Abstract
In Caenorhabditis elegans, a broad range of dietary restriction regimens extend life span to different degrees by separate or partially overlapping molecular pathways. One of these regimens, axenic dietary restriction, doubles the worm’s life span but currently, almost nothing is known about the underlying molecular mechanism. Previous studies suggest that mitochondrial stress responses such as the mitochondrial unfolded protein response (UPRmt) or mitohormesis may play a vital role in axenic dietary restriction–induced longevity. Here, we provide solid evidence that axenic dietary restriction treatment specifically induces an UPRmt response in C elegans but this induction is not required for axenic dietary restriction–mediated longevity. We also show that reactive oxygen species–mediated mitohormesis is not involved in this phenotype. Hence, changes in mitochondrial physiology and induction of a mitochondrial stress response are not necessarily causal to large increases in life span.
Keywords: Axenic culture, UPRmt, Longevity
Dietary restriction (DR), the restriction of nutrients without malnutrition, has been shown to extend life span in a wide variety of species (1). In Caenorhabditis elegans, a broad range of DR regimens has been proposed and they all extend life span to various degrees by separate or partially overlapping molecular pathways (2). DR-like phenotypes are also induced using sterile, chemically defined or undefined liquid media, called axenic media (3–5). Axenically cultured worms share many characteristic traits with worms subjected to other DR methods: slowed development, reduced fecundity, a slender appearance, and prolonged life span and hence the term axenic dietary restriction (ADR) is often applied (3).
Although the C elegans life span is doubled under ADR, the underlying molecular mechanisms supporting this robust longevity effect still remain enigmatic. Probably, this mechanism is distinct from that of most other DR regimens, as was recently shown in a genetic screen (6). Our previous findings suggest that increased stress resistance and enhanced mitochondrial function may play a vital role in ADR-induced longevity (3,7,8). These observations lead us to investigate the role of the mitochondrial unfolded protein response (UPRmt) and mitohormesis in ADR-mediated life-span extension.
The UPRmt is a highly conserved cellular stress response that activates nuclear-encoded mitochondrial chaperone genes to maintain protein homeostasis in the mitochondria (9). The UPRmt is activated upon different forms of mitochondrial stress including the misfolding of mitochondrial proteins or stoichiometric abnormalities of large multimeric complexes, such as electron transport chain (ETC) complexes (10,11). In C elegans, the UPRmt is essential for the life span–extending effect caused by inhibition of mitochondrial respiration (12). Mitonuclear protein imbalance by disturbance of mitochondrial protein synthesis also activates the UPRmt and extends C elegans life span (13). However, a more recent genome-wide RNAi screen showed that there is no straightforward correlation between UPRmt activation and life-span extension in C elegans. Constitutive activation of the UPRmt in the absence of mitochondrial stress also fails to extend life span (14,15). These findings cast doubt on the unique importance of the UPRmt in longevity. One possible explanation is that the nature of the mitochondrial proteotoxic stress may differ in various conditions. Nevertheless, the UPRmt has been implicated in life-span extension of worms, flies, and mice, suggesting a conserved role in cellular homeostasis (16).
The free radical theory of aging proposes that mitochondria are the main source and prime target of reactive oxygen species (ROS) and it is a widely held view that this might cause or at least contribute to the aging process (17). In recent years, a different view has gained popularity: ROS produced by mitochondria result in an adaptive response, called mitohormesis, which promotes cellular health and organismal longevity (18,19). The positive effect of mitohormesis on life span only occurs within a limited range of relatively low intracellular ROS levels. High ROS levels may not be compensated for by the mitohormetic response, which may explain the wide range of life spans observed for different respiratory chain mutants (20). Although the detailed mechanism of ROS-mediated mitohormesis has not been fully elucidated, several key factors, such as PMK-1 (p38 MAP kinase), HSF-1 (heat shock factor), SKN-1 (NRF-2), DAF-16 (FOXO), and PRDX-2 (peroxiredoxin) are involved for the mitohormetic response (18,19,21,22).
Here, we provide evidence that ADR treatment specifically induces a UPRmt response in C elegans but this induction is not required for the ADR-mediated longevity. We also show that ROS-mediated mitohormesis is probably not involved in this longevity phenotype.
Methods
C elegans and RNAi Strains
The wild-type C elegans used was Bristol N2 male stock (Caenorhabditis Genetics Center [CGC]). The mutant and transgenic strains VC289 prdx-2(gk169), VC1024 pdr-1(gk448), RB2547 pink-1(ok3538), SJ4100 zcIs13(hsp-6p::gfp), SJ4058 zcIs9(hsp-60p::gfp), CL2070 dvIs(hsp-16.2p::gfp), and SJ4005 zcIs4(hsp-4p::gfp) were also obtained from CGC. QC117 (atfs-1[et17]), QC118 (atfs-1[et18]), and atfs-1(tm4525) were provided by Dr. Matt Kaeberlein. dsRNA expressing bacterial strains targeting ubl-5 and hsp-6 were from the genomic RNAi library (produced by J. Ahringer). As a control, the bacterial strain containing the empty vector L4440 was used.
Worm Maintenance
Axenic and monoxenic culture conditions were adapted as previously described (6). To avoid contamination, 100 μg/mL ampicillin was added to both axenic and monoxenic cultures. The day of L4-to-adult transition was determined as Day 0 of adulthood. For monoxenic and axenic cultures, it took 3 and 6 days on average to reach this stage at 20°C, respectively. Worm samples were collected and cleaned by gravitational settling followed by three to five washes in S-basal.
Briefly, for monoxenic culture, eggs prepared by hypochlorite treatment of gravid adults were allowed to hatch overnight in S basal (23) and the resulting first stage larvae (L1s) were inoculated into Fernbach flasks containing 250 ml of growth medium (S-basal containing approximately 3 × 109 bacterial cells/mL and 5 µg/mL cholesterol). Bacteria were added as frozen beads consisting of equal volumes of pelleted bacteria and S basal. The flasks were shaken in a temperature controlled (20°C) gyrotory shaker at 120 oscillations/min. For axenic culture, the basal medium consists of 3% soy peptone (Sigma-Aldrich, St. Louis, MO) and 3% yeast extract (Becton-Dickinson, Franklin Lake, NJ), final concentrations (f.c.). Since C elegans is not capable of heme synthesis, after autoclaving the basal medium was supplemented with 0.05% hemoglobin f.c. (bovine; Serve, Heidelberg, Germany) diluted from a 100× stock in 0.1M KOH (autoclaved for 10 minutes). Axenic medium is very rich in nutrients and easily gets contaminated by microbes. Hence, all equipment and preparations should be handled in a laminar flow cabinet to ensure sterility.
