited to undergo the MRI portion. Of the 67, 42 participants qualified for and completed the MRI study procedures, but 15 were excluded for excessive motion (n = 10) or poor task compliance (n = 5). As a result, 27 participants were included in the statistical MRI analysis.
Research procedures were conducted within the Clinical Research Laboratory at the Hebrew SeniorLife Institute for Aging Research and the VA Boston Neuroimaging Research for Veterans (NeRVe) Center. All participants provided written informed consent as approved by both Institutional Review Boards.
Protocol
Study procedures were conducted during two visits, the first to the Hebrew SeniorLife Clinical Research Center where demographic, clinical, functional, gait, and TCD variables were collected, and the second to the VA NeRVe Center where MRI studies were performed.
N-back paradigm
During both TCD and fMRI procedures, participants performed a modified n-back task of executive function (6). Participants completed four 90-second blocks of testing: two blocks of Identify X (ie, control condition) and two blocks of a 2-back task (ie, experimental condition). The sequence of testing was fixed as one 90-second block of Identify X (IdX) followed by one 90-second block of the 2-back task, then repeated. Eighteen letters were presented in a single block, each for 3 seconds, with a 2-second inter-trial interval. For the IdX condition, participants were instructed to press the left key on a custom keypad when the target letter “X” was displayed and the right key when it was not displayed. For the 2-back condition, participants were instructed to press the left key if the present letter was seen two positions back and the right computer key if it was not. This requirement of consistent key press was implemented to encourage task engagement. Custom MATLAB scripts were generated to extract percent accurate target detections (rate %) and mean reaction time (RT) within and across conditions. Of note, mean RT results should be interpreted with caution, as participants were not instructed to answer as quickly as possible. Participants were also able to change their response within the allotted time, which could also affect RT.
TCD ultrasound
During the first visit, TCD (DWL Transcranial Doppler Systems, San Juan Capistrano, CA) was used to record CBF velocity according to a previously established MBS protocol (8). Briefly, while lying in a supine position, small, flat TCD probes were placed over the participant’s temporal bones and positioned to insonate the left and right MCA territories. Probes were held in place using a commercial probe holder (Marc 600 Headframe, Spencer Technologies). CBF velocity was first recorded during 300 seconds of rest, then 6 minutes of the n-back task.
The instantaneous CBF velocity (cm/s) within the left and right MCA was recorded during this procedure. The mean CBF was determined per condition by averaging across like blocks. Task-related ΔCBF was then quantified by calculating the percent change in mean CBF velocity between the IdX and the 2-back conditions, averaging across both the left and right MCA territories, that is, ([Mean CBF velocity2-back–Mean CBF velocityIdX]/Mean CBF velocityIdX) × 100. This phenomenon will be referred to as the task-related change in MCA CBF velocity (ie, ΔCBF), though in previous work it has been termed “neurovascular coupling” (6,16).
Walking assessment
Also during the first visit, standardized verbal instructions for the walking assessment were given, in which participants completed two trials of walking quietly at preferred speed. Each trial consisted of three separate passes over a 16-foot GAITRite mat (CIR Systems Inc., Havertown, PA), starting 4 feet before the mat from a quiet standing position. Average walking speed (m/s) was computed by dividing distance by time, which was quantified from each full step that occurred over the GAITRite mat within each trial. Walking speed was averaged within and then between trials.
MRI
Approximately 9 days following the first session (SD = 8; range = 1–37 days), qualifying participants attended a neuroimaging session, performed on a 3 Tesla Siemens TIM Trio with a 12-channel brain array. Functional data were acquired with a gradient-echo echo-planar sequence scan (TR = 2000 ms; TE = 30 ms; flip angle = 90°; 38 slices at 3.75 mm thickness; 3.0 × 3.0 mm in-plane resolution; 194 volumes). Two T1-weighted MPRAGE scans (T1 = 1000 ms; TR = 2730 ms; TE = 3.31 ms; flip angle = 7°; 128 slices at 1.3 mm thickness; FOV = 256 × 256 mm) were also collected and averaged for high-resolution neuroanatomy. Functional data were pre-processed in AFNI (17) with the following steps: volume registration; anatomical data alignment and warp into Talairach space for inter-subject normalization; smoothing with an 8-mm kernel; and scale to a percentage of the mean. Data were entered into a general linear model reflecting the n-back task conditions (ie, 2-back and IdX block regressors), while simultaneously modeling the effects of motion, nuisance cerebral spinal fluid and white matter signal, and censored time points with >0.5 mm in sudden movement. Events related to task performance (ie, correct or incorrect responses) were not modeled since TCD measures were similarly unadjusted. For quality assurance purposes, if a participant was found to have greater than 30% of time points censored, they were subsequently removed from the analysis.
Statistical Analyses
N-back task performance, TCD, and walking speed
To determine differences in task performance between conditions (ie, IdX, 2-back task) and, separately, between cohorts of Visit 1 and 2, we utilized matched pairs t-tests. To determine the association between task performance (ie, accuracy, mean RT) and our primary outcomes (ie, task-related ΔCBF, neural activation, walking speed), we utilized linear regression. Nonparametric tests were used when appropriate, for non-normally distributed data.
To determine potentially confounding variables (ie, age, sex, BMI, type 2 diabetes, hypertension, and atrial fibrillation) in the relationship between primary variables and walking speed, we utilized mixed stepwise regression (p = .05 threshold). The resulting best fit multiple linear regression model was then used to predict walking speed from task-related ΔCBF or neural activation. Standardized betas (β) are reported for effect size.
MRI analyses
To determine the regional changes in CBF associated with performing the n-back task, the 2-back and IdX condition deconvolve maps were contrasted for the 27 participants who completed both TCD and MRI protocols. To examine how these activation changes were associated with task-related ΔCBF, these whole-brain maps (2-back minus IdX) were then entered into a linear regression model with their associated TCD-derived measures. The resulting maps were then corrected for multiple comparisons using the spatial autocorrelation function (ACF) within AFNI’s 3dClustSim, based upon the individual model residuals. Cluster-corrected thresholds for p < .05 were given by nominal p = .02 and cluster size ≥ 247.
To determine how task-related ΔCBF and fMRI activation were associated with walking, a network-level approach was employed. First, activation was averaged across voxels within the dorsal attention (DAN), ventral attention (VAN), and executive networks of a previously defined 7-network parcellation (18). These estimates were then entered into multiple linear regression models to examine associations with both task-related ΔCBF and walking speed, adjusting for all confounders. These neural networks were selected a priori because they are associated with executive function and attentional control. Finally, to determine whether the association between task-related ΔCBF and walking speed was mediated by network activation, we utilized the Hayes’ PROCESS Procedure (19) (v. 2.16.3) in SPSS (SPSS Inc., Chicago IL) version 24.0.
Results
Table 1 summarizes the demographic, clinical, and task performance for the cohort at first visit, while Table 2 summarizes the cohort that underwent both visits. These samples (n = 67 and n = 27, respectively) did not differ across demographics, clinical characteristics or task performance. Additionally, within the larger cohort, there were no lateral differences between the left and right MCA task-related ΔCBF (paired t test: t = −1.18, p = .24) and these measures were significantly correlated (β = .81, p < .0001). Therefore, left and right CBF responses were not considered separately.
Table 1.