Life-Span Assay
Life-span assays were performed as described previously (6). ADR was performed in liquid axenic culture while fully-fed (FF) assays were carried out on standard solid nematode growth medium (NGM) plates seeded with bacteria (23). Briefly, gravid adults were subjected to a microbleaching procedure in which about 10 worms were brought in a 10 µL drop of sterile distilled water. Then 10 µL alkaline bleach (12° hypochlorite and 1M NaOH f.c.) was added and left to incubate until all adults were dissolved or for maximally 10 minutes. Then 5 mL of axenic medium containing 20% sterile skimmed milk was added. Milk was added to stimulate and synchronize larval growth and we confirmed that this component did not cause UPRmt (data not shown). The eggs were allowed to hatch and were incubated at 20°C until adulthood. At L4 stage (around 5 days after bleaching), 50 µM of 5-Fluoro-2′-deoxyuridine (FUdR) was added to prevent progeny production. At adulthood, worms were transferred and exposed to the experimental conditions. Worms were transferred to fresh NGM plates every 2 days and checked for survival. NGM plates with antioxidants were freshly prepared for every transfer, whereas for axenic cultures, antioxidants were added to final concentration only once. For butylated hydroxyanisole (BHA), the compound was added from a 1,000× concentrated stock in dimethyl sulfoxide (DMSO) and equal volume of DMSO was added to the respective controls.
For RNAi-based life-span assay, worms were cultured on standard NGM agar plates seeded with the Escherichia coli OP50 bacteria until adulthood. RNAi was carried out as previously described (6) and performed on adult worms for 5 days before they were transferred to the experimental conditions. For each strain, around 100 worms were placed on small NGM plates (10 per plate) seeded with E coli OP50 as FF control. For ADR, about 100 worms were transferred to small screw-cap tubes (three to five worms per tube) containing 0.3 mL of liquid axenic medium (ADR). Progeny production was avoided by the addition of FUdR at 100 and 50 µM f.c. for FF and ADR, respectively. Survival was scored at regular time intervals: daily for the FF condition, every other day for the ADR conditions. In solid conditions, worms were considered dead if they did not respond to gentle prodding with a platinum wire. In liquid conditions, worms were scored dead if no movement could be detected, even after gently tapping the tubes. Worms that died of protruding vulva or crawling off the plates were censored. All life-span assays were conducted at 20°C and were repeated at least twice independently (pooled data is shown).
Data were analyzed with the online application for survival analysis (OASIS) as described in Yang and colleagues (24). In all cases, life-span data is indicated as mean ± standard error of the mean and p values were calculated using the log-rank (Mantel–Cox) method. To evaluate the impact of genes or treatments on life-span extension, the relative importance for each experimental setup was calculated based on the formula previously described (6).
GFP Expression and Quantification
Eggs from gravid worms of the appropriate green fluorescent protein (GFP) reporter strains were collected and hatched overnight. L1 worms were grown monoxenically or axenically. For single-time point experiments, animals were harvested and tested at the first day of adulthood. For the longitudinal experiments, worms were collected and analyzed at regular time intervals.
Fluorimetry assays were performed using black 96-well plates in a Victor2 plate reader (Perkin Elmer, Waltham, MA). Fluorescence was normalized to protein content (standard bicinchoninic acid method) of the worms recovered from the fluorimetry plate. Each experiment was repeated independently at least three times. Images were taken from at least 10 randomly picked worms from each culture condition, using a Olympus SZX12 fluorescence stereomicroscope. Only for longitudinal experiments, FUdR was added (100 and 50 µM f.c. for FF and ADR, respectively) to prevent progeny production. Worms were paralyzed with 0.1 mg/ml levamisole (Sigma) for image capture.
Results
ADR Specifically Activates the Mitochondrial UPR
DR regimens, such as bacterial dilution and eat-2 mutation, were shown to increase life span independently from the UPRmt response (12). Also, the DR mimetic metformin operates independently of this response (21). Moreover, dietary interventions that suppress the reduced life span of phb-2 (RNAi) block UPRmt induction (25). These observations suggest that DR may not cause mitochondrial proteotoxic stress and its beneficial effect on life span is independent of the UPRmt. On the other hand, we found enhanced mitochondrial performance (8) and increased stress resistance (3) in ADR worms, hinting at enhanced proteostasis. Therefore, we reasoned that unfolded protein responses and in particular the UPRmt may underlie the life span–doubling effect of ADR. The bloated mitochondrial morphology of ADR worms may also reflect UPRmt activation (Supplementary Figure 1) (26).
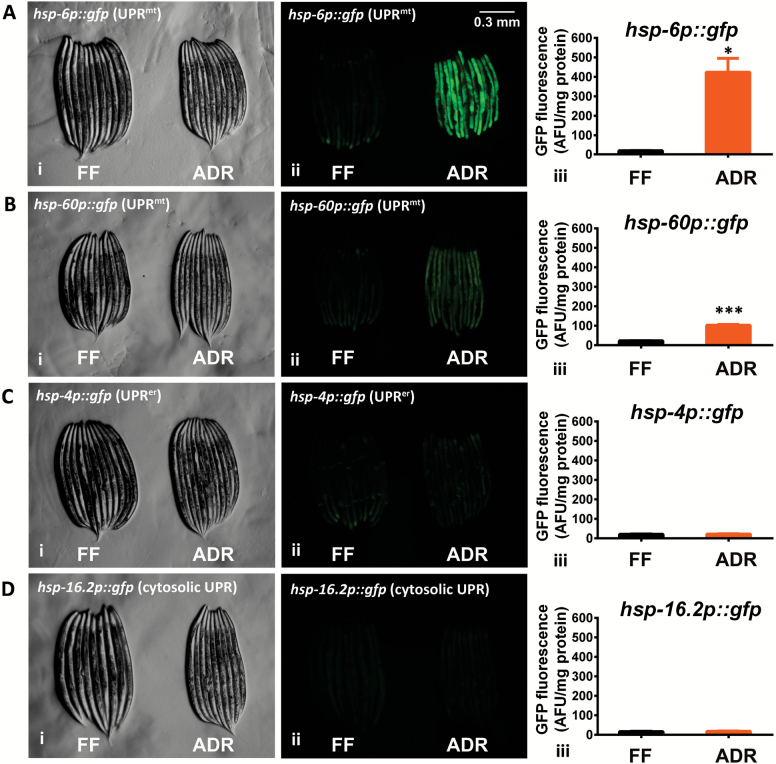
In order to test this hypothesis, worm strains, expressing specific reporters for UPRmt (hsp-6p::gfp and hsp-60p::gfp), UPRer (hsp-4p::gfp) and cytosolic UPR (hsp-16.2p::gfp) were subjected to ADR. The UPRmt response is clearly activated in ADR-treated worms compared to controls (Figure 1A and B), whereas the UPRer and cytosolic UPR reporter expression does not change upon exposure to axenic medium (Figure 1C and D). These observations show that ADR specifically activates the UPRmt.