Complete Population Demographic and Clinical Characteristics
| Visit 1 (TCD Protocol) | |
|---|---|
| N | 67 |
| Female (%) | 61 |
| Age | 85 ± 5 |
| BMI | 26 ± 5 |
| Mini-mental State Exam | 27 ± 2 |
| Trails A (s) | 46 ± 20 |
| Trails B (s) | 104 ± 50 |
| Trails B adjusted (s) | 61 ± 41 |
| Type 2 diabetes mellitus (%) | 8 |
| Hypertension (%) | 67 |
| Hyperlipidemia (%) | 52 |
| Atrial fibrillation (%) | 26 |
| Pacemaker (%) | 8 |
| Walking speed (m/s) | 1.0 ± 0.3 |
| Task-related ΔCBV (%) | 1.8 ± 3.3a |
| Mean CBF IdX (cm/s) | 51.7 ± 12.7 |
| Mean CBF 2-back (cm/s) | 52.7 ± 13.1 |
| N -back performance | Visit 1 |
| N | 55 |
| IdX correct target rate (%) | 99 ± 4 |
| IdX mean RT (s) | 0.9 ± 0.2 |
| 2-back correct target rate (%) | 74 ± 29 |
| 2-back mean RT (s) | 1.3 ± 0.3 |
| Diff correct target rate (%) | −25 ± 28 |
| Diff mean RT (s) | 0.41 ± 0.3 |
Notes: Data = mean ± SD or percentage; ∆CBF = “change in” Cerebral Blood Flow; BMI = body mass index; Diff = difference between the IdX and 2-back conditions; IdX = Identify X condition; RT = reaction time; TCD = Transcranial Doppler.
aThe increase in CBF for the 2-back condition compared to IdX is greater than 0 (p < .05).
Table 2.
Subset Demographic, Clinical, and Cognitive Performance Characteristics
| Visit 1 (TCD Protocol) | |||
|---|---|---|---|
| N | 27 | ||
| Female (%) | 74 | ||
| Age | 84 ± 4 | ||
| BMI | 24 ± 4 | ||
| Mini-mental State Exam | 28 ± 3 | ||
| Trails A (s) | 44 ± 19 | ||
| Trails B (s) | 95 ± 36 | ||
| Trails B adjusted (s) | 52 ± 31 | ||
| Type 2 diabetes mellitus (%) | 4 | ||
| Hypertension (%) | 56 | ||
| Hyperlipidemia (%) | 56 | ||
| Atrial fibrillation (%) | 26 | ||
| Pacemaker (%) | n/a | ||
| Walking speed (m/s) | 1.2 ± 0.3 | ||
| Task-related ΔCBV (%) | 2.4 ± 3 | ||
| Mean CBF IdX (cm/s) | 52 ± 9 | ||
| Mean CBF 2-back (cm/s) | 54 ± 10 | ||
| N -back performance | Visit 1 | Visit 2 (fMRI Protocol) | p |
| IdX correct target rate (%) | 99 ± 3 | 98 ± 5 | ns |
| IdX mean RT (s) | 0.8 ± 0.1 | 0.8 ± 0.2 | ns |
| 2-back correct target rate (%) | 72 ± 18 | 79 ± 22 | ns |
| 2-back mean RT (s) | 1.2 ± 0.3 | 1.2 ± 0.2 | ns |
| Diff correct target rate (%) | −28 ± 18 | −19 ± 23 | ns |
| Diff mean RT (s) | 0.43 ± 0.2 | 0.41 ± 0.2 | ns |
Note: Data = mean ± SD or percentage. ∆CBF = “change in” Cerebral Blood Flow; BMI = body mass index; Diff = difference between the IdX and 2-back conditions; fMRI = functional magnetic resonance imaging; IdX = Identify X condition; ns = nonsignificant; RT = reaction time; TCD = Transcranial Doppler.
It should be noted that task performance for the TCD Visit 1 cohort consists of 55 participants as additional participants (n = 12) were excluded from the task performance analysis due to the following reasons: very poor performance on the IdX and/or the 2-back (n = 7); no response at all—response keypad not recording (n = 2); participant tried but only pressed for some targets and not all of them (n = 2); and missing data—computer error (n = 1). Further, task performance data for the fMRI n-back task are reported for 22 participants, after data for five (n = 5) participants were excluded due to equipment failure. However, these participants exhibited activation patterns consistent with task engagement and had otherwise complete data.
Task Performance
N-back task performance during Visit 1 is presented in Table 1 for the entire cohort, while performance for the subset is presented in Table 2.
At Visit 1, differences were observed in target detection accuracy and mean RT between the IdX and 2-back conditions. During the IdX condition, participants had a higher target accuracy and faster mean RT, as compared to the 2-back condition (Z = −672, p < .0001 and Z = 216, p < .0001, respectively). These differences in task performance verified an increase in task difficulty and remained in the fMRI subset.
Correlation analyses determined that those with lower task-related ΔCBF took a longer time to identify targets during the IdX condition (rs = −.35, p = 0.01) and displayed a smaller change in mean RT from the IdX to the 2-back condition (β = .28, p = .03). Additionally, those with slower walking speed took more time to identify targets in both the IdX and 2-back conditions (rs = −.45, p = .001 and β = −.29, p = .03, respectively).
At Visit 2, we observed that greater executive network recruitment (2-back vs. IdX) was associated with greater 2-back accuracy (rs = .427, p = .047) and positively trended with the difference in accuracy (rs = .412, p = .057). There were no other associations between task performance (ie, target accuracy and mean RT) and our primary variables of interest (ie, task-related ΔCBF, neural activation, walking speed).
TCD-Derived Task-Related ΔCBF and Walking Speed
Linear regression revealed an association between task-related ΔCBF and walking speed. Those with lower task-related ΔCBF walked more slowly (β = .39, p = .001; see Supplementary Figure 1). To consider this relationship in the presence of all potential confounders, we employed a mixed stepwise regression analysis that ultimately included age (β = −.30, p = .001), sex (β = −.29, p = .003), BMI (β = −.33, p = .001) and hypertension (β = .34, p = .001) as covariates. The association between lower task-related ΔCBF and slower walking speed remained significant after adjustment (β = .28, p = .005).
Associations With Neural Recruitment
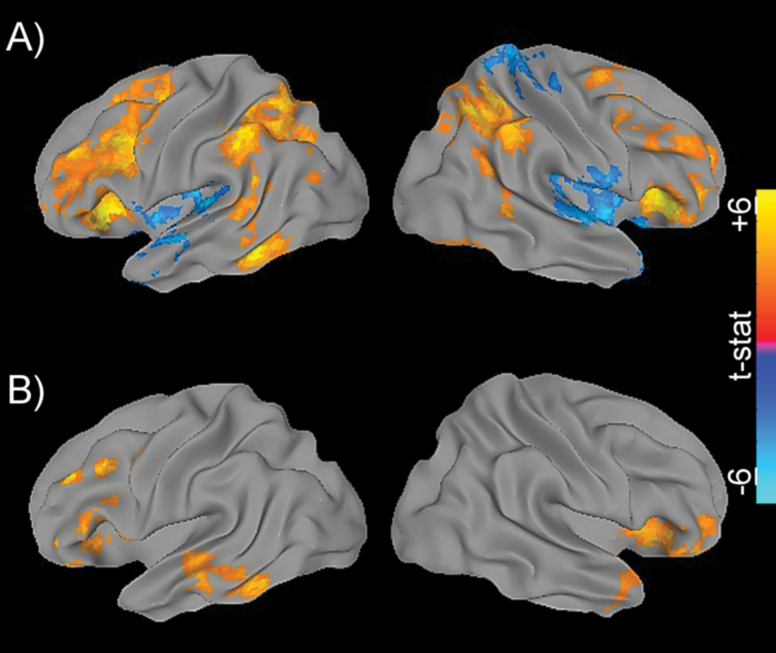
Within the smaller subset of participants, initial whole-brain group-level fMRI analyses revealed increased bilateral neural recruitment in the prefrontal and posterior parietal regions when performing the 2-back relative to IdX task (Figure 1A). This pattern of recruitment mirrored previous studies and largely overlapped with the frontoparietal working memory systems required for the more challenging 2-back condition (20). When TCD-derived task-related ΔCBF was correlated with this recruitment, significant voxel-wise clusters were found within the bilateral frontal and temporal regions spanning the MCA territory, as well as the right insula (Figure 1B).
Figure 1.
Whole-brain associations. (A) Differential activation maps comparing the 2-back and Identify X (2bk > IdX) task conditions. The positive tail (red to yellow) illustrates regions with greater activation during the 2-back condition. (B) Correlation map for 2bk > IdX neural activation and task-related ΔCBV. Maps are color-mapped according to voxel-wise t-statistics (range: −6 to +6).