Figure 1.
Axenic dietary restriction specifically induces UPRmt. The UPRmt reporter strains hsp-6p::gfp (A) and hsp-60p::gfp (B) show enhanced green fluorescent protein (GFP) expression under axenic dietary restriction (ADR) compared to fully-fed (FF) control. Strains carrying reporters for the UPRerhsp-4p::gfp (C) and cytosolic UPR hsp-16.2p::gfp (D) show no GFP induction when cultured in axenic medium. Panels (i) and (ii) show light and fluorescence images of the same worm clusters, respectively. Panels (iii) display microplate-based fluorimetric GFP quantification of worm populations, normalized to protein content. Error bars indicate standard error (standard error of the mean) of at least three independently grown replicates. *p < .05. **p < .01. *** p < .001. Student t test was used for statistical analysis.
ADR-Dependent Activation of UPRmt Peaks Near Juvenile-to-Adult Transition
Life-span extension by RNAi knockdown of electron transport chain components has a specific temporal requirement during the L3/L4 stages of larval development (27,28), a period that coincides with strong mitochondrial proliferation (29). Furthermore, the UPRmt marker hsp-6p::gfp shows its greatest activation at the L4 stage when worms are challenged with mitochondrial stress (11), we speculated that the ADR-dependent activation of the UPRmt also has a distinct temporal pattern that may underlie the longevity phenotype.
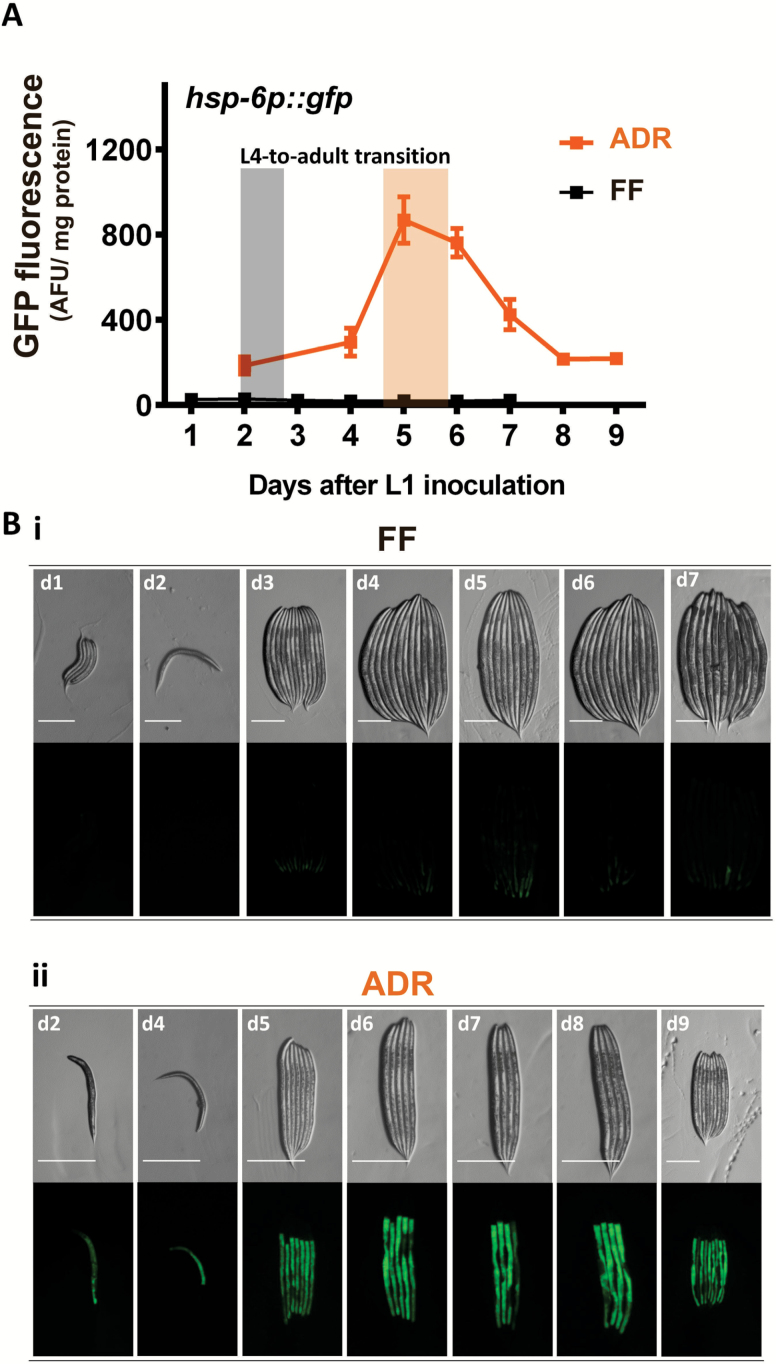
To study this, hsp-6p::gfp worms were cultured in both axenic and monoxenic medium from embryo to young adulthood. Worms were imaged and GFP fluorescence was measured for each culture condition at regular time intervals. Animals in monoxenic culture only showed background levels of GFP fluorescence, whereas worms collected from axenic medium increasingly expressed GFP up to L4 (Figure 2A and B), the stage which corresponds to the increased mitochondrial density. After the L4 peak, the UPRmt response decreased in young adult worms and stabilized from the second day of adulthood at a level which was still significantly higher than that of the monoxenic controls. This level was maintained throughout life (Supplementary Figure 2), suggesting that ADR causes mitochondrial stress. Hence, activation of UPRmt along life might promote longevity and knockdown of its components should abolish ADR-mediated life-span extension.
Figure 2.