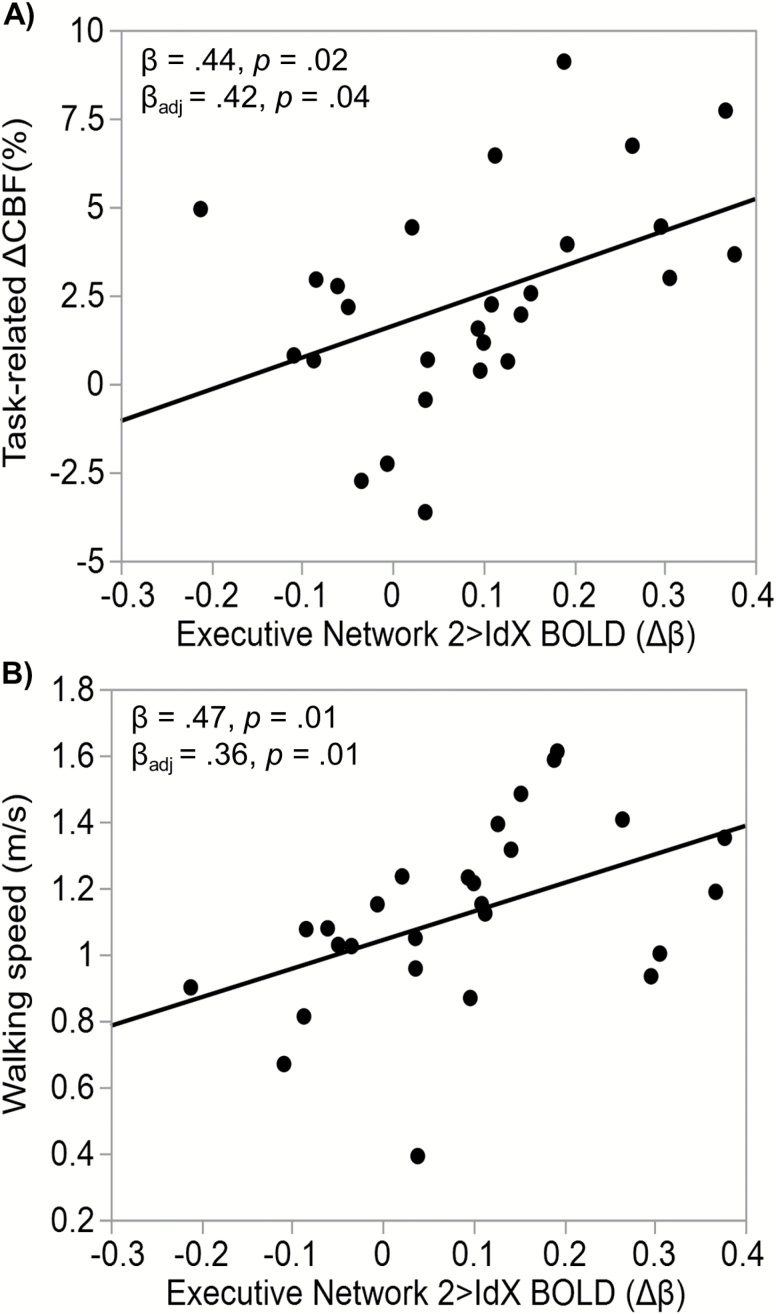
At the network-level, TCD-derived task-related ΔCBF was additionally predictive of the average DAN (β = .57, p = .009), VAN (β = .49, p = .017), and executive (β = 0.42, p = .036; Figure 2A) network recruitment. Significant associations were also revealed between walking speed and the DAN and executive networks during the n-back (β = .30, p = .031; β = .36, p = .01 [Figure 2B]); respectively). The VAN, though associated with task-related ΔCBF, was not associated with walking speed (β = .065, p = .686). These network-level associations were adjusted for age, sex, BMI, and hypertension status.
Figure 2.