ADR-dependent activation of UPRmt peaks near juvenile-to-adult transition. Expression of the UPRmt reporter hsp-6p::gfp was followed over time by collecting and analyzing worms regularly as indicated. (A) Microplate-based fluorimetric GFP quantification of worm populations, normalized to protein content. (B) Panels (i) and (ii) show light and fluorescence images of the same worm clusters from monoxenic culture and axenic culture, respectively. The days after L1 inoculation in each condition is indicated up-left. Error bars indicate standard error (standard error of the mean) of at least three independently grown replicates. *p < .05. **p < .01. ***p < .001. Scale bar: 0.3 mm. ADR = axenic dietary restriction; FF = fully fed; GFP = green fluorescent protein.
Activation of the UPRmt Is Not Required for Life-Span Extension by ADR
To assess whether induction of the UPRmt contributes to the strong longevity phenotype caused by ADR, we analyzed worm survival under FF and ADR conditions following RNAi knockdown of UPRmt pathway components.
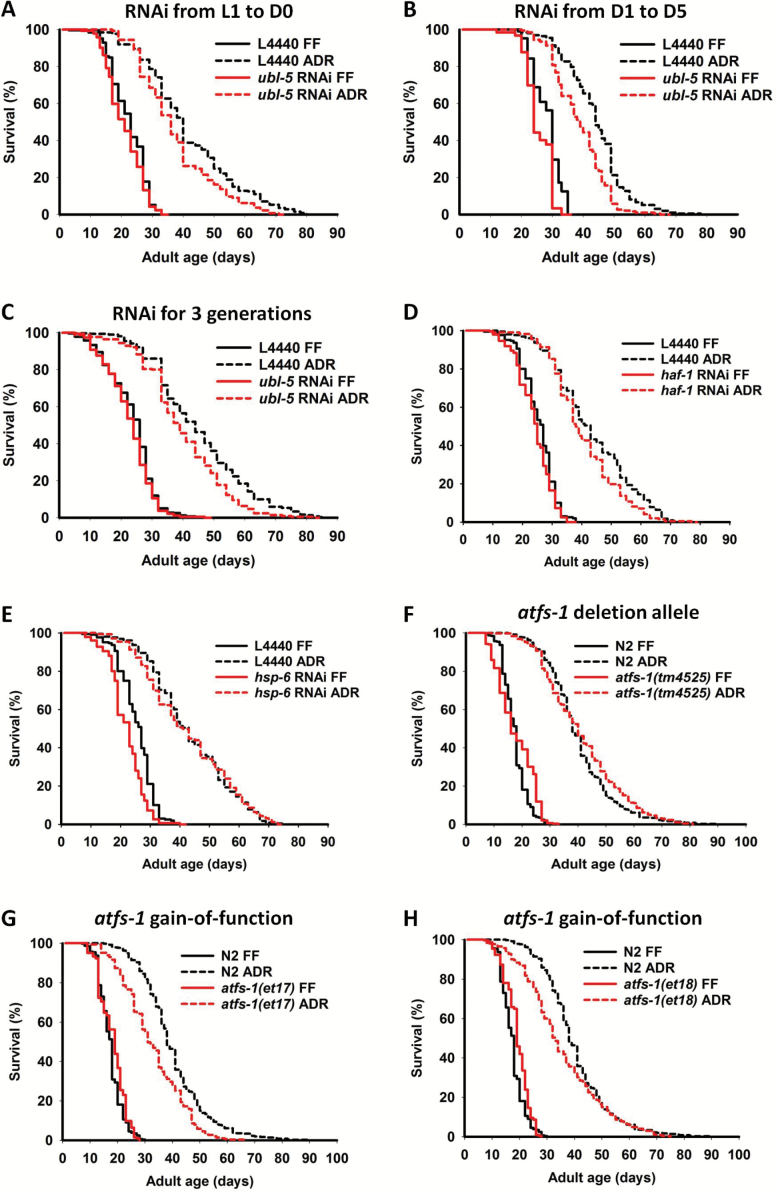
We found that RNAi knockdown of ubl-5 failed to prevent life-span extension by ADR, either treated from the L1 stage to early adulthood (Figure 3A; Supplementary Table 1) or during the first 5 days of adulthood (Figure 3B; Supplementary Table 1). Even a three-generation RNAi treatment failed to abolish life-span extension under ADR (Figure 3C; Supplementary Table 1). Although a minor reduction of ADR-induced longevity seems to occur in ubl-5 RNAi-treated worms, the relative importance of this gene for ADR longevity is not significant when RNAi was performed during juvenile stages or over three generations. Only for young adult RNAi treatment, the 10% rescue effect on ADR-induced life-span extension reaches borderline significance (Supplementary Table 2). Hence, ubl-5 seems not essential for life-span extension by ADR (Supplementary Tables 1 and 2). Inhibition of other UPRmt-related key factors by RNAi, such as haf-1 and hsp-6, also has no or limited effect on worm life span (Figure 3D and E; Supplementary Table 1). Again, the relative importance for each of these genes to the ADR-mediated longevity was not significant (Supplementary Table 2). Taken together, these observations provide primary evidence that induction of the UPRmt seems not to underlie the life-span extension caused by ADR.
Figure 3.
Inactivation of the UPRmt pathway by RNAi knockdown does not prevent ADR-induced life-span extension. (A) Effect of ubl-5 RNAi knockdown during worm development (L1 stage to first day of adulthood) on worm longevity under FF and ADR conditions. (B) Same as in (A) but ubl-5 knockdown was performed during the first 5 days of adulthood. (C) Same as in (A) with RNAi knockdown of ubl-5 for three generations. (D, E) Effect of haf-1 (D) and hsp-6 (E) knockdown during the first 5 days of adulthood on worm longevity under FF and ADR conditions. (F) Effect of the atfs-1 deletion allele tm4525 on life span in FF and axenic (ADR) condition. (G, H) Effect of constitutively active alleles of atfs-1(et17) and atfs-1(et18) on life span in FF and axenic (ADR) condition. All data are summarized in Supplementary Tables 1 and 2. ADR = axenic dietary restriction; FF = fully fed.
Since UPRmt activation cannot be blocked by RNAi knockdown of haf-1 (15) and inhibition of hsp-6 can induce UPRmt (11), we next set out to analyze the relation between UPRmt and ADR-mediated longevity using the atfs-1(tm4525) mutation, which has been shown to completely prevent induction of the UPRmt (30). No effect on life span was observed in the atfs-1(tm4525) background, as deletion of atfs-1 failed to significantly block the ADR-induced life-span extension (Figure 3F). Recently, constitutively active alleles of atfs-1 have been described, which result in induction of the UPRmt in the absence of exogenous mitochondrial stress (31). To determine whether constitutive activation of the UPRmt would further extend life span in ADR, we performed survival assays of strains carrying either the atfs-1(et17) or atfs-1(et18) gain-of-function alleles. Counter to expectation, the life span of atfs-1(et17) was slightly reduced under ADR, indicating that overactivity of UPRmt tends to decrease the life span–extending properties of ADR (Figure 3G, Supplementary Table 1). Similar results were obtained for atfs-1(et18) (Figure 3H).
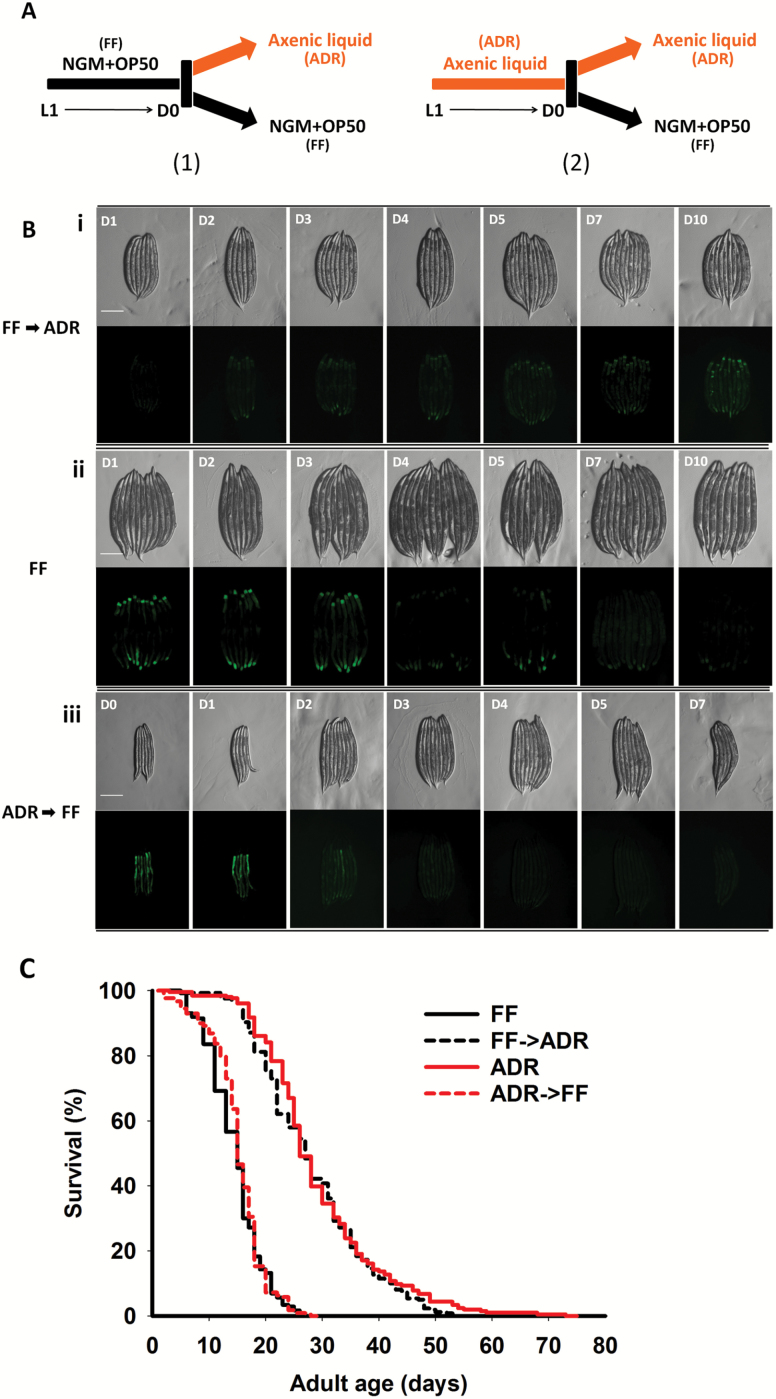
In order to further confirm our results, as an alternative approach to investigate the importance of the UPRmt for ADR longevity, we avoided early UPRmt induction by culturing juvenile worms on monoxenic plates. Once they reached adulthood, half of the population was switched to axenic medium, whereas the control worms were transferred to fresh monoxenic plates and life span was assessed. In a reverse setup, juveniles were grown under ADR, allowing UPRmt induction, and switched upon adulthood to monoxenic plates or fresh axenic cultures for the controls (Figure 4A).
Figure 4.
Activation of the UPRmt can be uncoupled from life-span extension by ADR. (A) Experimental setup: (1) hatched L1s were grown on solid nematode growth medium (NGM) plates seeded with Escherichia coli OP50 as fully-fed (FF) condition till young adulthood after which they were switched to either liquid axenic medium (ADR) or fresh E. coli plates (2); after microbleaching, hatched L1s were cultured in axenic medium until young adulthood. Afterwards, animals were transferred to fresh axenic medium or NGM plates seeded with E. coli OP50. (B) Images of worm clusters, grown according to the experimental setup in A. i: juveniles grown monoxenically and transferred to axenic medium at the first day of adulthood, ii: monoxenically grown worms, and iii: juveniles grown in axenic medium and transferred to monoxenic culture at the first day of adulthood. Top row: worms light image, bottom row: the same clusters imaged with fluorescence microscopy. The days of adult age are indicated in the upper left corners of the light images. (C) Survival of worms cultured according to experimental setup (1) and (2). Data are summarized in Supplementary Tables 1 and 2. Scale bar: 0.3 mm.
Interestingly, no obvious GFP expression was observed in animals grown monoxenically and subsequently transferred to axenic medium when reaching adulthood (Figure 4B), clearly demonstrating that the UPRmt is not activated in adults experiencing ADR. However, these worms still exhibit full life-span extension, comparable to those cultured axenically from L1 stage (Figure 4C). Besides, GFP expression gradually disappears once worms are transferred from axenic to monoxenic culture, indicating inactivation of UPRmt and alleviation of mitochondrial stress (Figure 4B). Despite their history in axenic medium and UPRmt induction during larval stages, these worms have a normal life span, comparable to monoxenically cultured worms (Figure 4C). Thus, activation of UPRmt during larval development by ADR is not sufficient to confer increased life span.
Overall, we conclude that UPRmt activation by ADR is specific and life stage-dependent but ADR-mediated longevity can be uncoupled from this stress response.