Executive network-level associations. Scatter plots with line of best fit demonstrating the linear associations between the 2bk > IdX neural activation (Δβ) within the executive network and (A) 2bk > IdX MCA blood flow velocity and (B) walking speed (m/s).
Finally, we examined whether task-related ΔCBF predicted walking speed when network activation was included as a potential mediator. While task-related ∆CBF was independently associated with both activation (β = .44, p = .02) and gait speed (β = .39, p = .02), it was no longer associated with gait speed when executive network activation was included in the regression model (β = .23, p = .23). The relationship between activation and gait speed, when adjusting for ∆CBF, still trended toward significant (β = .37, p = .07). The executive network was confirmed as a mediator with an indirect effect of 0.0146 (bootstrap 95% CI estimates: [0.0006, 0.0312]). This indirect path, however, was not significant when adjusting for age, sex, BMI, and hypertension as confounders (effect = 0.0131 [95% CI = −0.0012, 0.0273]), likely due to our small sample size. No evidence of mediation was found for the DAN.
Discussion
In the current study, we demonstrated, with regional specificity, how TCD-derived task-related ΔCBF during an executive function task is associated with neural recruitment and walking speed within a sample of community-dwelling older adults. To our knowledge, we are the first to report that TCD-derived measures reflect the neural recruitment of regions and networks supplied by the MCA during the n-back task. We also report that activation in the DAN and executive networks are associated with both task-related ΔCBF and walking speed. These findings support the importance of CBF in cognitive function and walking with older age. Mediation analyses supported our overall hypothesis that task-related ΔCBF is associated with executive network recruitment and that dysfunction of this specific network is associated with poorer mobility.
In our larger cohort, we confirmed a positive association between task-related ΔCBF and walking speed. Previously, it was shown in the MBS cohort of community-dwelling older adults that slow walkers (<0.67 m/s) had reduced task-related ΔCBF (ie, neurovascular coupling) when compared to fast walkers (≥0.67 m/s) (6). In the current study, we found a linear association such that those with lower task-related ΔCBF walked slower. This association was independent of age, sex, BMI, and hypertension—all of which were found to be associated with walking speed and were not included in prior models. This result highlights the important functional role of blood flow regulation within networks, particularly the frontal executive regions, and the need to meet the metabolic demands of walking - not only in the lower extremity musculature, but also in the brain.
As expected, we found task-related ΔCBF to reflect the neural recruitment of regions critical to the task at-hand. In this case, performing the 2-back task elicited increased blood flow velocity in the MCA domain for the majority of the cohort, and resulted in increased fMRI activation of the DAN, VAN, and executive networks (Figure 1A). These findings complement previous literature supporting the recruitment of task-positive neural regions as necessary for normal cognitive task performance in cognitively healthy adults (20). Considering, however, that the integrity of frontal regions generally declines with older age, it is possible that the combination of intact task-related ΔCBF, as well as increased recruitment likely reflects compensation for neuronal loss and the maintenance of cognitive reserve in our population (12,21). At this point, it is unclear why there would be a lateral difference in frontal activation and insular recruitment in the voxel-wise association between network recruitment and task-related ΔCBF, but lateral differences in the participation of these areas have been reported in higher level executive functioning (22).
Functional MRI also confirmed network-level recruitment associations with walking speed. While DAN, VAN, and executive network frontal areas were generally recruited to a greater extent for the 2-back experimental condition, with executive activation associated with better performance, activation of two MCA-supplied networks (ie, DAN and executive) was additionally associated with walking speed. This supports prior literature; frontal and parietal regions are critical to walking speed and other executive tasks (3,9,23,24). These regions are worthy of exploration for neuro-rehabilitation which has been found to improve functional mobility in older adults (25). Our finding that specific neural networks (ie, executive function and attention) is related to walking speed is also supported by previous literature suggesting that specific cognitive training interventions translates to improved mobility outcomes and even reduced fall-risk in older adults (26).