Mitohormesis Is Not Involved in the Life-Span Extension by ADR
There is a growing body of research emphasizing the importance of ROS as crucial cell signaling molecules rather than damaging agents (18,32,33). Our previous study indicated that young worms, exposed to ADR, show no increase in in vitro ROS production capacity as well as in vivo H2O2 levels compared to monoxenically grown controls (8), arguing against a role of mitohormesis in ADR longevity. However, it is possible that low or local levels of ROS may act as signaling molecules and potentially serve as the mitokine or intermediary signal to elicit a nuclear response (12).
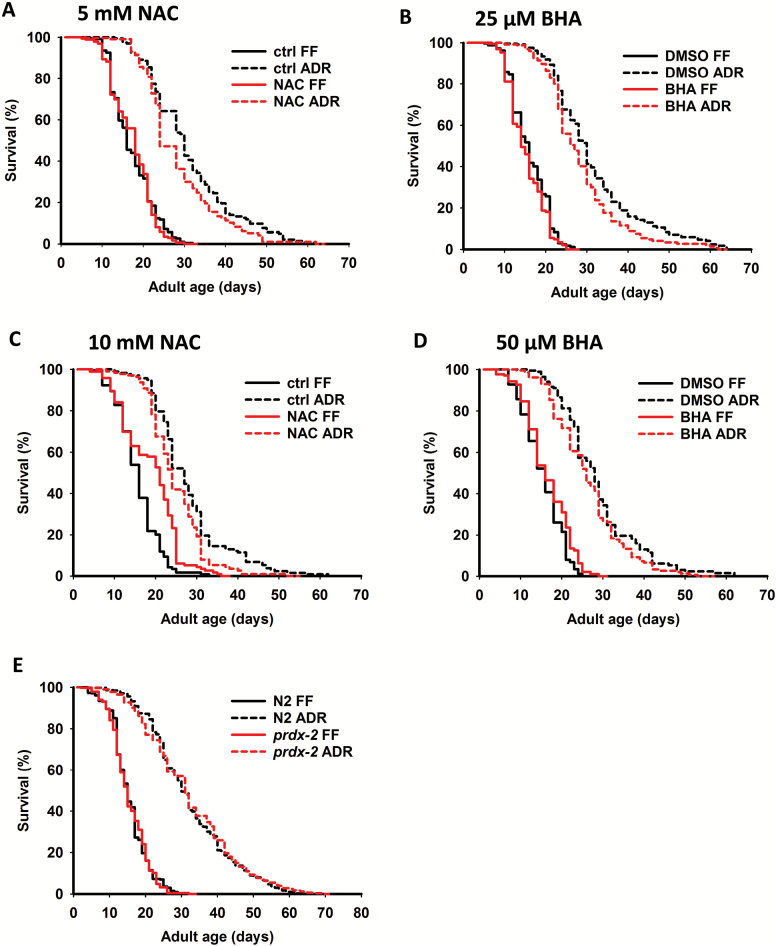
In order to further study whether a mitohormetic response contributes to the twofold life-span extension under ADR, worms were exposed to ROS scavengers in axenic medium. Life-long treatment with the antioxidants N-acetyl-l-cysteine (NAC, 5 mM) or BHA (25 µM) did not block ADR-induced life-span extension. However, a slight life-span reduction, of which the relative importance was not significant, was found in ADR animals treated with antioxidants (Figure 5A and B; Supplementary Tables 1 and 2). When antioxidant levels were doubled, still no clear effect was found for BHA (Figure 5D; Supplementary Tables 1 and 2). However, 10 mM NAC had opposite effects on life span in monoxenic and axenic conditions. Although this high dose of NAC extends life span in monoxenically cultured worms with 17%, it slightly shortens life span with 11% under ADR. In such case, it significantly decreased the life-span extension (p = .016) and the relative importance of 10 mM NAC treatment was 49.5% (Figure 5C; Supplementary Tables 1 and 2). Hence, where NAC treatment is beneficial to worms in bacterial culture, it seems harmful for worms under ADR.
Figure 5.
Mitohormesis is not involved in life-span extension by ADR. Survival analysis of worms treated with and without 5 mM NAC (A), 25 µM BHA (B), 10 mM NAC (C), and 50 µM BHA (D) under fully-fed (FF, full line) and axenic dietary restriction (ADR, dashed line) conditions. (E) Effect of prdx-2 mutation on life span under both FF and ADR conditions. Survival data are summarized in Supplementary Tables 1 and 2. ADR = axenic dietary restriction; BHA = butylated hydroxyanisole; DMSO = dimethyl sulfoxide; FF = fully fed; NAC = N-acetyl-l-cysteine.
NAC and BHA treatments did not interact with the UPRmt response as treatment of the axenically grown UPRmt reporter strain hsp-6b::gfp with NAC (5 mM) and BHA (25 µM) did not decrease reporter fluorescence (Supplementary Figure 3).
In C elegans, the peroxiredoxin PRDX-2 is involved in the prolongevity signal of the mitohormetic response (21). We did not observe life-span reduction in prdx-2 mutants compared to controls in axenic culture (Figure 5E; Supplementary Tables 1 and 2). Previously, we had shown that ADR-mediated longevity is independent of other players in the mitohormetic response such as DAF-16, HSF-1, and SKN-1 and it is only partially dependent of AAK-2 (6). Therefore, it is unlikely that ROS-mediated mitohormesis plays an important role in the life span–doubling effect by ADR in C elegans.
Discussion
Chemically defined axenic medium was established several decades ago, in a search for nematode nutritional requirements (5,34). An easy-to-prepare alternative, used in this study, is the undefined axenic medium based on a mixture of soy peptone and yeast extract, supplemented with heam (3). Axenically cultured worms show typical morphological features of DR and their life span is often doubled, making it the largest life-span increase of any DR method reported (2–4). A recent genetic screen for key regulators of ADR-mediated longevity showed that the Insulin/IGF pathway and oxidative stress response pathways are not involved in this remarkable phenotype (3,6). Mitochondria are often considered as the major culprits behind the aging process (17), yet their density and coupling were shown to be increased under ADR (8). We wondered whether mitochondrial stress responses such as the UPRmt and mitohormesis are required for ADR longevity.
In this study, we provided evidence that ADR, unlike other DR regimens, specifically activates the UPRmt while cytosolic and ER unfolded protein responses are unaffected. This suggests that axenic medium causes some sort of specific mitochondrial perturbation. In C elegans, the UPRmt can be triggered by ETC disturbance, by disrupted mitochondrial protein homeostasis or by mitonuclear protein imbalance (9,12–15). More recent studies indicate that both chromatin remodeling and histone modification are critical for the activation of UPRmt and its role for mitochondrial perturbation-mediated longevity (35,36).
Although UPRmt induction in axenic medium can be observed over the entire life span of the worms, it specifically peaked near L4-to-adult transition. This coincides with the period of rapid expansion of the mitochondrial compartment during development (29). It may seem plausible that the limited nutritional uptake under ADR cannot support the late juvenile mitochondrial expansion which in turn may trigger the UPRmt response. However, this scenario is unlikely as ADR worms have increased mitochondrial density, coupling and respiration (3,8). The link between juvenile UPRmt and longevity has also been found in worms suffering mitochondrial lesions such as knockdown of ETC subunits (12,13), although general causality has been questioned (15). Hence, an important question is whether juvenile UPRmt provokes the ADR longevity phenotype. A simple medium switch experiment showed that this is not the case: juveniles raised in axenic medium, showing induction of the UPRmt, were not long lived when switched to monoxenic medium at adulthood. On the other hand, worms that spent their juvenile stages on monoxenic plates and were transferred to axenic medium at adulthood displayed the full ADR longevity phenotype. Therefore, juvenile UPRmt induction and life-span extension can be uncoupled in ADR-treated worms.
Although UPRmt peaks at L4-to-adult transition in ADR worms, it is still activated during adulthood as well and may support ADR longevity at that stage. This assumption was tested by assessing life span of ADR worms in which key players of the UPRmt, such as ubl-5, haf-1, hsp-6, and atfs-1 (30,37) were knocked down or mutated. Worms unable to induce the UPRmt were still long lived under ADR conditions, showing that the ADR longevity phenotype does not rely on UPRmt at any life stage. As homozygous mutants of ubl-5, haf-1, and hsp-6 are not viable, the role of these UPRmt genes had to be assessed by RNAi knockdown. As there is currently no efficient sterile RNAi soaking procedure available, genes were knocked down by bacterial feeding during the first 5 days of adulthood, with the intention to knock down the UPRmt, after which worms were switched back to axenic medium with antibiotics. However, it must be noted that bacterial feeding by itself is sufficient to inhibit UPRmt (Figure 4B) and thus may act redundantly to genetic UPRmt knockdown. Nevertheless, this leaves the conclusion unaltered: UPRmt shutdown does not inhibit ADR longevity.
In testing the homozygous atfs-1 mutants, there were no bacterial feeding issues. The knockout allele tm4525, deleting almost the entire exons 2 to 4 and causing lack of UPRmt induction under spg-7 knockdown (38), did not influence ADR longevity. Also, two atfs-1 gain-of-function alleles failed to extend ADR life span. These data corroborate the finding that the UPRmt-related transcription factor ATFS-1 is not required for life-span extension in C elegans (15,30).
Our observations also raise the question whether axenic culture is stressing for larvae yet harmless for adult worms. It is commonly observed that worm development under ADR is slow (about 6 or more days compared to 3 days in monoxenic cultures), which may be result of dietary challenges experienced by the larvae. However, development in axenic medium can be restored to about 3–5 days by adding skimmed milk to the cultures, a treatment that does not quench the UPRmt response nor does it abolish life-span extension (data not shown). Hence, UPRmt activation is not related to slow development but rather to other physiological adaptations under ADR. It remains to be discovered whether the absence or relative overabundance of specific compounds in the axenic medium leads to UPRmt induction or increased life span. Likely, the absence of a putative nondiffusable heat-labile E coli factor is responsible for ADR longevity (39).
Next, we investigated whether mitohormesis is involved in ADR longevity, referring to the concept that an adaptive and protective antioxidant response is activated in mitochondria by moderate levels of ROS (33). This protective response may increase life span and can operate independently of the UPRmt (21). By treating worms with the ROS scavengers NAC or BHA, the mitohormesis effect can be blocked efficiently (18,22). We found that antioxidant treatment did not consistently abrogate ADR longevity, suggesting the absence of a clear mitohormetic effect. This is further supported by the fact that DAF-16 and SKN-1, two transcription factors that act in the mitohormesis pathway are not involved in ADR longevity, as was shown in our earlier studies (3,6). Furthermore, PRDX-2, known to mediate the beneficial effect of metformin treatment by activating mitohormesis (21), does not play a role in ADR longevity. Other genes involved in mitohormesis, such as pmk-1, contribute to the longevity of C elegans through their essential role in innate immunity against bacterial infection in the intestine (19). However, in axenic culture conditions, bacterial infections at late life stage are excluded because of sterility of the medium.
Interestingly, we found that 10 mM of NAC is beneficial to worms under monoxenic conditions (17% life-span increase), an effect that was not observed before (40), whereas this treatment is slightly toxic under axenic conditions. Dietary supplements of antioxidants were previously reported to have positive effects on longevity, whereas other studies reported controversial results (32). Catalase and superoxide dismutase activities are strongly upregulated in axenically cultured worms (3). Adding high levels of antioxidants to such conditions may cause reductive stress and mild life-span shortening.
Conclusion
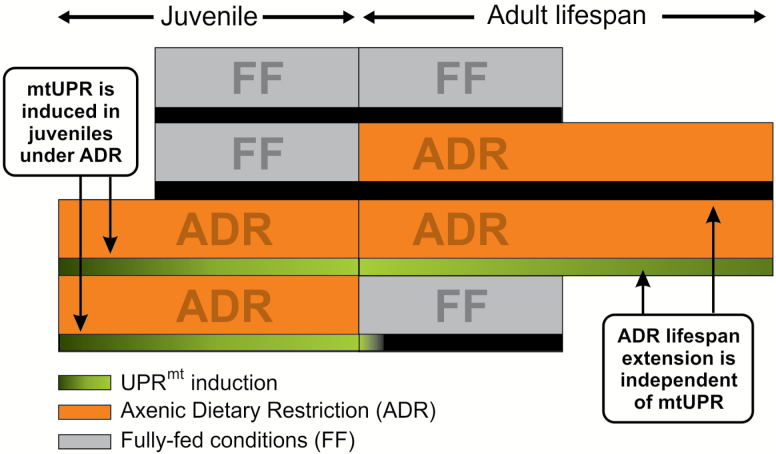
We provide solid evidence that, under ADR, the UPRmt is activated in C elegans larvae but this induction is not responsible for the extreme life-span extension (Figure 6). We further show that ROS-mediated mitohormesis is not required for this longevity phenotype, either. Despite clear improvements in mitochondrial function and changes in mitochondrial morphology in axenically cultured worms (7,8), UPRmt and mitohormesis do not seem to be involved in its life span–doubling effect. This leaves the dietary treatment with the largest life-span effect still the least characterized.