One possible explanation of our findings is that reduced brain activation was due to decreased n-back task performance. In our larger cohort, we found that those who took a longer time to identify targets during one or both conditions (ie, IdX, 2-back) also had lower task-related ΔCBF and slower walking speed. This finding is consistent with previous studies showing that RT, a measure of information processing, is associated with smaller prefrontal brain volume and slower walking speed in older adults (8). Sorond and colleagues (6) reported a positive association between performance on the n-back task and ΔCBF such that those with greater ΔCBF tended to perform better in accuracy on the 2-back. The current study replicated these findings within our fMRI cohort, showing that greater executive network recruitment is associated with 2-back accuracy.
Insights
Several insights are presented for older adults at risk for falls and mobility disability. First, fMRI confirms that functional TCD ultrasonography of the MCA can assess neurovascular health and function. Second, we provide evidence that increased blood flow velocity (and neural recruitment) to meet the increased difficulty of the 2-back condition is associated with n-back and mobility performance within this population. These findings suggest that methods to improve blood flow regulation and/or activation may prove useful to simultaneously enhance cognition, walking speed, and prevent falls. Multimodal interventions should include pharmacological agents already shown to improve CBF, including angiotensin converting enzyme inhibitors (27), angiotensin receptor blockers (28), cholinesterase inhibitors (29), intranasal insulin (30), deferoxamine (31), and cocoa (16). We also highlight that the executive and dorsal attention networks should more broadly be among neural targets for cognitive training and neuro-stimulation to potentially improve mobility in older adults.
Limitations
The reported results do not come without limitations. First, in the TCD cohort, 27% of the participants had what appeared to be a reduction in CBF during cognitive demand. This was due to the fact that after performance of the first experimental 2-back task, CBF levels remained elevated during the subsequent control IdX task and could not increase any further during performance of the n-back task. Although CBF was increased during performance of the 2-back task, it was not greater than the preceding IdX condition and calculation of the difference in CBF activation between 2-back and the IdX condition yielded a negative number. It is best to interpret the results in these participants as no response due to a “ceiling effect,” rather than a decrease in CBF during the executive task. For these individuals, we suspect that the 2-back task was more of a cognitive stressor when it was initially performed, resulting in a sustained elevation in CBF during IdX.
Secondly, we had a relatively small sample size with limited generalizability. Participants were required to be MRI-compatible and willing to lie within the scanner for an hour in order to qualify for the MRI protocol. We also excluded several participants who had excessive motion during the scan or did not adequately perform the task (ie, did not follow the instructions, failed to present effort). As a result, the study had only 27 participants with usable fMRI data, which limited our detection of associations at the voxel-level. Nevertheless, there was enough statistical power to uncover individual network-level activation differences in task-related ΔCBF and walking speed. Also, other tasks will need to be evaluated in the future as (a) non-normal distribution of 2-back performance indicated this particular condition was too challenging for some of the participants, and (b) it is possible that our observed relationships between task-related ΔCBF and walking speed are a function of task and are not limited to executive function alone.
Conclusions
In summary, the current study provides evidence that task-related ΔCBF is associated with activation in several neural networks. Of these networks, however, recruitment of the executive network underlies the association between task-related ΔCBF and walking speed. These findings warrant further investigation in future cognitive-motor intervention studies.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
A.J.J. was supported by the National Institutes of Health (grant numbers AG041785-02S1 and HL007374-36). V.N.P. was supported by the National Institutes of Health (grant numbers AG041785-04S1 and AG023480). B.M. was supported by the National Institutes of Health (grant number AG044543-01A1). M.E. was supported by Department of Veterans Affairs Clinical Sciences Research and Development (grant number 1IK2CX000706-01A2). L.A.L. designed the study, obtained funding from the National Institutes of Health (grant number AG041785 and AG023480), conducted experiments, and oversaw all aspects of the study, including data interpretation and manuscript preparation. W.M., M.E., and I.I. were also supported by grant number AG041785 from the National Institutes of Health.
Conflict of Interest
The authors report no disclosures.