Figure 6.
The relation between UPRmt activation and dietary regimen in juvenile and adult stages of Caenorhabditis elegans. ADR = axenic dietary restriction; FF = fully fed.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the China Scholarship Council (grant 201207650008 to H.C.) and Special Reserach Fund of Ghent University (BOF cofunding 01SC0313 to H.C.). The Caenorhabditis Genetics Center (CGC) is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440).
Conflict of Interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors thank Dr. Matt Kaeberlein for sharing the atfs-1 mutants and the Caenorhabditis Genetics Center (CGC) for providing the other strains used in this study. The authors also thank Myriam Claes for assisting with TEM imaging and Qing Xue for the help in graph editing. The authors thank other members of the Braeckman laboratory for helpful advice and discussion and the reviewers for insightful comments. Author Contributions: H.C. and B.P.B. designed the experiments. H.C. carried out the majority of the experiments. M.R., L.V., and L.M. helped in some survival assays. C.V. and I.D. helped in fluorescence microscopy. H.C., I.D., and B.P.B. analyzed, interpreted the data, and wrote the manuscript.
References
- 1. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi:10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi:10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houthoofd K, Braeckman BP, Lenaerts I, et al. Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp Gerontol. 2002;37:1371–1378. doi:10.1016/S0531-5565(02)00173-0. [DOI] [PubMed] [Google Scholar]
- 4. Szewczyk NJ, Udranszky IA, Kozak E, et al. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J Exp Biol. 2006;209(Pt 20):4129–4139. doi:10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- 5. Vanfleteren JR. Large scale cultivation of a free-living nematode (Caenorhabditis elegans). Experientia. 1976;32:1087–1088. doi:10.1007/BF01933985. [DOI] [PubMed] [Google Scholar]
- 6. Castelein N, Cai H, Rasulova M, Braeckman BP. Lifespan regulation under axenic dietary restriction: a close look at the usual suspects. Exp Gerontol. 2014;58:96–103. doi:10.1016/j.exger.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 7. Castelein N, Hoogewijs D, De Vreese A, Braeckman BP, Vanfleteren JR. Dietary restriction by growth in axenic medium induces discrete changes in the transcriptional output of genes involved in energy metabolism in Caenorhabditis elegans. Biotechnol J. 2008;3:803–812. doi:10.1002/biot.200800003. [DOI] [PubMed] [Google Scholar]
- 8. Castelein N, Muschol M, Dhondt I, et al. Mitochondrial efficiency is increased in axenically cultured Caenorhabditis elegans. Exp Gerontol. 2014;56:26–36. doi:10.1016/j.exger.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 9. Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123(Pt 22):3849–3855. doi:10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 10. Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics. 2006;174:229–239. doi:10.1534/genetics.106.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117(Pt 18):4055–4066. doi:10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- 12. Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi:10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houtkooper RH, Mouchiroud L, Ryu D, et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi:10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett CF, Kaeberlein M. The mitochondrial unfolded protein response and increased longevity: cause, consequence, or correlation? Exp Gerontol. 2014;56:142–146. doi:10.1016/j.exger.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett CF, Vander Wende H, Simko M, et al. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat Commun. 2014;5:3483. doi:10.1038/ncomms4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20:214–225. doi:10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barja G. Updating the mitochondrial free radical theory of aging: an integrated view, key aspects, and confounding concepts. Antioxid Redox Signal. 2013;19:1420–1445. doi:10.1089/ars.2012.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi:10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 19. Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi:10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dingley S, Polyak E, Lightfoot R, et al. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion. 2010;10:125–136. doi:10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Haes W, Frooninckx L, Van Assche R, et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci USA. 2014;111:E2501–E2509. doi:10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zarse K, Schmeisser S, Groth M, et al. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi:10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang JS, Nam HJ, Seo M, et al. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One. 2011;6:e23525. doi:10.1371/journal.pone.0023525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schleit J, Johnson SC, Bennett CF, et al. Molecular mechanisms underlying genotype-dependent responses to dietary restriction. Aging Cell. 2013;12:1050–1061. doi:10.1111/acel.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pimenta de Castro I, Costa AC, Lam D, et al. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 2012;19:1308–1316. doi:10.1038/cdd.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dillin A, Hsu AL, Arantes-Oliveira N, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi:10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 28. Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi:10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsang WY, Lemire BD. Mitochondrial genome content is regulated during nematode development. Biochem Biophys Res Commun. 2002;291:8–16. doi:10.1006/bbrc.2002.6394. [DOI] [PubMed] [Google Scholar]
- 30. Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science. 2012;337:587–590. doi:10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rauthan M, Ranji P, Aguilera Pradenas N, Pitot C, Pilon M. The mitochondrial unfolded protein response activator ATFS-1 protects cells from inhibition of the mevalonate pathway. Proc Natl Acad Sci USA. 2013;110:5981–5986. doi:10.1073/pnas.1218778110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Back P, Matthijssens F, Vanfleteren JR, Braeckman BP. A simplified hydroethidine method for fast and accurate detection of superoxide production in isolated mitochondria. Anal Biochem. 2012;423:147–151. doi:10.1016/j.ab.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33. Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45:410–418. doi:10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 34. Lu NC, Goetsch KM. Carbohydrate requirement of Caenorhabditis elegans and the final development of a chemically defined medium. Nematologica. 1993;1993:303–331. doi:10.1163/187529293X00259. [Google Scholar]
- 35. Merkwirth C, Jovaisaite V, Durieux J, et al. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell. 2016;165:1209–1223. doi:10.1016/j.cell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tian Y, Garcia G, Bian Q, et al. Mitochondrial stress induces chromatin reorganization to promote longevity and UPR(mt). Cell. 2016;165:1197–1208. doi:10.1016/j.cell.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell. 2010;37:529–540. doi:10.1016/j.molcel.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baker BM, Nargund AM, Sun T, Haynes CM. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 2012;8:e1002760. doi:10.1371/journal.pgen.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lenaerts I, Walker GA, Van Hoorebeke L, Gems D, Vanfleteren JR. Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. J Gerontol A Biol Sci Med Sci. 2008;63:242–252. doi:10.1093/gerona/63.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi:10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.