Supplementary Material
Acknowledgments
L.A.L. designed the study, obtained funding, oversaw all aspects of data collection and analysis, and helped write the manuscript. A.J.J. and V.N.P. contributed equal effort to the conduct, analysis, and writing of this research. Specifically, A.J.J. was responsible for the transcranial Doppler ultrasound (TCD) data acquisition and analysis. She contributed to the conduct, data collection, and statistical analyses of the TCD component, as well as the walking protocols, their interpretation, and manuscript preparation. V.N.P. was responsible for the fMRI aspects of the study. She contributed to the experimental conduct, n-back behavior and fMRI processing, fMRI data and statistical analyses, interpretation, and manuscript preparation. I.I. contributed to all other project data collection and management. W.M., B.M., and M.E. oversaw statistical analyses, data interpretation, and contributed to manuscript preparation.
References
- 1. Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi:10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi:10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: a systematic review. Neurosci Biobehav Rev. 2015;57:310–327. doi:10.1016/j.neubiorev.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 5. Nakamura T, Meguro K, Yamazaki H, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11:132–139. [DOI] [PubMed] [Google Scholar]
- 6. Sorond FA, Kiely DK, Galica A, et al. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol. 2011;70:213–220. doi:10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorond FA, Galica A, Serrador JM, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010;74:1627–1633. doi:10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi:10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jor’dan AJ, Manor B, Novak V. Slow gait speed - an indicator of lower cerebral vasoreactivity in type 2 diabetes mellitus. Front Aging Neurosci. 2014;6:135. doi:10.3389/fnagi.2014.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang D, Cabral D, Gaspard EN, Lipton RB, Rundek T, Derby CA. Cerebral hemodynamics in the elderly: a Transcranial Doppler Study in the Einstein Aging Study Cohort. J Ultrasound Med. 2016;35:1907–1914. doi:10.7863/ultra.15.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabayan B, Jansen S, Oleksik AM, et al. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev. 2012;11:271–277. doi:10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 12. Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi:10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13. Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- 14. Hall KS, Cohen HJ, Pieper CF, et al. Physical performance across the adult life span: correlates with age and physical activity. J Gerontol A Biol Sci Med Sci. 2017;72:572–578. doi:10.1093/gerona/glw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi:10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi:10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 18. Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi:10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. [DOI] [PubMed] [Google Scholar]
- 20. Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi:10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. [DOI] [PubMed] [Google Scholar]
- 22. Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM. Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb Cortex. 2011;21:307–317. doi:10.1093/cercor/bhq096. [DOI] [PubMed] [Google Scholar]
- 23. Venkatraman VK, Aizenstein H, Guralnik J, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–3442. doi:10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi:10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manor B, Zhou J, Jor’dan A, Zhang J, Fang J, Pascual-Leone A. Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J Cogn Neurosci. 2016;28:275–281. doi:10.1162/jocn_a_00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Segev-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother. 2011;11:1057–1075. doi:10.1586/ern.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipsitz LA, Habtemariam D, Gagnon M, et al. Reexamining the effect of antihypertensive medications on falls in old age. Hypertension. 2015;66:183–189. doi:10.1161/HYPERTENSIONAHA.115.05513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hajjar I, Kritchevsky S, Newman AB, et al. ; Health, Aging and Body Composition Study Renin angiotensin system gene polymorphisms modify angiotensin-converting enzyme inhibitors’ effect on cognitive function: the health, aging and body composition study. J Am Geriatr Soc. 2010;58:1035–1042. doi:10.1111/j.1532-5415.2010.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaudhary S, Scouten A, Schwindt G, et al. Hemodynamic effects of cholinesterase inhibition in mild Alzheimer’s disease. J Magn Reson Imaging. 2013;38:26–35. doi:10.1002/jmri.23967. [DOI] [PubMed] [Google Scholar]
- 30. Novak V, Milberg W, Hao Y, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–759. doi:10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorond FA, Tan CO, LaRose S, et al. Deferoxamine, cerebrovascular hemodynamics, and vascular aging: potential role for hypoxia-inducible transcription factor-1-regulated pathways. Stroke. 2015;46:2576–2583. doi:10.1161/STROKEAHA.115.009906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.